Abstract
Objective
Interleukin 1 Receptor 1 (IL1R1) and its ligand, IL1β, are upregulated in cardiovascular disease, obesity, and infection. Previously, we reported a higher level of IL1R1 transcripts in platelets from obese individuals of the Framingham Heart Study (FHS), but its functional effect in platelets has never been described. Additionally, IL1β levels are increased in atherosclerotic plaques and in bacterial infections. The aim of this work is to determine whether IL1β, through IL1R1, can activate platelets and megakaryocytes to promote atherothrombosis.
Approach and Results
We found that IL1β-related genes from platelets, as measured in 1819 FHS participants, were associated with increased body mass index, and a direct relationship was shown in wild-type mice fed a high-fat diet. Mechanistically, IL1β activated nuclear factor-κB and mitogen-activated protein kinase signaling pathways in megakaryocytes. IL1β, through IL1R1, increased ploidy of megakaryocytes to 64+ N by 2-fold over control. IL1β increased agonist-induced platelet aggregation by 1.2-fold with thrombin and 4.2-fold with collagen. IL1β increased adhesion to both collagen and fibrinogen, and heterotypic aggregation by 1.9-fold over resting. High fat diet-enhanced platelet adhesion was absent in IL1R1−/− mice. Wild-type mice infected with Porphyromonas gingivalis had circulating heterotypic aggregates (1.5-fold more than control at 24 hours and 6.2-fold more at 6 weeks) that were absent in infected IL1R1−/− and IL1β−/− mice.
Conclusions
In summary, IL1R1- and IL1β-related transcripts are elevated in the setting of obesity. IL1R1/IL1β augment both megakaryocyte and platelet functions, thereby promoting a prothrombotic environment during infection and obesity; potentially contributing to the development of atherothrombotic disease.
Keywords: blood platelets, diet, high-fat, IL1R1 protein, human, infection, megakaryocytes
A proinflammatory environment attributable to either an acute bacterial infection or a chronic disease, such as obesity, has effects on multiple cell types and organs beyond the immune system and infected tissue. Obesity, considered a chronic inflammatory state, is associated with an increase in circulating proinflammatory cytokines, including interleukin 1β (IL1β).1,2 Some bacterial infections, including Porphyromonas gingivalis (P gingivalis), are also associated with an increase in circulating IL1β.3,4 Finally, increased levels of this cytokine have also been found in atherosclerotic plaques.5 Strategies to reduce the expression or activity of IL1β or its receptor, interleukin 1 receptor 1 (IL1R1), have been shown to reduce plaque size in murine aortas.6–8 An ongoing phase III clinical trial, Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS; NCT01327846), uses an antibody against IL1β, previously used in autoinflammatory diseases, to reduce recurrent cardiovascular events in individuals with a recent myocardial infarction and elevated C-reactive protein (CRP).9 In the phase IIb trial, there was reduction in inflammation, as measured by IL6 and CRP, with no effect on lipids, in individuals given anti-IL1β antibody treatment.10 Interestingly, fibrinogen levels also decreased compared with placebo.10 New data from animal studies using IL1R1−/− apolipoprotein E−/− (ApoE−/−) mice show that although there was decreased plaques in certain regions, plaque stability and vessel remodeling were reduced in areas where hemorrhage was noted in the IL1R1−/− ApoE−/− mice.11 The vessel wall instability was associated in part with matrix metalloproteinase 3 (MMP3) expression.11 However, the presence of hemorrhage suggests that there could also be an alteration in hemostasis, such as a decrease in platelet function.
IL1R1 is expressed predominately on endothelial and immune cells.12 Its ligand, IL1β, signals through myeloid differentiation primary response gene 88 (MYD88) to activate the nuclear factor (NF)κB pathway, increasing the expression of other immune-related cytokines, growth factors, and adhesion molecules.12 Megakaryocytes were shown to respond to IL1β increasing platelet production,13–16 through thrombopoietin17 and transcription factors, including globin transcription factor-117 and NF-E2.17,18 In addition, megakaryocytes were shown to secrete IL1β, which could act on the bone marrow to increase megakaryopoiesis.19 Mice injected with IL1β, although showing an increase in the colony-forming units of the megakaryocytes,18,20 had reduced numbers of circulating platelets, which could be avoided by splenectomy before treatment.20
Unlike megakaryocytes, platelets have only been shown to release IL1β. Previously, activated platelets were shown to express active IL1β on their cell surface, as measured by proliferation of T cells that require IL1 to respond to growth factors.21 More recently, platelet-derived IL1β was shown to act on endothelial cells22,23 and vascular smooth muscle cells24 to stimulate inflammatory processes and promote adhesion.23 This platelet-derived IL1β was generated from pre-mRNA sequences in the platelet that were further processed and translated into protein upon activation.25,26
Specifically, how IL1β signals in the platelet and megakaryocyte is unknown. We hypothesized that IL1β and IL1R1 would regulate megakaryocyte maturation and platelet function. In the current study, we demonstrate that through known signaling pathways, megakaryocyte maturation and RNA production are altered by IL1β and IL1R1. Platelet function is enhanced through p38 mitogen activated protein kinase (MAPK) signaling pathway on IL1β stimulation. In particular, IL1β and IL1R1 increase platelet adhesion and heterotypic aggregate formation both in the setting of inflammation, high fat diet, and bacterial infection, that are reversed in IL1R1−/− and IL1β−/− mouse models. Therefore, IL1β, through IL1R1, promotes proinflammatory functions in both megakaryocytes and platelets that may contribute to the development of atherothrombotic diseases and is of immediate clinical relevance because this pathway has been targeted in a large ongoing clinical trial.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
IL1R1 Expression and Function on Megakaryocytes
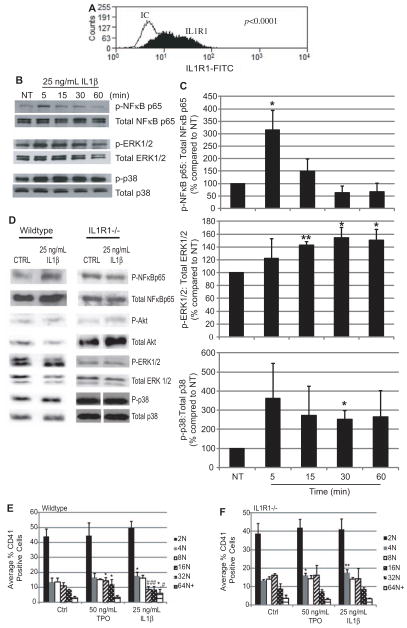
The presence of IL1R1 on the cell surface of megakaryocytes was determined with intact Meg-01 cells using flow cytometry. As seen in Figure 1A, this human megakaryocytic cell line expressed IL1R1 on its surface. To determine whether this receptor is functional, Meg-01 cells were treated with 25 ng/ mL IL1β for up to 1 hour. Activation of downstream signaling pathways of IL1R1 was measured through Western blotting for increased phosphorylation of each kinase. As seen in Figure 1B and 1C, there was a significant increase in NFκB p65 subunit phosphorylation after 5 minutes of IL1β treatment. MAPK pathways that are also activated downstream of IL1R1-tumor necrosis factor receptor–associated factor 6 (TRAF6), including extracellular signal-related kinase (ERK) and p38, showed significantly increased phosphorylation. ERK1/2 phosphorylation was increased significantly from 15 to 60 minutes of IL1β treatment. Phosphorylation of p38 was increased significantly at 30 minutes of IL1β treatment. Phosphorylation of Akt was increased, but not significantly (data not shown). The activation of these signaling pathways was confirmed to be through IL1R1 using wild-type (WT) and IL1R1−/− mice (Figure 1D). NFκB p65 and Akt phosphorylation was increased to 126% (± 28.7) and 213.9% (± 79.0), respectively, at 5 minutes in WT megakaryocytes. Both ERK and p38 phosphorylation were increased at 30 minutes to 210.1% (± 111.0) and 156.9% (± 54.1), respectively. There was no increase in NFκBp65, Akt, and p38 phosphorylation in the IL1R1−/− megakaryocytes. However, there was an increase in ERK phosporylation in the IL1R1−/− megakaryocytes that may be, in part, because of IL1β binding to IL1R2.18 ERK signaling is, therefore, not completely specific to IL1R1, but is for IL1β, because the increase in phosphorylation occurs compared with control, which contains buffer. These data suggest IL1R1 is present on megakaryocytes, and it can be activated by its ligand, IL1β, to signal through NFκB, Akt, and MAPK pathways.
Figure 1.
Expression of interleukin (IL)1R1 and function on megakaryocytes. Representative histogram of Meg-01 cells (A) stained with anti-human IL1R1 fluorescein isothiocyanate (FITC); n=5; IC indicates isotype control. B, Representative Western blots probed for phospho- and total NFκB p65, phospho- and total ERK, and phospho- and total p38 in Meg-01 cells. C, Quantification of the increases in phosphorylation of NFκB p65, ERK, and p38 after treatment with IL1β. n=4; *P<0.05, **P<0.01 compared with NT. D, Representative Western blots probed for phospho- and total NFκB p65, phospho- and total Akt, phospho-and total ERK, and phospho- and total p38 in isolated wild-type (WT) and IL1R1−/− mouse megakaryocytes; n=4. Isolated WT (E) or IL1R1−/− (F) mouse bone marrow cells were treated with vehicle (Control – Ctrl), thrombopoietin (TPO), or IL1β for 3 days. Megakaryocyte DNA content (2 N-64+N) was measured. n=18; *P<0.05, **P<0.01 compared with Control, #P<0.05, ##P<0.01 compared with TPO.
IL1R1 is structurally similar to toll-like receptor 2 (TLR2). As previously reported, TLR2 binding and activation induced megakaryocyte maturation, as shown with an increase in adhesion and ploidy.27 Treatment with 25 ng/mL IL1β significantly increased Meg-01 adhesion to fibrinogen by 286.7% ± 85.8 (Figure IA in the online-only Data Supplement) and with fibronectin by 236.3% ± 24.9 compared with No Treatment (Figure IB and IC in the online-only Data Supplement). IL1β (25 ng/mL) treatment ex vivo of mouse bone marrow for 3 days significantly increased ploidy in WT mouse megakaryocytes, particularly at the 4 N and 64+N population compared with control treatment (Figure 1E). Treatment with 50 ng/mL thrombopoietin (TPO), a regulator of megakaryocyte maturation, significantly increased 16 N and 32 N. IL1β treatment significantly reduced 16 N and 32 N compared with TPO treatment because of the movement of megakaryocytes into the more mature, higher ploidy 64+N that did not occur with TPO. This effect on megakaryocyte maturation was specific to IL1R1 because treatment of IL1R1−/− bone marrow ex vivo with IL1β did not yield the same results (Figure 1F). These data suggest that IL1R1 and IL1β increase megakaryocyte maturation to cells with a higher ploidy. The IL1R1−/− bone marrow cells did not respond like WT to TPO treatment, as previously seen with TLR2−/− megakaryocytes.27 These data suggest that the presence of IL1R1 may be necessary for megakaryocyte maturation. To determine whether this maturation leads to altered platelet numbers, platelet counts were performed in WT and IL1R1−/− mice. In Table I in the online-only Data Supplement, there were more platelets in the IL1R1−/− mice compared with WT at baseline. However, after challenge with an oral pathogen, the WT mice had an increase in circulating platelets that did not occur in IL1R1−/− mice. Therefore, although at baseline, the lack of IL1R1 does not affect platelet production, when challenged, platelet production is delayed.
IL1β Affects Megakaryocyte Inflammatory and Thrombotic Gene Expression
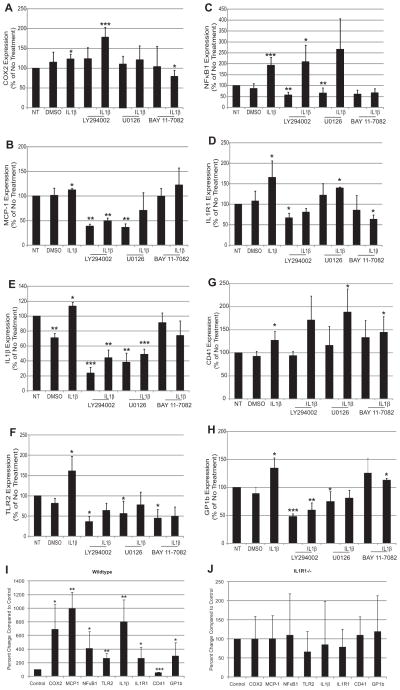
During megakaryocyte maturation, RNA production is increased for the production of platelets. Here, we tested whether IL1β/IL1R1-induced maturation could also affect gene expression. Several inflammatory and thrombotic genes, selected based on previous findings,27 were measured in Meg-01 cells treated with IL1β for 3 hours. In Figure 2A through 2H, IL1β treatment significantly increased both inflammatory and thrombotic gene expression in the megakaryocytic cells. Two downstream targets of NFκB are cyclooxygenase 2 (COX2) and monocyte chemotactic protein-1 (MCP-1). Both genes were upregulated with IL1β treatment to 123.1%±12.4 and 112.3%±3.5, respectively, compared with No Treatment (Figure 2A and 2B). Pretreatment with 50 μM LY294002, an inhibitor of phosphoinisitide 3-kinase (PI3K)/Akt pathway, reduced the effects of IL1β on MCP-1 gene expression (Figure 2B). U0126 (50 μmol/L), an MEK1/2 inhibitor, and BAY 11–7082 (50 μmol/L), an NFκB inhibitor, reduced the effects of IL1β on both COX2 and MCP-1 expression (Figure 2A and 2B). NFκB1 (p105/p50) was also upregulated by IL1β in the Meg-01 cells to 193.3%±35.9 compared with No Treatment (Figure 2C). Unlike COX2 and MCP-1, NFκB1 expression was upregulated by IL1β only through the NFκB pathway (Figure 2C). TLR2, a structurally similar receptor to IL1R1, was also upregulated by IL1β to 161.3%±36.0 compared with No Treatment, and its upregulation was signaled through PI3K, ERK, and NFκB pathways (Figure 2F). Finally, both IL1R1 and IL1β expressions were upregulated by IL1β treatment to 113.9%±5.6 and 165.7%±40.5 compared with No Treatment, respectively (Figure 2D and 2E). IL1β upregulated itself through NFκB, PI3K, and ERK pathways (Figure 2E). IL1R1 was upregulated by NFκB and PI3K pathways (Figure 2D).
Figure 2.
Interleukin (IL)1β alters megakaryocyte inflammatory- and thrombosis-related gene expression through NFκB, ERK, and PI3K/ Akt pathways. Gene expression of Meg-01 cells pretreated with 50 μmol/L LY294002, 50 μmol/L U0126, 50 μmol/L BAY 11–7082, or dimethyl sulfoxide (DMSO), then treated with IL1β. COX2 (A; n=8), monocyte chemotactic protein-1 (MCP-1; B; n=5), NFκB1 (C; n=8), IL1R1 (D; n=3), IL1β (E; n=4), TLR2 (F; n=7), CD41 (G; n=10), and GP1b (H; n=7). *P<0.05, **P<0.01, ***P<0.001 compared with NT (No Treatment). Gene expression in isolated wild-type (WT; I) and IL1R1−/− (J) mouse megakaryocytes treated with IL1β. n=3 for WT; n=3 for IL1R1−/−. *P<0.05, **P<0.01, ***P<0.001 compared with NT.
Two thrombotic genes were significantly upregulated by IL1β in Meg-01 cells. CD41, also known as integrin αIIb, which binds to fibrinogen, was upregulated to 133.0%±16.4 compared with No Treatment (Figure 2G). This gene was upregulated by IL1β only through the PI3K pathway (Figure 2G). GP1b, part of the von Willebrand Factor receptor, was also upregulated by IL1β to 134.3%±18.7 compared with No Treatment (Figure 2H). GP1b was upregulated by IL1β through both the PI3K and ERK pathways (Figure 2H). Neither one of the thrombotic genes was upregulated by IL1β through the NFκB pathway.
Expression of all the genes studied was confirmed to be regulated through IL1R1 by using WT and IL1R1−/− mice. As seen in Figure 2I, there was a significant increase in COX2, MCP-1, NFκB1, IL1β, IL1R1, TLR2, and GP1b compared with control in the WT mice. All of these genes were not increased in the IL1R1−/− mice (Figure 2J, note different scales for 2I and 2J), suggesting that the receptor is important for the effects of IL1β on gene expression. Baseline levels of each gene are comparable between WT and IL1R1−/− mice (Table II in the online-only Data Supplement), suggesting the loss of IL1R1 did not affect the baseline levels of the gene but the increase in gene expression. The only gene that was not consistent with the Meg-01 data was CD41, which could be related to differential effects seen between species.28
IL1R1 Expression and Function on Platelets
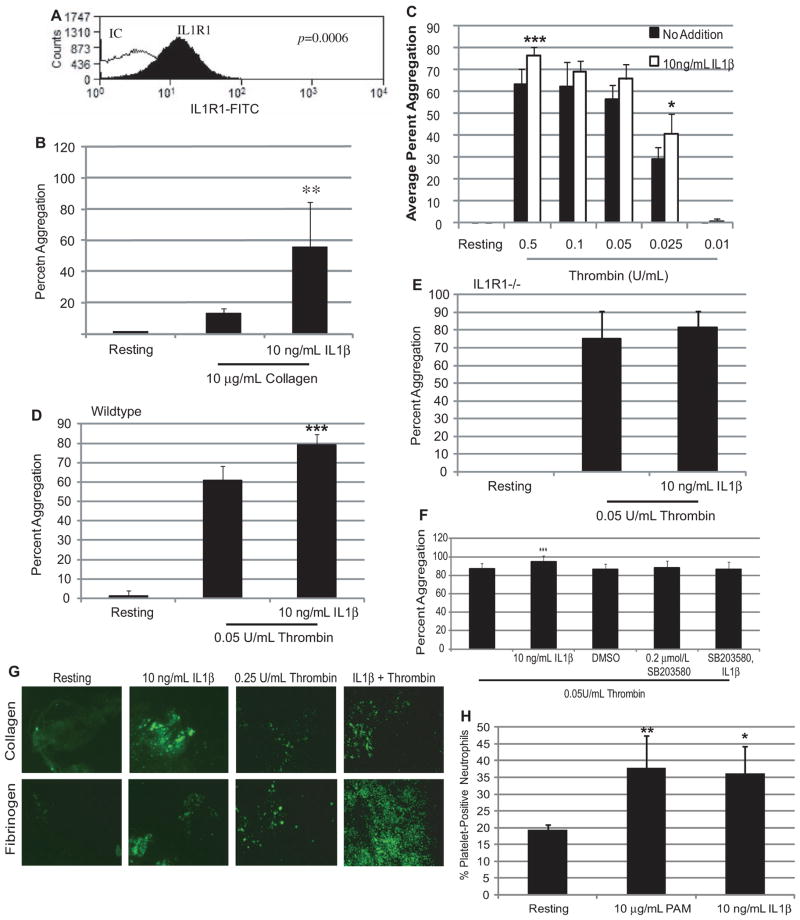
As shown in Figure 3A, human platelets express IL1R1 on their surface, as determined by flow cytometry. Treatment of human platelets with various concentrations of IL1β did not cause washed platelet aggregation (data not shown), unlike what was shown with TLR2.29 IL1β did enhance agonist- induced platelet aggregation. As shown in Figure 3B, pretreatment of IL1β increased the platelet response to collagen by ≈42% over collagen treatment alone. In the presence of increasing concentrations of thrombin, IL1β pretreatment also enhanced the response of the platelets by increasing aggregation on average 10.3% (13.3%–6.8%; Figure 3C). These data suggest that IL1β primes the platelets by activating signaling pathways to enhance their response to agonists.
Figure 3.
Interleukin (IL)1β affects platelet function. A, Representative histogram of isolated human platelets stained with anti-human IL1R1 FITC antibodies; n=5; IC indicates isotype control. B, Isolated human platelets aggregation was measured for 10 minutes in the presence of collagen. n=4; **P<0.01 compared with collagen alone. C, Isolated human platelet aggregation was measured for 10 minutes in the presence of increasing concentrations of thrombin. n=10; ***P<0.001, *P<0.05 compared with thrombin alone. Isolated WT (D; n=4) and IL1R1−/− (E; n=3) mouse platelet aggregation was measured in the presence of thrombin. ***P<0.001 compared with thrombin alone. F, Isolated human platelet aggregation was measured in the presence of thrombin for 10 minutes. n=15; ***P<0.001 compared with thrombin alone. G, Representative isolated human platelets treated with IL1β or thrombin, or IL1β, then thrombin adherent on collagen or fibrinogen; n=3. H, The percentage of platelet-positive neutrophils in the presence of Pam3CSK4 (PAM) or IL1β. n=6; *P<0.05, **P<0.01 compared with Resting.
Washed platelets from WT mice had an enhanced response to a low dose of thrombin (0.05 U/mL) by 18.5% when pretreated with 10 ng/mL IL1β as compared with thrombin alone (Figure 3D). This response was specific to IL1R1 because platelets from mice deficient in IL1R1 did not respond to IL1β pretreatment and had no enhanced response to thrombin (Figure 3E).
Megakaryocytes treated with IL1β resulted in the activation of multiple signaling pathways, including p38 MAPK. Platelets treated with IL1β leads to the phosphorylation of p38 (Figure IIA and IIB in the online-only Data Supplement). Pretreatment of platelets with IL1β followed by treatment with either thrombin or collagen also leads to enhanced phosphorylation of p38 (Figure IIA and IIB in the online-only Data Supplement). To understand whether this pathway was involved in IL1β enhancement of aggregation, human platelets were pretreated with 0.2 μmol/L SB203580, a selective p38 MAPK inhibitor, before treatment with IL1β and thrombin. As seen in Figure 3F, thrombin-stimulated aggregation was modestly enhanced with IL1β pretreatment by 8.1% over thrombin alone. In the presence of SB203580, IL1β was unable to enhance the platelet response to thrombin because aggregation returned to baseline level (86.4%±8.2 versus 86.9%±5.7, thrombin alone). Therefore, IL1β, through the activation of the p38 MAPK pathway, enhanced the response of platelets to thrombin.
Washed human platelets were labeled with calcein-AM for visualization purposes, and circulated over either collagen or fibrinogen. Unlike washed platelet aggregation, IL1β alone was able to induce adhesion to either substrate (Figure 3G). In addition, IL1β pretreatment enhanced thrombin-induced adhesion to either substrate, as seen in representative pictures in Figure 3G. These results suggest that IL1β directly activates and enhances platelet adhesion to various substrates.
A marker of platelet inflammatory response, heterotypic aggregate (platelet-positive neutrophils) formation was also tested using human whole blood. Treatment with IL1β (10 ng/mL) significantly induced the formation of heterotypic aggregates, as determined by flow cytometry (Figure 3H). This result was similar to what was shown with TLR2 and Pam3CysSerLys4 (Pam3CSK4),29 which was used as a positive control. These findings suggest that IL1β, through IL1R1, promotes both thrombotic and inflammatory functions in platelets.
IL1R1 Mediates Platelet Function In Vivo
Various disease processes induce the production of IL1β including obesity and infection. We have previously shown enhanced IL1R1 platelet expression associated with increased body mass index (BMI) in the Framingham Heart Study (FHS).30 Obesity has also been associated with inflammation and an increase in circulating IL1β levels,1 which could interact with platelets and affect their function. To test this hypothesis, WT and IL1R1−/− mice were placed on a high fat diet for 10 weeks. Both mouse models on the high fat diet gained weight compared with those on normal chow (Figure III in the online-only Data Supplement). WT mice gained 5.4 g on the high fat diet, whereas IL1R1−/− mice gained 8.7 g.
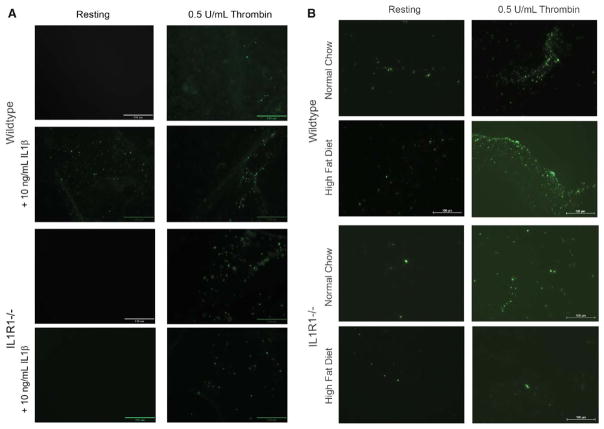
Platelets were isolated from these mice, and adhesion to collagen was tested. At baseline, platelets from WT mice adhere to collagen in the presence of 0.5 U/mL thrombin and 10 ng/mL IL1β (Figure 4A). Adhesion to collagen is enhanced in the presence of IL1β and thrombin, compared with thrombin alone. In platelets from IL1R1−/− mice, adhesion to collagen in the presence of thrombin or IL1β and thrombin is the same. IL1R1−/− platelets do not respond to IL1β. After being on a high fat or normal chow diet for 10 weeks, there was an increase in WT platelet adhesion in the presence of thrombin with a high fat diet compared with normal chow (Figure 4B), similar to baseline adhesion with IL1β and thrombin. There were fewer IL1R1−/− platelets adhering at baseline compared with WT. With a high fat diet, unlike the WT mice, there was no increase in adhesion in the IL1R1−/− mice, suggesting that the effects of a high fat diet on platelet adhesion are partially mediated through IL1R1. When this receptor is not expressed on the platelets, the prothrombotic effects of the diet were diminished.
Figure 4.
Interleukin (IL)1β affects adhesion in the setting of high fat diet. A, Representative photographs of isolated wild-type (WT) and IL1R1−/− platelets treated with IL1β, thrombin, or both and adherent on collagen at baseline; n=5 to 6 mice in each group. B, Representative photographs of isolated WT and IL1R1−/− platelets treated with thrombin and adherent to collagen; n=3 to 4 mice in each group.
To determine the role of infection on IL1R1 and platelets, WT, IL1R1−/−, and IL1β−/− mice were challenged orally with the periodontal pathogen P gingivalis. This bacterium has been previously shown to affect platelets29 and circulating IL1β levels.3,4 One day after the last oral challenge, there was a loss of the proform of IL1β into the active form in WT infected mice, but not in either the IL1R1−/− or IL1β−/− mice (Figure IVA and IVB in the online-only Data Supplement). A trend toward an increase in platelet counts with an acute infection in the WT mice (Table I in the online-only Data Supplement) was not seen in either knockout mouse model. WT mice (control) had a significant increase in platelet-positive neutrophils present in circulation (Figure IVC in the online-only Data Supplement) with an acute exposure to P gingivalis, which did not occur in the IL1R1−/− or IL1β−/− mice. Six weeks after the last P gingivalis challenge, there is again a loss of the proform of IL1β in the WT and IL1R1−/− infected mice, which does not occur in the IL1β−/− mice (Figure IVA and IVB in the online-only Data Supplement). Platelet counts increased slightly in the WT mice and to a lesser extent in the IL1β−/− mice, but there was a drop in platelets in the IL1R1−/− mice (Table I in the online-only Data Supplement). WT mice still had several circulating platelet-neutrophil aggregates (77.3%±6.7 versus 12.8%±2.2, Control; Figure IVD in the online-only Data Supplement). Again, both IL1R1−/− and IL1β−/− mice had no increase in circulating heterotypic aggregates compared with control. The least responsive of the mouse models was the IL1β−/− mice; however, ex vivo examination showed that platelets from these mice responded normally to both thrombin and IL1β in adhesion (Figure IVE and IVF in the online-only Data Supplement) and heterotypic aggregate (Figure IVG in the online-only Data Supplement) assays. Therefore, IL1R1 and the platelets were both responsive in the IL1β−/− mice, and the results seen in the infection model were attributable to the lack of IL1β production.
Inflammatory Genes Upregulated in Platelets from Mice on a High Fat Diet
Based on previously published results from the FHS,30 platelet RNA from mice fed a high fat or normal chow diet was tested for inflammatory and thrombotic transcripts. At 8 weeks, 19 genes had altered expression with a high fat diet (Table 1). Intercellular adhesion molecule 1 (ICAM1), phospholipase A2 group VII (PLA2G7), and toll-like receptor 1 (TLR1) were highly upregulated in high fat diet fed mice compared with normal chow. Nine of the 19 genes upregulated with a high fat diet in mice were also increased in obese and overweight individuals of the FHS.30 Two genes not upregulated in mice but increased in the FHS included interleukin 6 (IL6) and prostaglandin H2 synthase (PTGS2). Genes downregulated in mice fed a high fat diet included heparin-binding epidermal growth factor-like growth factor and tissue inhibitor of metalloproteinase 1 (TIMP1). These data suggest that at 8 weeks, the megakaryocytes are responding to the continued exposure to high fat and inflammation and increasing inflammatory and thrombotic transcripts.
Table 1.
Inflammatory and Thrombotic Genes in Murine Platelets Fed a High-Fat Diet Compared With Normal Chow
| Gene | Name | Function | Changes in Gene Expression at 8 Weeks
|
|
|---|---|---|---|---|
| ΔΔCT (SD) | Fold Change | |||
| CCL2 | Chemokine (C-C motif) ligand 2 (MCP-1) | Inflammatory chemokine | −0.90 (0.82) | 1.96 |
| CCR3 | Chemokine (C-C motif) receptor 3 | Receptor for various chemokines | 0.29 (1.95) | 1.39 |
| FCER1A | IgE Fc receptor subunit alpha | Inflammatory receptor involved in allergies | 0.32 (1.17) | 0.95 |
| FN1 | Fibronectin 1 | Extracellular matrix protein | −0.07 (1.49) | 1.68 |
| HBEGF | Heparin-binding EGF-like growth factor | Mitogen for fibroblasts, smooth muscle cells | 1.40 (0.90) | 0.42 |
| ICAM1 | Intercellular adhesion molecule 1 | Ligand for leukocyte adhesion | −0.39 (2.25) | 2.22 |
| IFNG | Interferon, gamma | Inflammatory cytokine | 0.59 (1.83) | 1.09 |
| IL1R1 | Interleukin 1 receptor 1 | Innate immune receptor | 1.07 (2.52) | 1.02 |
| IL6 | Interleukin 6 | Inflammatory cytokine | 3.68 (1.70) | 0.13 |
| MMP9 | Matrix metalloproteinase 9 | Breaks down type IV and V collagen | 0.76 (1.48) | 1.50 |
| MYD88 | Myeloid differentiation primary response gene (88) | TLR and ILR signaling mediator | 0.17 (1.72) | 1.16 |
| PLA2G7 | Phospholipase A2, group VII | Regulates platelet activating factor activity | −0.17 (1.60) | 2.16 |
| PTGS2 | Prostaglandin H2 synthase | Converts arachidonate to prostaglandin | 3.01 (1.40) | 0.62 |
| S100A9 | S100 calcium-binding protein A9 | Proinflammatory mediator of infections | 0.07 (1.83) | 1.75 |
| SELENBP1 | Selenium-binding protein 1 | – | −0.23 (1.79) | 1.78 |
| TIMP1 | Tissue inhibitor of metalloproteinase 1 | Inactivates various MMPs | 0.47 (1.70) | 0.91 |
| TIMP2 | Tissue inhibitor of metalloproteinase 2 | Inactivates various MMPs | 0.02 (1.07) | 1.30 |
| TLR1 | Toll-like receptor 1 | Innate immune receptor | −0.34 (1.71) | 2.59 |
| TLR2 | Toll-like receptor 2 | Innate immune receptor | 0.58 (2.74) | 1.21 |
Inflammatory- and thrombotic-related gene expression levels in platelets from mice fed a high-fat diet were compared with mice on normal chow at 8 wk (high-fat diet n=14; normal chow n=14). EGF indicates epidermal growth factor; Fc, fragment, crystallizable; IgE, immunoglobulin E; and MCP-1, monocyte chemotactic protein-1. SD=(standard deviation of target gene CT2 + standard deviation of actin [ACTB] CT2)^0.5).
Platelet RNA Transcripts From the FHS
IL1R1 expression in platelets was previously shown to be significantly higher in individuals with increased BMI in the FHS30 and in mice on a high fat diet (Table 1). To confirm the clinical relevance of our murine and in vitro findings, 6 additional inflammatory-related genes that are linked to IL1R1 and NFκB were examined. RNA from platelets isolated from participants of the FHS Offspring Cohort 8 was analyzed as previously described for white blood cell contamination.30 As reported, there was a <1/50 000 white blood cells in the platelet samples, and platelet gene expression varied from that of white blood cells.30 All data were normalized using 3 housekeeping genes, α-actin (ACTB), β2-microglobulin (B2M), and gylceraldehyde-3-phosphate dehydrogenase (GAPDH), which were found to be highly correlated.30 Clinical characteristics for the FHS participants are listed in Table 2. Of the 1819 participants, 51% were female, the average age was 67±9 years, and the average BMI was 28.3±5.3 kg/m2. The genes studied fell into 3 categories. Three genes were associated with IL1R1 and IL1β signaling, which included nucleotide-binding oligomerization domain, leucine rich repeat, and pyrin domain containing protein 3 (NLRP3),31 MYD88,32 and interleukin-1 receptor-associated kinase 1 (IRAK1).33 There were genes whose expression was both regulated by IL1β and found to be upregulated with IL1β in experimental models, which included interleukin 18 (IL18)34,35 and MMP9.4,36–38 Relative expression values (ΔCt) were evaluated in multivariable regression models including terms for age, sex, and additional clinical factors listed in Tables III and IV in the online-only Data Supplement. Three of these genes were nominally significantly associated with BMI-score (P<0.05; Tables III and IV in the online-only Data Supplement): IL1β regression β coefficient=0.029±0.015 (P=0.0490), MMP9 regression β coefficient=0.051±0.014 (P=0.0003), and MYD88 regression β coefficient=0.030±0.013 (P=0.0253). The association with MMP9 was the only one that survived correction for multiple comparisons. Of the other clinical covariates modeled, high-density lipoprotein level was associated with MMP9 expression, lipid treatment was associated with IL18, IL1β, and IRAK1 expression, and antihypertensive treatment was associated with IL1β, MYD88, and NLRP3 expression at P<0.05 (Table IV in the online-only Data Supplement); none of these additional associations survived correction for multiple corrections, however.
Table 2.
Characteristics of the Framingham Offspring Study Sample Participants
| Variables | Mean±SD/Number (%) |
|---|---|
| Sample size | 1819 |
| Female sex, n (%) | 993(51) |
| Age, y | 67±9 |
| BMI, kg/m2 | 28.3±5.3 |
| Lipid treatment, n (%) | 798(44) |
| Total cholesterol, mg/100 mL | 185±38 |
| HDL cholesterol, mg/100 mL | 57±19 |
| Triglyceride, mg/100 mL | 116±67 |
| Antihypertensive treatment, n (%) | 919(51) |
| Systolic blood pressure, mm Hg | 129±17 |
| Diastolic blood pressure, mm Hg | 73±10 |
| Glucose, mg/dL | 107±25 |
| Diabetes mellitus, n (%) | 255(14) |
| Prevalent coronary heart disease, n (%) | 199(11) |
| Aspirin (3 per week), n (%) | 823(45) |
| Current hormone replacement therapy, n (%) | 104(6) |
| Smoker, n (%) | 155(8.5) |
HDL indicates high-density lipoprotein.
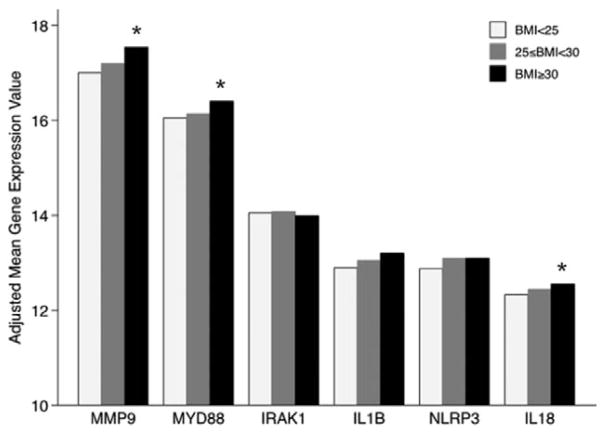
Gene expression values stratified by obesity status are presented in Figure 5. With the exception of IRAK1, all genes were upregulated in overweight or obese individuals in reference to normal weight individuals. The only associations statistically significant (P<0.05) were for MMP9, NLRP3, and MYD88 genes expression values contrasted between obese and normal weight individuals (results from full models are presented in Tables III and IV in the online-only Data Supplement).
Figure 5.
Platelet RNA expression in Framingham Heart Study (FHS) individuals associated with increased body mass index (BMI). The platelet RNA expression of IL1β-associated genes in FHS individuals stratified by BMI status adjusted by clinical covariates listed in Tables III and IV in the online-only Data Supplement. Relative expression values (ΔCt) corrected by the housekeeping genes (GAPDH, B2M, α-actin [ACTB]) were rescored so that larger values represent increased expression. MMP9, NLRP3, and myeloid differentiation primary response gene (MYD88) were all significantly increased in obese individuals (BMI≥30) compared with normal weight individuals (BMI<25); *P<0.05.
Further testing was run on each platelet gene with a significant association with BMI and inflammatory biomarkers measured from plasma from the same visit (Offspring, eighth visit). In Table V in the online-only Data Supplement, associations between each biomarker, CRP, ICAM1, IL6, MCP-1, osteoprotegerin, P-Selectin, and TNFR, and each gene are listed. Each gene associated with 2 or more measured biomarkers. IL18 and MMP9 both had associations with biomarkers that were significant after corrections for multiple comparisons. Platelet MMP9 gene expression associated with the most inflammatory biomarkers. To determine whether these biomarkers accounted for the MMP9–BMI association, additional analysis was performed adjusting for multiple corrections and various biomarkers (Table VI in the online-only Data Supplement). BMI was still significantly associated with MMP9 expression for all biomarkers, except CRP and IL6. These results further support that megakaryocytes respond to the increases in BMI and produce platelets with increased inflammatory transcripts, specifically related to IL1R1/IL1β signaling.
Discussion
Increasing BMI has been shown to correlate with significant increases in inflammatory and immunity-related genes in platelets.30 One transcript, IL1R1, although found to be significantly associated with obese and overweight individuals, had never been identified on platelets. Other IL1R1 and IL1β-related platelet transcripts were studied in the FHS and were shown to be increased with an increase in BMI (Figure 6A). These findings are consistent with previous observations that IL1β is increased in obese patients.1 These changes, although small, are significant and are consistent with data previously published on alterations in platelet transcripts in FHS.30,39 Mouse studies confirmed what has been shown in the FHS studies. TLR1, TLR2, and IL1R1 mRNA levels increased by 8 weeks in mice on a high-fat diet. ICAM1, PLA2G7, and Chemokine (C-C Motif) Ligand 2 all shown to be upregulated with an increase BMI in FHS, were also increased at 8 weeks in mice fed a high fat diet. MMP9 and MYD88, both shown in this study to be upregulated in the FHS, were also upregulated in mice on a high-fat diet. However, PTGS2 and IL6, which were both increased in the FHS, did not increase in the mice. The discrepancy between the mouse data and FHS may be because of the timing. It is possible that at 8 weeks, the mouse data do not precisely reflect what is occurring in humans, who have been exposed to a high fat diet for much longer.
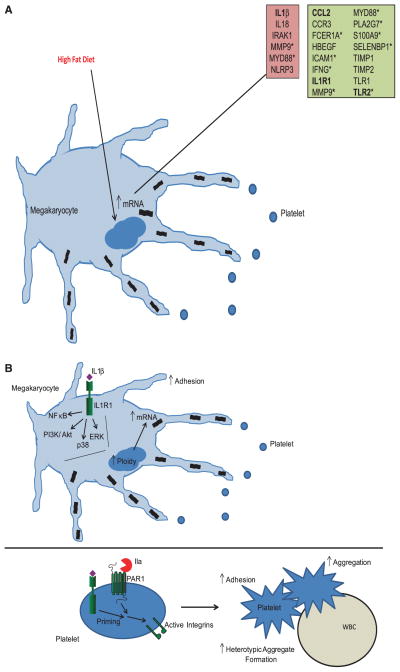
Figure 6.
Schematic of the effects of interleukin (IL)1β/IL1R1 on megakaryocyte and platelet function. A, A high fat diet will cause megakaryocytes to produce platelets with an increase in both inflammatory and thrombotic genes. Red highlighted data represent new Framingham Heart Study (FHS); green highlighted data represent mouse data; *confirmed in both human and mouse platelets; Bolded genes were confirmed to be upregulated in Meg-01 by IL1β. B, IL1β in circulation as a result of increased body weight will bind IL1R1 on megakaryocytes. This interaction leads to the activation of the nuclear factor (NF)κB, PI3K/Akt, and mitogen activated protein kinase (MAPK) (ERK and p38) pathways. As a result, there is an increase in megakarycoyte maturation, including increased adhesion, increases in ploidy, and increases in mRNA production of inflammatory and thrombotic genes. IL1β can also bind IL1R1 on platelets and either enhance aggregation induced by agonists or promote adhesion and heterotypic aggregate formation.
Work has shown the importance of TLRs in both platelets29,40 and megakaryocytes.27 However, little is known about IL1R1 and IL1β in regulating the function of both of these cells, in particular, in the setting of infection and obesity. Data presented here (summarized in Figure 6B) show that megakaryocytes express a functional IL1R1 that activates multiple signaling pathways, including NFκB, ERK MAPK, p38 MAPK, and PI3K/Akt pathways to increase both thrombotic and inflammatory gene expression. The changes in gene expression will result in altered transcript level in platelets. These transcripts can be translated into protein upon activation, as shown previously with IL1β transcripts in platelets,25,26 or can be transferred to other cells, such as immune or endothelial cells, during thrombosis or inflammation.41 Through IL1R1, IL1β regulates megakaryocyte maturation by increasing adhesion and ploidy. Baseline platelet counts in WT mice were slightly lower than in IL1R1−/− mice. When challenged with an infection, the P gingivalis infected WT platelet count rose, whereas the IL1R1−/− platelet count decreased. In response to an increase in circulating IL1β, megakaryocytes will produce more platelets that contain a higher amount of inflammatory and thrombotic genes, as seen in the FHS and mouse transcript data. Our data are similar to what was shown with TLR2.27 Both TLR2 and IL1R1 can respond to infection, affecting platelet production. Because both IL1R1−/− and IL1β−/− mice have functional TLR2 on their megakaryocytes, the data presented here along with what was previously published27 suggest that both receptors are necessary to respond to inflammatory stimuli.
The work presented here (summarized in Figure 6B) also shows how IL1R1 functions in platelets. IL1β does not cause aggregation, as seen with TLR229; however, it does enhance the response of platelets to both collagen and thrombin. This increased response is shown to be, in part, through p38 MAPK pathway. Interestingly, IL1β increased platelet adhesion to different substrates alone and in combination with thrombin and caused heterotypic aggregate formation. It is possible that p38 signaling could be involved in these functions as well. Recently, NADPH oxidase activity was shown to regulate collagen-induced platelet activation, through reactive oxygen species generation, and protein kinase C signaling.42 These pathways could also be involved in the effects of IL1R1 and IL1β on platelets. IL1R1 promotes the inflammatory function in platelets, which could enhance atherosclerosis and thrombosis. A high fat diet enhanced platelet adhesion in WT mice, which was abrogated in the IL1R1−/− mice. Overall, these data suggest that platelets contribute to the development of cardiovascular disease during obesity through IL1R1 and IL1β. Inhibition of IL1R1 would be desirable to alter disease progression.
Some bacterial infections, including P gingivalis, are associated with an increase in IL1β.3,4 When WT mice are infected acutely or chronically with P gingivalis, there is an increase in circulating heterotypic aggregates. Based on previous work,27,29,40 it would be hypothesized that this bacteria is being recognized by TLR2 on the megakaryocytes and platelets. However, IL1R1−/− and IL1β−/− mouse platelets, which still express TLR2, did not respond to the bacteria and form heterotypic aggregates. It is concluded that P gingivalis is recognized by TLR2 to increase IL1β levels, which, in turn, affects platelet–neutrophil interactions.
The work presented here uses 2 methods, epidemiological analyses and in vitro/in vivo experiments, to understand the role of IL1R1 and IL1β in megakaryocytes and platelets. There are some limitations of our approach. The FHS data were based on participants with a mean age of 67±9 years. The conclusions drawn from this epidemiological study may not reflect what occurs in young obese/overweight individuals. Additionally, participants of the FHS only report if they are on medications, including lipid-lowering drugs and aspirin, and did not report the specific type of medication, the dosage, and if they were on it at the time of examination. Platelet transcript studies were performed through a targeted approach and not based on an array. Although biased, this approach allowed us to study gene expression on 1819 individuals, reducing any issues derived from multiple testing. Platelets collected from healthy human volunteers were used for functional studies and not for comparison. Mice used in experiments were total knockout models and not platelet/megakaryocyte-specific knockout models, which are not commercially available. However, studying isolated platelets ex vivo allows us to see how IL1R1/IL1β affects these cells specifically, without any contaminating immune cells.
Our observations that IL1R1 modulates platelet processes may be clinically relevant. Previous work suggests a role for IL1R1 and IL1β in the development of cardiovascular disease. Increased levels of IL1β have been found in atherosclerotic plaques,5 and reduction of the expression or activity of IL1β and IL1R1 decreases plaque size in murine aortas.6–8 These results suggest that treatment that would reduce IL1β activity would be effective in reducing cardiovascular disease. Such a treatment could include the reduction in the levels or activation of IL1β or blockage of its receptor, including antibody therapy against IL1β or IL1R1. A clinical trial, CANTOS, has begun, which uses an antibody against IL1β to reduce cardiovascular events in individuals with coronary artery disease.9 Data from a recent study using IL1R1−/− ApoE−/− mice11 report that advanced atherosclerotic plaques are reduced in specific areas but are also showing signs of instability with increased intraplaque hemorrhage compared with IL1R1+/+ ApoE−/− mice. These data raise the possibility that plaque hemorrhage may be because of impaired hemostasis, that is, altered platelet function. While our model firmly establishes a role for IL1R1 in platelet function and its transcript present in human platelets in a large observational study, we cannot directly infer that the use of an antibody directed against IL1β will have clinical implications such as bleeding or vessel instability.
In conclusion, we show that IL1R1 and IL1β, associated with the innate immune system, regulate both megakaryocyte and platelet functions in murine and human models. IL1β through IL1R1 enhances megakaryocyte maturation and alters the RNA profiles in the developing platelets to be proinflammatory and prothrombotic. We provide evidence that megakaryocytes and platelets become activated in association with obesity and infections through IL1R1 and IL1β. Thus, in the circulation, platelets react to IL1β through IL1R1, which enhances its activation and stimulates the inflammatory function of platelets.
Supplementary Material
Significance.
A proinflammatory environment attributable to either an acute bacterial infection or a chronic disease such as obesity is associated with an increase in circulating interleukin (IL)1β. A current phase III clinical trial, Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS), is investigating the use of an antibody against IL1β to reduce recurrent cardiovascular events in individuals with a recent myocardial infarction and elevated C-reactive protein. However, new data from animal studies using IL1R1−/− ApoE−/− mice show plaque stability and vessel remodeling were reduced in areas where hemorrhage was noted, suggesting that this pathway may have effects beyond the vessel wall. The work presented here shows that IL1R1 and IL1β can influence platelet function and potentially augment the development of disease. These results are of future relevance as inflammatory receptors found in the vasculature are being targeted for therapeutic intervention but are not well characterized in the platelet.
Acknowledgments
L.M. Beaulieu was involved in experimental design, performing experiments, data analysis, writing, and editing of the article. E. Lin and M. Koupenova were involved in performing experiments, data analysis, and editing of the article. E.O. Weinberg and C.D. Kramer were involved in performing experiments. C.A. Genco was involved in experimental design and editing of the article. E.J. Benjamin, E. Mick, and M.G. Larson were involved with Framingham Heart Study sample collection, analysis, and editing of the article. K. Tanriverdi and J.E. Freedman were involved in experimental design, data analysis, and editing of the article.
Sources of Funding
This work was supported by grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Disease (P01 AI078894 to L.M. Beaulieu, E.O. Weinberg, C.D. Kramer, C.A. Genco, and J.E. Freedman) and the National Heart, Lung, and Blood Institute (HL087201 and RFA-HL-12-008 to J.E. Freedman, N01-HC 25195 and 1R01AG028321 to E.J. Benjamin, and T32 HL07224 to L.M. Beaulieu, M. Koupenova, and C.D. Kramer).
Nonstandard Abbreviations and Acronyms
- BMI
body mass index
- FHS
Framingham Heart Study
- IL1β
interleukin 1 beta
- IL1R1
interleukin 1 receptor 1
- IRAK1
interleukin 1 receptor-associated kinase 1
- MCP-1
monocyte chemotactic protein-1
- MMP
matrix metalloproteinase
- MYD88
myeloid differentiation primary response gene 88
- NLRP3
nucleotide-binding oligomerization domain, leucine rich repeat, and pyrin domain containing protein 3
- TRAF6
tumor necrosis factor receptor–associated factor 6
- TPO
thrombopoietin
- WT
wild-type
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.302700/-/DC1.
References
- 1.Faber DR, de Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009;10:554–563. doi: 10.1111/j.1467-789X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 2.Strandberg L, Verdrengh M, Enge M, Andersson N, Amu S, Onnheim K, Benrick A, Brisslert M, Bylund J, Bokarewa M, Nilsson S, Jansson JO. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One. 2009;4:e7605. doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao JJ, Feng XP, Zhang XL, Le KY. Effect of Porphyromonas gingivalis and Lactobacillus acidophilus on secretion of IL1B, IL6, and IL8 by gingival epithelial cells. Inflammation. 2012;35:1330–1337. doi: 10.1007/s10753-012-9446-5. [DOI] [PubMed] [Google Scholar]
- 4.Ozdemir SP, Kurtis B, Tuter G, Bozkurt S, Gultekin SE, Senguven B, Watanabe K, Aydin S. Effects of low dose doxycycline and bisphosphonate clodronate on gingival levels of matrix metalloproteinase-9, interleukin -1B and alveolar bone loss in diabetic rats. A histomorphometric and immunohistochemical study. J Periodontol. 2012;83:1172–7782. doi: 10.1902/jop.2012.110459. [DOI] [PubMed] [Google Scholar]
- 5.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ, Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011;216:313–320. doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/ or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 8.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T, Group CPI. Effects of interleukin-1beta inhibition with canakinumab on hemaglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 11.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 13.Nakai S, Aihara K, Hirai Y. Interleukin-1 potentiates granulopoiesis and thrombopoiesis by producing hematopoietic factors in vivo. Life Sci. 1989;45:585–591. doi: 10.1016/0024-3205(89)90043-x. [DOI] [PubMed] [Google Scholar]
- 14.Kimura H, Ishibashi T, Shikama Y, Okano A, Akiyama Y, Uchida T, Maruyama Y. Interleukin-1 beta (IL-1 beta) induces thrombocytosis in mice: possible implication of IL-6. Blood. 1990;76:2493–2500. [PubMed] [Google Scholar]
- 15.Furuya H, Ishibashi R, Wakayama T, Ohguni S, Notsu K, Takagi C, Kato Y. Effect of subcutaneous administration of interleukin-1 beta on blood platelet count and serum GM-CSF in patients with myelodysplastic syndrome and aplastic anemia. Rinsho Ketsueki. 1992;33:1172–1177. [PubMed] [Google Scholar]
- 16.Cobankara V, Oran B, Ozatli D, Haznedaroglu IC, Kosar A, Buyukasik Y, Ozcebe O, Dundar S, Kirazli S. Cytokines, endothelium, and adhesive molecules in pathologic thrombopoiesis. Clin Appl Thromb Hemost. 2001;7:126–130. doi: 10.1177/107602960100700209. [DOI] [PubMed] [Google Scholar]
- 17.Chuen CK, Li K, Yang M, Fok TF, Li CK, Chui CM, Yuen PM. Interleukin- 1beta up-regulates the expression of thrombopoietin and transcription factors c-Jun, c-Fos, GATA-1, and NF-E2 in megakaryocytic cells. J Lab Clin Med. 2004;143:75–88. doi: 10.1016/j.lab.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Li K, Chui CM, Yuen PM, Chan PK, Chuen CK, Li CK, Fok TF. Expression of interleukin (IL) 1 type I and type II receptors in megakaryocytic cells and enhancing effects of IL-1beta on megakaryocytopoiesis and NF-E2 expression. Br J Haematol. 2000;111:371–380. doi: 10.1046/j.1365-2141.2000.02340.x. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Levine JD, Fu Y, Deng B, London R, Groopman JE, Avraham H. Cytokine production by primary bone marrow megakaryocytes. Blood. 1994;84:4151–4156. [PubMed] [Google Scholar]
- 20.Williams DE, Morrissey PJ. Alterations in megakaryocyte and platelet compartments following in vivo IL-1 beta administration to normal mice. J Immunol. 1989;142:4361–4365. [PubMed] [Google Scholar]
- 21.Hawrylowicz CM, Santoro SA, Platt FM, Unanue ER. Activated platelets express IL-1 activity. J Immunol. 1989;143:4015–4018. [PubMed] [Google Scholar]
- 22.Kaplanski G, Porat R, Aiura K, Erban JK, Gelfand JA, Dinarello CA. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood. 1993;81:2492–2495. [PubMed] [Google Scholar]
- 23.Gawaz M, Brand K, Dickfeld T, Pogatsa-Murray G, Page S, Bogner C, Koch W, Schömig A, Neumann F. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148:75–85. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 24.Loppnow H, Bil R, Hirt S, Schönbeck U, Herzberg M, Werdan K, Rietschel ET, Brandt E, Flad HD. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood. 1998;91:134–141. [PubMed] [Google Scholar]
- 25.Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaulieu LM, Lin E, Morin KM, Tanriverdi K, Freedman JE. Regulatory effects of TLR2 on megakaryocytic cell function. Blood. 2011;117:5963–5974. doi: 10.1182/blood-2010-09-304949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman JE, Larson MG, Tanriverdi K, O’Donnell CJ, Morin K, Hakanson AS, Vasan RS, Johnson AD, Iafrati MD, Benjamin EJ. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the Framingham heart study. Circulation. 2010;122:119–129. doi: 10.1161/CIRCULATIONAHA.109.928192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184:5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Zienkiewicz J, Hawiger J. Interactive sites in the MyD88 Toll/interleukin (IL) 1 receptor domain responsible for coupling to the IL1beta signaling pathway. J Biol Chem. 2005;280:26152–26159. doi: 10.1074/jbc.M503262200. [DOI] [PubMed] [Google Scholar]
- 33.Song KW, Talamas FX, Suttmann RT, Olson PS, Barnett JW, Lee SW, Thompson KD, Jin S, Hekmat-Nejad M, Cai TZ, Manning AM, Hill RJ, Wong BR. The kinase activities of interleukin-1 receptor associated kinase (IRAK)-1 and 4 are redundant in the control of inflammatory cytokine expression in human cells. Mol Immunol. 2009;46:1458–1466. doi: 10.1016/j.molimm.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Dai SM, Shan ZZ, Nishioka K, Yudoh K. Implication of interleukin 18 in production of matrix metalloproteinases in articular chondrocytes in arthritis: direct effect on chondrocytes may not be pivotal. Ann Rheum Dis. 2005;64:735–742. doi: 10.1136/ard.2004.026088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaFramboise WA, Bombach KL, Dhir RJ, Muha N, Cullen RF, Pogozelski AR, Turk D, George JD, Guthrie RD, Magovern JA. Molecular dynamics of the compensatory response to myocardial infarct. J Mol Cell Cardiol. 2005;38:103–117. doi: 10.1016/j.yjmcc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Vikman P, Ansar S, Edvinsson L. Transcriptional regulation of inflammatory and extracellular matrix-regulating genes in cerebral arteries following experimental subarachnoid hemorrhage in rats. Laboratory investigation. J Neurosurg. 2007;107:1015–1022. doi: 10.3171/JNS-07/11/1015. [DOI] [PubMed] [Google Scholar]
- 37.Leviton A, Hecht JL, Allred EN, Yamamoto H, Fichorova RN, Dammann O ELGAN Study Investigators. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol. 2011;90:235–243. doi: 10.1016/j.jri.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Shchors K, Nozawa H, Xu J, Rostker F, Swigart-Brown L, Evan G, Hanahan D. Increased invasiveness of MMP-9-deficient tumors in two mouse models of neuroendocrine tumorigenesis. Oncogene. 2013;32:502–513. doi: 10.1038/onc.2012.60. [DOI] [PubMed] [Google Scholar]
- 39.McManus DD, Beaulieu LM, Mick E, Tanriverdi K, Larson MG, Keaney JF, Jr, Benjamin EJ, Freedman JE. Relationship among circulating inflammatory proteins, platelet gene expression, and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2013;33:2666–2673. doi: 10.1161/ATVBAHA.112.301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rex S, Beaulieu LM, Perlman DH, Vitseva O, Blair PS, McComb ME, Costello CE, Freedman JE. Immune versus thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein-protein interactions, and alpha-granule release. Thromb Haemost. 2009;102:97–110. doi: 10.1160/TH08-08-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Risitano A, Beaulieu LM, Vitseva O, Freedman JE. Platelets and platelet-like particles mediate intercellular RNA transfer. Blood. 2012;119:6288–6295. doi: 10.1182/blood-2011-12-396440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vara D, Campanella M, Pula G. The novel NOX inhibitor 2-acetlphenothiazine impairs collagen-dependent thrombus formation in a GPVI-manner. Br J Pharmacol. 2013;168:212–224. doi: 10.1111/j.1476-5381.2012.02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.