Abstract
Normal organismal physiology depends on the maintenance of proteostasis in each cellular compartment to achieve a delicate balance between protein synthesis, folding, trafficking, and degradation while minimizing misfolding and aggregation. Defective proteostasis leads to numerous protein misfolding diseases. Pharmacological chaperones are cell-permeant small molecules that promote the proper folding and trafficking of a protein via direct binding to that protein. They stabilize their target protein in a protein-pharmacological chaperone state, increasing the natively-folded protein population that can effectively engage trafficking machinery for transport to the final destination for function. Here, as regards the application of pharmacological chaperones, we focus on their capability to promote the folding and trafficking of lysosomal enzymes, G protein coupled receptors (GPCRs), and ion channels, each of which is presently an important drug target. Pharmacological chaperones hold great promise as potential therapeutics to ameliorate a variety of protein misfolding diseases.
Keywords: Pharmacological chaperone, proteostasis, chaperone, ERAD, protein misfolding disease, lysosomal storage disease, GPCR, ion channel
1. Proteostasis and proteostasis maintenance
1.1 Proteostasis in health and disease
Proteostasis represents an optimal state of the cellular proteome, in which a delicate balance between protein synthesis, folding, trafficking, aggregation and degradation is achieved for individual proteins that make up the proteome (1, 2). Normal organismal physiology depends on the maintenance of proteostasis in each cellular compartment. Proteostasis maintenance is challenged by intrinsic stress, such as inherited misfolding-prone proteins (3), environment (4, 5), and aging (6, 7), leading to loss-of-function and gain-of-toxic-function diseases. Loss-of-function diseases include ion channel diseases, such as cystic fibrosis (8, 9), cardiac arrhythmias (10), and idiopathic epilepsy (11), as well as G-protein-coupled receptor (GPCR) conformational diseases (12, 13), and lysosomal storage diseases (14). Loss-of-function diseases often result from the inheritance of a misfolding-prone mutant protein, which triggers the collapse of proteostasis and leads to disease. Gain-of-toxic-function diseases, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, are often associated with aggregation (15, 16).
Proteostasis deficiency can be corrected using two classes of mechanistically distinct small molecules: pharmacological chaperones and proteostasis regulators (17). Pharmacological chaperones bind to and stabilize a specific protein to enable its proper folding and trafficking, whereas proteostasis regulators increase the proteostasis network capacity in a general way to restore proteostasis (18, 19). In this review, we will focus on the application of pharmacological chaperones for lysosomal enzymes, ion channels, and GPCRs, which represent a potential therapeutic strategy to ameliorate a variety of protein misfolding diseases.
1.2 Proteostasis maintenance by the proteostasis network
The proteostasis network maintains the proteome integrity to achieve optimal concentrations, conformations, interactions, and locations of individual proteins in cells. This network is composed of a variety of subnetworks, including chaperone, degradation, and trafficking networks, as well as cellular signaling pathways. The signaling pathways include the heat shock response, which regulates cytosolic proteostasis (20); the unfolded protein response (UPR), which regulates proteostasis in the endoplasmic reticulum (ER) (21, 22); Ca2+-sensitive folding pathways (23, 24); ER-associated degradation (25); autophagy (26); and many others (16, 27).
The proteostasis network adapts by modifying its components. To understand how proteostasis is maintained by the proteostasis network in cells, it is critical to identify cellular proteostasis network components. Each specific protein uses only a subset of cellular proteostasis network components in a crowded cellular environment. These include chaperones/co-chaperones, folding enzymes, degradation factors and trafficking machinery. Thus, the identification of proteostasis network components that are specific for each protein will be important for understanding how to restore its proteostasis. Identification of a protein’s interactome in cells followed by bioinformatics analysis can effectively refine the potential proteostasis network components of the protein of interest. Examples include the identification of proteostasis network components for the cystic fibrosis transmembrane conductance regulator (CFTR) (28) and γ-aminobutyric acid type C (GABAC) receptors (29). Modern affinity purification (AP) coupled with tandem mass spectrometry (AP-MS/MS) technologies (30) were used to obtain a comprehensive understanding of the proteostasis network components for physiologically important ion channel proteins, which also contributed to the identification of novel pathways that might be useful in the clinic to ameliorate related ion channel diseases. Although it is certainly desirable to define each protein’s interactome as a potentially useful route for restoring proteostasis, pharmacological chaperones do not rely on the identification of a complex network of components, but instead focus in a specific manner to restore native folding and trafficking in a way that does not have the potential to alter complex networks.
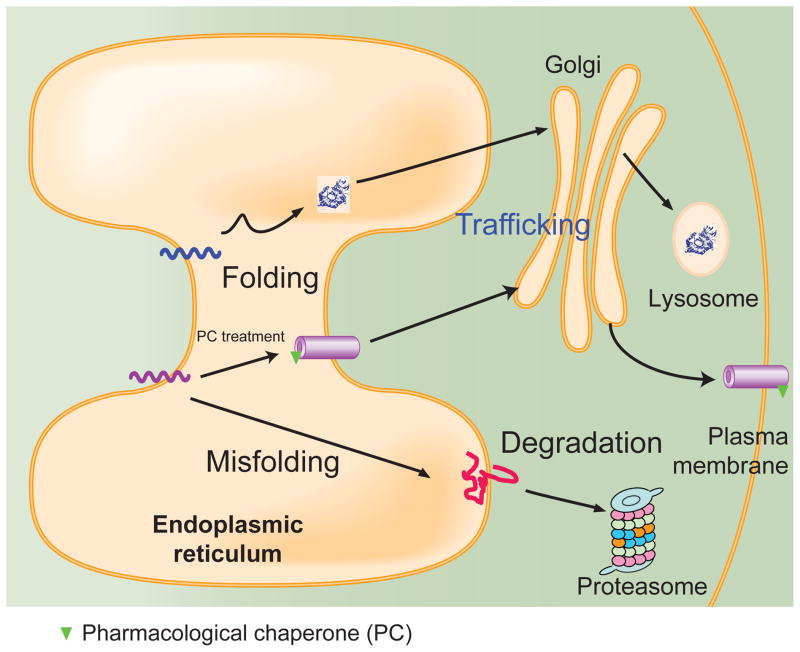
In this review we focus on pharmacological chaperones that target proteins synthesized in the ER. To function, proteins need to fold into their native structures and traffic efficiently to their final destination. Protein folding is a complicated process during which the polypeptide chain obtains its biologically active three-dimensional conformation. About 1/3 of the eukaryotic proteins are folded in the ER, including all membrane proteins, proteins in the secretory pathways, and proteins targeted to subcellular compartments such as the lysosome (Figure 1). Remarkably, over 10,000 different eukaryotic proteins are co-translationally translocated into the ER. Soluble proteins will enter the ER lumen, whereas membrane proteins, including ion channels and GPCRs, will reside on the ER membrane for folding.
FIGURE 1.
The proteostasis network maintains proteostasis in cells. About 1/3 of the eukaryotic proteins are folded in the ER, including all membrane proteins, proteins in the secretory pathways, and proteins targeted to subcellular compartments such as the lysosome. As illustrated examples, membrane proteins (purple line and purple cylinders) fold on the ER membrane, traffic through the Golgi, and reach the plasma membrane. Lysosomal enzymes (blue line and folds) fold in the ER lumen, traffic through the Golgi, and reach the lysosome. Misfolded proteins (red line) are subject to ER-associated degradation (ERAD), being retrotranslocated to the cytosol and degraded by the proteasome. The proteostasis network maintains the proteome integrity to achieve optimal concentrations, conformations, interactions, and locations of individual proteins in cells. Pharmacological chaperones (green triangles) are cell-permeant small molecules that bind to and stabilize a target protein. Pharmacological chaperone treatment stabilizes that membrane protein on the ER membrane, enabling more efficient trafficking from the ER through the Golgi and onward to the plasma membrane.
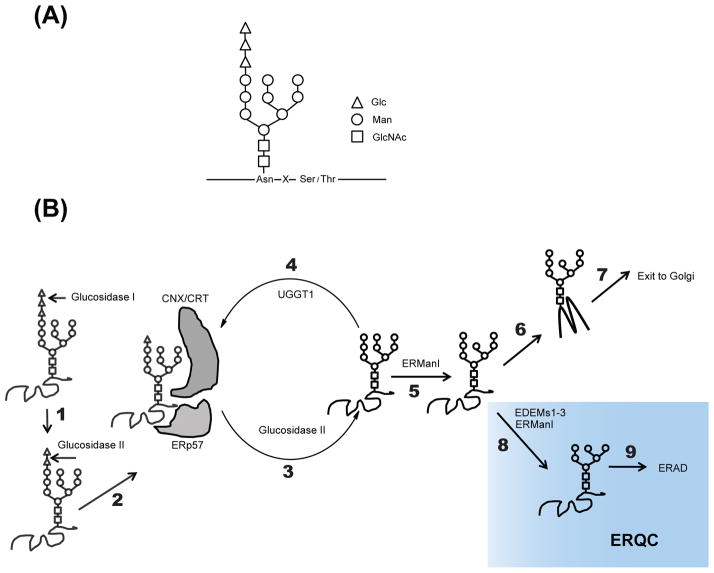
Asparagine N-linked glycosylation is the most common protein modification in the ER and occurs co-translationally, serving as a recognition tag for glycoprotein maturation (31). (Figure 2A) (32). The pathway of glycoprotein folding in the ER has been extensively studied (33–35). A glycoprotein is initially attached with Glc3Man9GlcNAc2 in its Asn residue in an Asn-X-Ser/Thr sequon (Figure 2A). N-glycan trimming serves as a crucial tag for protein maturation in the ER (Figure 2B). The two outermost glucose residues of a glycoprotein are cleaved by glucosidase I and glucosidase II before entering the calnexin/calreticulin folding cycles. The molecular chaperone lectins calnexin and calreticulin facilitate the folding of monoglucosylated glycocproteins. Additionally, folding enzymes such as protein disulfide bond isomerases (PDIases) catalyze disulfide bond formation, and peptidylprolyl isomerases (PPIases) assist proline isomerization. Removal of the terminal glucose residue by glucosidase II triggers the dissociation of the substrate from calnexin/calreticulin. At this point, this substrate can be reglucosylated by the folding sensor, UGGT1, and re-enter the calnexin/calreticulin folding cycles. Eventually, the substrate exits the calnexin/calreticulin cycles upon removal of mannose residues by ER-mannosidase I. When folded, the substrate will engage the trafficking machinery for export to the Golgi.
FIGURE 2.
Folding and degradation of glycoproteins in the ER. (A) Structure of N-linked glycans. Upon entering the ER, 14-monosaccharide residues Glc3Man9GlcNAc2 (Glc: glucose, Man: mannose, GlcNAc: N-acetylglucosamine) are attached to an Asn residue contained in an Asn-X-Ser/Thr sequon (where X is any residue except proline). (B) N-glycan trimming serves as a crucial tag for protein maturation in the ER. The two outermost glucose residues of a glycoprotein are cleaved by glucosidase I (step 1) and glucosidase II (step 2) before entering the calnexin (CNX)/calreticulin (CRT) folding cycles. CNX and CRT facilitate the folding of monoglucosylated glycocproteins. Removal of the terminal glucose residue by glucosidase II triggers the dissociation of the substrate from CNX/CRT (step 3). At this point, this substrate can be reglucosylated by the folding sensor, UGGT1 (step 4), and re-enter the CNX/CRT folding cycles. Eventually, the substrate exits the CNX/CRT cycles upon removal of mannose residues by ER-mannosidase I (ERManI) (step 5). When folded, the substrate will engage the trafficking machinery for export to the Golgi (step 6 to 7). Terminally misfolded glycoproteins will be further trimmed by ERManI and EDEM family proteins (step 8) and delivered to the ERAD pathway (step 9). Terminally misfolded proteins are segregated into a domain called the ER-derived quality-control compartment (ERQC), which is tightly linked to ERAD. The ERQC compartment is shadowed in cyan.
Beyond glycan-dependent, lectin-assisted chaperoning integral membrane proteins also undergo glycan-independent chaperoning by heat shock proteins (Hsp) (36). Hsp molecular chaperones play a central role in the maintenance of proteostasis (37, 38). They are universally present in all types of cells and in most of the cellular compartments, and they provide different pathways to assist peptide folding during different stages. Excellent reviews are present in the literature (39–41). The major Hsp chaperone machinery includes small Hsps, Hsp40, Hsp60, Hsp70, and Hsp90. During the protein folding process Hsps aid in disulfide bond formation, proline isomerization, and bind to hydrophobic patches of unfolded proteins to prevent aggregation.
After collaborative glycan-dependent and glycan-independent folding, the properly folded proteins exit the ER and traffic through the Golgi en route to their final destination. For protein trafficking, the COPII machinery is responsible for anterograde (forward) cargo protein vesicle transport from the ER to the Golgi (42, 43), whereas the COPI machinery is responsible for retrograde (backward) retrieval of cargo proteins (44). Terminally misfolded proteins are subjected to ER-associated degradation (ERAD): they are recognized, ubiquitinated, retrotranslocated into the cytosol, and degraded by the proteasome (25).
2. Using pharmacological chaperones to restore proteostasis
2.1. Overview of pharmacological chaperones
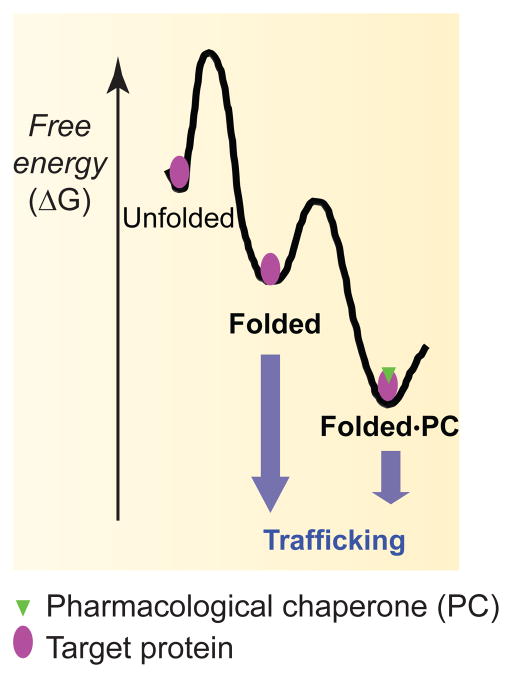
Pharmacological chaperones are cell-permeant small molecules that bind to and stabilize a target protein. In many protein misfolding diseases, a mutation in a protein leads to its misfolding and extensive degradation. The consequence is that few proteins reach their final destination for function. Pharmacological chaperone treatment stabilizes the target protein in the ER and enables its exit from the ER and trafficking through the Golgi and onward to its final destination (Figure 1 shows an example for an ion channel protein after pharmacological chaperone treatment). The mechanism of pharmacological chaperones is illustrated by using a free energy diagram (Figure 3). Without treatment, only a limited number of molecules of a target protein can achieve their folded state for trafficking. Pharmacological chaperone treatment transitions the target from the folded state to the folded protein-pharmacological chaperone state, where the free energy is more favorable. This will increase the concentration of the natively-folded protein that can successfully be recruited to the ER exit sites. A prominent advantage of pharmacological chaperones is their specificity. However, off-target effects have been reported for low potency pharmacological chaperones.
FIGURE 3.
A free energy diagram illustrates the mechanism of using pharmacological chaperones to restore proteostasis. Pharmacological chaperone (green triangle) treatment pulls the target protein (purple eclipse) from the folded state to the folded protein-pharmacological chaperone state, in which the free energy is more favorable. This will increase the folded protein population that can engage the trafficking machinery for transport to the final destination.
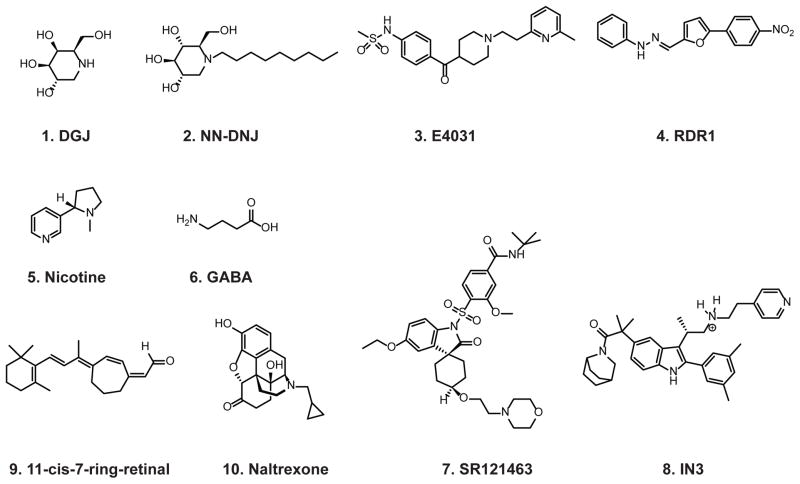
Pharmacological chaperones have been applied to enhance proteostasis of different types of proteins, including lysosomal enzymes (45), GPCRs (46), ion channels (47), transporters (48), and aggregation-prone proteins (49). Other reviewers of this issue have provided prominent examples. Here, we focus on their application on lysosomal enzymes, ion channels and GPCRs (Table 1). The chemical structures of the described pharmacological chaperones in Table 1 are shown in Figure 4.
Table 1.
Select pharmacological chaperones used to enhance proteostasis of lysosomal enzymes, GPCRs and ion channels. See Figure 4 for chemical structures.
| Protein type | Deficient protein | Disease | Pharmacological chaperones |
|---|---|---|---|
| Lysosomal enzymes | α-galactosidase A | Fabry disease | DGJ (56) |
| Acid β-glucosidase | Gaucher disease | NN-DNJ (63) | |
|
| |||
| Ion channels | hERG channel | Long QT syndrome | E4031 (69) |
| CFTR | Cystic fibrosis | RDR1 (78) | |
| nAChR | Many such as epilepsy | Nicotine (72, 73) | |
| GABAA receptor | Epilepsy | GABA (71) | |
|
| |||
| GPCRs | V2 vasopressin receptor | Nephrogenic diabetes insipidus | SR121463 (86) |
| GnRH receptor | Hypogonadotropic hypogonadsm | IN3 (90) | |
| Opsin | Retinitis pigmentosa | 11-cis-7-ring-retinal (94) | |
| δ-opioid receptor | Pain | Naltrexone (97) | |
FIGURE 4.
Chemical structures of select pharmacological chaperones, described in Table 1.
2.2. Application of pharmacological chaperones in lysosomal enzymes
The lysosome is an acidic organelle containing a variety of lysosomal enzymes that are responsible for the metabolic degradation of many complex molecules such as glycolipids, glycoproteins, and oligosaccharides. Defective lysosomal enzymes result in the accumulation of their substrates in the lysosome, causing lysosomal storage diseases (50, 51). To date, more than 50 lysosomal storage diseases have been described, including Gaucher disease, Fabry disease, and Pompe disease. In many cases, mutations in one lysosomal enzyme result in its degradation in the ER and thus its low concentrations in the lysosome, causing diseases (52). Application of pharmacological chaperones is expected to stabilize the target lysosome enzyme in the ER at neutral pH and enable its trafficking to the lysosome. Once the pharmacological chaperones reach the acidic lysosome, the binding between them and the lysosomal enzyme is weakened or released because of the acidic environment and the competition from natural substrates of the enzyme in the lysosome. Currently, pharmacological chaperones are under clinical trial for the treatment of Fabry disease (http://www.clinicaltrials.gov) (53).
Degradation of glycosphingolipids occurs in the lysosomes by numerous enzymes in a stepwise fashion. Fabry disease is the result of a defect in lysosomal α-galactosidase A (54), whereas Gaucher disease, the most common lysosomal storage disease, results from a defect in acid β-glucosidase (55). The earliest documented application of pharmacological chaperones to rescue lysosomal enzyme trafficking was on mutant lysosomal α-galactosidase A associated with Fabry disease (56). Since then, many pharmacological chaperones have been synthesized and tested in numerous disease-associated lysosomal enzymes (45, 57, 58). Here, we focus on introducing the application of pharmacological chaperones on Fabry disease and Gaucher disease.
Application of DGJ (Compound 1, Figure 4), a potent inhibitor of α-galactosidase A, promoted the trafficking of a Fabry disease-associated α-galactosidase A variant harboring the R301Q mutation in cells (56). Furthermore, administration of DGJ in a transgenic mouse restored the enzymatic activities of lysosomal α-galactosidase A (56). This discovery established a new paradigm for the amelioration of Fabry disease, and many other lysosomal storage diseases (45, 59). More potent pharmacological chaperones have been developed for α-galactosidase A (60). To confirm a direct stabilization effect of pharmacological chaperones on α-galactosidase A, a variety of biophysics assays, including urea-induced unfolding assays, have been developed (61, 62).
Application of NN-DNJ (Compound 2, Figure 4), a potent inhibitor of acid β-glucosidase, promoted the trafficking of disease-causing acid β-glucosidase mutants in Gaucher patient-derived fibroblasts (63). Further mechanism studies confirmed an increased stability of acid β-glucosidase in the presence of pharmacological chaperones (64). Many pharmacological chaperones effectively increase the trafficking of acid β-glucosidase harboring the N370S mutation. However, it is more difficult to rescue acid β-glucosidase harboring the L444P mutation using pharmacological chaperones, possibly because the L444P mutation leads to much faster degradation of acid β-glucosidase than the N370S mutation. More potent and specific pharmacological chaperones have been developed to enhance the trafficking of Gaucher disease-associated acid β-glucosidase (65–67).
2.3. Application of pharmacological chaperones in ion channels
Loss-of-function of ion channels leads to ion channel diseases called channelopathies (68). Misfolding of an ion channel leads to its reduced expression on the plasma membrane and thus loss of function. Pharmacological chaperones have been applied to promote the trafficking of numerous ion channels, including hERG (human ether-à-go-go-related gene) channels (69), CFTR (cystic fibrosis transmembrane conductance regulator) (70), and the Cys-loop superfamily of ligand-gated ion channels (71–73) (Table 1). For ion channel proteins, both agonists and antagonists are potential pharmacological chaperone candidates.
To our knowledge, the earliest documented application of pharmacological chaperones on ion channels was on trafficking-deficient mutant hERG channels (69). Loss of function of hERG channels causes type 2 long QT syndrome (LQT2), which is characterized by delayed cardiac repolarization and prolonged QT intervals on the electrocardiogram, leading to ventricular arrhythmias and sudden death (10). Most clinically important hERG mutations lead to protein trafficking defect: an insufficient amount of mutant hERG channels are trafficked to the plasma membrane (74). It was shown that E4031 (Compound 3, Figure 4), an antagonist of hERG channels, promoted the trafficking of hERG channels harboring the N470D mutation (69). Several other potent hERG channel blockers, such as astemizole and cisapride, were later reported to rescue the trafficking of LQT2-associated hERG channels harboring the G601S mutation (75). As a second example, cystic fibrosis results from loss of function of CFTR. In the literature, correctors are used to describe small molecules that promote the trafficking of CFTR, whereas potentiators are for small molecules that enhance CFTR channel function on the plasma membrane. Both correctors, including corr-4a, VRT-325, VRT-532 (76, 77), RDR1 (Compound 4, Figure 4) (78), and potentiators, including MPB compounds (79), could be pharmacological chaperone candidates. It was reported that RDR1 binds to the first nucleotide binding domain of ΔF508 CFTR directly. Therefore, RDR1 acted as a pharmacological chaperone to stabilize and rescue partial function of ΔF508 CFTR (78). A recent review summarized the current progress for the application of correctors and potentiators on CFTR (70). Thirdly, the Cys-loop superfamily of neurotransmitter-gated ion channels, which plays an important role for brain function, includes acetylcholine receptors, serotonin receptors, glycine receptors, and GABAA (γ-aminobutyric acid type A) receptors (80–82). It was reported that nicotine (Compound 5, Figure 4), an agonist of nicotinic acetylcholine receptors (nAChRs), acted as a pharmacological chaperone to increase the surface expression of wild-type nAChRs (72, 73). Furthermore, the nAChR upregulation by nicotine depends on specific subtypes, which possibly results from different nicotine binding affinity to nAChR subtypes (83, 84). Similar results were reported for GABAA receptors: GABA (Compound 6, Figure 4), an agonist of GABAA receptors, enhanced the trafficking of wild-type GABAA receptors (71). Enhanced trafficking of wild-type ion channels upon pharmacological chaperone treatment might indicate that even wild-type ion channels do not traffic at their optimal efficiency.
2.4. Application of pharmacological chaperones in GPCRs
Pharmacological chaperones have also been identified to restore proteostasis of numerous GPCRs associated with a variety of diseases (12, 13, 46, 85). Mutant V2 vasopressin receptors are retained in the ER causing nephrogenic diabetes insipidus. Their function can be effectively recovered if the mutant receptors are successfully trafficked to the membrane. It was shown that a cell-permeant antagonist of the V2 vasopressin receptor, SR121463 (Compound 7, Figure 4), promoted the trafficking and maturation of mutant receptors (86). Many other V2 vasopressin receptor antagonists, including VPA985, YM087, and OPC31260, were later used as pharmacological chaperones for V2 vasopressin receptors (87–89). Similarly, numerous mutations in gonadotropin-releasing hormone (GnRH) receptors are associated with congenital hypogonadotropic hypogonadism. Cell-permeant GnRH receptor antagonists, including IN3 (Compound 8, Figure 4), rescued the trafficking deficiency of mutant GnRH receptors in cells (90). Recently, it was demonstrated that in a knock-in mouse model expressing E90K GnRH, IN3 application restored the plasma membrane expression and partial function of E90K GnRH (91). Other structurally distinct pharmacological chaperones were later reported for GnRH receptors, including A177775, TAK-013, and Q89 (92). Another important example is the opsin variant harboring the P23H mutation, which is associated with autosomal dominant retinitis pigmentosa (93). Pro23 is located in the extracellular N-terminal domain, and P23H mutation resulted in the ER retention of P23H-opsin. The application of an inverse agonist, 11-cis-7-ring retinal (Compound 9, Figure 4), quantitatively rescued the P23H opsin trafficking deficiency and restored its transport to the plasma membrane (94). Other retinoids, including 9-cis-retinal and 11-cis-retinal, also rescued the trafficking of P23H opsin (95). Less misfolded opsin variants were more susceptible for pharmacological chaperoning (96). It is worth noting that pharmacological chaperones can also stabilize wild-type GPCRs and increase their surface expression. For example, membrane-permeant opioid antagonists, such as naltrexone (Compound 10, Figure 4), facilitated the transport of wild-type δ-opioid receptors (97). This indicates that for complicated GPCRs, even the wild-type proteins may not traffic at their optimal efficiency, which was also seen in ion channel protein cases.
3. Concluding remarks
The pharmacological chaperoning strategy is a promising one to rescue misfolded proteins in a specific manner. The approach has been applied to many protein targets both in vitro and in a few cases, in vivo, including for lysosomal enzymes, GPCRs, and ion channels. While pharmacological chaperones increase the pool of natively-folded proteins, proteostasis regulators function by increasing the proteostasis network capacity. Because of their distinct mechanisms, we expect that co-application of pharmacological chaperones and proteostasis regulators may yield additive or synergistic effects to restore proteostasis. Indeed, a synergistic restoration of mutant acid β-glucosidase function in Gaucher patient-derived fibroblasts was shown when a proteostasis regulator, MG-132, and a pharmacological chaperone, NN-DNJ, were co-applied (18). Because pharmacological chaperone treatment decreases the population of misfolded proteins in the ER, it is expected that they may also reduce ER stress under circumstances in which abnormally folded protein would accumulate in the ER. The interplay between pharmacological chaperones and proteostasis regulators may provide a powerful approach for treating protein folding disorders.
Supplementary Material
Acknowledgments
This work was supported by the Research Start-up Fund from Case Western Reserve University School of Medicine, Epilepsy Foundation of America (225243), and the Clinical Translational Science Collaborative of Cleveland CTSA (UL1RR024989) from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
Footnotes
Chemical compounds studied in this article
NN-DNJ (PubChem CID: 501640); E4031 (PubChem CID: 3185); nicotine (PubChem CID: 89594); GABA (PubChem CID: 119); SR121463 (PubChem CID: 9810773); naltrexone (PubChem CID: 5360515)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 2.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular Chaperone Functions in Protein Folding and Proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 3.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92:537–576. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers ET, Balch WE. Diversity in the origins of proteostasis networks - a driver for protein function in evolution. Nat Rev Mol Cell Biol. 2013;14:237–248. doi: 10.1038/nrm3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gidalevitz T, Prahlad V, Morimoto RI. The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb Perspect Biol. 2011;3:a009704. doi: 10.1101/cshperspect.a009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labbadia J, Morimoto RI. Proteostasis and longevity: when does aging really begin? F1000prime Rep. 2014;6:7. doi: 10.12703/P6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilchez D, Simic MS, Dillin A. Proteostasis and aging of stem cells. Trends Cell Biol. 2014;24:161–170. doi: 10.1016/j.tcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Balch WE, Roth DM, Hutt DM. Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harb Perspect Biol. 2011;3:a004499. doi: 10.1101/cshperspect.a004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukacs GL, Verkman AS. CFTR: folding, misfolding and correcting the DeltaF508 conformational defect. Trends Mol Med. 2012;18:81–91. doi: 10.1016/j.molmed.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias - Ion channel protein trafficking. Circ Res. 2004;94:1418–1428. doi: 10.1161/01.RES.0000128561.28701.ea. [DOI] [PubMed] [Google Scholar]
- 11.Steinlein OK. Ion Channel Mutations in Neuronal Diseases: A Genetics Perspective. Chem Rev. 2012;112:6334–6352. doi: 10.1021/cr300044d. [DOI] [PubMed] [Google Scholar]
- 12.Bernier V, Bichet DG, Bouvier M. Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol. 2004;4:528–533. doi: 10.1016/j.coph.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 14.Grabowski GA. Gaucher disease and other storage disorders. Hematology Am Soc Hematol Educ Program. 2012;2012:13–18. doi: 10.1182/asheducation-2012.1.13. [DOI] [PubMed] [Google Scholar]
- 15.Arosio P, Vendruscolo M, Dobson CM, Knowles TP. Chemical kinetics for drug discovery to combat protein aggregation diseases. Trends Pharmacol Sci. 2014;35:127–135. doi: 10.1016/j.tips.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and Chemical Approaches to Diseases of Proteostasis Deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 17.Lindquist SL, Kelly JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011;3:a004507. doi: 10.1101/cshperspect.a004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu TW, Ong DST, Wang YJ, Balch WE, Yates JR, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di X-J, Han D-Y, Wang Y-J, Chance MR, Mu T-W. SAHA Enhances Proteostasis of Epilepsy-Associated alpha1(A322D)beta2gamma2 GABAA Receptors. Chem Biol. 2013;20:1456–1468. doi: 10.1016/j.chembiol.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 21.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 22.Walter P, Ron D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 23.Mu TW, Fowler DM, Kelly JW. Partial restoration of mutant enzyme homeostasis in three distinct lysosomal storage disease cell lines by altering calcium homeostasis. PLoS Biol. 2008;6:e26. doi: 10.1371/journal.pbio.0060026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong DS, Mu TW, Palmer AE, Kelly JW. Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis. Nat Chem Biol. 2010;6:424–432. doi: 10.1038/nchembio.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodsky JL. Cleaning Up: ER-Associated Degradation to the Rescue. Cell. 2012;151:1163–1167. doi: 10.1016/j.cell.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klionsky DJ, Emr SD. Cell biology - Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong DS, Wang YJ, Tan YL, Yates JR, 3rd, Mu TW, Kelly JW. FKBP10 depletion enhances glucocerebrosidase proteostasis in Gaucher disease fibroblasts. Chem Biol. 2013;20:403–415. doi: 10.1016/j.chembiol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XD, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 29.Wang YJ, Han DY, Tabib T, Yates JR, Mu TW. Identification of GABAC Receptor Protein Homeostasis Network Components from Three Tandem Mass Spectrometry Proteomics Approaches. J Proteome Res. 2013;27:5570–5586. doi: 10.1021/pr400535z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Fonslow BR, Shan B, Baek MC, Yates JR., 3rd Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113:2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebert DN, Molinari M. Flagging and docking: dual roles for N-glycans in protein quality control and cellular proteostasis. Trends Biochem Sci. 2012;37:404–410. doi: 10.1016/j.tibs.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohorko E, Glockshuber R, Aebi M. Oligosaccharyltransferase: the central enzyme of N-linked protein glycosylation. J Inherit Metab Dis. 2011;34:869–878. doi: 10.1007/s10545-011-9337-1. [DOI] [PubMed] [Google Scholar]
- 33.Aebi M. N-linked protein glycosylation in the ER. Trends Cell Biol. 2013;1833:2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Ferris SP, Kodali VK, Kaufman RJ. Glycoprotein folding and quality-control mechanisms in protein-folding diseases. Dis Model Mech. 2014;7:331–341. doi: 10.1242/dmm.014589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lederkremer GZ. Glycoprotein folding, quality control and ER-associated degradation. Curr Opin Struct Biol. 2009;19:515–523. doi: 10.1016/j.sbi.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 36.van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol. 2005;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 37.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 38.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 39.Braakman I, Hebert DN. Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5:a013201. doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 42.Routledge KE, Gupta V, Balch WE. Emergent properties of proteostasis-COPII coupled systems in human health and disease. Mol Membr Biol. 2010;27:385–397. doi: 10.3109/09687688.2010.524894. [DOI] [PubMed] [Google Scholar]
- 43.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 44.Szul T, Sztul E. COPII and COPI Traffic at the ER-Golgi Interface. Physiology. 2011;26:348–364. doi: 10.1152/physiol.00017.2011. [DOI] [PubMed] [Google Scholar]
- 45.Boyd RE, Lee G, Rybczynski P, Benjamin ER, Khanna R, Wustman BA, Valenzano KJ. Pharmacological Chaperones as Therapeutics for Lysosomal Storage Diseases. J Med Chem. 2013;56:2705–2725. doi: 10.1021/jm301557k. [DOI] [PubMed] [Google Scholar]
- 46.Maya-Nunez G, Ulloa-Aguirre A, Janovick JA, Conn PM. Pharmacological chaperones correct misfolded GPCRs and rescue function: protein trafficking as a therapeutic target. Subcell Biochem. 2012;63:263–289. doi: 10.1007/978-94-007-4765-4_14. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan R, Richards CI, Xiao C, Rhee D, Pantoja R, Dougherty DA, Miwa JM, Lester HA. Pharmacological chaperoning of nicotinic acetylcholine receptors reduces the endoplasmic reticulum stress response. Mol Pharmacol. 2012;81:759–769. doi: 10.1124/mol.112.077792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loo TW, Bartlett MC, Clarke DM. Rescue of folding defects in ABC transporters using pharmacological chaperones. J Bioenerg Biomembr. 2005;37:501–507. doi: 10.1007/s10863-005-9499-3. [DOI] [PubMed] [Google Scholar]
- 49.Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol. 2012;421:185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prada CE, Grabowski GA. Neuronopathic lysosomal storage diseases: clinical and pathologic findings. Dev Disabil Res Rev. 2013;17:226–246. doi: 10.1002/ddrr.1116. [DOI] [PubMed] [Google Scholar]
- 51.Klein AD, Futerman AH. Lysosomal storage disorders: old diseases, present and future challenges. Pediatr Endocrinol Rev. 2013;11 (Suppl 1):59–63. [PubMed] [Google Scholar]
- 52.Bendikov-Bar I, Horowitz M. Gaucher disease paradigm: from ERAD to comorbidity. Hum Mutat. 2012;33:1398–1407. doi: 10.1002/humu.22124. [DOI] [PubMed] [Google Scholar]
- 53.Giugliani R, Waldek S, Germain DP, Nicholls K, Bichet DG, Simosky JK, Bragat AC, Castelli JP, Benjamin ER, Boudes PF. A Phase 2 study of migalastat hydrochloride in females with Fabry disease: selection of population, safety and pharmacodynamic effects. Mol Genet Metab. 2013;109:86–92. doi: 10.1016/j.ymgme.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Ishii S. Pharmacological chaperone therapy for Fabry disease. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:18–30. doi: 10.2183/pjab.88.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenbloom BE, Weinreb NJ. Gaucher disease: a comprehensive review. Crit Rev Oncog. 2013;18:163–175. doi: 10.1615/critrevoncog.2013006060. [DOI] [PubMed] [Google Scholar]
- 56.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 57.Fan JQ. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol Sci. 2003;24:355–360. doi: 10.1016/S0165-6147(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 58.Yu Z, Sawkar AR, Kelly JW. Pharmacologic chaperoning as a strategy to treat Gaucher disease. FEBS J. 2007;274:4944–4950. doi: 10.1111/j.1742-4658.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 59.Valenzano KJ, Khanna R, Powe AC, Boyd R, Lee G, Flanagan JJ, Benjamin ER. Identification and characterization of pharmacological chaperones to correct enzyme deficiencies in lysosomal storage disorders. Assay Drug Dev Technol. 2011;9:213–235. doi: 10.1089/adt.2011.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kato A, Yamashita Y, Nakagawa S, Koike Y, Adachi I, Hollinshead J, Nash RJ, Ikeda K, Asano N. 2,5-Dideoxy-2,5-imino-d-altritol as a new class of pharmacological chaperone for Fabry disease. Bioorg Med Chem. 2010;18:3790–3794. doi: 10.1016/j.bmc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 61.Andreotti G, Citro V, Correra A, Cubellis MV. A thermodynamic assay to test pharmacological chaperones for Fabry disease. Biochim Biophys Acta. 2014;1840:1214–1224. doi: 10.1016/j.bbagen.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guce AI, Clark NE, Rogich JJ, Garman SC. The molecular basis of pharmacological chaperoning in human alpha-galactosidase. Chem Biol. 2011;18:1521–1526. doi: 10.1016/j.chembiol.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S beta-glucosidase: A therapeutic strategy for Gaucher disease. Proc Natl Acad Sci USA. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sawkar AR, Schmitz M, Zimmer KP, Reczek D, Edmunds T, Balch WE, Kelly JW. Chemical chaperones and permissive temperatures alter localization of Gaucher disease associated glucocerebrosidase variants. ACS Chem Biol. 2006;1:235–251. doi: 10.1021/cb600187q. [DOI] [PubMed] [Google Scholar]
- 65.Diaz L, Bujons J, Casas J, Llebaria A, Delgado A. Click chemistry approach to new N-substituted aminocyclitols as potential pharmacological chaperones for Gaucher disease. J Med Chem. 2010;53:5248–5255. doi: 10.1021/jm100198t. [DOI] [PubMed] [Google Scholar]
- 66.Trapero A, Llebaria A. Glucocerebrosidase inhibitors for the treatment of Gaucher disease. Future Med Chem. 2013;5:573–590. doi: 10.4155/fmc.13.14. [DOI] [PubMed] [Google Scholar]
- 67.Yu Z, Sawkar AR, Whalen LJ, Wong C-H, Kelly JW. Isofagomine- and 2,5-anhydro-2,5-imino-D-glucitol-based glucocerebrosidase pharmacological chaperones for Gaucher disease intervention. J Med Chem. 2007;50:94–100. doi: 10.1021/jm060677i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashcroft FM. Ion Channels and Disease:Channelopathies. Academic press; 2000. [Google Scholar]
- 69.Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- 70.Hanrahan JW, Sampson HM, Thomas DY. Novel pharmacological strategies to treat cystic fibrosis. Trends Pharmacol Sci. 2013;34:119–125. doi: 10.1016/j.tips.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Eshaq RS, Stahl LD, Stone R, 2nd, Smith SS, Robinson LC, Leidenheimer NJ. GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABAA receptors. Brain Res. 2010;1346:1–13. doi: 10.1016/j.brainres.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha 4 beta 2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- 73.Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J. 2009;11:167–177. doi: 10.1208/s12248-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong QM, Zhou ZF, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 75.Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem. 2002;277:4989–4998. doi: 10.1074/jbc.M107345200. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Bartlett MC, Loo TW, Clarke DM. Specific rescue of cystic fibrosis transmembrane conductance regulator processing mutants using pharmacological chaperones. Mol Pharmacol. 2006;70:297–302. doi: 10.1124/mol.106.023994. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Loo TW, Bartlett MC, Clarke DM. Correctors promote maturation of cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by binding to the protein. J Biol Chem. 2007;282:33247–33251. doi: 10.1074/jbc.C700175200. [DOI] [PubMed] [Google Scholar]
- 78.Sampson HM, Robert R, Liao J, Matthes E, Carlile GW, Hanrahan JW, Thomas DY. Identification of a NBD1-Binding Pharmacological Chaperone that Corrects the Trafficking Defect of F508del-CFTR. Chem Biol. 2011;18:231–242. doi: 10.1016/j.chembiol.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 79.Galietta LJ, Springsteel MF, Eda M, Niedzinski EJ, By K, Haddadin MJ, Kurth MJ, Nantz MH, Verkman AS. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276:19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 80.Dacosta CJ, Baenziger JE. Gating of pentameric ligand-gated ion channels: structural insights and ambiguities. Structure. 2013;21:1271–1283. doi: 10.1016/j.str.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 81.Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Mu TW, Lester HA, Dougherty DA. Different binding orientations for the same agonist at homologous receptors: a lock and key or a simple wedge? J Am Chem Soc. 2003;125:6850–6851. doi: 10.1021/ja0348086. [DOI] [PubMed] [Google Scholar]
- 83.Henderson BJ, Srinivasan R, Nichols WA, Dilworth CN, Gutierrez DF, Mackey ED, McKinney S, Drenan RM, Richards CI, Lester HA. Nicotine exploits a COPI-mediated process for chaperone-mediated up-regulation of its receptors. J Gen Physiol. 2014;143:51–66. doi: 10.1085/jgp.201311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunham JH, Hall RA. Enhancement of the surface expression of G protein-coupled receptors. Trends Biotechnol. 2009;27:541–545. doi: 10.1016/j.tibtech.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morello JP, Salahpour A, Laperriere A, Bernier V, Arthus MF, Lonergan M, Petaja-Repo U, Angers S, Morin D, Bichet DG, Bouvier M. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernier V, Morello JP, Zarruk A, Debrand N, Salahpour A, Lonergan M, Arthus MF, Laperriere A, Brouard R, Bouvier M, Bichet DG. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 88.Los EL, Deen PM, Robben JH. Potential of nonpeptide (ant)agonists to rescue vasopressin V2 receptor mutants for the treatment of X-linked nephrogenic diabetes insipidus. J Neuroendocrinol. 2010;22:393–399. doi: 10.1111/j.1365-2826.2010.01983.x. [DOI] [PubMed] [Google Scholar]
- 89.Robben JH, Sze M, Knoers NV, Deen PM. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2007;292:F253–260. doi: 10.1152/ajprenal.00247.2006. [DOI] [PubMed] [Google Scholar]
- 90.Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–3262. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 91.Janovick JA, Stewart MD, Jacob D, Martin LD, Deng JM, Stewart CA, Wang Y, Cornea A, Chavali L, Lopez S, Mitalipov S, Kang E, Lee HS, Manna PR, Stocco DM, Behringer RR, Conn PM. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci USA. 2013;110:21030–21035. doi: 10.1073/pnas.1315194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conn PM, Ulloa-Aguirre A. Pharmacological chaperones for misfolded gonadotropin-releasing hormone receptors. Adv Pharmacol. 2011;62:109–141. doi: 10.1016/B978-0-12-385952-5.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hollingsworth TJ, Gross AK. Defective trafficking of rhodopsin and its role in retinal degenerations. Int Rev Cell Mol Biol. 2012;293:1–44. doi: 10.1016/B978-0-12-394304-0.00006-3. [DOI] [PubMed] [Google Scholar]
- 94.Noorwez SM, Kuksa V, Imanishi Y, Zhu L, Filipek S, Palczewski K, Kaushal S. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem. 2003;278:14442–14450. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noorwez SM, Malhotra R, McDowell JH, Smith KA, Krebs MP, Kaushal S. Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem. 2004;279:16278–16284. doi: 10.1074/jbc.M312101200. [DOI] [PubMed] [Google Scholar]
- 96.Krebs MP, Holden DC, Joshi P, Clark CL, 3rd, Lee AH, Kaushal S. Molecular mechanisms of rhodopsin retinitis pigmentosa and the efficacy of pharmacological rescue. J Mol Biol. 2010;395:1063–1078. doi: 10.1016/j.jmb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 97.Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.