Abstract
Validity of the central line-associated bloodstream infection (CLABSI) measure is compromised by subjectivity. We observed significant decreases in both CLABSI and total hospital-acquired bloodstream infection (BSI) following a CLABSI prevention intervention in adult intensive care units. Total hospital-acquired BSI could be explored as an adjunct, objective CLABSI measure.
Keywords: central line infection, assessment bias, surveillance, BSI, surrogate measure
Standardized definitions for the surveillance of healthcare-associated infections from the Centers for Disease Control and Prevention's (CDC) National Healthcare Safety Network (NHSN)1 are used by hospitals for internal use and mandatory public reporting. However, even these standardized definitions have subjective elements that lead to inter-observer variability, well exemplified in surveillance for central line-associated bloodstream infection (CLABSI).2–4 As hospitals are subjected to increasing pressure to achieve CLABSI rates of “zero”, experts continue to question the validity of the current CLABSI definition,3, 4 and the need for more objective outcome measures is evident.
The goal of this study was to evaluate “total hospital-acquired bloodstream infection (BSI)” as an adjunct, and more objective outcome measure, than the CDC NHSN definition for CLABSI.
Methods
We performed a longitudinal study of trends in hospital-acquired BSI over a 5-year period from July 2007 to June 2012, at the University of Maryland Medical Center, a 757-bed tertiary care hospital with 333 ICU beds. The study population included 8 adult ICUs (2 medical, 2 surgical, and 3 trauma units). This study was determined to be non-human subjects research by the University of Maryland Institutional Review Board.
Total hospital-acquired BSI was defined as all positive blood cultures with onset > 48 hours following admission. A single blood culture positive for Staphylococcus spp. (not S. aureus), Micrococcus spp., Bacillus spp. (not B. cereus or B. anthracis), Corynebacterium spp. (not C. jeikeium), or Propionibacterium spp, was considered a contaminant. CLABSI was defined as a positive blood culture in a patient with a central line in the absence of another site of infection using CDC-NHSN criteria.1 (All positive blood cultures are routinely reviewed by infection preventionists for possible CLABSI.) The above data were extracted from our hospital’s prospectively maintained Infection Prevention and Hospital Epidemiology database.
Comorbidity was measured by the Charlson score5 using discharge ICD-9 codes from the hospital’s Oracle-based clinical data repository. Severity of illness was measured using the case-mix index (CMI), an indicator of the resource intensity of treatment calculated using diagnosis-related groups used for reimbursement.6 We used the University HealthSystem Consortium CMI which compares the acuity of patients in our hospital to the average of all member hospitals.7
Multiple CLABSI prevention measures were implemented as an intervention “bundle” in all ICUs beginning July 2009. These included use of evidence-based central line insertion practices using checklists, chlorhexidine dressings at exit sites, rifampin-minocycline catheters, and neutral pressure needleless connectors, education on appropriate blood culture methodology, emphasis on aseptic central line maintenance, and removal of unnecessary central lines. Daily chlorhexidine bathing of all patients was introduced in one ICU (medical ICU) in July 2010. No other major infection prevention initiatives occurred during the study period.
Monthly rates of CLABSI (per 1000 central line-days), total and non-contaminant hospital-acquired BSI (per 1000 patient-days), and blood culture obtainment (number of blood cultures obtained per 1000 patient-days) were calculated. Trends in monthly rates were evaluated using Quasi-Poisson regression,8 comparing post-intervention to pre-intervention periods, having linear terms for month and a knot point at the start of the intervention. Analyses of BSI trends were adjusted for known potential confounders found to vary significantly over time, and for time since study onset to account for unmeasured confounders that could change with time. All analyses were performed using R statistical software.9
Results
From July 2007 to June 2012, there were 3948 total hospital-acquired BSIs, 3018 non-contaminant BSIs, and 565 CLABSIs. Therefore CLABSIs constituted 14.3% of the total hospital-acquired BSIs, and 18.7% of non-contaminant BSIs during the study period.
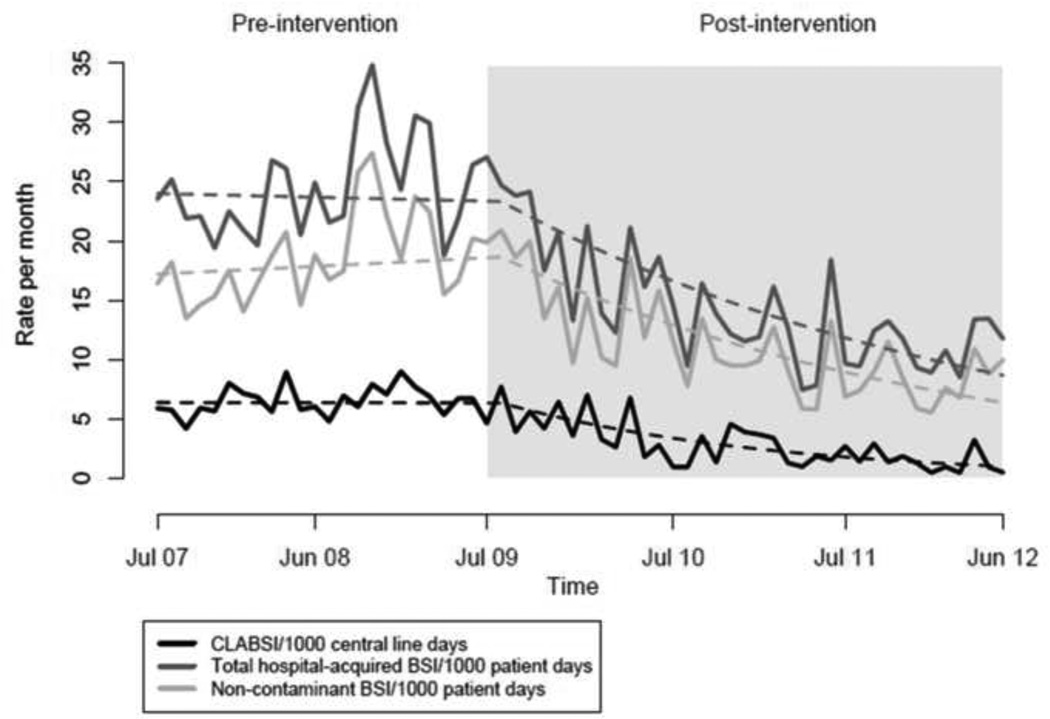
In the period prior to the CLABSI intervention (July 2007–June 2009), there was no significant change in monthly CLABSI, total hospital-acquired BSI, or non-contaminant BSI rates. Comparing post- to pre-intervention, there was a 5.1% per month relative mean decrease in CLABSI rates which was significant (95% confidence interval (CI): 3.0%–7.1%, P<.001). Comparing total hospital-acquired BSI rates post- to pre-intervention, there was a 2.7% relative decrease per month that was significant (95% CI: 1.4%–4.0%, P<.001). Similarly, there was a significant relative decrease in the non-contaminant BSI rate (3.3% decrease per month, CI: 1.9%–4.7%, P<.001) comparing post- to pre-intervention (Figure 1).
Figure 1.
Trends in Rates of Central Line-Associated BSI (CLABSI), Total Hospital-Acquired BSI, and Non-Contaminant BSI in Adult Intensive Care Units, July 2007–June 2012
There was no significant change in the blood culture obtainment rate prior to the intervention, and a 0.5% relative decrease per month in the blood culture obtainment rate comparing post- to pre-intervention (95% CI: 0.03%–0.98%, P=.037). The average monthly CMI in the pre-and post-intervention periods was 3.08 (range 2.79–3.36) and 2.45 (range 1.92–2.68) respectively, with an overall significant decrease during the study period (0.5% per month, 95% CI: 0.4%–0.6%, P<0.001). The average monthly Charlson score was 2.14 range 1.91–2.33) in the pre-intervention period, and 2.4 (range 1.48–2.68) in the post-intervention period with no significant trend over time.
Even after adjusting for blood culture obtainment rates, CMI, and time since study onset, comparing post- to pre-intervention, we observed significant decreases in CLABSI 5.2% per month, 95% CI: 2.7%–7.7%, P<.001), total hospital-acquired BSI (1.7% per month, 95% CI: 0.4%–3.1%, P=.015), and non-contaminant BSI (2.3% per month, 95% CI: 0.7%–3.8%, P = .004).
Discussion
We found that both the subjective CDC-NHSN CLABSI rate and the objective total hospital-acquired BSI rate decreased significantly and in parallel following implementation of a CLABSI intervention, independently of changes in the frequency of blood cultures and patient severity of illness. These findings suggest that total hospital-acquired BSI could be further evaluated as an adjunct measure in CLABSI surveillance.
The wide inter-reviewer variability and subjectivity in CLABSI assessment2 has significant implications for consumer perception and hospital reimbursement in the era of public reporting.3 Using computer-based algorithms to identify CLABSIs more objectively has been explored but is limited by differences in the elements and format of electronic data at different facilities.10 We selected total hospital-acquired BSI as the simplest informative measure as an adjunct to CLABSI surveillance because these data are routinely obtained in an automated manner at all facilities and are completely objective. We included potential contaminants because contaminant determination also involves subjective judgment, and reducing contaminants should be a goal of any intervention aimed at reducing BSI (notably, our results are similar whether contaminants are included or excluded).
Our study’s main limitation is that it was conducted at a single institution. The disadvantage of using the total BSI measure by itself is its breadth: because BSI may result from several sources, source identification where possible is important to identify intervention targets. Lastly, the reason for decline in total hospital-acquired BSI in our study is not clear. While education on appropriate blood culture methodology to decrease contaminants was part of the intervention, a significant reduction in the non-contaminant BSI was also observed. Avoidance of blood cultures via central lines could have decreased blood cultures positive for colonizers. Although adjusted for in the analysis, decrease in blood culture obtainment could still have contributed to the observed BSI reduction. Heightened attention to hand hygiene and aseptic technique along with promotion of a culture of patient safety might have played a role but this is speculative.
In summary, CLABSI prevention efforts were strongly associated with reduction in the rates of both CLABSI and total hospital-acquired BSI. The total hospital-acquired BSI measure should be explored further as an adjunct CLABSI measure, and a more objective measure than CLABSI as a potential indicator of hospital quality.
Acknowledgments
Financial Support: None
Footnotes
Potential Conflict of Interest: All authors report no conflicts of interest relevant to this article
Contributor Information
Surbhi Leekha, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, United States.
Shanshan Li, Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States.
Kerri A. Thom, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, United States.
Michael Anne Preas, Department of Infection Prevention, University of Maryland Medical Center, Baltimore, MD, United States.
Brian S. Caffo, Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States.
Daniel J. Morgan, Department of Epidemiology and Public Health, University of Maryland School of Medicine, and VA Maryland Healthcare System, Baltimore, MD, United States.
Anthony D. Harris, Department of Epidemiology and Public Health, University of Maryland School of Medicine, and VA Maryland Healthcare System, Baltimore, MD, United States.
References
- 1.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Mayer J, Greene T, Howell J, et al. Agreement in Classifying Bloodstream Infections Among Multiple Reviewers Conducting Surveillance. Clin Infect Dis. 2012;55(3):364–370. doi: 10.1093/cid/cis410. [DOI] [PubMed] [Google Scholar]
- 3.Sexton DJ, Chen LF, Moehring R, Thacker PA, Anderson DJ. Casablanca redux: we are shocked that public reporting of rates of central line-associated bloodstream infections are inaccurate. Infect Control Hosp Epidemiol. 2012;33(9):932–935. doi: 10.1086/667383. [DOI] [PubMed] [Google Scholar]
- 4.Lin MY, Hota B, Khan YM, et al. Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates. JAMA. 2010;304(18):2035–2041. doi: 10.1001/jama.2010.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 6.Fetter RB, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis-related groups. Med Care. 1980;18(2 Suppl):1–53. iii. [PubMed] [Google Scholar]
- 7.University HealthSystem Consortium. [Accessed December 12, 2012];Clinical Database/Resource Manager. https://www.uhc.edu/11536.htm.
- 8.McCullagh P, Nelder JA. Generalized linear models. Second ed. Vol 37. London: Chapman & Hall/CRC; 1989. [Google Scholar]
- 9.R Development Core Team. R: A language and environment for statistical computing. Austria: R Foundation for Statistical Computing, Vienna; 2011. ISBN 3-900051-07-0. Available at: http://www.R-project.org/ [Google Scholar]
- 10.Hota B, Lin M, Doherty JA, et al. Formulation of a model for automating infection surveillance: algorithmic detection of central-line associated bloodstream infection. J Am Med Inform Assoc. 2010;17(1):42–48. doi: 10.1197/jamia.M3196. [DOI] [PMC free article] [PubMed] [Google Scholar]