Abstract
The pharmacological and airways relaxant profiles of PL-3994 (Hept-cyclo(Cys-His-Phe-d-Ala-Gly-Arg-dNle-Asp-Arg-Ile-Ser-Cys)-Tyr-[Arg mimetic]-NH2), a novel natriuretic peptide receptor-A (NPR-A) agonist, were evaluated. PL-3994, a full agonist, has high affinity for recombinant human (h), dog, or rat NPR-As (Kis of 1, 41, and 10 nm, respectively), and produced concentration-dependent cGMP generation in human, dog and rat NPR-As (respective EC50s of 2, 3 and 14 nm). PL-3994 has a Ki of 7 nm for hNPR-C but was without effect on cGMP generation in hNPR-B. PL-3994 (1 µm) was without significant effect against 75 diverse molecular targets. PL-3994 or BNP, a natural NPR ligand, produced concentration-dependent relaxation of pre-contracted guinea-pig trachea (IC50s of 42.7 and 10.7 nm, respectively). PL-3994, and also BNP, (0.1 nm–100 µm) elicited a potent, concentration-dependent but small relaxation of pre-contracted human precision-cut lung slices (hPCLS). Intratracheal PL-3994 (1–1000 µg/kg) produced a dose-dependent inhibition of the bronchoconstrictor response evoked by aerosolized methacholine, but was without significant effect on cardiovascular parameters. PL-3994 was resistant to degradation by human neutral endopeptidase (hNEP) (92% remaining after 2 h), whereas the natural ligands, ANP and CNP, were rapidly metabolized (≤1% remaining after 2 h). PL-3994 is a potent, selective NPR agonist, resistant to NEP, with relaxant effects in guinea-pig and human airway smooth muscle systems. PL-3994 has the profile predictive of longer clinical bronchodilator activity than observed previously with ANP, and suggests its potential utility in the treatment of asthma, in addition to being a useful research tool to evaluate NPR biology.
Keywords: PL-3994, Natriuretic peptide receptors, Atrial natriuretic peptide, Brain natriuretic peptide, Neutral endopeptidase sensitivity, Bronchodilator
1. Introduction
Asthma, a disease characterized by airway inflammation and bronchoconstriction, remains a common cause of global morbidity and mortality. Despite advances in understanding the mechanisms underpinning the pathophysiology of asthma, therapeutic approaches for the most part remain inhaled corticosteroids and β2-adrenergic receptor agonists (β2-agonists), alone or in combination [1]. Concerning β2-agonist-mediated bronchodilation, evidence suggests that short- and long-acting β2-agonists induce tachyphylaxis of the β2-adrenergic receptor that limits their efficacy [2–5]. Other studies suggest that single nucleotide polymorphisms in the β2-adrenergic receptor may be associated with increased morbidity and mortality [1,6,7]. Accordingly, new bronchodilator approaches are an unmet therapeutic need in asthma and other lung diseases characterized by excessive bronchoconstriction.
The natriuretic peptides (NPs), including atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP), are distinct hormones that regulate a variety of physiological processes such as blood pressure, cardiac growth, and neural and skeletal development [8–10]. The biological actions of NPs are mediated by interaction with three cell surface natriuretic peptide receptors (NPRs), NPR-A, NPR-B and NPR-C [8–10]. NPR-A and NPR-B are the main functional receptors for the NPs, whose diverse effects in multiple cells and tissues are mediated via activation of membrane-associated guanylyl cyclases and production of the classical second (2nd) messenger cyclic guanosine monophosphate (cGMP). In contrast to NPR-A and NPR-B, NPR-C is not functionally linked to gunaylyl cyclase, and serves as the clearance receptor for the NPs, and also inhibits adenylyl cyclase [11]. ANP and BNP are potent agonists of the NPR-A receptor, whereas CNP binds selectively to NPR-B [8–10,12]. Naturally occurring NPs are rapidly degraded by neutral endopeptidases (NEP) and internalized via the clearance receptor NPR-C [10]. All three NPRs have been detected in the lung, where ANP is released [8–10,13].
In addition to their well characterized natriuretic and hemodynamic effects, NPs regulate a variety of mechanisms relevant to human lung diseases. These include: relaxation of airway smooth muscle (ASM), attenuation of airway inflammation and hyper-responsiveness, inhibition of proliferation of ASM, regulation of lung permeability, surfactant production and recovery from exercise [13–20]. Recent data link single nucleotide polymorphisms (SNPs) of NPPA, the gene coding for ANP, to asthma susceptibility [21,22]. Furthermore, preclinical in vitro and in vivo studies demonstrate beneficial bronchodilation and anti-inflammatory effects of ANP and BNP [13,15,19,20,23–25].
Hulks and co-workers were the first to report that ANP – delivered via intravenous infusion – produced bronchodilation in patients with asthma [26]. Other studies demonstrated subsequently that intravenous [27,28] or nebulized [29–31] ANP elicited a clinically significant, but short-acting bronchodilation. The short duration of action of ANP in the lung, which limits its clinical utility, may be linked to rapid degradation by NEP, as suggested by: (i) an increased duration of bronchodilation resulting from ANP inhalation and (ii) increased attenuation of histamine-induced bronchoconstriction after pretreatment with thiorphan, an NEP inhibitor [30,31].
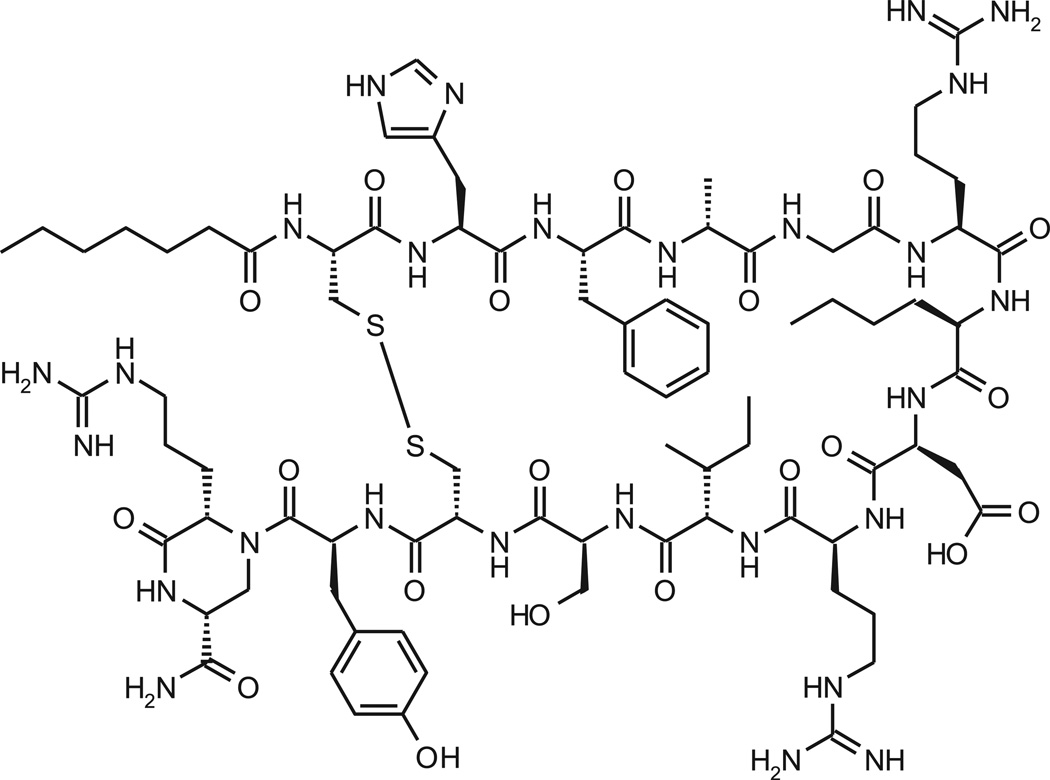
In this communication the in vitro and in vivo pharmacological profile of PL-3994, a novel cyclic peptide (Hept-cyclo(Cys-His-Phe-d-Ala-Gly-Arg-d-Nle-Asp-Arg-Ile-Ser-Cys)-Tyr-[Arg-mimetic]-NH2) NPR-A and NPR-C agonist (Fig.1) is described. Our data suggest that PL-3994 is a potent and selective NPR agonist that, unlike the natural NPR ligands, is resistant to degradation by NEP. PL-3994 produces relaxation in guinea-pig and human ASM and bronchodilation in guinea pigs in vivo, and may have utility in the treatment of patients with asthma, with the potential of a longer duration of action than observed previously with inhaled ANP. Furthermore, PL-3994 will be a valuable research tool to evaluate the biology of NPRs and their potential pathophysiological significance.
Fig. 1.
Structure of PL-3994 (C82H127N27O20S2; anhydrous, counter-ion free peptide).
2. Materials and methods
2.1. In vitro
2.1.1. Preparation of recombinant NP receptors
2.1.1.1. Human NPR-A, NPR-B and NPR-C receptor cloning and construction
Human natriuretic peptide receptor-A (NPR-A) cDNA clone was purchased from Bio S&T Inc. (Quebec, Canada). Human natriuretic peptide receptor-B (NPR-B) cDNA clone was purchased from OriGene Technologies (Catalog# SC117646; SC117646; Rockville, MD). Full-length human NPR-A and NPR-B were then reconstructed into a pcDNA 3.1(+) (Invitrogen Life Science, Catalog# V790-20, Carlsbad, CA). Human natriuretic peptide receptor-C (NPR-C) was cloned from human kidney total RNA. Briefly, cDNA of full length NPR-Cs were generated by reverse transcription-polymerase chain reaction, and then amplified by PCR with the 5′-sense primer 5′-CTCTTTCTTGCGGCACGATGCC and the 3′-antisense primer 5′-GTGGGGGGCTTCCTTTAAGCTACTG. The PCR product of human NPR-C was cloned into pCR TOPO vector (Invitrogen, Catalog# K4560-1). Once sequences were confirmed, human NPR-C was re-constructed into a pcDNA3.1 (+) vector.
2.1.1.2. Dog NPR-A cloning and construction
Dog natriuretic peptide receptor-A (NPR-A) was cloned from dog testis cDNA (Biochain, Hayward, CA). Based on the sequences homologies between human NPR-A and rat NPR-A, the 5′-fragment of dog NPR-A (2.6 kb) was amplified by PCR with sense primer, 5′-TCTCCCACCCTCCTCCTCCGACCGCT and antisenseprimer,5′-TGCGGCTCCTCAGCCCAGCAACGCT, which reached 290 base pairs above the start code. The second fragment of dog NPR-A (1.9 kb) was amplified by PCR with sense primer 5′-CGGGACTTCC CAAGAGCTGGTGGC and antisense primer, 5′-TCAGCCTCGAGTGCTG CTCCCCCG. There are 1086 base pairs that overlap between the first fragment and second fragment. The third fragment, 3′-end sequence of dog NPRA, was amplified through 3′-(RACE)-PCR with 3′-RACE primer 5′-CCTGGACAACCTGCTGTCCCGCATGGAGC and 3′-Nested primer 5′-CGCTACGTAACGGCATGACAGTG, which has 750 base pairs of overlap with the second fragment and reaches 400 base pair beyond the stop code. After dog NPR-A was completely sequenced, the full length dog NPRA was amplified with sense primer, 5′-AAGGGGCGGAGGAGGCC GTGTACGG and antisense primer, 5′-GGTCAGCCTCGAGTGCTGCTCCCC and cloned into a cloning vector first, and then re-constructed into pcDNA3.1(+).
2.1.1.3. Rat NPR-A cloning
Rat-NPRA was cloned from rat kidney total RNA, (Clontech Laboratories, Mountain View, CA Catalog# 636645). cDNA was generated by reverse transcription-polymerase chain reaction with oligo dT primer. Full length rat NPR-A cDNA was amplified by PCR using the following primers: 1) The 5′-sense primer (5′-GTTGCGGCTTCAACCCACCCCAGCTT) and 2) The 3′-antisense primer (5′-CAGGGCAGTAGGTCAGCCTCGAGTGCTACA). The PCR products of rat NPR-A were cloned into a cloning vector and were then re-constructed into pcDNA3.1(+) vector.
2.1.1.4. Establishment of stable expression human NPR-A, -B, -C, dog NPR-A and rat NPR-A cell lines
HEK293 cells (ATCC, Manasas, VA; Catalog# CRL-1573) were transfected separately with pcDNA3.1(+) containing human NPR-A, -B, or -C or dog NPR-A, or rat NPR-A using lipofectamine 2000 reagent medium (Invitrogen, Catalog# 11668-019). Twenty-four hours after the transfection, the medium was changed and the cells were allowed to recover in fresh growth medium for another 24 h. The cells were then split into 96-well plates with one cell per well, and selected in presence of G418 (0.5 mg/ml; Invitrogen, Catalog# 10131035). After selection and cell cloning, about 20 clones of each receptor were expanded and assayed for receptor expression using 125I-ANP binding, and high expression stable clones were selected. Functional activity of NPR-A (human, dog, rat) and NPR-B (human) receptors were measured by cGMP assays (see below in Section 2.1.3.).
2.1.2. Receptor binding studies
2.1.2.1. Recombinant receptors
2.1.2.1.1. Preparation of membranes
NPR-expressing cells were washed with ice-cold PBS (155 mm NaCl, 3.3 mm Na2HPO4, and1.1 mm KH2PO4, pH 7.4) buffer, and then lysed with hypotonic lysis buffer (10mm Tris, pH 7.4, 5mm EDTA) at 4 °C for 5- to 10-min. Cells were collected into polypropylene tubes, and 250 µl of 5× sucrose solution (1.25 m) was added to each 1-ml cell suspension solution. The cells were then homogenized with a Dounce homogenizer and then transferred to an Oakridge centrifuge tube and centrifuged for 10-min at 3000 rpm at 4 °C. Supernatants were collected into a clean centrifuge tube and centrifuged at 20,000 rpm for 30 min at 4 °C. The supernatants were then discarded and the pellet was resuspended in storage buffer (100 mm Tris, 10 mm MgCl2, pH 7.4) at 4 °C and stored on ice. Membrane homogenates were aliquoted into 1-ml vials and frozen at −80 °C. Protein concentrations were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL; Catalog# 23227) employing bovine serum albumin (BSA) as the standard.
2.1.2.1.2. Saturation binding studies
Cell membrane homogenates were re-suspended in assay buffer (25 mm Hepes, 100 mm NaCl, 2 mm CaCl2, 2 mm MgCl2, 0.1% BSA and 1 mm of 1, 10-phenanthroline (Sigma) and transferred into 96-well plates. Increasing concentrations of 125I-hANP were added and the cells were then incubated for 2-hr at 4 °C. At the end of the incubation, reactions were stopped by filtration using glass fiber filters (GF/B Unifilter plates, PerkinElmer, Waltham, MA). Non-specific binding was detected by measuring radioligand binding in the presence of 2 µm of unlabeled hANP. Filter plates were washed 3 times with icecold phosphate-buffered saline, air dried, and then the bottom of the plate was sealed with PerkinElmer bottom sealers. Thirty-five (35) µl of scintillation cocktail were added to each well and then read on a micro-beta counter (PerkinElmer, Model TRILUX6DET 1450021). Maximum binding (Bmax) and equilibrium dissociation constant (Kd) values were determined using GraphPad Software (version# 5.0).
2.1.2.1.3. Competitive binding studies
Cell membrane homogenates were incubated with 30–40 pm of 125I-ANP in the presence of various concentrations of compounds from 10 fm to 10 µm with 100 µl of assay buffer for 2-hr at 37 °C. At the end of the incubation the reactions were stopped by filtration with glass fiber filters (GF/B Unifilter plates, Perkin–Elmer). Filter plates were then washed 3 times with ice-cold phosphate-buffered saline. The plate bottoms were then sealed with Perkin–Elmer bottom sealers, 35 µl of scintillation cocktail were added to each well, and plates were counted on a micro-beta counter. The binding constant values (Ki) for compounds were determined using the one-site competition model in GraphPad Prism.
2.1.2.2. Guinea-pig lung tissue
2.1.2.2.1. Guinea-pig lung membrane preparation
Guinea-pig lung tissues (Bioreclamation, Hicksville, NY) were rinsed with phosphate-buffered saline (155 mm NaCl, 3.3 mm Na2HPO4, and 1.1 mm KH2PO4, pH 7.4), chopped with a razor blade on ice and homogenized in 10× tissue volume of homogenizing buffer (50 mm Tris and 1 mm NaHCO3, pH 7.4). The homogenized tissue was further diluted at 1:1 ratio with a diluting buffer (50mm Tris,1mm EDTA, and 1 mm MgCl2; pH 7.4) and centrifuged at 800 × g for 10 min at 4 °C. The supernatant was filtered through cheesecloth and centrifuged again for 30min at 20,000 × g. The pellet was re-suspended in 5mm Tris buffer (pH 7.4) containing 250 mm sucrose and stored at −70 °C.
2.1.2.2.2. Competition binding studies
Competition binding experiments were performed as described above for the recombinant receptors (Section 2.1.2.1.3.). However, IC50s rather than Ki values for compounds were determined, as the latter could not be assessed due to the technical difficulties in getting accurate Kd values owing to the mixed expression of NPR-A and NPR-C in the guinea pig lung tissue.
2.1.3. Functional studies: cGMP generation
HEK293 cells containing NPR-A (human, dog, rat) or NPR-B (human) were detached using an enzyme-free cell dissociation buffer (Invitrogen, Catalog# 13151014) and centrifuged at 1600 rpm for 5 min. Cells were re-suspended in assay buffer (Hank’s Balanced Salt Solution, 10 mm Hepes, pH 7.4, 5 mm MgCl2, 1 mm glutamine, 1 mg/ml BSA, and 0.5 mm 3-isobutyl-1-methyl-xanthine (IBMX), the non-selective PDE inhibitor) and plated in 96-well plates at a density of 5–10 × 104 cells per well. Cells were pre-incubated in assay buffer for 10 min at 37 °C and 5% CO2, and then exposed to the test compounds (10 fm to 10 µm) in a total assay volume of 200 µl for 15 min. At the end of the incubation period, the cells were lysed by adding 10 µl of lysis buffer (50mm Phosphate buffer, pH 7.0, 0.8 M kF, 1% Triton X100, 0.2% BSA) to each well. cGMP levels in the cell lysates were measured using the cGMP HTRF Kit (Cisbio, Bedford, MA; Catalog# 6261M2PEC) and read on a PerkinElmer Victor 3 plate reader at 665 and 620 nm. The measured fluorescence intensity values were converted to cGMP concentrations against a calibration curve generated on the day of the assay following the instructions provided with the kit. The EC50 values were determined using a sigmoidal dose response curve fit (GraphPad Prism 5.0). The maximum response to hANP was designated as 100% in each experiment, and the relative efficacies of agonists to hANP were calculated based on the ratio of the agonist maximum response relative to the hANP maximum response. For the guinea-pig lung tissue experiments, membranes were re-suspended in the assay buffer [50 mm Tris–HCl, 4 mm MnCl2, 10 mm creatinine phosphate, 100 U/ml creatine phosphokinase, 1 mm GTP, 1 mg/ml BSA, 2 mm IBMX (3-Isobutyl-1-methylxanthine)] to achieve a membrane protein concentration of 250 µg/ml.Ninety-nine (99) µl of membrane solution were then transferred to each tube in a 96-tube system (VWR, Radnor, PA; Catalog# 89005-568). The tubes were incubated at room temperature (~23 °C) for 15 min before 1 µl per tube of serially diluted compound was added to the tissue membrane tube to achieve final concentrations from 10 fm to 10 µm. The tubes were shaken gently and then incubated at room temperature for 15min. At the end of the incubation, the reaction was stopped by adding 10 µl of lysis buffer to each tube. The tubes were then centrifuged at 2300 × g for 15 min. Compound-induced cGMP production was measured by using the Cisbio cGMP HTRF Kit as described above.
2.1.4. Guinea-pig trachea experiments
Studies utilizing guinea-pig trachea were performed by Pneumolabs Limited (Harrow, Middlesex, United Kingdom).
2.1.4.1. Preparation of tissues and protocol
Male Dunkin Hartley guinea pigs were purchased from Harlan (Hillcrest, UK) and housed at the Pneumolabs facility for at least one week before use and were allowed free access to food and water. Guinea pigs (400–500 g) were killed with a blow to the head and exsanguination. The trachea was removed and cut into sections containing 4–5 cartilage rings; three rings were obtained from each animal. The resulting ring sections were opened by cutting through the cartilage on the opposite side of the ring to the muscle band. When preparing the tissue preparations care was taken not to remove the airway epithelium. Threads were attached to the cartilaginous ends of the resulting strips, which were then suspended in 10-ml water-jacketed organ baths in Krebs solution containing indomethacin (10 µm), gassed with CO2/O2 (5%/95%) and maintained at 37 °C. The composition of the Krebs solution was (mm): NaCl 118.4, KCl 4.8, NaHCO3 25.0, MgSO4 1.2, KH2PO4 1.2, glucose 11.1, CaCl2 2.5. Tension was monitored using isometric force displacement transducers and a Biopac Systems data acquisition system (AcqKnowledge ® III; Goleta, CA). A resting tension of 1 g was applied to the tissues and a stabilization period allowed before the experiment began during which time the tissues were washed every 10 min. After 20-min stabilization an EC70 concentration of carbachol (0.5 µm) was added to each organ bath to evoke contraction of the tissues. When the carbachol-induced contractions had reached a plateau (after about 20 min) one preparation from each animal was exposed tocumulatively increasing concentrations of PL-3994, BNP or vehicle (Krebs solution); a contact time of 5–20-min per concentration was used. After the final concentration of test drug was added and the response had plateaued, salbutamol (0.5mm)was added to all baths to abolish any remaining tone. Both PL-3994 and BNP were dissolved in Krebs solution to give a 1 mm solution which was diluted serially with Krebs solution. Carbachol and salbutamol were also dissolved in Krebs solution at concentrations of 50 µm and 50mm so that the final organ bath concentrations were 0.5 µm and 0.5 mm, respectively. Indomethacin was dissolved in 1% Na2CO3 solution at a concentration of 10 mm and added directly to the Krebs solution reservoir to give a final concentration of 10 µm.
2.1.4.2. Data analysis
After exposure to carbachol the tension was noted in all tissues immediately before addition of the first concentration of test drug, and this was used as the zero inhibition value. The relaxation effect of each concentration of the test compound was measured and expressed as a % of the maximum relaxation produced by salbutamol (0.5 mm), which was taken as 100%. Pooled concentration-response data for each treatment group was analyzed using GraphPad Prism 4.0 with curve fitting to a sigmoidal, variable slope model. Results are expressed as mean ± SEM.
2.1.5. Rat trachea experiments
Studies utilizing rat trachea were performed by Ricerca Biosciences, LLC (Taipei, Taiwan).
2.1.5.1. Preparation of tissues and protocol
Male Wistar rats (from BioLASCO, Tapei, Taiwan) weighing 250 ± 20 g were used. The animals were killed by CO2 overexposure and 3 tracheal rings were isolated from each animal. When preparing the ring preparations care was taken to keep the airway epithelium intact. Each tissue preparation was placed under 1 g tension – recorded using an isometric transducer and amplifier (Harvard Instruments, Holliston, MA, USA) and two-pen recorder (Sekonic SS-250F, Sekonic Corporation, Tokyo, Japan) – in a 10-ml water-jacketed organ bath containing Krebs solution which included indomethacin (2.8 µm) and was gassed with CO2/O2 (5%/95%) and maintained at pH 7.4 at 37 °C; the composition of the Krebs solution was (mm): NaCl 118, KCl 4.7, NaHCO3 25.0, MgSO4 1.2, KH2PO4 1.18, glucose 10, CaCl2 2.52. Sub-maximal tonic contraction was induced by carbachol (1 µm), which was set as the 100% control response. Relaxation in tracheal rings pre-contracted with carbachol was evaluated for each test substance concentration, allowing the response to reach a maximal, plateau effect in order to generate a cumulative concentration-response curve (1 nm–10 µm in 1-log increments). A maximum concentration of salbutamol (100 µm) was added to each tissue at the end of the concentration–response curve to determine the maximum relaxation. IC50 values were obtained by non-linear regression analysis. All aspects of this work including housing, experimentation and animal disposal were performed in general accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996).
2.1.6. Human precision-cut lung slice (hPCLS) experiments
2.1.6.1. Preparation of tissues and protocol
PCLS from healthy whole human lungs (received from National Disease Research Interchange (NDRI), Philadelphia, PA) were prepared as described previously [32,33]. When preparing the PCLS care was taken to ensure that the airway epithelial layer was left intact. Following their preparation, the lung slices were placed in a 12-well plate in 1.0 ml Ham’s F-12 medium, held in place using a platinum weight with nylon attachments and viewed under a microscope (Magnification: 40×). A baseline image was taken (0% contraction) and airways were contracted (approximately 80–90%) with 1.0 µm carbachol, followed by the addition of the lowest concentration of the test compound to begin the concentration response (0.1 nm–100 µm) in the continued presence of carbachol. The positive control β2-agonist isoproterenol (100 µm) was added to each tissue at the end of the concentration-response curve. Images were collected 10 min after addition of each concentration of test compound. Previous experiments have determined that these time points are sufficient to allow maximal effect at each concentration (data not shown). Airway lumen area was measured using a macro written within Image Pro-Plus (version 6.0) software (Media Cybernetics, Bethesda, MD) and given in units of µm2. An IC50 and Emax value for each airway was derived from a concentration-response curve. In all instances, inclusion and exclusion criteria for use of PCLS required that all slices contract 80%–90% in response to carbachol and that slices were sensitive to the β2-agonist bronchodilators, albuterol or isoproterenol that typically reverse 80% of the carbachol-induced contraction. At least four airways from each donor were used per condition; n-values in the text indicate the number of individual lungs used. The relaxation effect of each concentration of the test compound was measured and indicated as a % of the relaxation produced by isoproterenol (100 µm), which was taken as 100%. Data are expressed as the mean ± SEM. Statistical difference was determined by utilizing a non-paired Student’s t-test, with P < 0.05 regarded as significant.
2.1.7. Neutral endopeptidase sensitivity experiments
All compounds were dissolved in DMSO to 5 mm as stock solution, and then diluted in 0.1 m Tris–HCL buffer (pH 7.4) to 100 µm. Forty (40) µl of diluted compound, producing a concentration of 50 µm, were added to tubes which were kept on ice. Forty (40) µl of diluted human recombinant NEP (R&D Systems, Inc., Minneapolis, MN; Catalog# 1182-ZN) at a concentration of 8 ng/µl were added to each tube. The tubes were mixed gently and spun down, then incubated at 37 °C for 0, 0.5, 1 or 2 h. At the end of incubation, the reaction was stopped by adding 5 µl of 10% TFA to each tube. All the solution samples were then transferred to HPLC tubes for HPLC or UPLC analysis. The percentages of test compound remaining after exposure to hNEP for various times were calculated.
2.2. In vivo
The in vivo studies were performed by Pneumolabs Limited (Harrow, Middlesex, United Kingdom).
2.2.1. Animal preparation
Each guinea-pig (obtained from Harlan, Hillcrest, UK) was anaesthetized with urethane (1.75 g/kg, i.p.). The trachea was cannulated so that each animal could be artificially ventilated via a small animal ventilator (10% oxygen in ambient air, 55 breath/min). The tracheal cannula was also connected to a physiological pressure transducer (SP844, Memscap AS, Skoppun, Norway). Changes in pulmonary inflation pressure (Ppi) were recorded at the trachea using a Power lab data acquisition system (AD Instruments, Bella Vista, NSW, Australia) and displayed in real time on a personal computer. Bronchoconstriction was measured as the increase in the maximum Ppi above the basal inflation pressure produced by the positive pressure, constant volume respiratory ventilator. The carotid artery was also cannulated for concomitant recording of blood pressure and heart rate and the jugular vein cannulated for intravenous administration of test materials.
2.2.2. Experimental protocol: methacholine, PL-3994 and salbutamol challenges
Following surgery the animals were allowed to stabilize for 20min before initial baseline readings were taken. Bronchoconstriction was evoked with aerosolized methacholine (10 µg/ml for 20 s). After the recorded parameters returned to baseline (usually 5–10 min) the methacholine challenge was repeated until a consistent bronchoconstrictor response was obtained (usually after 3 challenges). Once baseline levels recovered following the final methacholine challenge each animal received a single dose (200 µl dose volume) of vehicle, PL-3994 or salbutamol. Changes to baseline parameters were recorded throughout the post-treatment period. Animals treated with PL-3994 received subsequent methacholine challenges (10 µg/ml for 20 s) at +15 min, +60 min, (vehicle- and 1 µg/kg, 10 µg/kg, 100 µg/kg and 1000 µg/kg PL-3994-dosed animals) and also at + 120 min and +240 min (for vehicle- and 10 µg/kg and 100 µg/kg PL-3994-dosed animals only) after PL-3994 administration. Animals treated with salbutamol received subsequent methacholine challenges (10 µg/ml for 20 s) at +60 min after treatment.
2.2.3. Data analysis
Baseline values were taken 1 min before the administration of methacholine and bronchoconstriction results are expressed as changes from baseline values. Alterations in pulmonary inflation pressure, blood pressure and heart rate evoked by methacholine in the presence of PL-3994 or salbutamol are expressed as a percentage difference to the mean control methacholine responses observed before drug treatment. All values have been expressed as the mean ± SEM. Statistical analysis was conducted using either ANOVA followed by Dunnett’s test or Student’s t-test, as appropriate, with P < 0.05 regarded as being significant.
2.3. Reagents
The natriuretic peptides, hANP (H-2095), hBNP (H-9060), hCNP (H-1296), hANP 1–28, porcine BNP-26, hBNP-32, mini-ANP, rat ANF, hANF 4–28 were obtained from Bachem (Hayward, CA). 125I-hANP was purchased from GE Healthcare (Piscataway, NJ). Rat BNP-32 was purchased from Sigma–Aldrich (St. Louis, MO), dog BNP-32 and mouse BNP-45 were purchased from Phoenix Pharmaceuticals (Burlingame, CA). All reagents were obtained from Sigma–Aldrich (St. Louis, MO or Gillingham, Dorset, UK), unless otherwise stated. PL-3994 was synthesized by Medicinal Chemists at Palatin Technologies, Inc. (Cranbury, New Jersey). The salts for the Krebs’ solution used in the studies conducted by Pneumolabs were ANALAR® grade from BDH (Lutterworth, Leicestershire, UK).
3. Results
3.1. In vitro
The in vitro pharmacological profile of PL-3994 was assessed in various binding and functional assays and usually compared with the activities of the natural NP ligands, ANP, BNP and/or CNP.
3.1.1. Affinities and potencies for NPRs
The NP receptor binding affinities (Ki) of PL-3994 (0.01 nm–1 µm) were evaluated using recombinant human (h), dog, or rat NPRs expressed in human embryonic kidney (HEK-293) cell lines. The Kis of PL-3994 for human, dog, and rat NPR-A were 1, 41, and 10 nm, respectively (Table 1), indicating that the affinity of PL-3994 is greatest for the hNPR-A, with ≥10-fold lower affinity at the rat and dog NPR-A receptors.
Table 1.
Comparison of the affinities (Kis) and functional potencies (cGMP generation; EC50s and Emaxs) of PL-3994 for human, dog and rat NPR-As (expressed in HEK cell lines). Results are given as the mean ± SEM.
| Receptor | Binding | Function (cGMP) | ||||
|---|---|---|---|---|---|---|
| Ki (nM) | (n) | EC50 (nm) |
Emax (% hANP Response) |
(n) | ||
| Human NPR-A | 1 ± 0.2 | (45) | 2 ± 0.6 | 95 ± 1 | (57) | |
| Dog NPR-A | 41 ± 11 | (6) | 3 ± 0.6 | 94 ± 5 | (5) | |
| Rat NPR-A | 1 ± 0.2 | (4) | 14 ±1 | 98± 1 | (53) | |
The functional potency of PL-3994 was assessed by measuring its ability to generate cGMP in human, dog, and rat NPR-As in HEK cell lines. PL-3994 produced a concentration-dependent generation of cGMP in human, dog and rat NPR-As with respective EC50 values of 2, 3 and 14 nm (Table 1). PL-3994 was a full agonist, relative to ANP, in the NPR-As from different species. In this assay, unlike the results of binding studies, PL-3994 has equivalent functional potency in human and dog NPR-A. We hypothesize that the discrepancy between binding affinity (Ki) and functional potency (EC50) is due to the large number of spare receptors in the dog NPRA expressing cell line. Similar to binding assay results, PL-3994 has about 10-fold lower functional potency (cGMP generation) in rat versus human NPR-A.
With respect to the selectivity of PL-3994 for NPR-A versus the other NP receptors, NPR-B and NPR-C, in binding studies in HEK cell lines PL-3994 has a Ki of 7 ± 0.6 nm (n = 4) for human NPR-C (the “clearance” receptor), i.e., 7-fold lower affinity than for the NPR-A receptor. PL-3994 was without effect, in concentrations up to 10 µm, in the NPR-B functional assay (cGMP generation; n = 8). Thus, the relative potencies or affinities of PL-3994 for the NPRs is NPR-A > NPR-C ⋙ NPR-B.
3.1.2. Comparison of potencies and affinities of PL-3994, ANP and BNP for hNPRs
PL-3994 has similar affinity and potency as BNP for hNPR-A, whereas ANP has about 20-fold higher affinity (from binding experiments) and 5-fold great potency (cGMP generation) than PL- 3994 at this receptor (Table 2). Similar to PL-3994, ANP and BNP (up to 10 µm) had no functional activity (cGMP generation) at the hNPRB. At hNPR-C PL-3994 has lower affinity (Ki = 7 nm) than BNP (Ki = 0.7 nm) and especially ANP (Ki = 0.05 nm). Thus, unlike PL-3994, the natural NP ligands, hANP and hBNP have equivalent or higher affinity, respectively, for NPR-C than NPR-A. These differential receptor affinity ratios may have implications for the relative clearances and durations of action of these compounds in vivo.
Table 2.
Comparison of the affinities (Kis) and functional potencies (cGMP generation; EC50s and Emaxs) of PL-3994, hANP and hBNP for hNPR-A, hNPR-B and hNPR-C (expressed in HEK cell lines). Results are given as the mean ± SEM.
| Compound | hNPR-A | hNPR-B | hNPR-C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Binding | Function (cGMP) | Function (cGMP) | Binding | |||||||
| Ki (nm) | (n) | EC50 (nm) | Emax (% hANP) | (n) | EC50 (nm) | Emax (% hCNP) | (n) | Ki (nm) | (n) | |
| PL-3994 | 1 ± 0.2 | (45) | 2 ± 0.6 | 95 ± 1 | (57) | Not calculable | 1 ± 0.5 | (8) | 7 ± 0.6 | (4) |
| hANP | 0.05 ± 0.01 | (6) | 0.4 ± 0.04 | 100 ± 0 | (64) | Not calculable | 6 ± 0.8 | (5) | 0.05 ± 0.03 | (5) |
| hBNP | 3 ± 1 | (4) | 2 ± 0.6 | 96 ± 2 | (11) | Not calculable | 9 ± 1 | (7) | 0.7 | (2) |
3.1.3. Guinea-pig lung: tissue binding and functional studies
A comparison of the ability of PL-3994, hANP and hBNP to inhibit 125I-hANP binding in guinea-pig lung tissue preparations was evaluated. In guinea-pig lung samples PL-3994 had much lower affinity than ANP (>1000-fold) and to a lesser extent BNP (about 5-fold), with respective Kis of 494 ± 106 nm, 0.13 ± 0.03 nm and 86 ± 11 nm (n = 5). This discrepancy from the results of binding studies using specific recombinant NPRs in HEK cells – albeit from species other than guinea pig – (described above in Section 3.1.2.; Table 1) is probably, in part, due to 125I-hANP binding to multiple NP receptors, including NPR-C for which PL-3994 has a lower affinity than for NPR-A.
PL-3994 produced a concentration-dependent elevation in cGMP generation in guinea-pig lung membrane preparations with an EC50 of 22 ± 7 nm (n = 4). PL-3994 was a full agonist with a similar efficacy as hANP (98 ± 2.7% of the response to hANP; n – 4), although it was about 50-fold less potent than the natural ligand (EC50 for hANP = 0.48 ± 0.2 nm; n = 4).
3.1.4. Selectivity: non-NPR molecular targets
The receptor selectivity of PL-3994 for NPR-A and NPR-C was evaluated by determining its effects against 75 diverse molecular targets in a standard profiling screen conducted by CEREP (Poitiers, France). PL-3994 (1 µm) had no significant effect (<20% inhibition) against 66 of the 75 targets examined, and against the following 9 targets demonstrated >20% but <50% inhibition: neurokinin-2 receptor (hNK-2), kappa opioid receptor, opioid receptor-like 1 (hORL1), non-selective somatostatin receptor (sst), dopamine (transporter (h), γ-aminobutyric acid receptor (GABA), muscarinic receptor-1 (hM-1), neuromedin-preferring bombesin receptor (BB), and pituitary adenylate cyclase-activating polypeptide receptor (hPACAP). Follow-up studies determined that the Kis of PL-3994 for inhibition of hNK-2, kappa, hORL1, and sst were 0.4, 0.88, 0.95, and 4.2 µm, respectively. The effects of PL-3994 against these targets occurred at concentrations approximately 3-orders of magnitude greater than those that interact with NPR-A and confirms the selectivity of PL-3994 for NPRs versus other non-NPR targets.
3.1.5. Relaxation of isolated lung tissues
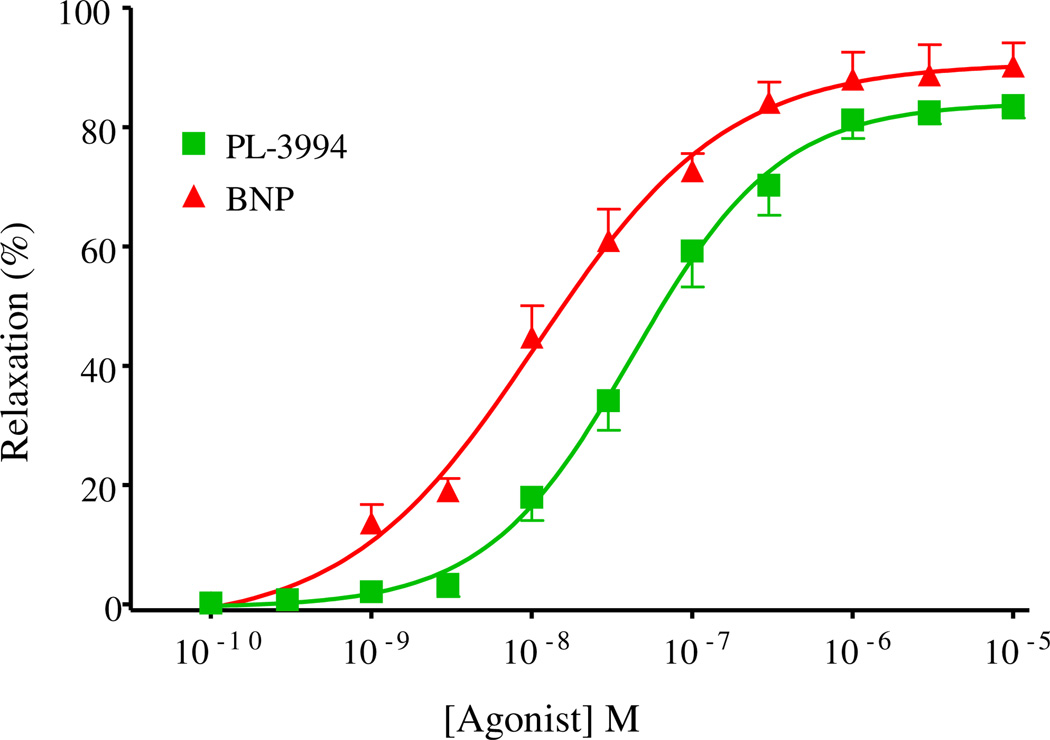
In guinea-pig trachea PL-3994 (0.1 nm–10 µm) produced a concentration-dependent relaxation of preparations precontracted with the muscarinic receptor agonist, carbachol (0.5 µm), with an IC50 of 42.7 nm (n = 3). The maximum relaxation elicited by PL-3994 was 83.6% of that produced by the positive control, β2-agonist, salbutamol (0.5 mm) (Fig. 2). BNP (0.1 nm–10 µm) also produced a concentration-dependent relaxation of precontracted guinea-pig trachea, with an IC50 of 10.7 nm, and a maximum relaxation that was 90.3% of that produced by salbutamol (n = 3; Fig. 2). The potency difference between BNP and PL-3994 in this assay (BNP is about 4-fold more potent) is similar to the differences in binding affinities determined in guinea-pig lung preparations (BNP had about 5-fold higher affinity: 86 nm vs. 494 nm for PL-3994; see Section 3.1.3. above).
Fig. 2.
Concentration-dependent relaxant effects of PL-3994 ( ) or BNP (
) or BNP ( ) in guinea-pig trachea contracted with carbachol (0.5 µm). Results are expressed as a % of the relaxation produced by salbutamol (0.5 mm) and are given as the mean ± SEM; n = 3.
) in guinea-pig trachea contracted with carbachol (0.5 µm). Results are expressed as a % of the relaxation produced by salbutamol (0.5 mm) and are given as the mean ± SEM; n = 3.
In rat trachea PL-3994 (1 nm–100 µm; n = 3–6) produced minimal relaxation of tissues pre-contracted with 1 µm carbachol except in high concentrations; PL-3994 10 µm elicited 17.0 ± 6.1% relaxation; n = 6). Thus, the IC50 for PL-3994 for relaxation of precontracted rat trachea was >100 µm, which is more than 200-fold greater than the PL-3994 IC50 for relaxing guinea-pig trachea (about 40 nm; see Section 3.1.5. above). Salbutamol (100 µm) produced 70.0 ± 10.4% relaxation of carbachol-precontracted rat trachea (n = 6).
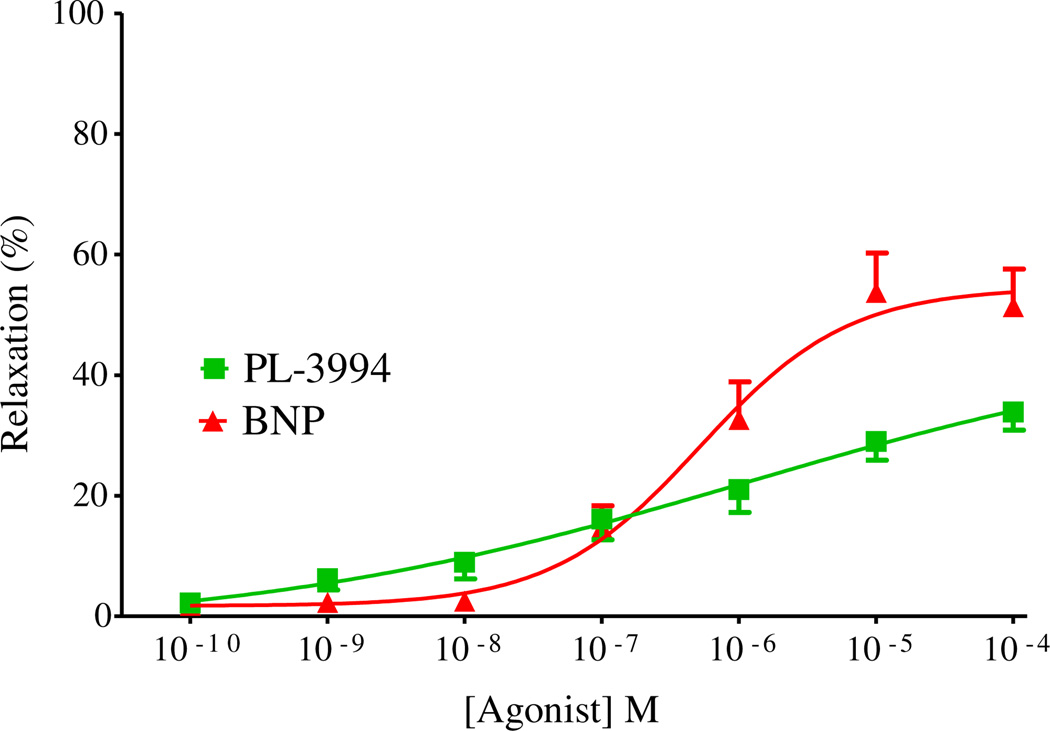
The relaxant effects of PL-3994 were examined in a novel human precision-cut lung slice (hPCLS) preparation [32,33]. In these experiments, conducted in non-paired tissue samples from 3 human lungs, the response to the contractile agonist, carbachol(1 µm), in vehicle-treated tissues was maintained throughout the study (<5% loss in tone). PL-3994 (0.1 nm–100 µm) produced a potent, concentration-dependent but small relaxation of tissues precontracted with carbachol (Fig. 3). The relaxation elicited by the highest concentration of PL-3994, 100 µm, was about 30% of that produced by the positive control β2-agonist isoproterenol (100 µm). BNP (0.1 nm–100 µm) also relaxed precontracted human lung preparations with a maximal relaxation about 50% of the isoproterenol-induced relaxation, which was significantly larger than that produced by PL-3994 (n = 3; Fig. 3).
Fig. 3.
Concentration-dependent relaxation by PL-3994 ( ) or BNP (
) or BNP ( ) in human PCLS preparations (containing small airways) contracted with carbachol (1 µm). Results are expressed as a % of the relaxation produced by isoproterenol (100 µm) and are given as the mean ± SEM from at least 4 preparations from 3 separate lungs.
) in human PCLS preparations (containing small airways) contracted with carbachol (1 µm). Results are expressed as a % of the relaxation produced by isoproterenol (100 µm) and are given as the mean ± SEM from at least 4 preparations from 3 separate lungs.
3.1.6. Sensitivity to degradation via neutral endopeptidase (NEP)
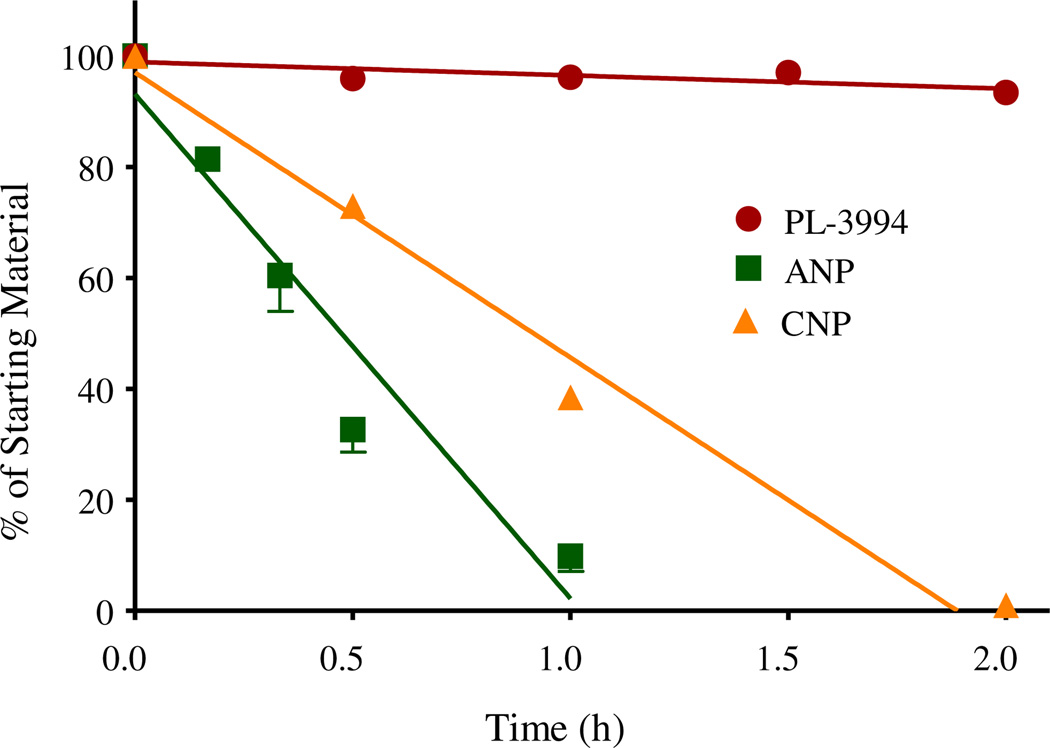
The natural NPs, ANP, BNP and CNP, are rapidly degraded by NEP. For example, the human plasma half-life for ANP, BNP and CNP is about 2 min, 20 min and 2 min, respectively [10]. A comparison was made between the sensitivity of PL-3994, ANP and CNP (substrate concentration of 50 µm) to metabolism by hNEP. After 2-hr exposure to hNEP solution at 37 °C, there was minimal degradation of PL-3994 (92% remaining; n = 5). In contrast, under these conditions about 10% of ANP remained after 60 min and there was complete degradation after 2 h (n = 4). Similarly, for CNP there was 39% remaining after 60-min incubation with hNEP, and 1% after 2 h (n = 2). For technical reasons BNP could not be evaluated. These data demonstrate that PL-3994 is resistant to metabolism by NEP, especially in relation to ANP and CNP (Fig. 4).
Fig. 4.
Comparison of the sensitivity of PL-3994 ( ), ANP (
), ANP ( ) and CNP (
) and CNP ( ) to degradation by hNEP solution in vitro. Results are expressed as % of the starting material and are the mean or mean ± SEM of 2–5 experiments. PL-3994 was resistant to degradation by hNEP (92% remaining after 2-h exposure at 37 °C), whereas the natural ligands, ANP and CNP, were rapidly metabolized by hNEP (≤1% remaining after 2 h).
) to degradation by hNEP solution in vitro. Results are expressed as % of the starting material and are the mean or mean ± SEM of 2–5 experiments. PL-3994 was resistant to degradation by hNEP (92% remaining after 2-h exposure at 37 °C), whereas the natural ligands, ANP and CNP, were rapidly metabolized by hNEP (≤1% remaining after 2 h).
3.2. In vivo
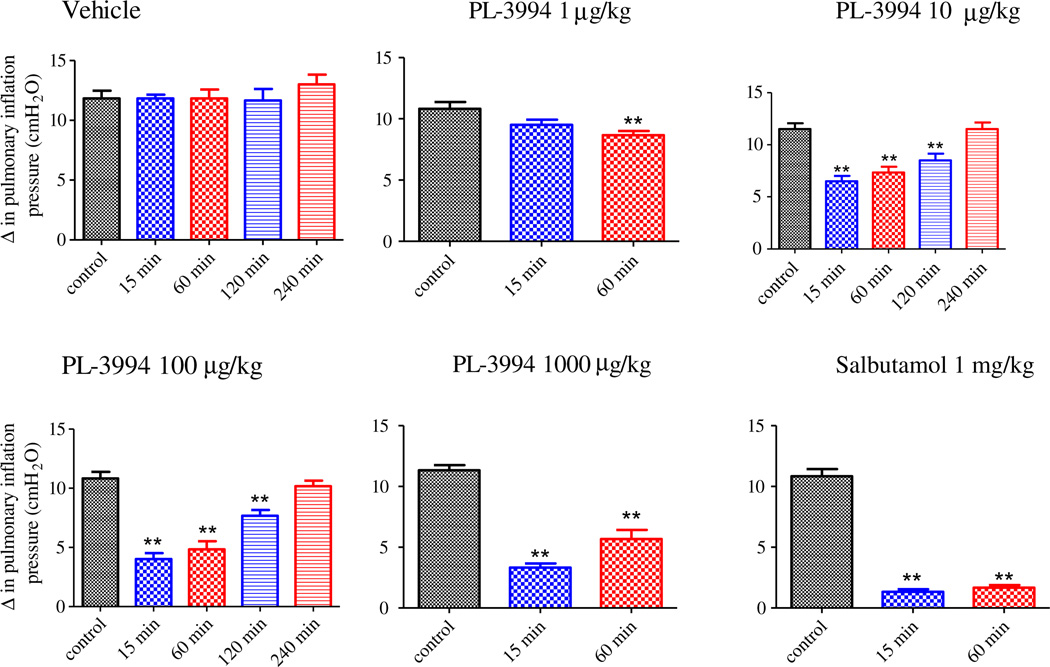
The ability of local delivery (intratracheal; i.t.) of PL-3994 to produce bronchodilation and influence CV parameters was examined in anesthetized guinea-pigs challenged with the muscarinic receptor agonist, methacholine. Intratracheal PL-3994 (1–1000 µg/kg; n = 6) produced a dose-dependent inhibition of the bronchoconstrictor response to aerosolized methacholine (10 µg/ml) (Fig. 5). For doses of 10, 100 and 1000 µg/kg a significant (P < 0.05) maximum inhibition of 43 ± 4%, 63 ± 5% and 70 ± 3%, respectively, was observed after 15-min pretreatment with PL-3994. The inhibition was maintained for all doses after the 60-min pretreatment time. The duration of action of the 10 µg/kg and 100 µg/kg doses of PL-3994 was explored. For both PL-3994 doses the inhibition extended to 120 min post-administration, although it was reduced, and the inhibitory effect was absent after 240-min pretreatment (Fig. 5). Administration of the positive control β2-agonist, salbutamol (1000 µg/kg, i.t., n = 6) significantly inhibited the bronchoconstrictor response evoked by methacholine (10 µg/ml) challenge, reaching a maximum inhibition of 87 ± 2% after 15 min. This inhibition was maintained over the duration of the study (60 min).
Fig. 5.
Bar graphs showing the change in pulmonary inflation pressure from baseline evoked by methacholine (10 µg/ml) aerosol exposure in anaesthetized guinea pigs treated intra-tracheally (i.t.) with vehicle, PL-3994 (1–1000 µg/kg; n = 6) or salbutamol (1000 µg/kg; n = 6). Results are expressed as the mean ± SEM. The methacholine-induced response was assessed in all treatment groups 15- and 60-min after compound or vehicle administration. PL-3994 produced dose-dependent inhibition of methacholine-induced bronchoconstriction. The duration of action of 10 µg/kg and 100 µg/kg doses of PL-3994 was explored further. For both PL-3994 doses the inhibition extended to 120 min post-administration.
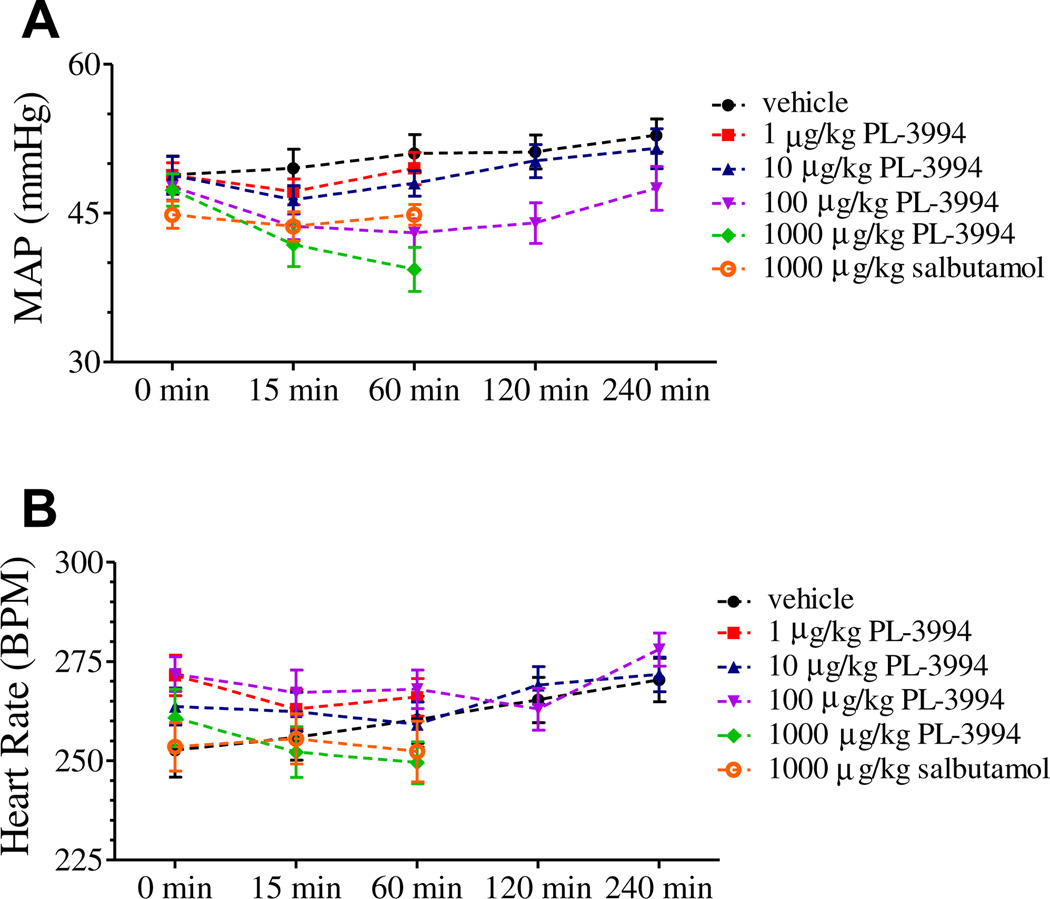
Although i.t. PL-3994 (1–1000 µg/kg; n = 6) caused a dose-dependent trend toward a fall in mean arterial blood pressure and heart rate, there was no significant effect when compared to baseline (Fig. 6). Salbutamol (1000 µg/kg, i.t.; n = 6) had no significant effect on baseline mean arterial blood pressure and heart rate (Fig. 6).
Fig. 6.
Time-course of (A) mean arterial blood pressure (MAP, mmHg) and (B) heart rate (HR, beats min−1) changes in anaesthetized guinea pigs recorded immediately prior to dosing with PL-3994 (1–1000 µg/kg, i.t., n = 6) or salbutamol (1000 µg/kg, i.t., n = 6) and after dosing but immediately prior to subsequent methacholine challenges. Each point represents the mean ± SEM. There were no significant effects of PL-3994 or salbutamol on blood pressure (A) or heart rate (B) compared to baseline values.
4. Discussion
PL-3994 is a novel, potent and selective NPR agonist. The results indicate: 1) PL-3994 is a full agonist and has high potency (cGMP generation) and affinity for recombinant human, dog or rat NPR-As; 2) PL-3994 has high affinity for hNPR-C but was without effect in the hNPR-B functional assay (cGMP generation); 3) PL-3994, unlike the natural NP ligands, ANP and BNP, has lower affinity for the clearance receptor, NPR-C, than NPR-A; 4) PL-3994 was without significant effect against 75 diverse molecular targets; 5) PL-3994 produced concentration-dependent relaxation of precontracted hPCLC preparations and guinea-pig trachea; 6) Intratracheal (i.t.) PL-3994 produced a dose-dependent inhibition of the bronchoconstrictor response evoked by aerosolized methacholine in guinea pigs; 7) PL-3994 was resistant to degradation by hNEP whereas the natural ligands, ANP and CNP, were rapidly metabolized. ANP and BNP produce a short-acting bronchodilation in asthmatics [27–31]. The differentiated profile of PL-3994, combining resistance to degradation by NEP with lower relative affinity for the clearance receptor, NPR-C, provides the promise that PL-3994 may produce a longer-lasting and more effective bronchodilation in asthma patients than observed with the native NP ligands.
4.1. Receptor pharmacology
A feature of PL-3994 that distinguishes it from natural NPs is its differential NP receptor pharmacological profile, including reduced affinity for binding to the scavenger receptor, NPR-C. PL-3994 is a full agonist at NPR-A. Compared to the natural NPs, PL-3994 has lower affinity and potency (about 7- to 20-fold) than ANP at hNPRA, with affinity and potency similar to that of BNP. PL-3944, and also ANP and BNP, did not engage the NPR-B receptor functionally to produce cGMP. Importantly, of the three ligands, PL-3994 has the lowest affinity for NPR-C relative to NPR-A: PL-3994 has higher affinity (7-fold) for hNPR-A vs. hNPR-C, whereas ANP has equivalent affinity for both receptors and BNP has lower affinity (about 4- fold) for hNPR-A vs. hNPR-C. Although originally described as a scavenger receptor for NPs, subsequent research demonstrated that NPR-C is linked to inhibition of adenylyl cyclase activity, augmentation of phospholipase C activity, stimulation of eNOS or inhibition of MAPK activity in various systems [11]. Given its lower absolute and relative affinity for NPR-C, PL-3994 may have distinct PK characteristics, a longer duration of action and perhaps a different in vivo pharmacological profile (via its impact on intracellular 2nd messenger pathways) than the natural NPs.
4.2. Resistance to NEP
Another characteristic of PL-3994 compared with the natural NPs is its markedly increased resistance to proteolytic inactivation by NEP. Prior clinical studies have generally shown an effective but short-lasting bronchodilation of inhaled ANP [28–31]. This short duration of action of ANP is probably linked in large part to the presence of metabolic mechanisms in the airway, in particular NEP. Angus and colleagues demonstrated that pre-treatment of asthmatics with thiorphan (an NEP inhibitor) extended the duration, but not the magnitude of the bronchodilator effect of ANP inhalation [30,31]; the authors speculated that only partial NEP inhibition was achieved in these studies. In addition to NEP, other proteases may contribute to the degradation of naturally occurring NPs. One candidate is an arginine-specific protease isolated from ragweed pollen, which was reported to rapidly hydrolyze ANP, suggesting the possible involvement of ANP inactivation in the human lung after ragweed exposure [34]. The resistance of PL-3994 to degradation by NEP in vitro provides the potential that the clinical bronchodilator effect, including the duration of action, with PL- 3994 may be superior to the native peptides.
4.3. Bronchodilator activity in animal and human models
PL-3994 demonstrated concentration-dependent relaxation of isolated, carbachol-contracted guinea pig tracheal strips, producing maximum relaxation that was >80% of that produced by the positive control β2-agonist, salbutamol. PL-3994 was approximately 4-fold less potent than BNP in this assay system, which correlates with the results of the binding studies in guinea-pig lung membranes. Furthermore, in vivo experiments in anesthetized, methacholine-challenged guinea pigs, indicated that intratracheal administration of PL-3994 caused dose-dependent inhibition of the bronchoconstrictor response, with a maximal inhibition of 70%; whichwas in the similar range to that produced by salbutamol (87% inhibition). Importantly, the PL-3994-induced bronchodilator activity was not associated with a significant effect on cardiovascular parameters.
The bronchodilator effect of PL-3994 was further characterized in the hPCLS preparation [32,33]. PL-3994 produced potent, concentration-dependent, but modest relaxation of hPCLS, with a maximal effect of 33% of the isoproterenol-induced response. Similar effects were observed with BNP, although BNP produced a significantly greater maximal relaxant effect than did PL-3994 in these preliminary experiments in 3 human lungs. Further studies are required to evaluate more comprehensively the relaxant profile of PL-3994 in this human airways preparation, including in sensitized tissues, and especially relative to the natural ligands ANP and BNP.
ANP and BNP were reported initially to have little relaxant effect on human isolated bronchus, either on basal tone or after stimulation with a contractile agonist [25,35]. However, in later studies ANP produced concentration-dependent, full relaxation of methacholine-contracted human bronchus, with an IC50 of about 1 µm [36], and BNP elicited >40% relaxation of non-sensitized sensitized human bronchus contracted with histamine [37]. The variability in the results obtained in human isolated airways may be in part due to experimental protocol differences (e.g., the presence or absence of NEP inhibitor; the level of airway tone produced by the contractile agonist, the contractile agonist used (for example, histamine vs. carbachol [37]) and the source and quality of the lung tissue). Although our data demonstrate that NPR activation of human airways promotes bronchodilation, significant variability of responses was noted, perhaps related to donor genetic heterogeneity. Notwithstanding the smaller effects of ANP or BNP alone observed in human isolated airways compared to isoproterenol, both compounds have demonstrated significant bronchodilator activity in humans, with efficacies similar to that observed with the β2-agonist, albuterol [26–31,38]. This would suggest that a significant component of the clinical effects on airways of the natural NPs, and by inference PL-3994, may be due to indirect effects, in addition to direct activity on airway smooth muscle. Collectively, the data above with PL-3994, ANP and BNP in isolated guinea pig and human airways, concomitant with the clinical bronchodilator activity produced by ANP and BNP, suggests that PL-3994 will produce significant beneficial effects on pulmonary function when administered to humans.
4.4. Species differences
In contrast to guinea-pig trachea, and despite having high affinity for the recombinant rat NPR-A receptor (Ki = 10 nm), PL-3994 did not produce significant bronchorelaxation in pre-contracted rat trachea, except in high concentrations (≥10 µm). The reason for the limited response of rat airways to PL-3994 may reflect the paucity of NPRs in the trachea compared to other tissues in this species. In support of this, it was reported by Bianchi and colleagues that no specific binding sites for 125I-ANF(5–28) were detected in rat airway smooth muscle or epithelium [39]. In contrast, Fernandes et al. demonstrated specific 125I-ANF(1–28) binding in guinea-pig airway smooth muscle (and epithelium) [24], a tissue that is sensitive to the relaxant effects of PL-3994 (IC50 = 40 nm). In addition, it is possible that PL-3994 is sensitive to rat NEP, in contrast to the resistance of the compound to human NEP, which may impact the sensitivity of rat airways to the compound. The airway epithelium, which is a source of NEP [40], has been determined to influence the relaxant responses to the NPs and analogs in human [41], rat [24] and guinea-pig isolated tissues [23–25], with evidence that the role of the epithelium may depend upon the species and NP ligand examined. In the current series of studies, experiments using airway preparations were all conducted with intact epithelium. Additional experiments would be required to systematically assess the potential influence of the airway epithelium on the relaxant responses of PL-3994, and ANP and BNP, in different species.
4.5. Novel NPR analogs
Burnette and colleagues have synthesized and profiled some peptide analogs of the NPs, most notably CD-NP a novel chimeric 37-amino acid peptide based upon CNP [42], and M-ANP, a 40-amino acid peptide derived from ANP [43]. Assessment of cGMP generation from HEK cells determined that CD-NP was about 200-fold more potent than CNP for activation of NPR-A. However, based upon these results and the data described in this manuscript, CD-NP is > 100-fold less potent than PL-3994, or ANP [42,44]. In contrast to PL-3994 or ANP, CD-NP produced a concentration-dependent activation of NPR-B (EC50 < 1 µm), and is a dual activator of NPR-A and NPR-B. Furthermore, unlike PL-3994 which is a full agonist relative to ANP, CD-NP was reported to be a partial agonist at NPR-A [42,44]. Information was not provided on the sensitivity of CD-NP to NEP. M-ANP had a similar potency and efficacy as ANP for NPR-A, and like the native ligand had limited ability to activate NPR-B [42,45]. M-ANP was more resistant to degradation by hNEP than ANP, with about 65% remaining after 30 min, while ANP was essentially fully metabolized after that time [42,45]. It will be of interest in the future to compare the biological and NEP degradation profiles of PL-3994, CD-NP and M-ANP.
In summary, PL-3994 is a potent NPR-A agonist, which also has high affinity for NPR-C (the clearance receptor) but not NPR-B, and potently relaxes guinea-pig and human ASM. Intratracheal PL-3994 produced a dose-dependent bronchodilation in guinea pigs challenged with aerosolized methacholine, but was without significant effect on mean arterial blood pressure and heart rate. PL-3994 was chemically engineered to be resistant to degradation by hNEP, which effectively metabolizes the natural ligand NPs. The overall profile of PL-3994, combining high potency for NPR-A, resistance to NEP and lower potency for the clearance receptor, NPR-C, than the native NPs, concomitant with potent airway relaxant effects, suggests that PL-3994 will likely be a valuable research tool toevaluate the biology of NPRs and their potential pathophysiological significance. In addition, PL-3994 has the potential to exhibit more effective and longer-lasting bronchodilator activity in subjects with asthma than observed previously with ANP.
Acknowledgments
PL-3994 was synthesized at Palatin Technologies, Inc. by Marguerita Bastos, Ph.D.
Dr. Panettieri’s lab received support from Palatin in the form of an unrestricted research grant.
Abbreviations
- ANP
atrial natriuretic peptide
- ASM
airway smooth muscle
- BNP
brain natriuretic peptide
- BSA
bovine serum albumin
- β2-agonists
β2-adrenergic receptor agonists
- hPCLS
human precision-cut lung slices
- HEK cells
human embryonic kidney cells
- h
human
- i.t.
intratracheal
- NEP
neutral endopeptidase
- NK-2
neurokinin-2 receptor
- NPR-A
natriuretic receptor-A
- NPR-B
natriuretic receptor-B
- NPR-C
natriuretic receptor-C
- ORL1
opioid receptor-like 1
- SNP
single nucleotide polymorphism
- sst
non-selective somatostatin receptor
Footnotes
Disclosure statement
Dr. Hay has been paid as a consultant by Palatin.
References
- 1.Panettieri RA, Jr, Covar R, Grant E, Hilyer EV, Bacharier L. Natural history of asthma: persistence versus progression – does the beginning predict the end? J Allergy Clin Immunol. 2008;121:607–613. doi: 10.1016/j.jaci.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Clark DJ, Lipworth BJ. Dose-response of inhaled drugs in asthma. Update Clin Pharmacokinet. 1997;32:58–74. doi: 10.2165/00003088-199732010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Shore SA, Drazen JM. Beta-agonists and asthma: too much of a good thing? J Clin Invest. 2003;112:495–497. doi: 10.1172/JCI19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson HS. Is there a problem with inhaled long-acting beta-adrenergic agonists? J Allergy Clin Immunol. 2006;117:3–16. doi: 10.1016/j.jaci.2005.10.013. [quiz 17]. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, et al. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol. 1989;122:921–928. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega VE, Peters SP. Beta-2 adrenergic agonists: focus on safety and benefits versus risks. Curr Opin Pharmacol. 2010;10:246–253. doi: 10.1016/j.coph.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigo GJ, Castro-Rodriguez JA. Safety of long-acting beta agonists for the treatment of asthma: clearing the air. Thorax. 2012;67(4):342–349. doi: 10.1136/thx.2010.155648. [DOI] [PubMed] [Google Scholar]
- 8.Pandey KN. Biology of natriuretic peptides and their receptors. Peptides. 2005;26:901–932. doi: 10.1016/j.peptides.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 10.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handbook Exp Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;6:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Barr CS, Rhodes P, Struthers AD. C-type natriuretic peptide. Peptides. 1996;17(7):1243–1251. doi: 10.1016/s0196-9781(96)00110-6. [DOI] [PubMed] [Google Scholar]
- 13.Perreault T, Gutkowska J. Role of atrial natriuretic factor in lung physiology and pathology. Am J Respir Crit Care Med. 1995;151:226–242. doi: 10.1164/ajrccm.151.1.7812560. [DOI] [PubMed] [Google Scholar]
- 14.Rubinstein I, Reiss TF, Gardner DG, Liu J, Bigby BG, Boushey HA., Jr Effect of exercise, hyperpnea, and bronchoconstriction on plasma atrial natriuretic peptide. J Appl Physiol. 1989;67(6):2565–2570. doi: 10.1152/jappl.1989.67.6.2565. [DOI] [PubMed] [Google Scholar]
- 15.Ohbayashi H, Suito H, Takagi K. Compared effects of natriuretic peptides on ovalbumin-induced asthmatic model. Eur J Pharmacol. 1998;346:55–64. doi: 10.1016/s0014-2999(98)00014-4. [DOI] [PubMed] [Google Scholar]
- 16.Torphy TJ. Phosphodiesterase Isozymes. Molecular targets for novel antiasthma agents. Am Rev Resp Crit Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- 17.Hamad AM, Johnson SR, Knox AJ. Antiproliferative effects of NO and ANP in cultured human airway smooth muscle. Am J Physiol. 1999;277(5 Pt 1):L910–L918. doi: 10.1152/ajplung.1999.277.5.L910. [DOI] [PubMed] [Google Scholar]
- 18.Hamad AM, Clayton A, Islam B, Knox AJ. Guanylyl cyclases, nitric oxide, natriuretic peptides, and airway smooth muscle function. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L973–L983. doi: 10.1152/ajplung.00033.2003. [DOI] [PubMed] [Google Scholar]
- 19.Mohapatra SS, Lockey RF, Vesely DL, Gower WR., Jr Natriuretic peptides and genesis of asthma: an emerging paradigm? J Allergy Clin Immunol. 2004;114:520–526. doi: 10.1016/j.jaci.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Mohapatra SS. Role of natriuretic peptide signaling in modulating asthma and inflammation. Can J Physiol Pharmacol. 2007;85:754–759. doi: 10.1139/Y07-066. [DOI] [PubMed] [Google Scholar]
- 21.Lima J, Mohapatra S, Feng H, Lockey R, Jena PK, Castro M, et al. A polymorphism in the NPPA gene associates with asthma. Clin Exp Allergy. 2008;38:1117–1123. doi: 10.1111/j.1365-2222.2008.02955.x. [DOI] [PubMed] [Google Scholar]
- 22.Rogers AJ, Raby BA, Lasky-Su JA, Murphy A, Lazarus R, Klanderman BJ, et al. Assessing the reproducibility of asthma candidate gene associations, using genomewide data. Am J Respir Crit Care Med. 2009;179:1084–1090. doi: 10.1164/rccm.200812-1860OC. [Erratum in: Am J Respir Crit Care Med 2010; 181:96]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell M, Garippa R, Welton AF. Relaxant activity of atriopeptins in isolated guinea pig airway and vascular smooth muscle. Peptides. 1985;6(4):597–601. doi: 10.1016/0196-9781(85)90159-7. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes LB, Preuss JMH, Goldie RG. Epithelial modulation of the relaxant activity of atriopeptides in rat and guinea-pig tracheal smooth muscle. Eur J Pharmacol. 1992;212:187–194. doi: 10.1016/0014-2999(92)90328-2. [DOI] [PubMed] [Google Scholar]
- 25.Candenas M-L, Naline E, Puybasset L, Devillier P, Advenier C. Effect of atrial natriuretic peptide and on atriopeptins on the human isolated bronchus. Comparison with the reactivity of the guinea-pig isolated trachea. Pulmon Pharmacol. 1991;4:120–125. doi: 10.1016/0952-0600(91)90062-8. [DOI] [PubMed] [Google Scholar]
- 26.Hulks G, Jardine A, Connell JMC, Thomson NC. Bronchodilator effect of atrial natriuretic peptide in asthma. Br Med J. 1989;229:1081–1082. doi: 10.1136/bmj.299.6707.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chanez P, Mann C, Bousquet J, Chabrier PE, Godard P, Braquet P, et al. Atrial natriuretic factor (ANF) is a potent bronchodilator in asthma. J Allergy Clin Immunol. 1990;86:321–324. doi: 10.1016/s0091-6749(05)80094-6. [DOI] [PubMed] [Google Scholar]
- 28.Angus RM, McCallum MJA, Hulks G, Thomson NC. Bronchodilator, cardiovascular, and cyclic guanylyl monophosphate response to high-dose infused atrial natriuretic peptide in asthma. Am Rev Respir Dis. 1993;147:1122–1125. doi: 10.1164/ajrccm/147.5.1122. [DOI] [PubMed] [Google Scholar]
- 29.Hulks G, Thomson NC. High dose inhaled atrial natriuretic peptide is a bronchodilator in asthmatic subjects. Eur Respir J. 1994;7(9):1593–1597. doi: 10.1183/09031936.94.07091593. [DOI] [PubMed] [Google Scholar]
- 30.Angus RM, Millar EA, Chalmers GW, Thomson NC. Effect of inhaled atrial natriuretic peptide and a neutral endopeptidase inhibitor on histamineinduced Bronchoconstriction. Am J Respir Crit Care Med. 1995;151(6):2003–2005. doi: 10.1164/ajrccm.151.6.7767551. [DOI] [PubMed] [Google Scholar]
- 31.Angus RM, Millar EA, Chalmers GW, Thomson NC. Effect of inhaled thiorphan, a neutral endopeptidase inhibitor, on the bronchodilator response to inhaled atrial natriuretic peptide (ANP) Thorax. 1996;51(1):71–74. doi: 10.1136/thx.51.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 33.Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, et al. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L530–L537. doi: 10.1152/ajplung.00133.2009. [DOI] [PubMed] [Google Scholar]
- 34.Bagarozzi DA, Jr, Potempa J, Travis J. Purification and characterization of an arginine-specific peptidase from ragweed (Ambrosia artemisiifolia) pollen. Am J Respir Cell Mol Biol. 1998;3:363–369. doi: 10.1165/ajrcmb.18.3.2825. [DOI] [PubMed] [Google Scholar]
- 35.Labat C, Norel X, Benveniste J, Brink C. Vasorelaxant effects of atrial peptide II on isolated human pulmonary muscle preparations. Eur J Pharmacol. 1988;150:397–400. doi: 10.1016/0014-2999(88)90027-1. [DOI] [PubMed] [Google Scholar]
- 36.Nally JE, Clayton RA, Thomson NC, McGrath JC. The interaction of human atrial natriuretic peptide (ANP) with salbutamol, sodium nitroprusside and isosorbide dinitrate in human bronchial smooth muscle. Br J Pharmacol. 1994;113:1328–1332. doi: 10.1111/j.1476-5381.1994.tb17143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matera MG, Calzetta L, Parascandolo V, Curradi G, Rogliani P, Cazzola M. Relaxant effect of brain natriuretic peptide in nonsensitized and passively sensitized isolated human bronchi. Pulmon Pharmacol Therap. 2009;22:478–482. doi: 10.1016/j.pupt.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Akerman MJ, Yaegashi M, Khiangte Z, Murugan AT, Abe O, Marmur JD. Bronchodilator effect of infused B-type natriuretic peptide in asthma. Chest. 2006;130(1):66–72. doi: 10.1378/chest.130.1.66. [DOI] [PubMed] [Google Scholar]
- 39.Bianchi C, Gutkowska J, Thibault G, Garcia R, Genest J, Cantin M. Radioauto-graphic localization of 125I-atrial natriuretic factor (ANF) in rat tissues. Histochemistry. 1985;82:441–452. doi: 10.1007/BF02450479. [DOI] [PubMed] [Google Scholar]
- 40.Nadel JA, Borson DB. Modulation of neurogenic inflammation by neutral endopeptidase. Am Rev Respir Dis. 1991;143(3 Part 2):S33–S36. doi: 10.1164/ajrccm/143.3_Pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 41.Matera MG, Calzetta L, Passeri D, Facciolo F, Rendina EA, Page C, et al. Epithelium integrity is crucial for the relaxant activity of brain natriuretic peptide in human isolated bronchi. Br J Pharmacol. 2011;163:1740–1754. doi: 10.1111/j.1476-5381.2011.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKie PM, Sangaralingham SJ, Burnett JC., Jr CD-NP: an innovative designer natriuretic peptide activator of particulate guanylyl cyclase receptors for cardiorenal disease. Curr Heart Fail Rep. 2010;7:93–99. doi: 10.1007/s11897-010-0016-6. [DOI] [PubMed] [Google Scholar]
- 43.McKie PM, Ichiki T, Burnett JC., Jr M-Atrial natriuretic peptide: a novel antihypertensive protein therapy. Curr Hyper Rep. 2012;14:62–69. doi: 10.1007/s11906-011-0244-5. [DOI] [PubMed] [Google Scholar]
- 44.Dickey DM, Burnett JC, Jr, Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–35009. doi: 10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickey DM, Yoder AR, Potter LR. A familial mutation renders atrial natriuretic peptide resistant to proteolytic degradation. J Biol Chem. 2009;284:19196–19202. doi: 10.1074/jbc.M109.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]