Abstract
Objective
To determine the frequency of significant liver injury and acute liver failure (ALF) in patients with ornithine transcarbamylase deficiency (OTCD), the most common urea cycle defect (UCD).
Study design
A historical cohort study was performed. Charts were reviewed at two centers to assess the proportion of 71 individuals with OTCD who had evidence of ALF (INR ≥ 2.0), liver dysfunction (INR 1.5–1.99), or hepatocellular injury (AST/ALT≥ 250 IU/L).
Results
57% of the 49 patients with symptomatic OTCD had liver involvement: 29% met the criteria for ALF, 20% had liver dysfunction, and 8% had isolated hepatocellular injury. The proportion with ALF was greatest in those with more severe OTCD, including neonates with markedly elevated ammonia levels (> 1,000 μmol/L). Some patients with severe liver involvement (INR ≥ 2.0 and AST/ALT > 1,000 IU/L) had only moderate hyperammonemia (100 – 400 μmol/L). ALF was the initial presenting symptom of OTCD in at least 3 of 49 symptomatic OTCD patients.
Conclusions
Episodes of hepatocellular injury, liver dysfunction, and ALF were identified in a high proportion of individuals with symptomatic OTCD. The more severely affected OTCD patients had a higher likelihood of ALF. The diagnosis of a UCD should be considered in unexplained ALF, liver dysfunction or hepatocellular injury.
Keywords: Urea cycle defects, hyperammonemia, acute liver failure, elevated AST/ALT, Reye syndrome, orotic acid
Accurate diagnosis of an underlying etiology is crucial to outcome in acute liver failure (ALF)1. Up to 50% of children with ALF2, and ~15% of adults with ALF1, are of “indeterminate” cause. Genetic metabolic disorders are an important cause of ALF in children, comprising 9.7% of final diagnoses in a large international multi-site observational study2, and 42.5% of final diagnoses in patients presenting at less than one year to a single center3. Metabolic disorders presenting with ALF include galactosemia, tyrosinemia type 1, fatty acid oxidation defects, Wilson disease, mitochondrial hepatopathies, and others4,5. Narkewicz et al2 emphasized that a systematic evaluation for treatable causes in children with ALF, including metabolic diseases, does not occur routinely in many centers.
Urea cycle defects (UCDs) occur in approximately 1/30,000 live births6. These disorders are not considered prominent among metabolic diseases that cause severe hepatic dysfunction and ALF4,5, despite past reports of hepatocellular injury and ALF in individuals with ornithine transcarbamylase deficiency (OTCD)7–11. OTCD is the most common UCD, and is an X-linked genetic disorder that affects both males and females. Severely affected males present with marked hyperammonemia in the newborn period. There is variable clinical expression in heterozygote females and in males with residual OTC enzyme activity; individuals of either sex may remain asymptomatic throughout their lifetime with no episodes of hyperammonemia, and symptomatic individuals, with at least one episode of hyperammonemia, can present at any age12. Hepatic histology in OTCD may show microvesicular steatosis, focal cell necrosis, aggregates of clear hepatocytes, portal to portal bridging fibrosis, abnormal mitochondria, abnormal peroxisomes, or may appear normal13–19. Other UCDs have been associated with hepatocellular injury and liver failure as well20–28. Sundaram et al29 reported that 2 of 148 infants less than 3 months of age (1.4%) with ALF were diagnosed with a UCD, one with OTCD. Despite these reports, OTCD and other UCDs are rarely considered in children and adults presenting with severe liver injury or ALF unless profound hyperammonemia is present; this has resulted in delayed or post-mortem diagnosis11. The goal of this study was to determine the frequency of significant liver injury and ALF in patients with OTCD.
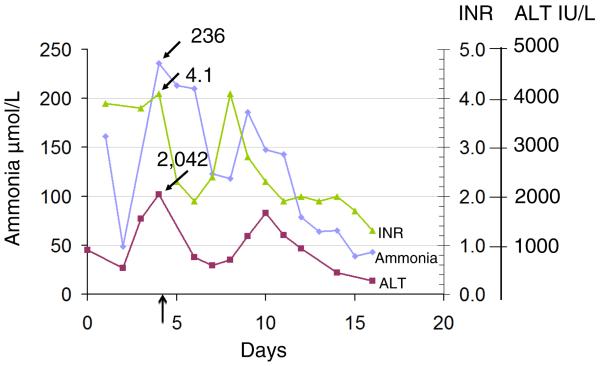
An illustrative case is a 19-month-old female who was transferred to UCLA Medical Center for evaluation for liver transplant because of ALF of unknown origin. She had presented to an outside hospital with fever, vomiting, and lethargy, and was found to have an ALT of 906 IU/L, an INR of 3.9, a PTT of 46, and ammonia of 161 μmol/L. Evaluation for infectious hepatitis, autoimmune hepatitis, Wilson disease, and acetaminophen toxicity was negative. Liver biopsy showed acute hepatocellular injury with mild lobular necrosis. Upon further testing it was noted that orotic acid and uracil were elevated in the patient's urine. Pharmacologic treatment of OTCD was initiated on day 10 of hospitalization. Laboratory abnormalities normalized after treatment (Figure 1). The diagnosis of OTCD was confirmed by genotyping which identified a heterozygous c.67C>T (p.R23X) mutation. Liver tissue was not available for enzyme analysis (part 2, case 8, Table III; available at www.jpeds.com).
Figure 1.
Time course of plasma ammonia, ALT, and INR in a severe OTCD female during her initial hospitalization at 19 months of life. Dietary treatment was instituted on day 7, and full medical therapy on day 10. The vertical black arrow indicates the time point collected for the chart review in this hyperammonemic episode (part 2, case 8; Table III).
Table 3.
Part 1 OTCD Cases at Children's Hospital Colorado
| Case No. Current status/Rx Liver Injury |
Gender | Age | Presentation | AST IU/L |
ALT IU/L |
PT sec |
INR | PTT sec |
Bili | NH3 umol/L |
V | VII | VIII | Liver histology | Fibrinogen | D- dimers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neonatal Males | ||||||||||||||||
| 1 Deceased ALF |
M | 4 d | 3 day h/o increased RR, progressive encephalopathy | 104 | 81 | 37.6 | 3.83 | 65 | 6.0 | 2634 | ND | ND | ND | ND | 71 | 5.73 |
| 2 Deceased ALF |
M | 2 d | Increased RR, jitteriness, progressive encephalopathy | 91 | 22 | 35.6 | 3.3 | >250 Dialysis | 10.0 | 2125 | ND | ND | ND | ND | 119 | 1.62 |
| 3 Deceased ALF |
M | 2 d | Respiratory distress, lethargy | ND | ND | 24.3 | ND | 39.3 | 5.1 | 1398 | ND | ND | ND | Normal | 140 | ND |
| 4 Deceased ALF |
M | 2 d | Increased RR, twitching, apnea | 45 | 32 | 24.0 | 2.1 | 45 | 8.4 | 1308 | ND | ND | ND | OTC enzyme activity 2%. Microvesicular steatosis, mild cholestasis | 112 | 3.64 |
| 5 Deceased LD |
M | 2 d | Poor feeding, lethargy | 37 | 130 | 17.0 | 1.57 | 109 | 3.1 | 24 (peak 1939) | ND | ND | ND | ND | 108 | 1017 |
| 6 Tx LD HCI |
M | 2 d | Emesis | 467 | 256 | 17.8 | 1.43 | 39 | 5.4 | 1152 | ND | ND | ND | Unremarkable | 214 | 1.79 |
| Severe OTCD | ||||||||||||||||
| 7 Tx ALF HCI |
F | 1 year | Liver failure, h/o emesis, “reflux” (Had multiple later HA events without LF) |
4139 | 2089 | 63.7 | 5.7 | 49.9 | 0.4 | 207 | ND | ND | ND | Microvesicular steatosis, mild inflammation, mild portal fibrosis | ND | ND |
| 8 Tx ALF HCI |
F | Prenatal | Known family history, liver failure at 2 years | 2232 | 2127 | 23.8 | 2.52 | 45 | 0.8 | 49 (was > 100) | 55 (low) | 9.2 (low) | 230 | OTC enzyme activity 4% Increased glycogen, no fibrosis, diffuse microvesicular steatosis | 263 | 333 |
|
ALF
HCI |
Same pt as above | Same pt as above | Same pt as above | 1538 | 1165 | 21.5 | 1.8 | 45.4 | 1.2/0.5 | 40 (was 178) | ND | ND | ND | Same pt as above | ND | ND |
|
ALF
HCI |
Same pt as above | Same pt as above | Same pt as above | 2401 | 1692 | 23.6 | 1.97 | 43.6 | 0.5 | 216 | 39.9 (low) | 17.7 (low) | 130.2 | Same pt as above | ND | < 200 |
|
ALF
HCI |
Same pt as above | Same pt as above | Same pt as above | 74 (went up to 869) | 178 (went up to 997) | 52.3 | 3.76 | 58.5 | 0.4 | 171 | 60.4 (low) | <4.0 (low) | 192 | Same pt as above | 227 | < 230 |
| 9 Tx ALF HCI |
F | 5 years | Emesis, lethargy with UTIs | 377 | 1083 | 19 | 2.4 | ND | ND | ND | ND | ND | ND | OTC enzyme activity 7.8% Diffuse glycogenation of hepatocytes with minimal portal fibrosis | ND | ND |
|
ALF
HCI |
Same pt as above | Same pt as above | Same pt as above | 57 | 446 | 18.8 | 2.66 | ND | ND | ND | ND | ND | ND | Same pt as above | ND | ND |
|
ALF
HCI |
Same pt as above | Same pt as above | Same pt as above | 1212 | 1749 | 28.4 | ND | ND | 1.1/0.4 | 41 (was 167) | 49 (low) | 19 (low) | ND | Same pt as above | ND | ND |
| LD | Same pt as above | Same pt as above | Same pt as above | ND | ND | 15.7 | 1.52 | ND | ND | 88 (was 118) | ND | ND | ND | Same pt as above | ND | ND |
| 10 Diet/Bu/Cit LD HCI |
F | 3 years | 2 year h/o emesis, AMS, recurrent vision loss | 2039 | 1605 | 17.5 | 1.39 | 40 | 0.8 | 111 (was 410) | ND | ND | ND | ND | 227 | 1.09 |
| 11 Tx No liver injury |
F | 4 years | Mother died of OTCD | 20 | 41 | 14.9 | 1.29 | 37.3 | 0.4 | 106 | ND | ND | ND | Path at tx – mild peripoprtal fibrosis, patchy centrilobular architectural collapse | ND | ND |
| 12 Tx No liver injury |
F | 3 d | Lethargy and poor feeding, progressive encephalopathy | 80 | 169 | ND | ND | ND | 0.4 | 192 | ND | ND | ND | OTC activity very low Ballooning hepatocytes, multinucleation granular cytoplasm, minimal periportal fibrosis | ND | ND |
| Moderate OTCD | ||||||||||||||||
| 13 Diet/Bu/Arg ALF HCI |
M | 3 years | Recurrent emesis | 615 | 1938 | 22.8 | 1.84 | 38 | 0.2/0.0 | 46 (was 160) | ND | ND | ND | OTC activity 10% Patchy ballooning hepatocytes, glycogneated nuclei | ND | ND |
|
ALF
HCI |
Same pt as above | Same pt as above | See above | ND | 1088 | ND | 2.1 | ND | ND | ND | ND | ND | ND | Same pt as above | ND | ND |
| HCI | Same pt as above | Same pt as above | See above | ND | 1530 | ND | ND | ND | ND | ND | ND | ND | ND | Same pt as above | ND | ND |
| 14 Diet/Bu/Arg ALF HCI |
M | 3 years | Illness, affected sib | 785 | 1223 | 34.9 | 3.1 | 48 | 0.3 | 108 | ND | ND | ND | ND | ND | ND |
| Mild OTCD | ||||||||||||||||
| 15 Diet/Be/Cit LD |
F | 2 years | Altered mental status | 41 | 67 | 17.6 | 1.4 | 55 | 0.4 | 198 | ND | ND | ND | ND | ND | ND |
| 16 LD Arg |
M | 11 y | Valproate induced hyperammonema h/o autism, delay, recurrent emesis | 14 | 14 | 15.6 | 1.21 | 29 | 0.1 | 144 | ND | ND | ND | ND | ND | ND |
| LD | Same pt as above | Same pt as above | Same pt as above | 23 | 10 | 16 | 1.28 | 33 | 0.4 | <9 | ND | ND | ND | Same pt as above | 232 | ND |
| 17 Cit LD |
F | NA | Family history, brother with neonatal OTC | 18 | 27 | 15.4 | 1.16 | 30 | 0.6 | 91 | ND | ND | ND | ND | ND | ND |
| LD | Same pt as above | Same pt as above | Same pt as above | 17 | 27 | 15.8 | 1.28 | 32 | 0.7 | 99 | ND | ND | ND | Same pt as above | 236 | ND |
| LD | Same pt as above | Same pt as above | Same pt as above | 21 | 28 | 15.3 | 1.18 | 31 | 0.9 | 35 | ND | ND | ND | Same pt as above | ND | ND |
| LD | Same pt as above | Same pt as above | Same pt as above | 26 | 43 | 15.6 | 1.22 | 30 | 1.2 | ND | ND | ND | ND | Same pt as above | 281 | ND |
| 18 Bu/Cit LD |
F | 2 y | Intermittent ataxia, abnormal eye movements, family history, brother with neonatal OTC | 43 | 31 | 15.1 | 1.16 | 39 | 0.3 | 48 | ND | ND | ND | ND | ND | ND |
| LD | Same pt as above | Same pt as above | Same pt as above | 25 | 18 | 15.2 | 1.14 | 33 | 0.5 | 56 | ND | ND | ND | Same pt as above | ND | ND |
| LD | Same pt as above | Same pt as above | Same pt as above | 26 | 14 | 15.4 | 1.19 | 37 | 0.4 | 36 | ND | ND | ND | Same pt as above | 175 | ND |
| 19 Arg HCI |
M | 1st year | Recurrent emesis, increased AST/ALT | 519 | 689 | ND | ND | ND | 0.2/0.8 | ND | ND | ND | ND | Scattered individual hepatocytes with nonspecific degeneration | ND | ND |
| 20 No Rx HCI |
M | Neonatal presentation, suspected mosaic due to current status | 150 | 317 | 13.6 | 1.26 | 35 | 0.5 | 33 | ND | ND | ND | ND | ND | ND | |
| 21 Bu/Arg No liver injury |
F | 27 y | Son with neonatal OTCD | 30 | 14 | 14.0 | 1.04 | 28 | 0.6 | 23 | ND | ND | ND | ND | 208 | ND |
| 22 No current Rx, was on Bu/Arg No liver injury |
F | 37 y | Family history, possible seizures | 23 | 39 | 13 | 0.95 | 29 | 0.2 | 29 | ND | ND | ND | ND | 271 | ND |
| No Symptoms/Treatment | ||||||||||||||||
| 23 No Rx LD |
F | NA | Family history | 12 | 14 | 16.2 | 1.27 | 32 | 0.4 | 57 | ND | ND | ND | ND | 195 | ND |
| 24 No Rx LD |
F | NA | Family history | 51 | 44 | 15.6 | 1.21 | 33 | 0.4 | 40 | ND | ND | ND | ND | 201 | ND |
| 25 No Rx No liver injury |
M | 10 y | Brother with hyperammonemia | 37 | 19 | 14.2 | 1.09 | 32 | 0.7 | 22 | ND | ND | ND | ND | 252 | ND |
| 26 No Rx No liver injury |
F | NA | Family history | 34 | 49 | 13.3 | 0.98 | 30 | 0.3 | <9 | ND | ND | ND | ND | 304 | ND |
| 27 No Rx No liver injury |
F | NA | Family history | 19 | 11 | 12.7 | 0.92 | 29 | 0.3 | 13 | ND | ND | ND | ND | 369 | ND |
| 28 Cit since birth, Asx No liver injury |
F | NA | Affected brother | 62 | 21 | 13.4 | 0.98 | 30 | 1.2 | 20 | ND | ND | ND | ND | 249 | ND |
| 29 No Rx No liver injury |
F | NA | Three affected children | 20 | 12 | 13.1 | 0.96 | 32 | 0.5 | 57 | ND | ND | ND | ND | 351 | ND |
| 30 No Rx No liver injury |
F | 37 | Affected son | 34 | 19 | 13.6 | 1.01 | 29 | 0.5 | 39 | ND | ND | ND | ND | 285 | ND |
| 31 No Rx No liver injury |
F | 21 | Affected son | 15 | 12 | 12.8 | 0.94 | 29 | 0.4 | < 9 | ND | ND | ND | ND | 342 | ND |
| Table 3 Part 2. OTCD Cases at UCLA. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. Current status/Rx Liver Injury |
Gender | Age | Presentation | AST IU/L |
ALT IU/L |
PT sec |
INR | PTT sec |
Bili | NH3 umol/L |
V | VII | VIII | Liver histology |
Fibrinogen | D- dimers |
| Neonatal Males | ||||||||||||||||
| 1 Deceased ALF |
M | 2 d | Poor feeding, lethargy, then seizure, then apnea, and then NH3 = 1734. | 202 | 188 | 32.9 | 3.2 | 151.2 | 14.1/1.9 | 1293 | ND | ND | ND | ND | 67 | ND |
| 2 Deceased ALF |
M | 9 d | Pulmonary hemorrhage on DOL 3, hyperammonemia on DOL 9. | 138 | 94 | 22.8 | 2.3 | 38.7 | 25.2/14.6 | 1744 | 19.9% act. | 19.6% act. | ND | Hepatomegaly, splenomegaly; green, homogeneous liver parenchyma, geographic fibrosis with bile stasis noted. | 158 | ND |
| 3 Tx ALF HCI |
M | 1 d | Brother had passed away of OTC, lethargy on DOL 1, needed hemodialysis. | 406 | 242 | 32.6 | 6.4 | >180 | 8.2/5.8 | 256 | ND | ND | ND | Diffuse macro and micro vesicular steatosis, moderate cholestasis, no evidence of hepatitis. | 66 | ND |
| 4 Tx No Liver Injury |
M | 2 d | Poor feeding, irritable, hyperammonemia, coma by 36 hours of life | 93 | 93 | 14.3 | 1.1 | 41 | 4.3 | 143 | ND | ND | ND | ND | ND | ND |
| 5 Diet/Bu/Be No Liver Injury |
M | prenatal | Diagnosed in utero (mother is OTC carrier), neonatal seizures. | 49 | 39 | 10.8 | 1.1 | 31.3 | 0.6 | 41 | ND | ND | ND | ND | 159 | ND |
| 6 Tx No Liver Injury |
M | 1 d | Poor feeding and lethargy at 24–30 hours of life. | 25 | 22 | 8.6 | 0.9 | 26 | 2.2/1.0 | 754 | ND | ND | ND | 233 | ND | |
| Severe OTCD | ||||||||||||||||
| 7 Diet/cit/arg/Be/Ac ALF HCI |
F | 5 y | Increased irritability with ketosis. | 419 | 900 | 37.7 | 3.5 | 62 | 0.6 | 133 | ND | ND | ND | ND | ND | ND |
|
ALF
HCI |
Same pt as above | Same pt as above | See above | 3204 | 4407 | 26.3 | 2.4 | 43 | 1.4 | 492 | ND | ND | ND | ND | 176 | 2 |
| 8 Diet/Bu/Cit ALF HCI |
F | 1 yo 7 m | Presented with h/o chronic n/v, with acute fever, vomiting, seizures found to be in acute liver failure. | 1932 | 2042 | 39.5 | 4.1 | 39.9 | 0.4/0.1 | 220 | 70% | 11% act. | 211% act | Acute hepatocellular injury with mild lobular necrosis, paucity of inflammatory infiltrate. | 245 | ND |
|
ALF
HCI |
Same pt as above | Same pt as above | Same pt as above | 6299 | 5519 | 21.4 | 2.1 | 35.1 | 0.3 | 443 | 39% | 13% | ND | ND | 228 | ND |
| 9 Diet/Bu/Arg Carnitine No Liver Injury |
F | 6 y | Reccurent abd pain/n/v/ataxia. | 16 | 16 | 10.6 | 1 | 27.8 | 0.4 | 174 | ND | ND | ND | ND | 242 | ND |
| Moderate OTCD | ||||||||||||||||
| 10 Diet/Bu/Cit HCI |
F | 3 yo 7 m | AGE with persistently elevated AST/ALT | 59 | 216 | 11.9 | 1.2 | 31.3 | 0.5 | 253 | ND | ND | ND | ND | 236 | ND |
| HCI | Same pt as above | Same pt as above | Same pt as above | 309 | 65 | ND | ND | ND | 0.6 | ND | ND | ND | ND | ND | ND | ND |
| 11 Diet/Be LD) |
F | 12 m | Lethargy, hyperammonemia, left sided weakness, multiple, bilateral foci of stroke R>L. | 123 | 167 | 17.5 | 1.8 | 30.2 | 0.5 | 97 | ND | ND | ND | ND | 181 | ND |
| 12 Diet/Bu LD |
M | 20 m | Chronic emesis, elevated ammonia. | 21 | 25 | 15.2 | 1.5 | 26 | 0.5 | 235 | ND | ND | ND | ND | 206 | ND |
| 13 Diet/Be/Cit LD HCI |
F | 13 m | Fever, emesis × 5 mo, lethargy, irritability, elevated liver enzymes, coagulopathy. | 337 | 959 | 16.6 | 1.7 | 53 | 0.2 | 159 | ND | ND | ND | ND | 305 | ND |
| 14 Diet/Bu No Liver Injury |
F | 4 y | N/v + FH of OTC. in brother | 11 | 13 | 11.6 | 1.1 | 26.3 | 0.4 | 213 | ND | ND | ND | ND | 192 | ND |
| 15 Diet/Bu No Liver Injury |
M | 9 y | Occasional lethargy and inability to function, hyperammonemia. | 14 | 25 | 10.6 | 1 | 27.2 | 0.6 | 132 | ND | ND | ND | ND | 222 | ND |
| 16 Diet No Liver Injury |
M | 13 m | URI + lethargy, hyperammonemia. | 102 | 191 | 11.4 | 1.1 | 28 | 0.5 | 214 (later 332) | ND | ND | ND | ND | 198 | ND |
| 17 Diet/Cit No Liver Injury |
M | 12 y | Hyperammonemia with coma. | 18 | 28 | 11.4 | 1.1 | 30 | 0.8 | 55 | ND | ND | ND | ND | 165 | ND |
| 18 Diet/BuCit No Liver Injury |
F | ND | ND | 20 | 13 | 10.4 | 1 | 26 | 0.4 | 38 | ND | ND | ND | ND | 226 | ND |
| 19 Diet/Cit/Arg Carnitine No Liver Injury |
F | 16–20 m | Hyperammonemia and coma. | 14 | 21 | 10.8 | 1.1 | 29.6 | ND | 86 | ND | ND | ND | ND | 285 | ND |
| Mild OTCD | ||||||||||||||||
| 20 Diet/Bu HCI |
F | 7 yo 2 m | Frank confusion and hyperammonemia with h/o intermittent abdominal pain and nausea. | 97 | 252 | 11.3 | 1.1 | ND | 0.3 | 27 (was 297) | ND | ND | ND | ND | ND | ND |
| 21 Diet/Be/Cit No Liver Injury |
F | 1 y | Nausea/vomiting + FH with sister with OTC. | 41 | 14 | ND | ND | ND | 0.4 | 35 | ND | ND | ND | ND | 226 | ND |
| 22 Diet/BuArg No Liver Injury |
F | 2 m | FH of brother with OTC | 25 | 20 | 11.3 | 1.2 | ND | ND | 55 | ND | ND | ND | ND | ND | ND |
| 23 Diet/Be/Cit/Carnitine No Liver Injury |
M | 3 y 2 m | Lethargy, hyperammonemia, nausea, vomiting, and history of headache head hitting and decreased attention. | 24 | 11 | 10.7 | 1 | 29.6 | 0.7 | 42 | ND | ND | ND | ND | 253 | ND |
| 24 Deceased No Liver Injury |
M | 13 y 11 m | H/o learning disabilities, and schizoaffective disorder, lethargy, coma, hyperammonemia, brain herniation | 78 | 63 | 13.4 | 1.4 | 43 | 0.4/0.1 | 2372 | ND | ND | ND | Hepatomegaly (3030g), diffuse micro and macrovesicular steatosis. | ND | ND |
| 25 Diet/Bu No Liver Injury |
F | 9 m | Stroke and hyperammonemia | 14 | 10 | ND | ND | ND | 0.3 | 63 | ND | ND | ND | ND | ND | ND |
| 26 No Rx No Liver Injury |
M | 30 y | Coma with Meclizine + FH (brother with OTC) | 14 | 22 | 10.4 | 1 | 30.5 | ND | 32 | ND | ND | ND | ND | 335 | ND |
| 27 Diet/Cit No Liver Injury |
F | ND | Infrequent irritability, + FH (3 sisters with OTC) | 23 | 18 | 11.8 | 1.1 | 30.5 | 2.1 | 60 | ND | ND | ND | ND | ND | ND |
| No Symptoms/Treatment | ||||||||||||||||
| 28 No Rx No Liver Injury |
F | N/A | Family History | 12 | 11 | 10.8 | 1.1 | 27 | 0.4 | 65 | ND | ND | ND | ND | 282 | ND |
| 29 No Rx No Liver Injury |
F | N/A | Family History | 15 | 15 | 10.4 | 1 | 27.9 | 0.4 | 39 | ND | ND | ND | ND | 245 | ND |
| 30 No Rx No Liver Injury |
F | N/A | Family History | 12 | 13 | 10.4 | 1 | 25.9 | 0.4 | 43 | ND | ND | ND | ND | 319 | ND |
| 31 No Rx No Liver Injury |
F | N/A | ADHD and Family History | 17 | 26 | 10.6 | 1 | 28 | 0.7 | 49 | ND | ND | ND | ND | 249 | ND |
| 32 No Rx No Liver Injury |
F | N/A | Family History | 10 | 11 | 11 | 1.1 | 29.1 | 0.5 | 40 | ND | ND | ND | ND | 230 | ND |
| 33 No Rx No Liver Injury |
F | N/A | Family History | 21 | 18 | 10.1 | 1 | 28.3 | 0.5 | 37 | ND | ND | ND | ND | 298 | ND |
| 34 No Rx No Liver Injury |
F | N/A | Family History | 10 | 11 | 10.7 | 1 | 28.9 | 0.3 | 23 | ND | ND | ND | ND | 341 | ND |
| 35 No Rx No Liver Injury |
F | N/A | Family History (son) | 14 | 17 | 11 | 1.1 | 27.5 | 0.5 | 26 | ND | ND | ND | ND | 310 | ND |
| 36 No Rx No Liver Injury |
F | N/A | Family History (son) | 24 | 22 | 10.5 | 1 | 25.9 | 0.5 | 34 | ND | ND | ND | ND | 217 | ND |
| 37 No Rx No Liver Injury |
F | N/A | Family History | 20 | 16 | 11.2 | 1.1 | 24.5 | 0.6 | 25 | ND | ND | ND | ND | 142 | ND |
| 38 No Rx No Liver Injury |
F | N/A | Family History | 14 | 12 | 10.8 | 1 | 26.7 | 0.5 | 105 | ND | ND | ND | ND | 250 | ND |
| 39 No Rx No Liver Injury |
F | N/A | Family History | 20 | 20 | 10.6 | 1 | 28.7 | 1.1 | 32 | ND | ND | ND | ND | 256 | ND |
| 40 No Rx No Liver Injury |
M | N/A | Family History (daughters) | 24 | 25 | 11.7 | 1.2 | 29.4 | 1 | 78 | ND | ND | ND | ND | 212 | ND |
Legend: LF: Acute liver failure. LD: Liver dysfunction. HCI: Hepatocellular injury. RR: Respiratory rate. Rx: Treatment. Tx: Liver transplant. ND: Not done. AMS: Altered mental status. Diet: Dietary therapy, restriction of natural protein +/− use of essential amino acids. Arg: Arginine therapy. Cit: Citrulline therapy. Bu: Sodium phenylbutyrate. Be: Sodium benzoate. Asx: Asymptomatic. NH3: Ammonia. HA: Hyperammonemic.
Legend: ALF: Acute liver failure. LD: Liver dysfunction. HCI: Hepatocellular injury. RR: Respiratory rate. Rx: Treatment. Tx: Liver transplant. ND: Not done. AMS: Altered mental status. Diet: Dietary therapy, restriction of natural protein +/− use of essential amino acids. Arg: Arginine therapy. Cit: Citrulline therapy. Bu: Sodium phenylbutyrate. Be: Sodium benzoate. Asx: Asymptomatic. NH3: Ammonia. HA: Hyperammonemic. N/v: Nausea and vomiting. DOL: day of life. FH: Family History. URI: Upper respiratory tract infection. ADHD: Attention deficit hyperactivity disorder.
METHODS
A historical cohort study was conducted at two large metabolic disease centers (Children's Hospital Colorado and UCLA Medical Center). The study was approved by the Institutional Review Board at each center. Records were reviewed of all individuals with OTCD who were followed at these centers between the years 2000 and 2011. They were identified by site records and ongoing clinical care, and through enrollment in the NIH funded, multi-site Longitudinal Study of Urea Cycle Disorders at these two centers, which is an IRB approved, natural history study30, for which written informed consent was obtained. Many individuals followed clinically for OTCD were also subjects in the NIH study, some asymptomatic individuals were not seen clinically, but were subjects in the NIH study. These two groups constituted all known individuals followed for OTCD at the two centers. OTCD was established in each subject by biochemical test results, molecular diagnosis, or by enzymology. For each subject the following historical information was recorded: age at presentation, sex, OTC mutation if known, clinical presentation, OTC enzyme activity in liver, and liver histology. To assess liver injury the following tests were recorded at least once for every subject: AST, ALT, prothombin time (PT), PTT, and INR. Values were obtained from clinic visits, Longitudinal Study research visits, or hospitalizations. If available, concurrent total and direct bilirubin, Factor V, Factor VII, Factor VIII, D-dimers and fibrinogen were also recorded, as was plasma ammonia. For each subject the time point at which the available liver injury related laboratory results were collected was at the time of the highest recorded PT or INR (or AST/ALT if PT/INR was not performed). Some of the recorded tests may have been obtained at an earlier or later time point that same day. Liver injury related tests were also recorded on every identified occasion that the AST/ALT or PT/INR values met the criteria defined in this study for ALF, liver dysfunction or hepatocellular injury, see below. When possible, outside medical records, including evaluations prior to the identification of a UCD, were reviewed.
For the purposes of this study, acute liver failure (ALF) was defined as acute liver injury with an INR ≥ 2.0, or PT ≥ 20 seconds in the absence of disseminated intravascular coagulation2,29; liver dysfunction as INR ≥ 1.5 and < 2.0 or PT ≥ 15 seconds and < 20 seconds; and hepatocellular injury as AST or ALT ≥ 250 IU/L. Lack of response of elevated INR or PT to vitamin K was not included as a criterion for ALF, as this is a retrospective study and vitamin K was not given uniformly.
For the purposes of this study individuals were placed in one of five groups of clinical severity with respect to their urea cycle defect. Individuals were considered to be Asymptomatic with respect to OTCD if they had not had episodes of hyperammonemia requiring medical intervention. Individuals with symptomatic OTCD were classified into four groups: Neonatal Males who lack residual enzyme activity are the most severe and developed symptoms of hyperammonemia in the first two days of life; Severe males and females developed symptoms of hyperammonemia after two days of life, required maximal medical and dietary therapy, and had frequent hospitalizations; Moderate males and females required medical and/or dietary therapy, and had less frequent hospitalizations; Mild males and females did not have recurrent hyperammonemia after establishment of the diagnosis of OTCD.
Statistical Analyses
A two sample test of proportions was performed comparing the proportion of symptomatic versus asymptomatic OTCD subjects who had ALF, liver dysfunction, or hepatocellular injury. Descriptive statistics (mean, standard deviation, and range for AST, ALT, ammonia, PT and INR) were performed using the STATA program.
RESULTS
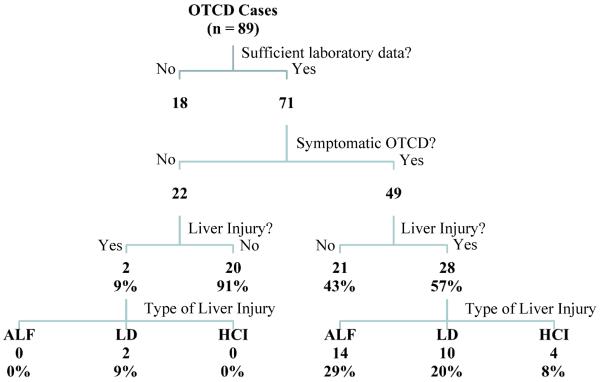
Charts of 89 subjects were reviewed from the two centers, and 71 subjects were included in the analysis; 18 subjects were excluded due to lack of sufficient laboratory data, 12 of thom were asymptomatic (Table III; online). For each of the 71 subjects, available liver injury related laboratory tests were recorded at least once. Additional events of ALF, liver dysfunction or hepatocellular injury were observed in several cases and these data were recorded on separate rows. For each individual the most severe liver injury identified (ALF> liver dysfunction>hepatocellular injury) was used for Tables I and II); each individual is included only once in the summary tables.
Table 1.
Liver Laboratory Values in 71 Individuals with OTCD
| Clinical Classification (N) | Ammonia μmol/L | ALT IU/L | AST IU/L | PT seconds | INR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Neonatal Male (12) | 1232 | 817.6 | 41.1, 2634 | 109 | 85.6 | 22, 256 | 151 | 151 | 25, 467 | 23 | 9.8 | 8.6, 37.6 | 2.48 | 1.64 | 0.9, 6.41 |
| Severe (9) | 202 | 92.1 | 106, 410 | 923 | 827.6 | 16, 2089 | 1063 | 1405 | 16, 4139 | 32 | 19.4 | 10.6, 63.7 | 2.92 | 1.64 | 1.0, 5.7 |
| Moderate (12) | 155 | 92.4 | 37.6, 332 | 331 | 467.1 | 13, 1223 | 137 | 236 | 11, 785 | 14.8 | 7.4 | 10.4, 34.9 | 1.48 | 0.62 | 1.0, 3.1 |
| Mild (15)* | 72.2 | 79.7 | 9, 297 | 102 | 187.6 | 10, 689 | 71.1 | 129 | 14, 519 | 13 | 2.4 | 10.4, 17.6 | 1.15 | 0.14 | 0.95, 1.4 |
| Asymptomatic (22) | 39 | 22.97 | 9, 104.5 | 19 | 10.04 | 11, 49 | 23 | 13.5 | 10, 62 | 12 | 1.8 | 10.1, 16.2 | 1.04 | 0.09 | 0.92, 1.27 |
One individual was classified as mild but did not survive the initial presentation, his values are not included here.
Normal values: Ammonia: < 50 μmol/L (< 80 μmol/L in Neonates ); ALT: 10 – 35 IU/L; AST: 15 – 40 IU/L; PT: 12.0 – 15.0 seconds; INR: < 1.5
Table 2.
Acute Liver Failure, Liver Dysfunction, and Hepatocellular Injury in 71 Individuals with OTCD
| Clinical Classification (N) | Acute Liver Failure | Liver Dysfunction | Hepatocellular Injury Only | No Known Liver Lab Abnormality | Percent with either ALF/LD/HCI |
|---|---|---|---|---|---|
| Neonatal Male (12) | 58% | 17% | 0% | 25% | 75% (9/12) |
| Severe (9) | 56% | 11% | 0% | 33% | 67% (6/9) |
| Moderate (12) | 17% | 25% | 8% | 50% | 50% (6/12) |
| Mild (16) | 0% | 25% | 19% | 56% | 44% (7/16) |
| Asymptomatic (22) | 0% | 9% | 0% | 91% | 9% (2/22) |
ALF: Acute Liver Failure: INR ≥ 2.0, or PT ≥ 20 LD: Liver Dysfunction: INR ≥ 1.5 and < 2.0, or PT ≥ 15 and < 20 HCI: Hepatocellular Injury: AST or ALT ≥ 250 IU/L
In this historical cohort of 71 individuals with OTCD, the severity of hepatic synthetic dysfunction, reflected by the PT and INR, correlated with the severity of the OTCD (Table I). The mean PT and INR were higher in Neonatal Males and Severe Females than in those classified as Moderate and Mild. Markedly elevated AST (1,063 ± 1,405 IU/L) and ALT (923 ± 828 IU/L) were identified in individuals classified as Severe, all of whom were female. Elevations of AST and ALT were moderate in Neonatal Males (AST 151 ± 151, ALT 109 ± 86). Mild elevations of ammonia, minimal elevations of AST and ALT, and mild elevations of PT and INR were identified in Asymptomatic individuals. Symptomatic individuals classified as Moderate or Mild had intermediate elevations of ammonia, ALT, AST, PT and INR.
The risk of ALF, liver dysfunction and isolated hepatocellular injury varied with OTCD clinical severity (Table II); 75% of Neonatal Males and 67% of Severe females showed evidence of liver involvement. Those with less severe OTCD had lower frequencies of liver involvement. ALF was identified most often in individuals classified as Neonatal Male (7/12, 58%), or Severe (5/9, 56%). Almost half of individuals classified as Mild OTCD showed evidence of liver dysfunction or isolated hepatocellular injury at least once in their clinical course. In some cases ALF or liver dysfunction was associated with hepatocellular injury as defined for this study. All six Severe females with ALF or liver dysfunction had concurrent elevated AST or ALT ≥ 250 IU/L and met criteria for hepatocellular injury. Two of nine Neonatal Males, with ALF or liver dysfunction had concurrent elevated AST or ALT ≥ 250 IU/L. The two Moderate individuals with ALF met had concurrent elevated AST or ALT ≥ 250 IU/L, as did one of three with liver dysfunction. Mild individuals with liver dysfunction did not have concurrent AST or ALT ≥ 250 IU/L.
Type of liver injury in symptomatic versus asymptomatic individuals with OTCD is shown in Figure 2. In those individuals with liver injury, serum bilirubin was normal for age, or only mildly elevated (Table III). The proportion of OTCD subjects who had ALF, liver dysfunction or hepatocellular injury was significantly higher in the symptomatic (28 of 49) compared with the asymptomatic (2 of 22) group (p=0.0002).
Figure 2.
Summary of types of liver injury present in cases of OTCD followed at the two metabolic disease centers.
ALF was the initial clinical presentation of at least three individuals (part 1, cases 7 and 8; part 2, case 8; Table III). ALF was documented to be recurrent in five individuals (part 1, cases 8, 9 and 13; part 2, cases 7 and 8; Table III). Liver dysfunction and hepatocellular injury were also recurrent in some cases. Liver laboratory abnormalities were not always recurrent in subsequent episodes of hyperammonemia, though this was not formally assessed in this study. Two females with severe OTCD had recurrent hyperammonemia, but never had documented liver test abnormalities using the criteria established for this study. Notably, liver histology was abnormal in these two subjects (part 1, cases 11 and 12; Table III).
DISCUSSION
We report the results of a historical cohort study conducted at two large genetic metabolic disease centers, which has identified the presence of significant liver injury and dysfunction in over 50% of individuals with symptomatic OTCD at some time in their clinical history. This report represents a systematic investigation of this association and suggests that a clinical liver presentation, including ALF, is not uncommon among patients with OTCD.
The most severe clinical presentations of UCDs occur in the newborn period, and later-onset presentations may be precipitated by infection, the post-partum period, valproate therapy, or corticosteroid use12,24,31,32. As these are treatable conditions in which delay of treatment can result in irreversible neurologic injury or death, testing for UCDs is critical when indicated. Although the urea cycle enzymes are active in the liver, standard liver function has generally been considered to be largely unaffected in UCDs4,5,33. However, elevated AST and ALT and coagulopathy were identified in the first reported cases of OTCD in the 1960s and 1970s34–38, and a Reye syndrome presentation of OTCD was recognized in the 1970s and 1980s39–42 (Table IV; available at www.jpeds.com). In the late 1980s and early 1990s, neurologic presentations of OTCD were emphasized, with little focus on hepatic injury43–47. Since the 1990s there have been rare reports of significant clinical liver disease in patients with OTCD8,10,48.
Table 4.
Twenty-Four Literature Cases of OTCD with Acute Liver Failure, Liver Dysfunction or Hepatocellular Injury
| Sex Liver Injury |
Age | Presentation | AST IU/L | ALT IU/L | PT | INR | PTT | Bili | NH3 μmol/L | V | Author | Year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F HCI |
4 y | Recurrent emesis, lethargy, hemiparesis stupor (Case 10 in literature) | 2,232 | 2,610 | NR | NR | NR | NR | 658 | NR | Sunshine38 | 1972 |
| M HCI |
10 m | Emesis, hypotonia, brother died at 7 months | 1,590 | 740 | NR | NR | NR | NR | NR | NR | Van der Heiden53 | 1978 |
| F HCI |
14 m | Episodic emesis, loss of milestones | 600 | NR | NR | NR | NR | NR | Unknown (later 315 and 407) | NR | La Brecque13 | 1979 |
| M HCI |
15 m | Recurrent “Reye syndrome” | 542 | 481 | NR | NR | NR | NR | 29 | NR | Yokoi41 | 1981 |
| M ALF HCI |
9m | Emesis, seizures, encephalopathy | NR | 1,200 | 28% | NR | NR | NR | 406 | NR | Landrieu15 | 1982 |
| M HCI |
21 y | Emesis, nausea, progressive encephalopathy, death, brother died at 10 years | 720 | 561 | NR | NR | NR | Nl | 1,012 | NR | Tallan16 | 1983 |
| F HCI |
2 y | Emesis, lethargy, “Reye syndrome” | 679 | 976 | NR | NR | NR | NR | 113 | NR | Hayasaka54 | 1987 |
| M HCI |
6 m | Emesis, episodic lethargy, Coagulation “severely disturbed” | 900 | 600 | NR | NR | NR | NR | NR | NR | Wendel55 | 1989 |
| M LD |
12.5 years | Emesis, lethargy, altered mental status – combative | 90 | 86 | 17.2 | NR | NR | 6.4 | 282 | NR | Capistrano-Estrada56 | 1994 |
| F HCI |
23 m | Recurrent emesis, lethargy, | 777 | 1,213 | NR | NR | NR | NR | 1026 | NR | Pridmore46 | 1994 |
| extensor plantar reflexes | ||||||||||||
| F HCI |
22 m | Recurrent emesis, lethargy, delay, acute cerebellar ataxia with increased AST/ALT, “atypical Reye syndrome” | 1,052 | 275 | NR | NR | NR | NR | 218 | NR | Pridmore46 | 1994 |
| M HCI LD |
13 y | Emesis, ataxia, altered mental status, clonus | 68 | 347 | 16.5 | NR | NR | 2.4 (0.1) | 408 | NR | Myers47 | 1995 |
| F HCI |
3 y | Emesis | 1,206 | 1,663 | 42% | NR | 43 | NR | 294 | NR | Zammarchi48 | 1996 |
| F ALF HCI |
5 m | Emesis | 1,692 | 2,328 | 18% | NR | 48 | NR | 150 | NR | Zammarchi48 | 1996 |
| F LD |
3 y | Coma in illness | 187 | 114 | 17.6 | NR | NR | NR | 343 | NR | Inui57 | 1996 |
| F ALF |
3 d | Hypoglycemia, lactic acidemia, seizures, hypotonia | NR | NR | NR | NR | NR | NR | 2301 | 10% | Klowsowski58 | 1998 |
| F HCI |
3 y | Aggression, confusion, abnormal movements | NR | 299 | NR | NR | NR | NR | NR | NR | Schultz59 | 2000 |
| F HCI |
28 y | GI bleed | 196 | 466 | 15 | 1.3 | NR | 1.1 | 247 | NR | Trivedi7 | 2001 |
| M HCI |
15 m | Recurrent emesis, elevated AST/ALT, FTT, normal ammonia, Gln, orotic, possible HFI | 300 | 1,727 | NR | NR | NR | NR | 40 | NR | Burlina60 | 2006 |
| F ALF HCI |
14 m | Emesis | 2,212 | 3,609 | NR | 5.1 | 67 s | Nl | 74 | NR | Mustafa8 | 2006 |
| M HCI |
36 y | Lethargy post steroid treatment | 295 | 317 | NR | 1.1 | NR | 3.1/0.9 | 494 | NR | Atiq9 | 2008 |
| F ALF HCI |
3 y | Emesis, lethargy, aggression | 7,900 | 5,000 | NR | 3.1 | NR | NR | 161 | 25% | Teufel10 | 2009 |
| M ALF |
24 y | Emesis, hallucinations, h/o ADHD, growth retardation | 114 | NR | NR | 2.7 | NR | Nl | 348 | Low | Thurlow11 | 2010 |
| F ALF |
1.5 y | Cyclic vomiting, lethargy | NR | 3,500 | 55 | NR | NR | NR | 207 | NR | Mira61 | 2011 |
Legend: ALF: Acute liver failure. LD: Liver dysfunction. HCI: Hepatocellular injury. V: Factor V. RR: Respiratory rate. Rx: Treatment. Tx: Liver transplant. ND: Not done. AMS: Altered mental status. HFI: Hereditary fructose intolerance. ADHD: Attention deficit hyperactivity disorder. FTT: Failure to thrive. NR: Not recorded. Gln: Glutamine F: Female. M: Male, m: Months old. y: Years old. Nl: Normal
As in the case presented here, liver injury in OTCD is often seen in association with elevated ammonia levels (Figure 1). Our study, therefore, required review of the highest recorded liver laboratory tests (AST, ALT, PT, PTT and INR) during hyperammonemic episodes. These data were not collected through the Longitudinal Study, and required local chart review. A limitation of this study is that liver tests were collected for some subjects during hospitalizations, and for others, largely those asymptomatic with respect to OTCD, at study visits in the outpatient setting. This is due to the fact that asymptomatic individuals were not hospitalized frequently, therefore, in some cases the only available liver test results were obtained during study visits. However, in symptomatic individuals, many of whom who were hospitalized, the highest mean PT and INR were in those more severely affected, and the frequency of liver test abnormalities correlated with the severity of OTCD (Tables I and II). This suggests the underlying OTCD as the cause of, or a predisposing factor to, the liver injury. The pathogenesis of liver injury in OTCD is unknown and this study suggests that further work is indicated. It is possible that the liver injury is caused by direct toxicity of ammonia; toxicity of carbamoyl phosphate has been proposed, but this is speculative49,50.
The observation that liver blood tests may be elevated in individuals with OTCD raises the question of whether regular assessment of liver tests, especially during episodes of hyperammonemia, should be performed. These abnormal tests may resolve with treatment (Figure 1)25 and may not change initial care, however, in at least one reported case urgent liver transplantation for acute liver failure in OTCD was performed10. In addition, the finding of histological abnormalities on liver biopsy13–19 and reports of hepatocellular carcinoma (HCC) in OTCD patients49 suggest that recurrent liver injury and chronic inflammation could predispose to the development of HCC, as in other genetic metabolic liver diseases51. An increased risk of HCC has implications for long-term treatment and monitoring of patients with OTCD49. There are currently no recommendations for HCC screening in OTCD.
The finding of an ALF presentation in OTCD raises the question as to whether undiagnosed OTCD may be responsible for a significant number of indeterminate pediatric ALF patients. In the past decade, a renewed interest in pediatric ALF revealed that an appropriate evaluation to define treatable underlying genetic and metabolic etiologies is not completed for most patients2. As a result, patients with metabolic disorders, such as UCDs, may have gone undiagnosed and labeled indeterminate ALF. Indeed, in the Pediatric ALF Study Group registry, only 1 of 148 infants less than 3 months of age had the diagnosis of OTCD (another had an unspecified urea cycle defect) and 56 (38%) were labeled indeterminate29. However, OTCD was not investigated in the majority of the indeterminate cases. OTCD can also be a cause of liver failure in adults11. Therefore, the current study suggests that OTCD should be considered in all patients with indeterminate ALF, especially those with normal, or mildly elevated, serum bilirubin concentration.
In conclusion, this study demonstrates that clinical hepatic presentations of OTCD may include ALF, liver dysfunction and hepatocellular injury as defined in this report, which may be associated with only moderate elevations of blood ammonia levels that may not call attention to a UCD. To investigate a UCD in a patient with ALF, laboratory evaluation should be expedited6. Initial testing should include quantitative plasma amino acids, urine organic acids and a quantitative urine orotic acid. Specific metabolic treatment can be life-saving, and may reverse ALF, obviating consideration of emergency liver transplantation23. The failure to correctly diagnose and treat OTCD may result in irreversible neurologic disability or death, and is a lost opportunity to evaluate family members and to provide genetic counseling52.
ACKNOWLEDGMENTS
We thank the members of the Urea Cycle Disorders Consortium, particularly the local site study coordinators: Curtis Coughlin II, MS, MBe (Colorado), Shannon Scrivner, MS (Colorado), and Nagmeh Dorrani, MS (UCLA). We thank Robert McCarter, DSc, for critical reading of the manuscript. All of the acknowledged individuals are funded by NIH (U54HD061221).
Appendix
Funded by the National Institute of Child Health and Human Development (U54HD061221 to R.G.) and the National Institutes of Health (NIH) Office of Rare Diseases Research. The Longitudinal Study of Urea Cycle Disorders is conducted by the Urea Cycle Disorders Consortium, which is a part of the NIH Rare Diseases Clinical Research Network. The Urea Cycle Disorders Consortium is supported by the O'Malley Foundation, the Rotenberg Family Fund, the Dietmar-Hopp Foundation, and the Kettering Fund; Children's Hospital Colorado received funding from the O'Malley Foundation, and the Kettering Family Fund. R.G. and D.W. served as local site principal investigators for a clinical trial of a novel medication for urea cycle disorders, sponsored by Hyperion Therapeutics (registered with ClinicalTrials.gov: <<>>) The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the US Government. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding and conflict of interest information available at www.jpeds.com (Appendix).
Portions of the study have been presented at the annual Meeting of the Urea Cycle Disorder Consortium <<>>, as well as a poster at the meet of the Society for Inherited Metabolic Disease, March 2012.
REFERENCES
- 1.Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142–52. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- 2.Narkewicz MR, Dell Olio D, Karpen SJ, Murray KF, Schwarz K, Yazigi N, et al. Pattern of diagnostic evaluation for the causes of pediatric acute liver failure: an opportunity for quality improvement. J Pediatr. 2009;155:801–806. e1. doi: 10.1016/j.jpeds.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durand P, Debray D, Mandel R, Baujard C, Branchereau S, Gauthier F, et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139:871–6. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 4.Clayton PT. Inborn errors presenting with liver dysfunction. Semin Neonatol. 2002;7:49–63. doi: 10.1053/siny.2001.0086. [DOI] [PubMed] [Google Scholar]
- 5.Clayton PT. Diagnosis of inherited disorders of liver metabolism. J Inherit Metab Dis. 2003;26:135–46. doi: 10.1023/a:1024429032116. [DOI] [PubMed] [Google Scholar]
- 6.Lanpher B, Gropman Andrea, Chapman Kimberly, Lichter-Konecki U, Urea Cycle Disorder Consortium. Summar ML. Urea cycle disorders overview [Internet] GeneReviews. [cited 2012 Sep 21]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1217/
- 7.Trivedi M, Zafar S, Spalding MJ, Jonnalagadda S. Ornithine transcarbamylase deficiency unmasked because of gastrointestinal bleeding. J Clin Gastroenterol. 2001;32:340–3. doi: 10.1097/00004836-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Mustafa A, Clarke JTR. Ornithine transcarbamoylase deficiency presenting with acute liver failure. J Inherit Metab Dis. 2006;29:586. doi: 10.1007/s10545-006-0303-2. [DOI] [PubMed] [Google Scholar]
- 9.Atiq M, Holt AF, Safdar K, Weber F, Ravinuthala R, Jonas ME, et al. Adult onset urea cycle disorder in a patient with presumed hepatic encephalopathy. J Clin Gastroenterol. 2008;42:213–4. doi: 10.1097/01.mcg.0000225628.84168.25. [DOI] [PubMed] [Google Scholar]
- 10.Teufel U, Weitz J, Flechtenmacher C, Prietsch V, Schmidt J, Hoffmann GF, et al. High urgency liver transplantation in ornithine transcarbamylase deficiency presenting with acute liver failure. Pediatr Transplant. 2011;15:E110–115. doi: 10.1111/j.1399-3046.2009.01171.x. [DOI] [PubMed] [Google Scholar]
- 11.Thurlow VR, Asafu-Adjaye M, Agalou S, Rahman Y. Fatal ammonia toxicity in an adult due to an undiagnosed urea cycle defect: under-recognition of ornithine transcarbamylase deficiency. Ann Clin Biochem. 2010;47:279–81. doi: 10.1258/acb.2010.009250. [DOI] [PubMed] [Google Scholar]
- 12.Summar ML, Barr F, Dawling S, Smith W, Lee B, Singh RH, et al. Unmasked adult-onset urea cycle disorders in the critical care setting. Crit Care Clin. 2005;21:S1–8. doi: 10.1016/j.ccc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.LaBrecque DR, Latham PS, Riely CA, Hsia YE, Klatskin G. Heritable urea cycle enzyme deficiency-liver disease in 16 patients. J Pediatr. 1979;94:580–7. doi: 10.1016/s0022-3476(79)80014-1. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro JM, Schaffner F, Tallan HH, Gaull GE. Mitochondrial abnormalities of liver in primary ornithine transcarbamylase deficiency. Pediatr Res. 1980;14:735–9. doi: 10.1203/00006450-198005000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Landrieu P, François B, Lyon G, Van Hoof F. Liver peroxisome damage during acute hepatic failure in partial ornithine transcarbamylase deficiency. Pediatr Res. 1982;16:977–81. doi: 10.1203/00006450-198212000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Tallan HH, Schaffner F, Taffet SL, Schneidman K, Gaull GE. Ornithine carbamoyltransferase deficiency in an adult male patient: significance of hepatic ultrastructure in clinical diagnosis. Pediatrics. 1983;71:224–32. [PubMed] [Google Scholar]
- 17.Latham PS, LaBrecque DR, McReynolds JW, Klatskin G. Liver ultrastructure in mitochondrial urea cycle enzyme deficiencies and comparison with Reye's syndrome. Hepatology. 1984;4:404–7. doi: 10.1002/hep.1840040308. [DOI] [PubMed] [Google Scholar]
- 18.Badizadegan K, Perez-Atayde AR. Focal glycogenosis of the liver in disorders of ureagenesis: its occurrence and diagnostic significance. Hepatology. 1997;26:365–73. doi: 10.1002/hep.510260217. [DOI] [PubMed] [Google Scholar]
- 19.Yaplito-Lee J, Chow C-W, Boneh A. Histopathological findings in livers of patients with urea cycle disorders. Mol Genet Metab. 2013;108:161–5. doi: 10.1016/j.ymgme.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Morrow G, 3rd, Barness LA, Efron ML. Citrullinemia with defective urea production. Pediatrics. 1967;40:565–74. [PubMed] [Google Scholar]
- 21.Ito S, Kurasawa G, Yamamoto K, Furuta I, Ishihara F, Kobayashi K, et al. A pregnant patient with fulminant hepatic failure was found to carry a novel missense mutation in the argininosuccinate synthetase gene. J Gastroenterol. 2004;39:1115–7. doi: 10.1007/s00535-004-1455-1. [DOI] [PubMed] [Google Scholar]
- 22.Güçer S, Aşan E, Atilla P, Tokatli A, Cağlar M. Early cirrhosis in a patient with type I citrullinaemia (CTLN1) J Inherit Metab Dis. 2004;27:541–2. doi: 10.1023/b:boli.0000037401.63596.de. [DOI] [PubMed] [Google Scholar]
- 23.De Groot MJ, Cuppen M, Eling M, Verheijen FW, Rings EHHM, Reijngoud D-J, et al. Metabolic investigations prevent liver transplantation in two young children with citrullinemia type I. J Inherit Metab Dis [Internet] 2010 doi: 10.1007/s10545-010-9207-2. [cited 2012 Jan 23];Available from: http://www.ncbi.nlm.nih.gov/pubmed/20852933. [DOI] [PMC free article] [PubMed]
- 24.Salek J, Byrne J, Box T, Longo N, Sussman N. Recurrent liver failure in a 25-year-old female. Liver Transpl. 2010;16:1049–53. doi: 10.1002/lt.22118. [DOI] [PubMed] [Google Scholar]
- 25.Faghfoury H, Baruteau J, De Baulny HO, Häberle J, Schulze A. Transient fulminant liver failure as an initial presentation in citrullinemia type I. Mol Genet Metab. 2011;102:413–7. doi: 10.1016/j.ymgme.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Fecarotta S, Parenti G, Vajro P, Zuppaldi A, Della Casa R, Carbone MT, et al. HHH syndrome (hyperornithinaemia, hyperammonaemia, homocitrullinuria), with fulminant hepatitis-like presentation. J Inherit Metab Dis. 2006;29:186–9. doi: 10.1007/s10545-006-0120-7. [DOI] [PubMed] [Google Scholar]
- 27.Mhanni AA, Chan A, Collison M, Seifert B, Lehotay DC, Sokoro A, et al. Hyperornithinemiahyperammonemia-homocitrullinuria syndrome (HHH) presenting with acute fulminant hepatic failure. J Pediatr Gastroenterol Nutr. 2008;46:312–5. doi: 10.1097/MPG.0b013e318145a8e5. [DOI] [PubMed] [Google Scholar]
- 28.Mori T, Nagai K, Mori M, Nagao M, Imamura M, Iijima M, et al. Progressive liver fibrosis in late-onset argininosuccinate lyase deficiency. Pediatr Dev Pathol. 2002;5:597–601. doi: 10.1007/s10024-002-0109-7. [DOI] [PubMed] [Google Scholar]
- 29.Sundaram SS, Alonso EM, Narkewicz MR, Zhang S, Squires RH. Characterization and outcomes of young infants with acute liver failure. J Pediatr. 2011;159:813–818. e1. doi: 10.1016/j.jpeds.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seminara J, Tuchman M, Krivitzky L, Krischer J, Lee H-S, LeMons C, et al. Establishing a Consortium for the Study of Rare Diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab. 2010;100:S97–S105. doi: 10.1016/j.ymgme.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celik O, Buyuktas D, Aydin A, Acbay O. Ornithine transcarbamylase deficiency diagnosed in pregnancy. Gynecol Endocrinol. 2011;27:1052–4. doi: 10.3109/09513590.2011.569787. [DOI] [PubMed] [Google Scholar]
- 32.Smith W, Kishnani PS, Lee B, Singh RH, Rhead WJ, Sniderman King L, et al. Urea cycle disorders: clinical presentation outside the newborn period. Crit Care Clin. 2005;21:S9–17. doi: 10.1016/j.ccc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Sze YK, Dhawan A, Taylor RM, Bansal S, Mieli-Vergani G, Rela M, et al. Pediatric liver transplantation for metabolic liver disease: experience at King's College Hospital. Transplantation. 2009;87:87–93. doi: 10.1097/TP.0b013e31818bc0c4. [DOI] [PubMed] [Google Scholar]
- 34.Levin B, Dobbs RH, Burgess EA, Palmer T. Hyperammonaemia. A variant type of deficiency of liver ornithine transcarbamylase. Arch Dis Child. 1969;44:162–9. doi: 10.1136/adc.44.234.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin B, Abraham JM, Oberholzer VG, Burgess EA. Hyperammonaemia: a deficiency of liver ornithine transcarbamylase. Occurrence in mother and child. Arch Dis Child. 1969;44:152–61. doi: 10.1136/adc.44.234.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin B, Oberholzer VG, Sinclair L. Biochemical investigations of hyperammonaemia. Lancet. 1969;2:170–4. doi: 10.1016/s0140-6736(69)91419-6. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins IJ, Connelly JF, Dawson AG, Hird FJ, Maddison TG. Hyperammonaemia due to ornithine transcarbamylase deficiency. Arch Dis Child. 1969;44:143–8. doi: 10.1136/adc.44.234.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunshine P, Lindenbaum JE, Levy HL, Freeman JM. Hyperammonemia due to a defect in hepatic ornithine transcarbamylase. Pediatrics. 1972;50:100–11. [PubMed] [Google Scholar]
- 39.Thaler MM, Hoogenraad NJ, Boswell M. Reye's syndrome due to a novel protein-tolerant variant of ornithine-transcarbamylase deficiency. Lancet. 1974;2:438–40. doi: 10.1016/s0140-6736(74)91819-4. [DOI] [PubMed] [Google Scholar]
- 40.Thaler MM. Letter: Role of ornithine transcarbamylase in Reye's syndrome. N Engl J Med. 1974;291:797. doi: 10.1056/nejm197410102911522. [DOI] [PubMed] [Google Scholar]
- 41.Yokoi T, Honke K, Funabashi T, Hayashi R, Suzuki Y, Taniguchi N, et al. Partial ornithine transcarbamylase deficiency simulating Reye syndrome. J Pediatr. 1981;99:929–31. doi: 10.1016/s0022-3476(81)80025-x. [DOI] [PubMed] [Google Scholar]
- 42.Finkelstein JE, Hauser ER, Leonard CO, Brusilow SW. Late-onset ornithine transcarbamylase deficiency in male patients. J Pediatr. 1990;117:897–902. doi: 10.1016/s0022-3476(05)80129-5. [DOI] [PubMed] [Google Scholar]
- 43.DiMagno EP, Lowe JE, Snodgrass PJ, Jones JD. Ornithine transcarbamylase deficiency--a cause of bizarre behavior in a man. N Engl J Med. 1986;315:744–7. doi: 10.1056/NEJM198609183151207. [DOI] [PubMed] [Google Scholar]
- 44.Coskun T, Ozalp I, Mönch S, Kneer J. Lethal hyperammonaemic coma due to ornithine transcarbamylase deficiency presenting as brain stem encephalitis in a previously asymptomatic ten-year-old boy. J Inherit Metab Dis. 1987;10:271. doi: 10.1007/BF01800076. [DOI] [PubMed] [Google Scholar]
- 45.Christodoulou J, Qureshi IA, McInnes RR, Clarke JT. Ornithine transcarbamylase deficiency presenting with strokelike episodes. J Pediatr. 1993;122:423–5. doi: 10.1016/s0022-3476(05)83432-8. [DOI] [PubMed] [Google Scholar]
- 46.Pridmore CL, Clarke JT, Blaser S. Ornithine transcarbamylase deficiency in females: an often overlooked cause of treatable encephalopathy. J Child Neurol. 1995;10:369–74. doi: 10.1177/088307389501000506. [DOI] [PubMed] [Google Scholar]
- 47.Myers JH, Shook JE. Vomiting, ataxia, and altered mental status in an adolescent: late-onset ornithine transcarbamylase deficiency. Am J Emerg Med. 1996;14:553–7. doi: 10.1016/S0735-6757(96)90097-2. [DOI] [PubMed] [Google Scholar]
- 48.Zammarchi E, Donati MA, Filippi L, Resti M. Cryptogenic hepatitis masking the diagnosis of ornithine transcarbamylase deficiency. J Pediatr Gastroenterol Nutr. 1996;22:380–3. doi: 10.1097/00005176-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Wilson JM, Shchelochkov OA, Gallagher RC, Batshaw ML. Hepatocellular carcinoma in a research subject with ornithine transcarbamylase deficiency. Mol Genet Metab. 2012;105:263–5. doi: 10.1016/j.ymgme.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes Metab. 2009;11:823–35. doi: 10.1111/j.1463-1326.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- 51.Erez A, Shchelochkov OA, Plon SE, Scaglia F, Lee B. Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism. Am J Hum Genet. 2011;88:402–21. doi: 10.1016/j.ajhg.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batshaw ML, Msall M, Beaudet AL, Trojak J. Risk of serious illness in heterozygotes for ornithine transcarbamylase deficiency. J Pediatr. 1986;108:236–41. doi: 10.1016/s0022-3476(86)80989-1. [DOI] [PubMed] [Google Scholar]
- 53.Van der Heiden C, Bakker HD, Desplanque J, Brink M, De Bree PK, Wadman SK. Attempted dietary treatment of a boy with hyperammonemia due to ornithine transferase deficiency. Eur J Pediatr. 1978;128:261–72. doi: 10.1007/BF00445611. [DOI] [PubMed] [Google Scholar]
- 54.Hayasaka K, Metoki K, Ishiguro S, Kato S, Chiba T, Hirooka M, et al. Partial ornithine transcarbamylase deficiency in females: diagnosis by an immunohistochemical method. Eur J Pediatr. 1987;146:370–2. doi: 10.1007/BF00444940. [DOI] [PubMed] [Google Scholar]
- 55.Wendel U, Wieland J, Bremer HJ, Bachmann C. Ornithine transcarbamylase deficiency in a male: strict correlation between metabolic control and plasma arginine concentration. Eur J Pediatr. 1989;148:349–52. doi: 10.1007/BF00444132. [DOI] [PubMed] [Google Scholar]
- 56.Capistrano-Estrada S, Marsden DL, Nyhan WL, Newbury RO, Krous HF, Tuchman M. Histopathological findings in a male with late-onset ornithine transcarbamylase deficiency. Pediatr Pathol. 1994;14:235–43. doi: 10.3109/15513819409024257. [DOI] [PubMed] [Google Scholar]
- 57.Inui A, Fujisawa T, Komatsu H, Tanaka K, Inui M. Histological improvement in native liver after auxiliary partial liver transplantation for ornithine transcarbamylase deficiency. Lancet. 1996;348:751–2. doi: 10.1016/S0140-6736(05)65637-1. [DOI] [PubMed] [Google Scholar]
- 58.Klosowski S, Largilliere C, Storme L, Rakza T, Rabier D, Lequien P. Lethal ornithine transcarbamylase deficiency in a female neonate: a new case. Acta Paediatr. 1998;87:227–8. doi: 10.1080/08035259850157723. [DOI] [PubMed] [Google Scholar]
- 59.Schultz R, Salo M. Under recognition of late onset ornithine transcarbamylase deficiency. Arch Dis Child. 2000;82:390–1. doi: 10.1136/adc.82.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burlina AB, Peduto A, Di Palma A, Bellizzi A, Sperlì D, Morrone A, et al. An unusual clinical and biochemical presentation of ornithine transcarbamylase deficiency in a male patient. J Inherit Metab Dis. 2006;29:179–81. doi: 10.1007/s10545-006-0193-3. [DOI] [PubMed] [Google Scholar]
- 61.Mira, Valerie B, Richard Liver Failure with Coagulopathy, Hyperammonemia and Cyclic Vomiting in a Toddler Revelaed to Have Combined Heterozygosity for Genes Invovled with Ornithine Transcarbamylase Deficiencyand Wilson Disease. JIMD Reports. 2011;3:1246–7. doi: 10.1007/8904_2011_70. [DOI] [PMC free article] [PubMed] [Google Scholar]