Abstract
Stroke is one of the most disabling complications of sickle cell anemia (SCA). The molecular mechanisms leading to stroke in SCA or by which packed red blood cell (PRBC) transfusion prevents strokes are not understood. We investigated the effects of PRBC transfusion on serum biomarkers in children with SCA who were at high-risk for stroke. Serum samples from 80 subjects were analyzed, including baseline, study exit time point and 1 year after study exit. Forty of the 80 samples were from subjects randomized to standard care and 40 from transfusion arm. Samples were assayed for levels of BDNF, sVCAM-1, sICAM-1, MPO, Cathepsin-D, PDGF-AA, PDGF-AB/BB, RANTES (CCL5), tPAI-1, and NCAM-1 using antibody immobilized bead assay. Significantly lower mean serum levels of sVCAM-1 (2.2 × 106 ± 0.8 × 106 pg/mL vs. 3.1 × 106 ± 0.9 × 106 pg/mL, P < 0.0001), Cathepsin-D (0.5 × 106 ± 0.1 × 106 pg/mL vs. 0.7 × 106 ± 0.2 × 106 pg/mL, P < 0.0001), PDGF-AA (10556 ± 4033 pg/mL vs. 14173 ± 4631 pg/mL, P = 0.0008), RANTES (0.1 × 106 ± 0.07 × 106 pg/mL vs. 0.2 × 106 ± 0.06 × 106 pg/mL, P < 0.006), and NCAM-1 (0.7 × 106 ± 0.2 × 106 pg/mL vs. 0.8 × 106 ± 0.1 × 106 pg/mL, P < 0.0006) were observed among participants who received PRBC transfusion, compared to those who received standard care. Twenty or more PRBC transfusion over 4 years was associated with lower serum levels of sVCAM-1 (P < 0.001), PDGF-AA (P = 0.025), and RANTES (P = 0.048). Low baseline level of BDNF (P = 0.025), sVCAM-1 (P = 0.025), PDGF-AA (P = 0.01), t-PAI-1 (P = 0.025) and sICAM-1 (P = 0.022) was associated with higher probability of stroke free survival. Beyond improving hemoglobin levels, our results suggest that the protective effects of PRBC transfusion on reducing stroke in SCD may result from reduced thrombogenesis and vascular remodeling.
Introduction
Sickle cell disease (SCD) remains a significant public health problem, affecting over 100,000 people in the United States (US). It is a huge burden to the healthcare system and a significant source of health disparity [1–4]. The most common form is sickle cell anemia (SCA, Hemoglobin SS), which results from a point mutation in both human β-globin genes, leading to the substitution of valine for glutamic acid at the sixth position of the β-globin chains [5]. SCD is associated with global activation of inflammatory and coagulation factors [6,7], and individuals with SCA have variable clinical severity [8]. Stroke due to cerebral artery stenosis is one of the most severe complications. By age 18 years, about 11% of children with SCA experience at least one symptomatic stroke [9]. Recognized risk factors for development of stroke in children with SCA include high transcranial Doppler (TCD) ultrasonography velocity [10], anemia [11], relative hypertension [12] cerebral vasculopathy [13–15], and presence of extracranial vasculopathy [16].
Chronic packed red blood cell (PRBC) transfusion therapy is effective for primary prevention of stroke among most children with SCA and high TCD velocity [17] and for secondary prevention of recurrent symptomatic stroke [17,18]. There is no safe time to discontinue chronic PRBC transfusion because the stroke risk reverts to pre-intervention levels [19]. Chronic PRBC transfusion is associated with significant iron overload [20] and currently, hydroxyurea is being investigated as an alternative in order to mitigate the complications of iron overload [20]. In addition, chronic PRBC transfusions cannot effectively prevent silent cerebral infarcts (SCI) in subjects with advanced cerebral vasculopathy [21]. Although cerebral vasculopathy is a major herald for stroke in individuals with SCA, factors that determine its onset, evolution and progression are not well understood [11]. Inflammation [22,23], vascular remodeling [24], and anemia leading to a hyperdynamic cerebral circulation [25,26] are associated with an increased risk for developing cerebral vasculopathy and stroke in children with SCA. However, the mechanisms by which chronic PRBC transfusion reduces stroke risk and incidence are largely unknown. In the STOP study, chronic PRBC transfusions were found to reduce free plasma hemoglobin, which would be expected to improve cerebrovascular function by increasing nitric oxide bioavailability [27].
We have previously demonstrated a positive association between TCD velocity and serum concentrations of brain derived neurotropic factor (BDNF) and platelet derived growth factor (PDGF)-AA (a potent mitogen affecting vascular endothelial cells) in subjects of the Stroke Prevention in Sickle cell anemia (STOP) study [28]. Elevated pre-treatment concentrations of PDGF-AA predicted the risk for developing stroke among SCA subjects with high TCD velocity. In the current study, we evaluated the relationship between type of treatment (standard care or chronic PRBC transfusion) and serum concentrations of biomarkers of neuroischemia, endothelial activation, inflammation, and thrombosis among subjects who participated in the STOP study. We hypothesized that these biomarkers will be lower in those subjects who were treated with frequent PRBC transfusion compared to standard care, and that the biomarker levels will be correlated with clinical outcome.
Methods
Sample processing and analysis
This study is ancillary to the STOP study [NCT00000592], a randomized phase III clinical trial investigating the efficacy of regular PRBC transfusion for the prevention of stroke in children with SCA and high TCD velocity. A total of 130 children ages 2–16 years, with sickle cell anemia (Hemoglobin SS or S-β0 thalassemia) and high TCD ultrasound velocity (≥ 200 cm/s), were randomly assigned to receive either standard care (SC, n = 67) or chronic PRBC transfusion (n = 63). The study was terminated early because of the overwhelming evidence of benefit to the transfusion arm, with a 92% reduction in stroke incidence compared to the SC arm [17]. Subjects in the SC arm were then allowed to crossover to chronic PRBC transfusion and followed for 1 year, after which blood samples, TCD velocity and other clinical and hematological evaluations were obtained (post-study/trial period).
Our laboratory obtained and analyzed stored frozen serum samples from three time points (baseline, study exit and 1-year post-trial), from the STOP Study group at the Medical University of South Carolina (MUSC). Two hundred and forty serum samples from 80 subjects (40 each from the SC and transfusion arms of STOP) were obtained. To be included, the subject must have at least one sample aliquot at each time point. The analytes of interest, brain-derived neurotrophic factor (BDNF), soluble vascular cell adhesion molecule (sVCAM)-1, soluble intercellular adhesion molecule (sICAM)-1, myeloperoxidase (MPO), Cathepsin-D, platelet derived growth factor subtypes (PDGF-AA and PDGF-AB/BB), released upon activation normal T-cell expressed and secreted (RANTES, CCL5), tissue plasminogen activator inhibitor (tPAI)-1, and neural cell adhesion molecule (NCAM)-1, were measured in duplicate using the Human Neurodegenerative Panel of antibody immobilized bead kit (Millipore, Billerica, MA). The mean fluorescent intensity (MFI) was measured on a Bioplex reader powered by Luminex technology (Bio-Rad, Hercules, CA) and concentrations calculated using an interpolated 5PL logistic curve and the Bioplex software. These values, together with TCD velocity, anthropometric and hematological data were exported to Microsoft Excel and analyzed using IBM SPSS 20 for Windows and GraphPad prism.
Data analysis
The characteristics of the STOP participants have been previously reported [15]. Serum concentrations of biomarkers are expressed in box and whisker plots to show the median, maximum and minimum values. The difference in mean serum biomarker levels between groups was estimated using An ANOVA test with adjustment for serum biomarker levels at baseline and for multiple comparisons. Partial correlation was conducted to investigate the relationship between number of transfusions over the period of the entire study (STOP and post-trial) and serum levels of biomarkers. Only biomarkers with significant partial correlations are presented as scatter plots with regression line and 95% confidence interval.
Our analyses were done and presented in the following steps; first, serum concentrations of the biomarkers at study exit were compared between SC and transfusion arms. Plots shown are only for those biomarkers with statistically significant differences between the groups. Next, since some SC arm subjects received PRBC transfusions for acute illnesses, we also investigated the relationship between number of blood transfusions received over four years (3 years of STOP plus 1 year of post-STOP) and the serum biomarker concentrations. These results are expressed using scatter plots, with best-fit correlation lines and also lines for 95% confidence intervals.
Finally, using receiver operator characteristic curves, we estimated the serum concentration of biomarkers at baseline that, together with high TCD velocity, could enable prediction of the incidence of stroke with ≥80% sensitivity and specificity. Using this level (which fell between the 65th–95th percentile depending on the biomarker), the serum concentration of biomarkers in this study was categorized into high or low depending on whether they were above or below this set value. The probability of a stroke free survival based on whether the subject had high or low serum level of that biomarker at baseline was then estimated, using a Kaplan–Meier survival plot with Log Rank test (Mantel–Cox). Only biomarkers with a statistically significant probability of stroke free survival are shown. A P-value of <0.05 was considered statistically significant.
Results
Serum biomarker levels
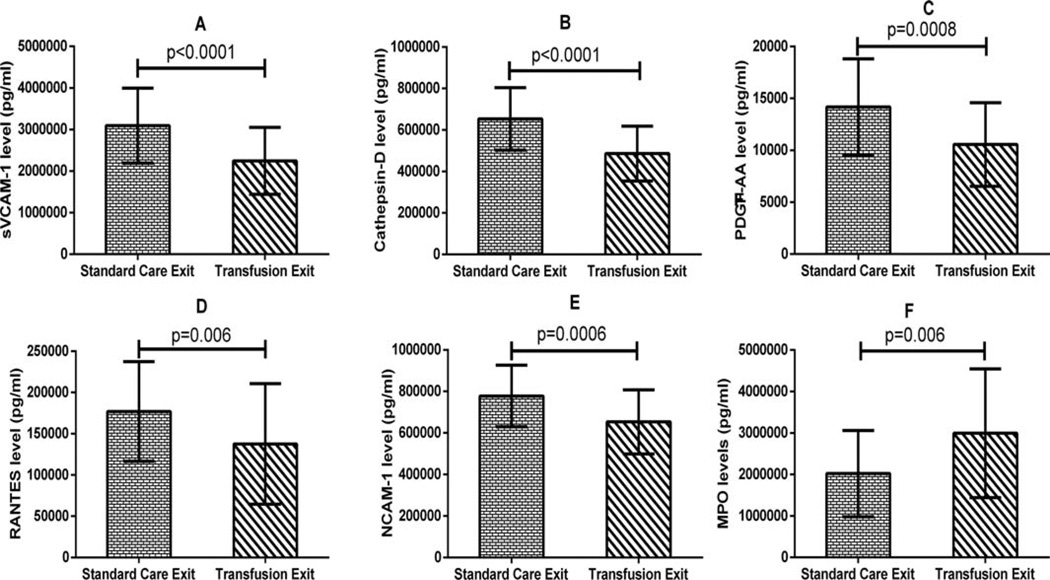
A total of 240 samples were analyzed; 80 each from baseline, study exit and 1 year post-trial time points. There was no significant difference in baseline TCD velocity, hematological parameters (except Hb level) or anthropometric measurements between subjects randomized to transfusion versus “standard care (SC)” for those included in this study. Similarly, except for MPO levels the biomarker levels were significantly higher among those randomized to SC compared with those randomized to transfusion (Table I) at baseline. At STOP study exit, significantly lower mean serum levels of sVCAM-1 (2.2 × 106 ± 0.8 × 106 pg/mL vs. 3.1 × 106 ± 0.9 × 106 pg/mL, P <0.0001), Cathepsin-D (0.5 × 106 ± 0.1 × 106 pg/mL vs. 0.7 × 106 ± 0.2 × 106 pg/mL, P <0.0001), PDGF-AA (10556 ± 4033 pg/mL vs. 14173 ± 4631 pg/mL, P = 0.0008), RANTES (0.1 × 106 ± 0.07 × 106 pg/mL vs. 0.2 × 106 ± 0.06 × 106 pg/mL, P < 0.006), and NCAM-1 (0.7 × 106 ± 0.2 × 106 pg/mL vs. 0.8 × 106 ± 0.1 × 106 pg/mL, P < 0.0006) were observed among participants who received PRBC transfusion, compared to those who received standard care (Fig. 1A–E). On the other hand, the mean serum MPO level (Fig. 1F) was significantly higher for those who received PRBC transfusion compared with those who received standard care (3.0 × 106 ± 1.5 × 106 pg/mL vs. 2.0 × 106 ± 1.0 × 106 pg/mL, P < 0.006).
TABLE I.
Baseline Hemoglobin, Fetal Hemoglobin Percent, and Serum Biomarkers Levels in Children with Sickle Cell Disease and High TCD
| Baseline serum biomarkers levels | Standard care | N | Transfusion | N | P-values for comparison |
|---|---|---|---|---|---|
| Hb F (%) | 9.4 ± 5.1 | 40 | 9.2 ± 5.3 | 40 | 0.873 |
| Hb (g/dL) | 7.7 ± 0.8 | 40 | 7.2 ± 0.9 | 40 | 0.021 |
| BDNF (×104 pg/mL) | 3.4 ± 0.9 | 40 | 1.8 ± 0.6 | 39 | <0.001 |
| sVCAM-1 (×104 pg/mL) | 261.5 ± 103.2 | 40 | 165.0 ± 39.4 | 39 | <0.001 |
| sICAM-1 (×104 pg/mL) | 17.1 ± 6.5 | 40 | 9.9 ± 6.7 | 39 | <0.001 |
| MPO (×104 pg/mL) | 217.9 ± 146.9 | 40 | 176.9 ± 115.5 | 39 | 0.172 |
| Cathepsin-D (×104 pg/mL) | 51.1 ± 22.4 | 40 | 36.7 ± 17.7 | 39 | 0.001 |
| PDGF-AA (×104 pg/mL) | 1.5 ± 0.6 | 40 | 0.7 ± 0.2 | 39 | <0.001 |
| PDGF-AB/BB (×104 pg/mL) | 9.1 ± 2.9 | 40 | 4.5 ± 1.9 | 39 | <0.001 |
| RANTES (CCL5) (×104 pg/mL) | 18.1 ± 7.0 | 40 | 10.6 ± 3.7 | 39 | <0.001 |
| tPAI-1 (×104 pg/mL) | 10.0 ± 4.6 | 40 | 6.3 ± 2.1 | 39 | <0.001 |
| NCAM-1 (×104 pg/mL) | 67.5 ± 15.7 | 40 | 43.9 ± 10.9 | 39 | <0.001 |
Except for MPO and HbF, the mean levels of Hb and biomarkers were significantly higher among subject randomized to “standard care” compared with those randomized to transfusion.
Values are Means ± SD.
HbF, fetal hemoglobin; Hb, hemoglobin; BDNF, brain derived neurotropic factor; sVCAM-1, soluble vascular cell adhesion molecule-1; sICAM-1, soluble intercellular adhesion molecule-1; MPO, myeloperoxidase; PDGF, platelet derived growth factor; RANTES, regulated on activation normal T-cell expressed and secreted; tPAI-1, total plasminogen activator inhibitor; NCAM-1, neural cell adhesion molecule-1.
Figure 1.
Comparison of serum levels of sVCAM-1, MPO, Cathepsin-D, PDGF-AA, RANTES, and NCAM-1 at study exit between subjects randomized to standard care or transfusion. Except for MPO, there was a significantly lower serum level of each of these biomarkers among subjects in the transfusion arm, after adjusting for pre-transfusion levels compared to the SC arm.
Relationship between serum biomarkers and transfusion frequency
At the post-STOP time point, there was a significant negative correlation between serum concentrations of sVCAM-1 (r = −0.323, P = 0.012), PDGF-AA (r = −0.316, P = 0.014) and tPAI-1 (r = −0.293, P = 0.023) and the number of PRBC transfusions received (Supplemental Figure, Fig. 1A–C). Serum BDNF (r = −0.221, P = 0.090) and NCAM-1 (r = −0.236, P = 0.070) levels showed similar trends for negative correlation with number of PRBC transfusions, but were not statistically significant. There was no correlation between baseline biomarkers levels and baseline hematological variables.
Prediction of stroke-free survival based on pre-treatment biomarker concentrations
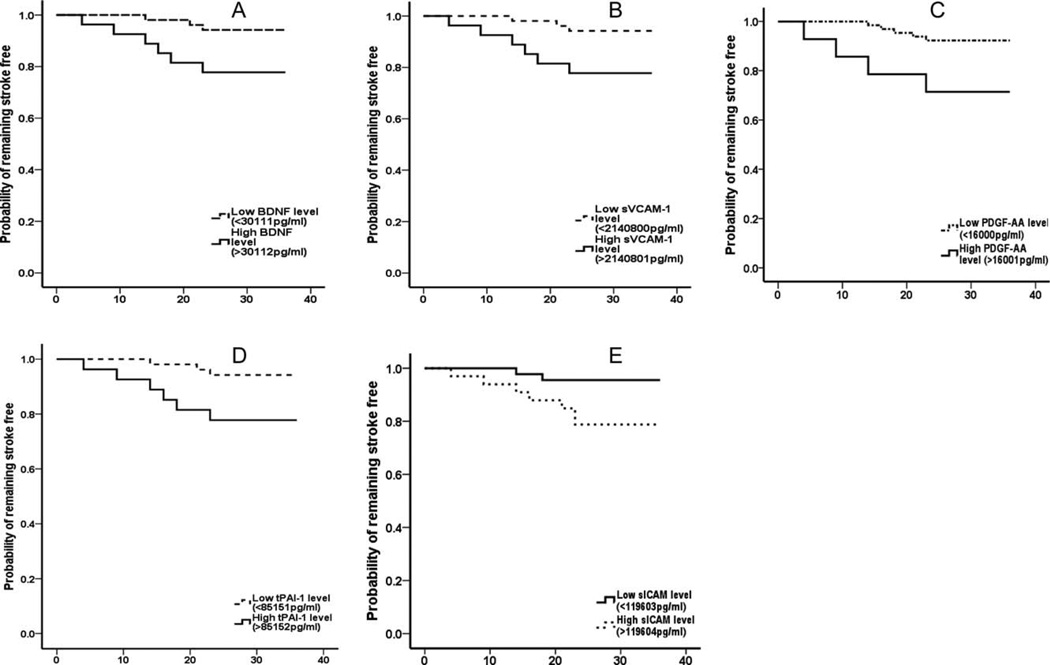
Serum biomarker concentrations were defined as either high or low as previously stated. This level was noted to be similar in value to the 65th–90th percentile of the serum concentration for similar biomarkers from our previous study [24]. A Kaplan–Meier survival analysis and Log Rank test showed that subjects with a low serum level of BDNF (<30.1 ng/mL, P = 0.025), sVCAM-1 (<2142 ng/mL, P = 0.025), PDGF-AA (<16.0 ng/mL, P = 0.01), tPAI-1 (<85 ng/mL, P = 0.025), and sICAM-1 (<119.6 ng/mL) had ≥92% probability of stroke free survival, while those with serum levels above these levels had a lower probability (≤78%) of stroke free survival (Fig. 2A–E).
Figure 2.
Probability of stroke-free survival. Subjects with low baseline levels of BDNF (P = 0.025), sVCAM-1 (P = 0.025), PDGF-AA (P = 0.01), tPAI-1 (P = 0.025), and sICAM-1 (P = 0.022) had a significantly higher probability of stroke free survival.
Discussion
We have previously demonstrated an association between high TCD velocity and elevated baseline serum concentrations of BDNF and PDGF-AA among children with SCA, and that elevated baseline serum concentration of PDGF-AA was a sensitive biomarker for predicting stroke development in children with SCA and high TCD velocity [28]. These findings suggest that PDGF-AA may be both a biomarker and mediator of cerebral artery remodeling that leads to symptomatic stroke in SCA.
In the current study, we extend our investigation to determine the effect of PRBC transfusions on the serum concentrations of biomarkers of endothelial activation, coagulation and neuroischemia, and the relationship of these biomarkers to stroke free survival. We found that serum concentrations of sVCAM-1, Cathepsin-D, PDGF-AA, RANTES (CCL5), and NCAM-1 were lower among subjects who receive PRBC transfusions in the STOP study, compared to those who received standard care. Soluble VCAM-1 and RANTES are both involved in endothelial activation, inflammatory cell adhesion, endothelial cell mediated thrombosis, and platelet activation [29,30]. NCAM-1 is associated with neuronal development, differentiation, and plasticity, and is elevated in response to cerebral ischemia [31,32], which is reported to be a feature and possible mediator of sickle cell associated cerebrovascular disease [33]. Cathepsin-D is involved in the propagation of endothelial inflammation by facilitating intimal infiltration by activated macrophages. In addition, it is thought to promote the release of pro-angiogenic factors from the extracellular matrix, contributing to angiogenesis via endothelial proliferation [34,35]. Our data suggests that transfusions have an anti-inflammatory effect, and reduces cerebral ischemia, endothelial activation and potential for thrombosis. However, serum MPO (a marker of neutrophil-mediated inflammation) was higher among the transfused subjects, compared to the SC arm. Since other markers of inflammation/endothelial activation, sICAM-1, sVCAM-1, and RANTES were lower in the transfused group, it is possible that PRBC transfusion might have less effect on neutrophil activation compared with other inflammatory and/or endothelial cells. Furthermore, a recent study showed that blood products (packed cells and fresh frozen plasma) accentuate expression of inflammatory markers and neutrophil transmigration through endothelial monolayers, which was associated with the fatty acid content of the blood product [36]. It is possible that neutrophils could also be activated in this setting and among the subjects that were on chronic PRBC transfusion.
Serum concentrations of biomarkers of endothelial activation and proliferation (sVCAM-1 and PDGF-AA) and thrombophilia (tPAI-1) were significantly negatively correlated with the number of PRBC transfusion received by subjects over the period of 4 years, with similar trends for biomarkers of cerebral tissue ischemia and adaptation (BDNF and NCAM-1). Adjusted for multiple comparisons and baseline levels, serum sVCAM-1 (P < 0.001), PDGF-AA (P = 0.025), and RANTES (P = 0.048) were significantly elevated among post-STOP subjects who received fewer than 19 transfusions over the 4-year period, compared with those who received more frequent (20 or more) transfusions over the same period. These results suggest that the protective effects of PRBC transfusions in preventing stroke in SCA are through reduction of endothelial activation, vascular cell proliferation, and thrombogenesis.
Subjects with high TCD velocities and low pre-intervention serum concentrations of BDNF, sVCAM-1, PDGF-AA, and tPAI-1 had a higher probability for stroke free survival, compared with those having high concentrations of these biomarkers. Conversely, the combination of high TCD velocity and high biomarker concentrations above the cut-off values was associated with higher likelihood of stroke. Individuals with high levels of these biomarkers may have had more severe vasculopathy at the start of the study and thus were more likely to develop stroke. Correlation of biomarker values with the original neuroimaging studies is needed to confirm this possibility. Such an effort is currently in progress by our group.
High biomarker concentrations in the presence of elevated TCD velocities show that these are concomitant processes at the time the subjects were diagnosed with high TCD velocities. The potential role of each protein as a mediator of cerebrovascular disease cannot yet be determined. To define the time course of events, we would need to prospectively and sequentially measure the biomarkers prior to the development of elevated TCD velocities. A project of this nature is currently pending IRB approval. It would be predicted that endothelial activation (sVCAM) and thrombogenesis (tPAI-1) would be elevated earliest, vascular cell proliferation (PDGF-AA) occurring afterwards, and cerebral ischemia (BDNF) occurring later.
Despite a relatively small sample size of 40 subjects per treatment group, our findings have strong statistical significance and exhibit a pattern that is patho-physiologically plausible. The four key biomarkers in our study represent patho-biological processes that have been described to be associated with cerebrovascular disease in SCA, i.e., cerebral ischemia, endothelial activation, vascular cell proliferation, and thrombosis [37–39]. Our data also support the concepts outlined by Hebbel et al. [20] and Kato et al. [33], that sickle cell-associated vasculopathy is mediated by endothelial dysregulation and inflammation and that mitigating these pathologies (in this case, using PRBC transfusion) will result in improved clinical outcome, as was observed in the STOP study. A surprising finding for us was the observation of higher baseline biomarkers levels among subjects randomized to SC compared with those randomized to receive transfusion. The reason is unclear given that the treatment group assignment was done randomly. However, conclusions regarding the effect of blood transfusion on serum biomarkers are still valid since our statistical analysis adjusted for baseline biomarkers levels.
In summary, this study has defined four biomarkers of cerebral ischemia, endothelial activation, vascular cell proliferation, and thrombosis that are associated with high TCD velocity and elevated stroke risk. Three of the biomarkers were significantly reduced in subjects who received frequent PRBC transfusions. Stroke free survival was significantly longer among subjects with high TCD velocities who also had lower pre-intervention biomarker levels. Our results illustrate potential pathophysiologic mechanisms of SCA-associated cerebrovascular disease and the beneficial role of PRBC transfusion in reducing the effects of these processes. Future prospective studies are warranted to validate these findings, with larger subject groups and beginning before the onset of high TCD velocities. Identifying key circulating mediators in the development of cerebral vasculopathy and stroke in SCA may allow for earlier diagnosis of stroke risk, more refined risk prediction and personalized medical therapy, and the potential for developing therapies that directly target mechanistic pathways.
Supplementary Material
Acknowledgments
We are grateful to Ellen Debenham, RN; Judy Luden; Corinne Hilbert and staffs of Dr. Kindy’s lab (all at MUSC) and Jane Chu from the Protein Profiling Core at MSM for their logistical support in retrieving the samples and equipment setup. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Department of Defense.
Contract grant sponsor: National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health; Contract grant number: UL1TR000454.
Contract grant sponsor: National Institute on Minority Health and Health Disparities (NIMHD); Contract grant number: Contract grant number: 8 U54 MD007588-04.
Contract grant sponsor: National Center for Research Resources (NCRR); Contract grant number: U54 RR026137.
Contract grant sponsor: NIMHD; Contract grant number: Contract grant number: S21 MD000101-05.
Contract grant sponsor: Children’s Healthcare of Atlanta Friends Pilot Project Award, the MUSC/Department of Defense Cooperative Agreement (SE VIEW); Contract grant number: Contract grant number: W81XWH-10-2-0057.
Contract grant sponsor: Cooperative Agreements with the National Heart, Lung, and Blood Institute; Contract grant number: Contract grant numbers: U10 HL 52193 and U10 HL 52016.
Contract grant sponsor: NIH/NHLBI; Contract grant number: Contract grant numbers: R21HL092358 and NIH/NCRR, 5P20RR0111044 pilot.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to report
References
- 1.Centers for Disease Control and Prevention. Atlanta: Centers for disease Control and Prevention; 2012. Children with sickle cell disease had significantly higher medical costs than those without sickle cell disease. [Google Scholar]
- 2.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prevent Med. 2010;38:S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Smith LA, Oyeku SO, Homer C, et al. Sickle cell disease: A question of equity and quality. Pediatrics. 2006;117:1763–1770. doi: 10.1542/peds.2005-1611. [DOI] [PubMed] [Google Scholar]
- 4.Weatherall DJ. Hemoglobinopathies worldwide: Present and future. Curr Mol Med. 2008;8:592–599. doi: 10.2174/156652408786241375. [DOI] [PubMed] [Google Scholar]
- 5.Pauling L, Itano HA. Sickle cell anemia a molecular disease. Science (New York, NY) 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 6.Noubouossie DF, Lê PQ, Corazza F, et al. Thrombin generation reveals high procoagulant potential in the plasma of sickle cell disease children. Am J Hematol. 2012;87:145–149. doi: 10.1002/ajh.22206. [DOI] [PubMed] [Google Scholar]
- 7.Hibbert JM, Hsu LL, Bhathena SJ, et al. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med. 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driss A, Asare KO, Hibbert JM, et al. Sickle cell disease in the post genomic era: A monogenic disease with a polygenic phenotype. Genom Insights. 2009;2:23–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 10.Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 11.Jordan LC, Casella JF, DeBaun MR. Prospects for primary stroke prevention in children with sickle cell anaemia. Br J Haematol. 2012;157:14–25. doi: 10.1111/j.1365-2141.2011.09005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoppe C. Defining stroke risk in children with sickle cell anaemia. Br J Haematol. 2005;128:751–766. doi: 10.1111/j.1365-2141.2004.05310.x. [DOI] [PubMed] [Google Scholar]
- 13.Dobson SR, Holden KR, Nietert PJ, et al. Moyamoya syndrome in childhood sickle cell disease: A predictive factor for recurrent cerebrovascular events. Blood. 2002;99:3144–3150. doi: 10.1182/blood.v99.9.3144. [DOI] [PubMed] [Google Scholar]
- 14.Gebreyohanns M, Adams RJ. Sickle cell disease: Primary stroke prevention. CNS Spectrum. 2004;9:445–449. [PubMed] [Google Scholar]
- 15.Hulbert ML, Scothorn DJ, Panepinto JA, et al. Exchange blood transfusion compared with simple transfusion for first overt stroke is associated with a lower risk of subsequent stroke: A retrospective cohort study of 137 children with sickle cell anemia. J Pediatr. 2006;149:710–712. doi: 10.1016/j.jpeds.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Deane CR, Goss D, Bartram J, et al. Extracranial internal carotid arterial disease in children with sickle cell anemia. Haematologica. 2010;95:1287–1292. doi: 10.3324/haematol.2010.022624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 18.Ware RE, Helms RW. Stroke with transfusions changing to hydroxyurea (SWiTCH) Blood. 2012;119:3925–3932. doi: 10.1182/blood-2011-11-392340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams RJ, Brambilla D. Optimizing primary stroke prevention in sickle cell anemia (STOP 2) trial investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski JL, Cohen AR, Garro J, et al. Transfusional iron overload in children with sickle cell anemia on chronic transfusion therapy for secondary stroke prevention. Am J Hematol. 2012;87:221–223. doi: 10.1002/ajh.22228. [DOI] [PubMed] [Google Scholar]
- 21.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117:772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asare K, Gee BE, Stiles JK, et al. Plasma interleukin-1β concentration is associated with stroke in sickle cell disease. Cytokine. 2010;49:39–44. doi: 10.1016/j.cyto.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato GJ, Hebbel RP, Steinberg MH, et al. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkel K, Ginsberg P, Parker J, et al. Cerebrovascular disease in sickle cell anemia: A clinical, pathological and radiological correlation. Stroke. 1978;9:45–52. doi: 10.1161/01.str.9.1.45. [DOI] [PubMed] [Google Scholar]
- 25.Prohovnik I, Hurlet-Jensen A, Adams R, et al. Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. J Cereb Blood Flow Metab. 2009;29:803–810. doi: 10.1038/jcbfm.2009.6. [DOI] [PubMed] [Google Scholar]
- 26.Prohovnik I, Pavlakis SG, Piomelli S, et al. Cerebral hyperemia, stroke, and transfusion in sickle cell disease. Neurology. 1989;39:344. doi: 10.1212/wnl.39.3.344. [DOI] [PubMed] [Google Scholar]
- 27.Lezcano NE, Odo N, Kutlar A, et al. Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 2006;37:1424–1426. doi: 10.1161/01.STR.0000221173.97108.01. [DOI] [PubMed] [Google Scholar]
- 28.Hyacinth HI, Gee BE, Adamkiewicz TV, et al. Plasma BDNF and PDGF-AA levels are associated with high TCD velocity and stroke in children with sickle cell anemia. Cytokine. 2012;60:302–308. doi: 10.1016/j.cyto.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weyrich AS, Prescott SM, Zimmerman GA. Platelets, endothelial cells, inflammatory chemokines, and restenosis: Complex signaling in the vascular play book. Circulation. 2002;106:1433–1435. doi: 10.1161/01.cir.0000033634.60453.22. [DOI] [PubMed] [Google Scholar]
- 30.Jin C, Lu L, Zhu ZB, et al. Increased serum vWF and sVCAM-1 levels are associated with late or very late angiographic stent thrombosis after sirolimus-eluting stent implantation. Coron Artery Dis. 2010;21:273–277. doi: 10.1097/MCA.0b013e32833b20f1. [DOI] [PubMed] [Google Scholar]
- 31.Axell MZ, Zlateva S, Curtis M. A method for rapid derivation and propagation of neural progenitors from human embryonic stem cells. J Neurosci Methods. 2009;184:275–284. doi: 10.1016/j.jneumeth.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Seki T, Sato K, et al. Expression of polysialylated neural cell adhesion molecule in rat brain after transient middle cerebral artery occlusion. Brain Res. 2001;907:130–133. doi: 10.1016/s0006-8993(01)02543-4. [DOI] [PubMed] [Google Scholar]
- 33.Nur E, Kim Y-S, Truijen J, et al. Cerebrovascular reserve capacity is impaired in patients with sickle cell disease. Blood. 2009;114:3473–3478. doi: 10.1182/blood-2009-05-223859. [DOI] [PubMed] [Google Scholar]
- 34.Briozzo P, Badet J, Capony F, et al. MCF7 mammary cancer cells respond to bFGF and internalize it following its release from extracellular matrix: A permissive role of cathepsin D. Exp Cell Res. 1991;194:252–259. doi: 10.1016/0014-4827(91)90362-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.