Abstract
Background
Restless legs syndrome (RLS) is a common neurologic syndrome and is associated with iron deficiency in many patients. It is unclear whether iron therapy is effective treatment for RLS.
Objectives
The objective of this review was to assess the effects of iron supplementation (oral or intravenous) for patients with RLS.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Jan 1995 to April 2011); EMBASE (Jan 1995 to April 2011); PsycINFO (Jan 1995 to April 2011); and CINAHL (Jan 1995 to April 2011). Corresponding authors of included trials and additional members of the International Restless Legs Syndrome Study Group were contacted to locate additional published or unpublished trials.
Selection criteria
Controlled trials comparing any formulation of iron with placebo, other medications, or no treatment in adults diagnosed with RLS according to expert clinical interview or explicit diagnostic criteria.
Data collection and analysis
Two review authors extracted data and at least two authors assessed trial quality. We contacted trial authors for missing data.
Main results
Six studies (192 total subjects) were identified and included in this analysis. The quality of trials was variable. Our primary outcome was restlessness or uncomfortable leg sensations, which was quantified using the IRLS severity scale in four trials and another RLS symptom scale in a fifth trial. Combining data from the four trials using the IRLS severity scale, there was no clear benefit from iron therapy (mean difference in IRLS severity scores of -3.79, 95% CI: -7.68 to 0.10, p = 0.06). However, the fifth trial did find iron therapy to be beneficial (median decrease of 3 points in the iron group and no change in the placebo group on a 10 point scale of RLS symptoms, p = 0.01). Quality of life was improved in the iron group relative to placebo in some studies but not others. Changes in periodic limb movements were not different between groups (measured in two studies). Objective sleep quality, subjective sleep quality and daytime functioning were not different between treatment groups in the studies that assessed them. The single study of subjects with end stage renal disease did show a benefit of therapy. Most trials did not require subjects to have co-morbid iron deficiency and several excluded patients with severe anemia. The single study that was limited to iron deficient subjects did not show clear benefit of iron supplementation on RLS symptoms. There was no clear superiority of oral or intravenous delivery of iron. Iron therapy did not result in significantly more side effects than placebo (RR 1.39, 95% CI 0.85 to 2.27).
Authors' conclusions
There is insufficient evidence to determine whether iron therapy is beneficial for the treatment of RLS. Further research to determine whether some or all types of RLS patients may benefit from iron therapy, as well as the best route of iron administration, is needed.
Medical Subject Headings (MeSH): Iron [deficiency; *therapeutic use]; Iron, Dietary [therapeutic use]; Quality of Life; Randomized Controlled Trials as Topic; Restless Legs Syndrome [*drug therapy; etiology]; Severity of Illness Index; Sleep [physiology]; Treatment Outcome
MeSH check words: Humans
Background
Description of the condition
Restless legs syndrome (RLS) is a common condition consisting of four cardinal features: an urge to move the legs (which is usually but not invariably associated with unpleasant leg sensations); at least transient relief with movement; worsening with rest; and a predilection to occur during the evening and night. The diagnosis is one of clinical judgment, with no definitive confirmatory testing presently available. However, the presence of periodic limb movements, a response to dopaminergic medications, a positive family history, and the presence of sleep disruption can all provide supportive evidence for the diagnosis (Allen 2003). Periodic limb movements are stereotyped, repetitive flexions of the great toe and ankle that occur with a periodicity of between 5 and 90 seconds. Periodic limb movements are seen in 80 to 90% of patients with RLS (Montplaisir 1997, Trotti 2009), but they are not specific to RLS because they are also found in a variety of other sleep disorders and in aging in the absence of sleep disorders (Hornyak 2006). RLS is more common in women than in men (Berger 2004) and the prevalence of RLS increases with age (Bliwise 2006). RLS has a prevalence of 5 to 15% in populations of western European descent but lower prevalence in other investigated populations such as subsets of Asia (0.1 to 5%) (Stefansson 2007). RLS has long been suspected to have a genetic basis because of the high proportion of sufferers who have a positive family history. Familial linkage studies have identified numerous potentially causative regions (Trotti 2008). Three recent genome-wide association studies have found a total of four single nucleotide polymorphisms that conferred increased risk for RLS or periodic limb movements, located in BTBD9 (on chromosome 6), in MEIS1 (on chromosome 2), in a region on chromosome 15 containing the genes MAP2K5 and LBXCOR1, and in PTPRD (on chromosome 9) (Stefansson 2007; Winkelmann 2007; Schormair 2008). Collectively, the population attributable risk for the variants at BTBD9, MEIS1, and MAP2K5/LBXCOR1 is approximately 70% (Winkelmann 2007).
Despite this genetic advance, the pathophysiology of RLS remains somewhat unclear. What is known about the functions of the recently implicated genes does not yet explain RLS pathology. From a clinical standpoint, a state of dopaminergic dysfunction is often suspected tocause RLS because patients with RLS frequently improve when givenlowdoses of dopaminergic medications (Allen 2004).
How the intervention might work
Iron deficiency has also been implicated in the pathophysiology of RLS, based on the clinical findings that patients with iron deficiency have a higher frequency of RLS (30%) than those without and that severity of iron deficiency correlates with the severity of RLS symptoms (Earley 2000). Treatment with dopamine agonists is sometimes complicated by a phenomenon known as augmentation, in which symptoms return with earlier time of onset, greater severity, and more extensive bodily involvement (Allen 2003); this treatment complication appears more likely to occur in those patients who have iron deficiency Trenkwalder 2008). As with dopamine, iron metabolism exhibits a circadian pattern similar to RLS symptoms; serum iron nadirs occur between 8 pm and midnight, in line with the peak time of RLS symptom severity (Earley 2000). Two of the most common causes of secondary RLS, pregnancy and end-stage renal disease, are also associated with iron deficiency (Allen 2004). Studies of iron in patients with RLS have demonstrated low cerebrospinal iron levels (Earley 2000), low iron concentrations in the substantia nigra on magnetic resonance imaging (Allen 2004), and decreased substantia nigra iron stores on autopsy specimens (Connor 2003). Additionally, iron has known interactions with dopamine, acting as a cofactor for tyrosine hydroxylase, the rate-limiting enzyme in dopamine production. Iron deficient rats have been shown to have altered dopamine functioning, with decreased D2 receptors, decreased dopamine transporter functioning, and elevated levels of extracellular dopamine in the caudate nucleus and putamen (Earley 2000; Nelson 1997).
Based on this presumed pathophysiology, iron therapy has been used for RLS patients, both those with and those without documented iron deficiency. Iron supplementation can be provided orally or intravenously, with several formulations available for each method of delivery. Intravenous iron provides the advantage of replenishing iron stores more quickly than oral therapy, which can take weeks to months, but intravenous therapy carries the added risk of severe complications such as anaphylaxis (Silverstein 2004). Current consensus guidelines for RLS put forth by the RLS Foundation's Medical Advisory Board recommend iron therapy for patients with a low ferritin (usually less than 20 nanograms per milliliter) and consideration of iron therapy, on a case-by-case basis, for those with a ferritin level below 45 to 50 nanograms per milliliter (Silber 2004). The optimal timing to assess response to such therapy is not known.
Objectives
To evaluate the efficacy and safety of oral or parenteral iron for the treatment of RLS when compared with placebo or other therapies.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all controlled trials investigating the treatment of RLS with oral or parenteral iron versus placebo, versus another drug, or with a no-intervention control group. We planned to include controlled trials regardless of whether or not they were randomized or blinded. We planned to include parallel and cross-over trials, but not the second phase of cross-over trials because washout periods are not universally used or may not be long enough. Trials that allowed concurrentuse of other medications such as dopamine agonists, anticonvulsants or others were considered suitable for inclusion if they allowed equal access to such medications for patients in the iron and control groups.
Types of participants
We sought trials on adult patients (18 years or over) of either sex, in whom RLS was diagnosed according to expertclinical interview or to explicit diagnostic criteria such as those defined by the International Restless Legs Syndrome Study Group (IRLSSG) (Allen 2003). We planned to include trials on pregnant women or patients with renal disease (including patients with end stage renal disease who are on dialysis) and use these in the investigation of study heterogeneity.
Types of interventions
Therapy with any dose or regimen of oral or parenteral iron-containing compounds compared with placebo, other drugs or no intervention.
Types of outcome measures
Primary outcomes
The primary outcome considered in this review was restlessness or unpleasant sensations as experienced subjectively by the patient. We did not pre-specify a rating scale or other measure of restlessness, although the majority of studies employed the International Restless Legs Syndrome Study Group severity scale (Walters 2003). This 10 question, 40 point scale addresses the severity and impact of RLS symptoms.
Secondary outcomes
Secondary outcomes included the following:
Efficacy related outcomes:
quality of life measures;
patient satisfaction with treatment;
PLM index (number of periodic limb movements per hour of sleep);
sleep quality (subjective and objective);
daytime functioning;
decreased occurrence of augmentation (according to the definition in Allen 2003).
Safety related outcomes:
adverse events during treatment;
discontinuation rate.
Search methods for identification of studies
Electronic searches
We searched the following databases: The Cochrane Movement Disorders Group's Trials Register (Jan 1995 to April 2011); MEDLINE (Jan 1995 to April 2011); EMBASE (Jan 1995 to April 2011); PsycINFO (Jan 1995 to April 2011); CINAHL (Jan 1995 to April 2011); the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, most recently available issue at the time of the search). The lower time period limit was set to 1995 because generally accepted diagnostic criteria did not exist until that date (see Walters 1995).
Our MEDLINE search used the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (2008 revision) along with MESH headings and free-text searches designed to identify studies of iron administration and of patients with restless legs syndrome with high sensitivity (Appendix 1). The MEDLINE search was adapted as necessary for use in the other databases searched.
Searching other resources
We checked the reference lists of all included studies for other potentially relevant publications in any language. We contacted the corresponding authors of randomized controlled trials and selected members of the International Restless Legs Syndrome Study Group to attempt to find additional published and unpublished trials. We searched the US National Institutes of Health database of ongoing trials (at: clinicaltrials.gov) for ongoing or unpublished trials.
Data collection and analysis
Selection of studies
Two reviewers (LMT and SB) used the titles and abstracts of the identified citations to exclude trials that clearly did not meet the inclusion criteria of the review. If either reviewer thought that the trial might possibly meet the criteria, the full paper was obtained for further examination. The same two reviewers then reviewed articles that passed this initial screen to determine their fit with the review inclusion criteria. Authorship and results were not blinded. All types of disagreement were resolved by discussion and consensus.
Data extraction and management
We developed a data extraction form to aid in the collection of relevant and important data from included studies. As described above in'Criteria for considering studies for this review', we abstracted data on participants, interventions, and outcomes. We also abstracted on to this form the trial characteristics to be used in our assessment of methodological quality. In addition, we collected data on potential effect modifiers, including co-morbidities such as end stage renal disease, baseline iron status, and pregnancy. Two reviewers (LMT and SB) extracted and cross-checked the data. We contacted study authors to obtain unpublished information, including outcome data not explicitly stated in the published papers. Disagreements concerning data extraction were resolved through the third reviewer (LAB).
Assessment of risk of bias in included studies
Two reviewers (LMT and SB) assessed the methodological quality of each trial, using the domain-based evaluation outlined in the chapter'Assessing risk of bias in included studies' in the Cochrane Handbook for Systematic Reviews of Interventions version 5 (Higgins 2008). We used the kappa statistic to assess agreement among the reviewers regarding quality of the retrieved articles, resolving disagreements by discussion and consensus among all three reviewers.
Measures of treatment effect
Where appropriate, we combined treatment/control differences in the outcomes across studies using random-effects meta-analyses. We analyzed continuous data using weighted mean differences where possible and performed standardized mean difference analyses when different measurements were used among studies. We calculated risk ratios for dichotomous data using the Mantel-Haen-szel method and 95% confidence intervals. Where possible, we performed intention to treat or per protocol analysis to control attrition bias.
Assessment of heterogeneity
We analyzed study heterogeneity using Cochrane's Q-test and I-square statistics.
We planned to investigate the following as potential sources of heterogeneity when sufficient data were available:
different formulations of oral or parenteral iron;
comparator;
duration of treatment;
patient characteristics, including pregnancy or end stage renal disease;
baseline levels of ferritin or other documentation of iron deficiency.
Assessment of reporting biases
We planned to construct a funnel plot to identify possible publication bias.
Sensitivity analysis
We planned to conduct sensitivity analyses for high- versus low-quality trials and for parallel group versus cross-over trial designs. We prespecified subgroup analyses to examine the effects of different types of patients (e.g., pregnant women or patients with ESRD) and different formulations of iron (oral versus parenteral).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
The initial searches yielded fourteen potentially relevant trials, one of which (Birgegard 2010) was published during the preparation of this review and one (Allen 2009) was initially published only in abstract form. Of these, six studies met criteria for inclusion (Allen 2009, Davis 2000, Earley 2009, Grote 2009, Sloand 2004, Wang 2009), for a total of 192 subjects. Eight trials were excluded because of 1) lack of a non-iron control group (Birgegard 2010, Earley 2004, Earley 2005, O'Keeffe 1994, Simakajornboon 2003, Mohri 2008, Zilberman 2010), and/or 2) subject age younger than 18 (Simakajornboon 2003, Konofal 2008, Mohri 2008), and/or 3) study performed prior to standard RLS criteria definition in 1995 (O'Keeffe 1994). All six included studies were randomized, placebo-controlled, parallel group trials. In the included studies, RLS was defined using the 1995 IRLSSG criteria (Walters 1995) in one study (Sloand 2004), the 2003 IRLSSG criteria (Allen 2003) in three studies (Allen 2009; Grote 2009, Wang 2009), the Johns Hopkins telephone diagnostic interview for RLS in one study (Earley 2009), and by clinical interview by a neurologist in one study (Davis 2000). In this latter study, 24 of 28 subjects met all four diagnostic criteria for RLS using the 1995 criteria. One study (Grote 2009) included only subjects who were iron deficient (defined as a serum ferritin ≤ 45) and a second study included only those with low or low-normal iron stores (serum ferritins ranging from 15-75) (Wang 2009). One study (Sloand 2004) included only patients on dialysis (hemodialysis or peritoneal dialysis), while renal disease was an exclusion criteria for four of the remaining studies (Allen 2009, Earley 2009, Grote 2009, Wang 2009). No studies included pregnant women. Two studies administered oral iron (both as ferrous sulfate 325 mg twice a day) (Davis 2000, Wang 2009), while the remaining four used intravenous iron preparations, two of which were iron sucrose (Earley 2009, Grote 2009), one of which was iron dextran (Sloand 2004), and one of which was ferric carboxymaltose (Allen 2009). Our primary endpoint of restlessness or unpleasant sensations was evaluated in five of the included studies, using the International Restless Legs Syndrome Study Group severity scale (IRLS) in four studies (Allen 2009, Earley 2009, Wang 2009, Grote 2009) and a scale developed at the University of Rochester in the fifth (Sloand 2004).
In the first trial (Davis 2000), 28 patients were randomized to receive either placebo or oral ferrous sulfate liquid, 325 mg twice a day for 16 weeks, at which point subjects who wished to continue the study drug as monotherapy were followed for an additional ten weeks. Exclusion criteria included anemia with a hemoglobin less than 10, pregnancy, and current or recent treatment with iron sulfate. Iron status and renal function at baseline were not part of the inclusion or exclusion criteria. RLS symptoms were measured with two visual analog scales, the first which quantified the extent to which RLS symptoms interfered with sleep and the second which quantified how RLS symptoms affected each subject's life. In the second trial (Sloand 2004), 25 subjects with end-stage renal disease on dialysis (22 on hemodialysis, 3 on peritoneal dialysis) were randomized to receive either intravenous iron dextran or placebo. Both interventions were given as a 15 mL test dose (30 mg of iron dextran) over three minutes followed by 1 hour of observation for adverse reactions; if no adverse reactions were noted, the remaining 485 mL (970 mg of iron dextran) was infused over three hours. RLS symptoms were measured at baseline, 1 week, 2 weeks, and 4 weeks after infusion using the University of Rochester RLS severity scale developed for this study. This three-question scale assesses frequency of RLS symptoms within the last two days, the amount of distress caused by RLS symptoms, and the duration of symptoms to generate a score from 0 to 10 point scale (with higher numbers corresponding to increased RLS severity).
Allen et al (Allen 2009) enrolled 46 subjects, of whom 43 received all doses of study medication. Subjects were randomized to receive either intravenous ferric carboxymaltose or placebo. Iron was delivered as 500 mg of ferric carboxymaltose in 100 mL of normal saline, given on day 0 and day 5. Normal saline was given using the same volume and schedule to subjects in the placebo group. Following day 28, subjects were assigned to receive ferric carboxymaltose or normal saline based on their serum ferritin and IRLS scores at day 28. Because the treatment assignation at day 28 was not randomized (although it remained blinded), we only considered data gathered before the treatment given on day 28. IRLS scores were measured at baseline, day 14, and day 28. Other outcome measures included the RLS Quality of Life scale (RLS-QoL), the Medical Outcomes Study sleep scale (a subjective measure of sleep quality), periodic limb movements (measured via leg actigraphy), the Clinical Global Inventory of Change, a patient global rating of change, and the Fatigue Severity Scale. The full manuscript (Allen 2011) was published during revision of this review, so data analysis was performed on pre-publication data provided by the authors. In the trial by Earley et al (Earley 2009), 21 subjects were randomized to receive either intravenous iron or placebo. A prespecified interim analysis was performed after 18 subjects had been enrolled, at which point the study was discontinued because of lack of effect of the intervention, so three of the randomized subjects did not complete the protocol. Intravenous iron was given as iron sucrose, 500 mL (containing 500 mg iron sucrose) over 10 hours, followed by a second infusion over 12 hours the following day. The same infusion schedule of saline was given to the placebo group. IRLS scores were measured at baseline and two weeks following intervention. Other outcome measures included a global rating scale of RLS severity, periodic limb movements during wake and sleep, changes in cerebrospinal fluid ferritin and transferrin levels, objective sleep quality (total sleep time and sleep efficiency during polysomnography), and changes in iron index within the substantia nigra on MRI.
Grote et al (Grote 2009) randomized60subjects, all of whom were iron-deficient (based on a serum ferritin less than or equal to 45), to receive either intravenous iron or placebo. Iron was delivered as 200 mg of iron sucrose (10 mL of 20 mg/mL Venofer), given five times over a three week period. The placebo group received the same volume and dosing schedule of 0.9% NaCl. The IRLS scale was measured at baseline, week 3, week 7, week 11, 5 months, 8 months, and 12 months. Serum ferritin and the Epworth Sleepiness Scale were both measured as well.
Wang et al (Wang 2009) enrolled 18 subjects with low or low-normal ferritin levels (serum ferritin 15-75) to receive either oral iron (ferrous sulfate 325 mg bid) or matched placebo for twelve weeks. IRLS scores were measure datbase line, six weeks, and twelve weeks after intervention. Serum ferritin was also measured at 12 weeks, as was a single question regarding change in quality of life compared to prior to beginning the study medication (rated as improved, stayed the same, or worsened). Compliance (based on manual pill count) was measured at 6 and 12 weeks.
Risk of bias in included studies
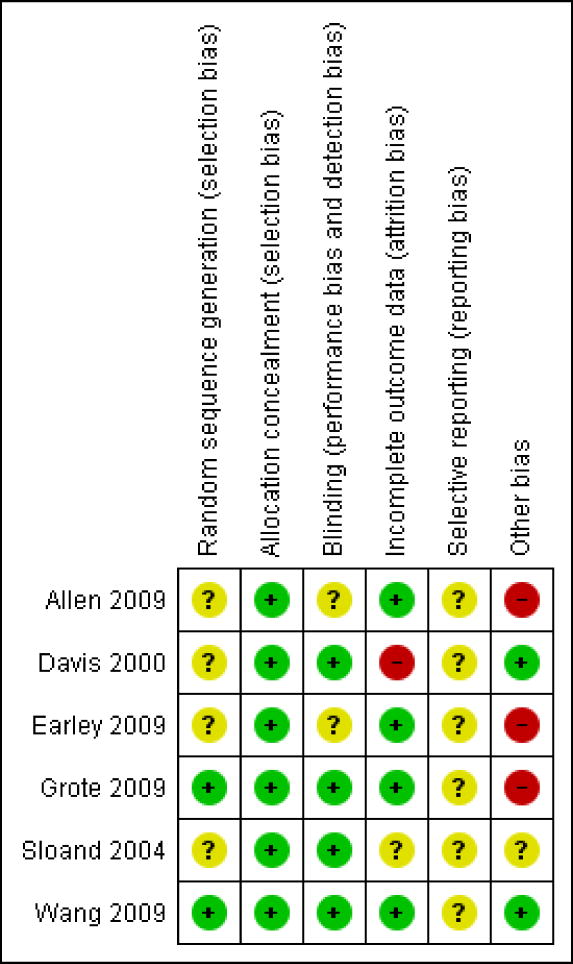
Several of the studies had inadequate reporting, which limited our assessment of potential bias (Figure 1). Sequence generation was not clearly described in four of the studies (Allen 2009, Davis 2000, Earley 2009, Sloand 2004). Two studies were subject to possible unblinding because a study nurse or physician who interacted with subjects (although did not assess outcomes) were aware of allocation status (Allen 2009, Earley 2009). Incomplete outcome data were a potential problem in two studies (Davis 2000, Sloand 2004). In the study by Davis et al, 6 of 14 iron-treated subjects but only one of 14 placebo-treated patients withdrew before analysis at 14 weeks (the time of primary outcome measurement) and no imputation of missing values was performed. Sloand et al reported two drop-outs in the placebo group but reported outcome data for the entire group, without a clear discussion of how outcome data was imputed for the subjects who dropped out. It is difficult to fully rule out the possibility of selective outcome reporting in any of the trials without published protocols (Allen 2009, Davis 2000, Earley 2009, Sloand 2004, Wang 2009). Only one trial (Grote 2009) was registered in a publicly accessible trial registry (www.controlled-trials.com). This registration specified the same primary and secondary outcomes as reported in the published paper, but the date listed as the date on which the trial number was assigned was almost three years after the anticipated end date of the trial. Other biases were possible or probable in four studies. The intervention groups were imbalanced with respect to important potential confounders in one study (Sloand 2004), with mean baseline RLS severity scores being two points higher (out of a possible 10) in the placebo group and with duration of dialysis being longer in the placebo group (3.3 vs 2.5 years). Both of these could potentially indicate more severe disease in the placebo group, which might be more refractory to therapy. Two studies (Allen 2009, Grote 2009) were funded by the pharmaceutical companies selling the iron formulations tested in the studies. One study (Earley 2009) stopped early based on a pre-specified interim analysis of the data, which suggested that continuing the study to the planned enrollment targets was unlikely to show significant differences between the intervention groups. Kappa for agreement between two reviewers about risk of bias was 0.32.
Figure 1. Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
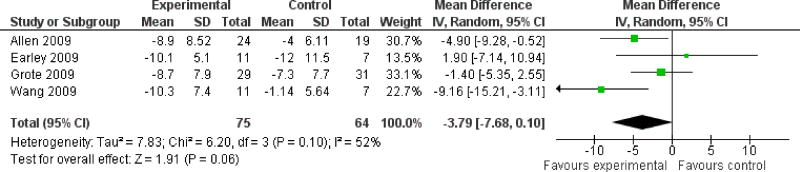
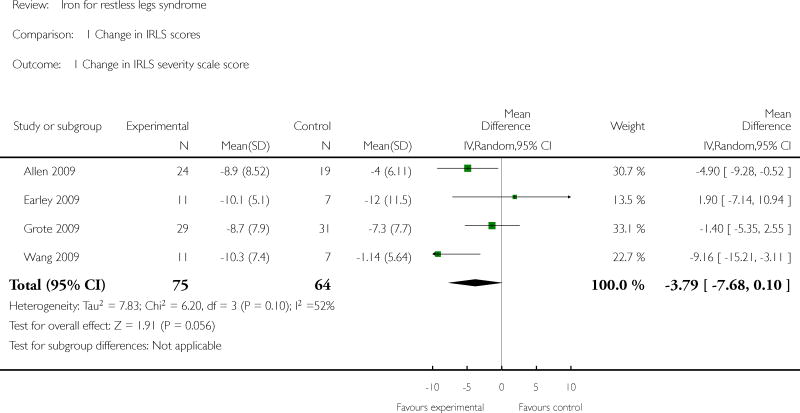
The included studies did not all report the same outcome measures. For our primary outcome measure, subjective restlessness or leg discomfort experienced by the patient, data were available from five studies. Four of these studies (Allen 2009, Earley 2009, Grote 2009, Wang 2009)used the IRLS scale and the fifth(Sloand 2004) used a severity scale developed for use in the trial. Of the three studies using the IRLS and IV delivery of iron, Allen reported data for 9 and 23 days following completion of infusions, Earley reported data for two weeks after infusion, and Grote for three, seven, and eleven weeks after infusion, as well as 5, 8, and 12 months after infusion. Wang reported changes in IRLS at 6 and 12 weeks after beginning twice daily oral iron. For the purpose of comparing changes in IRLS scores across studies, we analyzed changes in IRLS scores at 2 weeks in the Earley study, as this was the only time point available, 3 weeks in the Grote study (as this was the closest time point to that used the Earley study, which was also an intravenous iron study), 3 weeks (23 days) in the Allen study (to be consistent with the Grote study and because the majority of outcome measures were only measured at this time point), and 12 weeks in the Wang study (the duration of the intervention). Combining these four studies, there was no significant difference in change in IRLS scores between iron treatment groups and placebo (mean difference -3.79, 95% CI: -7.68 to 0.10, p = 0.06) (Figure 2). Standardized mean differences including data from the Sloand study could not be computed because the Sloand study reported results as medians rather than means, but this study did find a significant difference in change in RLS severity scores between the iron and placebo groups at 2 weeks (median decrease of 3 points in the iron group and no change in the placebo group, p = 0.01).
Figure 2. Change in IRLS severity scale score.
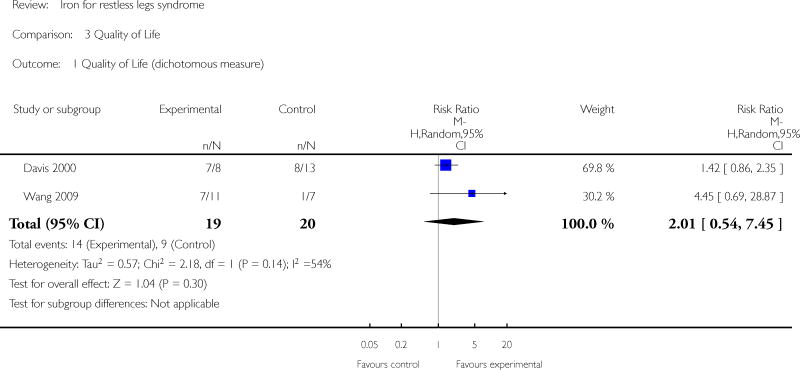
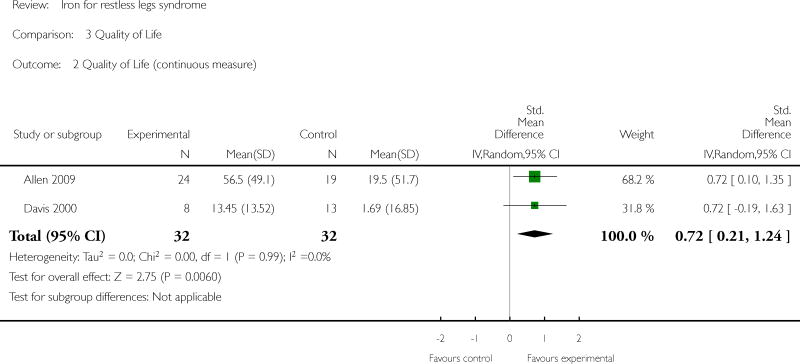
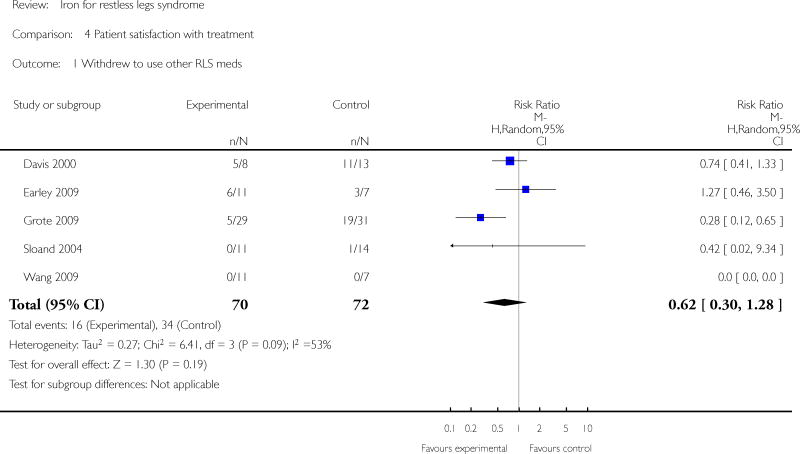
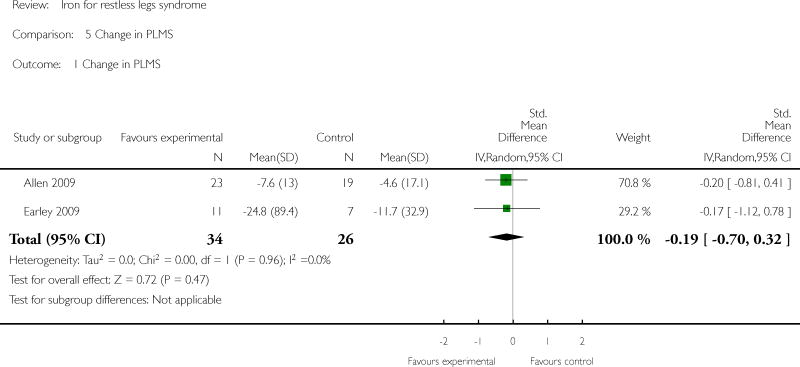
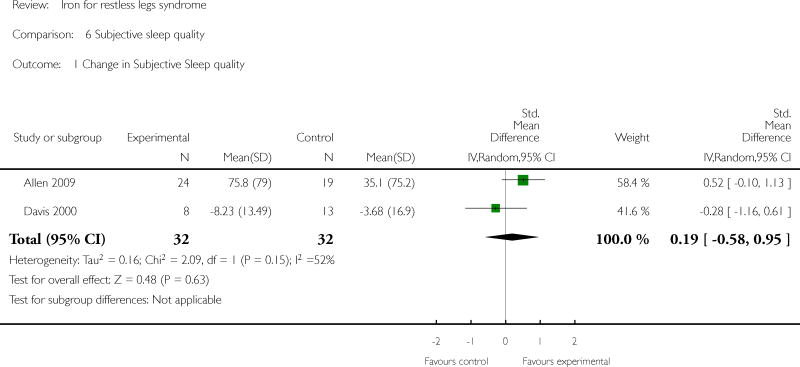
Changes in quality of life with treatment were assessed in three studies (Allen 2009, Davis 2000, Wang 2009). There was no difference in change in quality of life between the treatment and placebo groups using a dichotomized measure of improved versus unchanged or worsened (RR 2.01, 95% CI: 0.54 to 7.45, I2 = 54%), but there was an improvement in quality of life measured as a continuous variable (SMD 0.72, 95% CI 0.21 to 1.24, I2 = 0%); each measurement of quality of life was available from two of the three trials. All studies assessed patient satisfaction with treatment, either directly or indirectly. In three of the studies, satisfaction was measured directly at a certain time point, when subjects were asked if they would prefer to remain in the study or leave the study to start different therapy for RLS (Allen 2009, Davis 2000, Earley 2009). In the remaining three studies, subject drop outs due to lack of efficacy of therapy were reported. Combining these two measures to determine the proportion of subjects who chose to leave the study due to lack of efficacy, there was no difference between iron and placebo groups (RR 0.62, 95% CI 0.30, 1.28, I2 = 53%). The Allen study was not included in this summary measure, because satisfaction was assessed after day 28 (at which time some subjects received additional, non-randomly allocated iron treatment). Periodic limb movements of sleep were measured in two studies (Allen 2009, Earley 2009), by actigraphy or polysomnography. The decrease after treatment was not significantly different between iron and placebo groups (SMD -0.19, 95% CI -0.70 to 0.32, I2 = 0%). Subjective sleep quality was assessed in two studies (Allen 2009, Davis 2000), using different measures. Sleep quality was not found to be different after treatment with iron versus placebo (SMD 0.19, 95% CI -0.58 to 0.95, I2 = 52%). Objective sleep quality, measured as sleep efficiency during polysomnography, was not significantly different between iron and placebo groups (-35.5 +/- 92.0 versus -41.4 +/-98.2) in the single study that measured it (Earley 2009). Daytime functioning was assessed in one study using the Epworth Sleepiness Scale, a subjective measure of propensity to fall asleep during routine activities (Grote 2009). Epworth scores were not given for the treatment groups, but were noted not to be significantly different from placebo at any time point. Changes in the rate of augmentation was not explicitly addressed in any of the included studies.
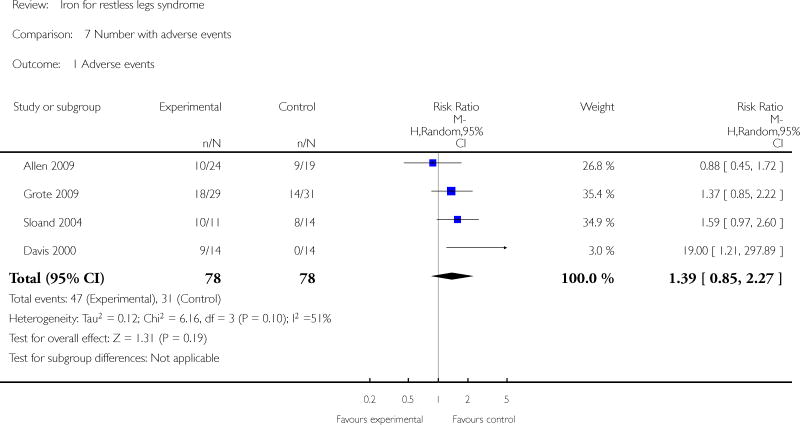
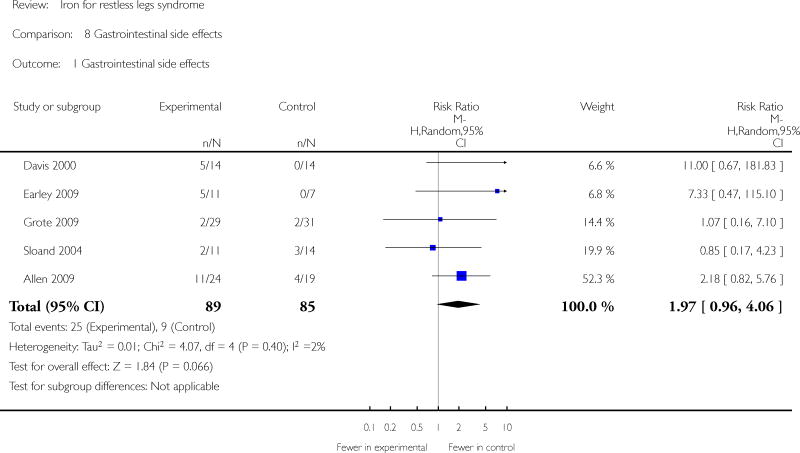
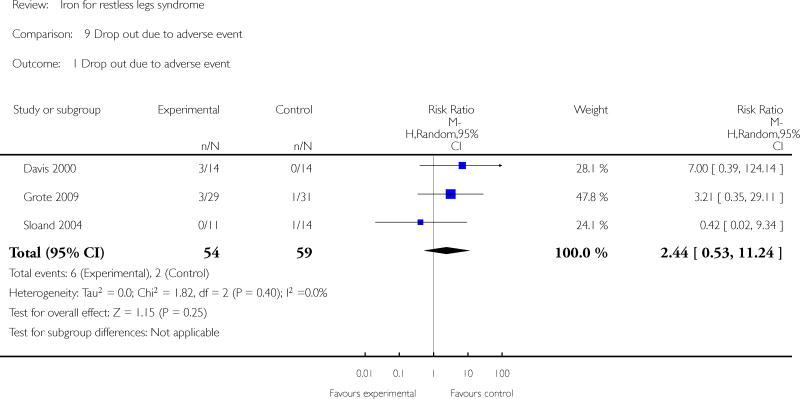
Adverse events were noted for five of the six studies. In four of the studies, data was reported (Davis 2000, Sloand 2004) or available from the authors (L. Grote, R. Allen) by number of subjects in each group to experience any adverse event. In the other (Earley 2009), adverse events were listed but it was not clear whether adverse events were unique occurences or whether an individual subject may have had more than one adverse event. For the purpose of our meta-analysis, therefore, only data from the first four studies were included. The risk of having any adverse event was no different between iron and placebo groups (RR 1.39, 95% CI 0.85 to 2.27, I2 = 51%). Gastrointestinal side effects (nausea/vomiting, constipation, diarrhea, and abdominal pain) were considered as unique events (i.e., counted as separate occurences even if they occurred in the same patient) and were no more common in iron than placebo groups (RR 1.97, 95% CI 0.96 to 4.06, I2 = 2%). Subjects were no more likely to drop out of the study due to adverse events in the iron than the placebo group (RR 2.44, 95% CI 0.53 to 11.24, I2 = 0%); no drop outs due to adverse events occurred in three of the studies (Allen 2009; Earley 2009; Wang 2009).
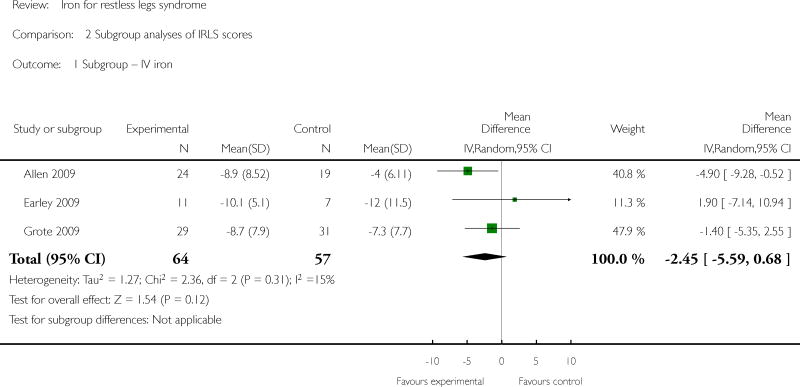
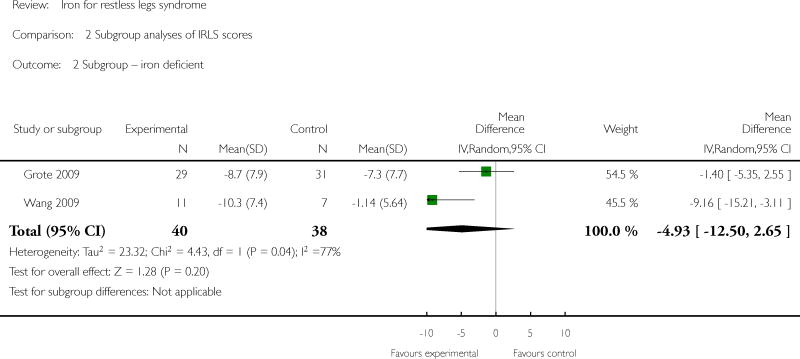
Subgroup analyses by patient type were possible for the subgroups of ESRD and baseline iron deficiency. Only one study explicitly included patients with ESRD (Sloand 2004), and this study did show a significant improvement in RLS severity scores at 2 weeks. One study included only subjects who were iron deficient (as defined by a serum ferritin ≤ 45) (Grote 2009), with an additional study limited to those with low and low-normal iron store (as defined by serum ferritin levels between 15 and 75) (Wang 2009). Neither the Grote study alone nor both studies in combination found an improvement in IRLS scores in the treated group compared to placebo for our specified time points, although the Grote study did find a significant improvement at another time point (albeit not their prespecified primary endpoint). Two studies (Allen 2009, Davis 2000) reported that baseline iron stores were not different between subjects who responded to iron therapy and those who did not. Considering type of iron delivery (oral versus intravenous), the one study to use both oral iron and a measure of RLS severity (Wang 2009) found a significant improvement in RLS severity with treatment, while intravenous iron was associated with significant improvements in two of three studies (Allen 2009, Sloand 2004). Sufficient data were not available to conduct sensitivity analyses for low versus high quality trials nor parallel group versus crossover trials.
There was moderate heterogeneity for our primary outcome measure (I2 = 52%). Heterogenity decreased when only IV studies were considered together, suggesting that method of iron administration explained some of the observed heterogeneity. All studies used a placebo comparator, so this was not a source of heterogeneity. When studies reported multiple time points for outcome measurement, we chose the time point closest to that reported in other studies to minimize heterogeneity from duration of therapy. The IV studies measured outcomes at 2-3 weeks and the oral studies at 12 weeks, so heterogeneity caused by difference in time of outcome assessment cannot be separated from difference in method of administration. Baseline severity of PLMS might have contributed to heterogeneity, as PLMS greater than 15/hour were required for inclusion in Allen 2009 and Earley 2009 but PLMS causing significant sleep disruption were an exclusion criterion in Wang 2009. Considering only those studies in which subjects had low or low-normal iron status at baseline resulted in greater heterogeneity (I2 = 77%), suggesting that baseline iron stores did not account for observed heterogeneity.
Discussion
There is insufficient evidence to conclude whether iron is beneficial for the treatment of restless legs syndrome. In our meta-analysis of the four studies that reported mean change in IRLS scores, the mean improvement in IRLS was 3.8 points higher in those subjects on iron than those taking placebo. This estimate is lower than the 6 point difference that has been proposed as a clinically meaningful improvement in IRLS scores (Trenkwalder 2007) and the 5.7 point difference observed with dopamine agonist therapy (Scholz 2011). However, due to the small number of included subjects (n = 139), the confidence interval around this estimate is broad and includes estimates that would be clinically meaningful. We were not able to include results from two of the available trials in this estimate, one of which found a significant improvement in subjective RLS symptoms. Additionally, several trials measured outcomes at multiple time points. For consistency, we used data from 2-3weeksafter IV iron and12 weeks after oral iron. However, the optimal time after iron administration to measure RLS-related outcomes is not known, and in the case of studies that showed significant benefit of iron at only some time points (e.g., the study by Grote et al, Grote 2009), this choice may have obscured a transient effect of iron.
While adverse events were no more common with iron therapy, there may be still be differences in tolerability between oral and intravenous iron for the treatment of RLS. Of the studies that could be included in our analysis of any adverse event, three used intravenous iron and one oral iron. The only one of these studies to show a significantly higher risk of adverse events in the treated group was the oral iron study. Tolerability may be influenced by factors other than route of administration (e.g., formulation of iron).
A lack of power in the primary studies may also have limited our ability to draw conclusions about the efficacy of iron therapy for RLS. Power analyses were only mentioned in four of the included studies (Allen 2009, Davis 2000, Grote 2009, Wang 2009). Of these, the studies by Allen and Grote had sufficient power (80-90%) to detect a difference in IRLS scores. However, the study by Davis et al was clearly underpowered, with only a 25% power to detect a difference in their primary outcome (improvement in average quality of sleep). The study by Earley et al may have been underpowered as well, as 36 subjects were originally planned to provide 80% power, but the study was stopped after only 18 subjects had completed the protocol due to apparent lack of effect. Furthermore, although randomized, the two treatment arms were unbalanced at baseline in the Sloand group (Sloand 2004), with more severely affected patients in the placebo group, which may have decreased the power to see an effect in this study.
The role of baseline iron deficiency in the response to iron therapy cannot be fully determined from the available evidence. Stratified response data by baseline iron status were not available for the majority of studies, although two studies found that responders and non-responders did not differ by baseline iron status. Additionally, several of the studies had exclusion criteria that would serve to exclude the most iron deficient subjects from participating. The study by Wang et al excluded those with a percent saturation < 15% or anemia (defined as hemoglobin less than 11.1 in women and 14 in men), the study Earley et al excluded those with hemoglobin less than 12, and the study by Davis et al excluded those with hemoglobin less than 10. Given that iron deficiency is one of the most common causes of anemia, excluding anemic patients would serve to exclude a number of severely iron deficient patients.
Authors' Conclusions
Implications for practice
There is insufficient evidence to determine whether iron is effective therapy for restless legs syndrome or whether particular subsets of patients (e.g., those with end-stage renal disease on dialysis, those who are iron deficient) are more likely to benefit. Both oral and intravenous formulations of iron were reasonably well-tolerated in the included studies.
Implications for research
Larger, high quality studies are needed to determine whether iron therapy is an effective therapy for RLS. Future studies should also be designed to identify specific patient characteristics (e.g., baseline iron stores, renal function) that may be related to response to treatment. If iron ultimately proves to be beneficial in RLS patients, the question of which formulation of iron is most effective and best tolerated will also need to be answered.
Plain Language Summary.
Iron for restless legs syndrome
Restless legs syndrome (RLS) is a common medical condition that results in uncomfortable urges to move the legs, especially in the evening and at night, and often interferes with sleep. Low blood levels of iron are frequently seen in people who have RLS and the lack of iron may be part of the cause of RLS. Iron can be supplemented either in pill form or through injections into the bloodstream. This review was performed to see if iron supplements are effective in reducing the symptoms of RLS. Six studies of iron were included, which together involved only 192 subjects. Results from the studies were conflicting, with some studies showing that iron was not effective but others showing some help for patients' feelings of restlessness or discomfort. Because of the different ways in which the studies were done, we could not combine results from all of the studies to come up with an overall judgement of whether or not iron is effective. Two of the studies were limited to specific sub-groups of RLS patients, who might be expected to respond to iron differently than would the RLS group as a whole. The study of RLS patients with severe kidney disease showed a benefit of iron therapy. The study of RLS patients with low blood levels of iron did not consistently show a benefit of iron therapy at all time points. Iron did not cause any more side effects than the placebo medication. More studies are needed before we will be able to determine whether iron therapy should be used for patients with RLS.
Acknowledgments
We wish to thank the investigators of the studies included in this review, especially Richard Allen, Bradley Davis, and Ludger Grote, who provided us with unpublished data or manuscript clarifications. We also wish to thank Jan Ulfberg and his coauthors for providing us access with their then-unpublished manuscript comparing oral and intravenous iron in blood donors.
Sources of Support
Internal sources
No sources of support supplied
External sources
Jazz Pharmaceuticals Fellowship Training Grant in Sleep Medicine, USA. Partial funding for LM Trotti
PHS Grant KL2 RR025009 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources, USA. Partial funding for LM Trotti
Characteristics of Studies
Characteristics of included studies [ordered by study ID]
Allen 2009.
| Methods | Randomized, controlled trial | |
| Participants | 46 adult men and women with RLS, enrolled from multiple sites | |
| Interventions | Intervention: ferric carboxymaltose 500 mg in 100 mL normal saline on days 0 and 5 Placebo: 100 mL normal saline with same dosing schedule | |
| Outcomes | IRLS, RLS-QoL, CGI-1, PGI-1, MOS sleep scale, FSS, PLMS measured by actigraphy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | study randomized but method of sequence generation not specified |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and
detection bias) All outcomes |
Unclear risk | Study nurse not blinded and interacted with subjects (not an outcome assessor) |
| Incomplete outcome data (attrition
bias) All outcomes |
Low risk | |
| Selective reporting (reporting bias) | Unclear risk | no study protocol available |
| Other bias | High risk | funded by pharmaceutical company |
Davis 2000.
| Methods | Randomized, controlled trial | |
| Participants | 28 adult men and women with symptomatic RLS currently on treatment, enrolled from a single neurology clinic (USA) | |
| Interventions | Intervention: Ferrous sulfate liquid 325 mg bid. Placebo: a 50:50 mixture of water and 2% carboxymethlycellulose with a “slightly disagreeable taste, similar to liquid ferrous sulfate” Study drug continued for 16 weeks, then extended an additional 10 weeks if patients preferred study drug to their previous RLS medication | |
| Outcomes | Visual analog scale (VAS) of the extent that RLS interferes with sleep; VAS of how RLS affects life, ferritin, GI side effects | |
| Notes | Power for primary outcome was 25% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | block randomization but method not specified |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and
detection bias) All outcomes |
Low risk | |
| Incomplete outcome data (attrition
bias) All outcomes |
High risk | 6 of 14 treated pts left before wk 14, which was primary endpoint; this data only reported for the people who remained |
| Selective reporting (reporting bias) | Unclear risk | no study protocol available |
| Other bias | Low risk | |
Earley 2009.
| Methods | Randomized, controlled trial | |
| Participants | 18 adult men and women with RLS and PLMS >= 15/hr, enrolled from a single medical center (USA) | |
| Interventions | Intervention: Iron sucrose, given as a 500 mL infusion (containing 500 mg iron sucrose) over 10 hours, then another 500 mL over 12 hours the following day. Placebo: NaCl, given in the same infusion volume & with the same dosing schedule | |
| Outcomes | IRLS (a secondary outcome), PLMS/hr by 2nd night PSG, ferritin, global rating scale (7 pt scale of symptom severity completed by the patient), sleep diary, PLMW on SIT, change in CSF ferritin, change in MRI iron index in substantia nigra | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized 2:1 but doesn't specify method of sequence generation |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and
detection bias) All outcomes |
Unclear risk | Nurse & MD knew treatment status; they were not the outcome assessors but did interact with some subjects |
| Incomplete outcome data (attrition
bias) All outcomes |
Low risk | |
| Selective reporting (reporting bias) | Unclear risk | no study protocol available |
| Other bias | High risk | Stopped early due to data-dependent process (i.e., a stopping rule) |
Grote 2009.
| Methods | Randomized, controlled trial | |
| Participants | 60 adult men and women meeting all 4 RLS diagnostic criteria who had IRLS scores >= 10 and s-ferritin < 45, recruited from three separate hospitals (Sweden) | |
| Interventions | Intervention: 200 mg iron sucrose IV (10mL of 20mg/mL iron (III) as iron (III)-hy-droxide sucrose complex) given 5 times over 3 weeks Placebo: NaCl given with same timing as intervention | |
| Outcomes | IRLS (BL, wk 3, wk 7, wk 11, mo 5, mo 8, mo 12, with difference at wk 11 set as primary outcome), ferritin, ESS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and
detection bias) All outcomes |
Low risk | |
| Incomplete outcome data (attrition
bias) All outcomes |
Low risk | Intention to treat analysis with last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Study protocol registered but registration date appears to be after study completion |
| Other bias | High risk | Funded by pharmaceutical company |
Sloand 2004.
| Methods | randomized, placebo-controlled trial | |
| Participants | 25 adult men and women with ESRD (on either hemodialysis or peritoneal dialysis) who also had RLS by IRLSSG criteria, recruited from the dialysis units of a single medical center (USA) | |
| Interventions | Active: IV iron dextran given as: 30 mg test dose over 3 min, followed by 970 mg (in 485 mL volume) over 3 hrs if no reaction to the test dose Placebo: NaCl given in the same volume & and with the same timing as the intervention group | |
| Outcomes | ferritin, Hct, GI side effects, U of R severity score (10 pt scale where 0=none, 10=bad) at 0, 1, 2, 4 wks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of randomization not specified |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and
detection bias) All outcomes |
Low risk | |
| Incomplete outcome data (attrition
bias) All outcomes |
Unclear risk | two placebo patients dropped out after infusion, but Figure 1 reports data on all 14 placebo patients |
| Selective reporting (reporting bias) | Unclear risk | no study protocol available |
| Other bias | Unclear risk | baseline imbalance of 2 points in RLS severity score; there was also a broader range of scores in the treated group; imbalance in duration of dialysis |
Wang 2009.
| Methods | Randomized, controlled controlled | |
| Participants | 18 adult men and women with RLS who had ferritin 15-75 and IRLS scores >= 11 | |
| Interventions | Intervention: ferrous sulfate 325 mg bid in non-descript capsules Placebo: lactose, appearanced matched Study drug continued for 12 weeks | |
| Outcomes | IRLS (BL, 6 wks, 12 wks), ferritin (BL, 12 wks), single question on change in QOL at 12 wks (improved, same, worsened), compliance at 6 & 12 wks (by manual pill count) | |
| Notes | authors raise issue of iron causing stool discoloration and therefore inadvertent unblind-ing, although this is speculative | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and
detection bias) All outcomes |
Low risk | |
| Incomplete outcome data (attrition
bias) All outcomes |
Low risk | |
| Selective reporting (reporting bias) | Unclear risk | no study protocol available |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Birgegard 2010 | No control group |
| Earley 2004 | No control group |
| Earley 2005 | No control group |
| Konofal 2008 | Included children |
| Mohri 2008 | No control group; included children |
| O'Keeffe 1994 | Performed prior to 1995 (non-standard diagnostic criteria for RLS used); no control group |
| Simakajornboon 2003 | No control group; included children |
| Zilberman 2010 | No control group |
Characteristics of ongoing studies [ordered by study ID]
Earley NCT00685815.
| Trial name or title | Intravenous Iron Metabolism in Restless Legs Syndrome |
| Methods | Double-blind, randomized controlled trial |
| Participants | 36 adults with RLS |
| Interventions | 500 mg of IV ferric carboxymaltose given on two consecutive days |
| Outcomes | RLS symptoms |
| Starting date | November 2006 |
| Contact information | Christopher J. Earley, MD, PhD, Johns Hopkins University |
| Notes | registered at www.clinicaltrials.gov |
Data And Analyses
Comparison 1. Change in IRLS scores.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in IRLS severity scale score | 4 | 139 | Mean Difference (IV, Random, 95% CI) | -3.79 [-7.68, 0.10] |
Comparison 2. Subgroup analyses of IRLS scores.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subgroup -- IV iron | 3 | 121 | Mean Difference (IV, Random, 95% CI) | -2.45 [-5.59, 0.68] |
| 2 Subgroup -- iron deficient | 2 | 78 | Mean Difference (IV, Random, 95% CI) | -4.93 [-12.50, 2.65] |
Comparison 3. Quality of Life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of Life (dichotomous measure) | 2 | 39 | Risk Ratio (M-H, Random, 95% CI) | 2.01 [0.54, 7.45] |
| 2 Quality of Life (continuous measure) | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.72 [0.21, 1.24] |
Comparison 4. Patient satisfaction with treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrew to use other RLS meds | 5 | 142 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.30, 1.28] |
Comparison 5. Change in PLMS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in PLMS | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | -0.19 [-0.70, 0.32] |
Comparison 6. Subjective sleep quality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Subjective Sleep quality | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [-0.58, 0.95] |
Comparison 7. Number with adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 4 | 156 | Risk Ratio (M-H, Random, 95% CI) | 1.39 [0.85, 2.27] |
Comparison 8. Gastrointestinal side effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal side effects | 5 | 174 | Risk Ratio (M-H, Random, 95% CI) | 1.97 [0.96, 4.06] |
Comparison 9. Drop out due to adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Drop out due to adverse event | 3 | 113 | Risk Ratio (M-H, Random, 95% CI) | 2.44 [0.53, 11.24] |
Analysis 1.1. Comparison 1 Change in IRLS scores, Outcome 1 Change in IRLS severity scale score.
|
Analysis 2.1. Comparison 2 Subgroup analyses of IRLS scores, Outcome 1 Subgroup -- IV iron.
|
Analysis 2.2. Comparison 2 Subgroup analyses of IRLS scores, Outcome 2 Subgroup -- iron deficient.
|
Analysis 3.1. Comparison 3 Quality of Life, Outcome 1 Quality of Life (dichotomous measure).
|
Analysis 3.2. Comparison 3 Quality of Life, Outcome 2 Quality of Life (continuous measure).
|
Analysis 4.1. Comparison 4 Patient satisfaction with treatment, Outcome I Withdrew to use other RLS meds.
|
Analysis 5.1. Comparison 5 Change in PLMS, Outcome 1 Change in PLMS.
|
Analysis 6.1. Comparison 6 Subjective sleep quality, Outcome 1 Change in Subjective Sleep quality.
|
Analysis 7.1. Comparison 7 Number with adverse events, Outcome 1 Adverse events.
|
Analysis 8.1. Comparison 8 Gastrointestinal side effects, Outcome 1 Gastrointestinal side effects.
|
Analysis 9.1. Comparison 9 Drop out due to adverse event, Outcome 1 Drop out due to adverse event.
|
Appendix 1. Search Strategy
Our MEDLINE search used the following strategy:
RANDOMIZED CONTROLLED TRIAL [pt]
CONTROLLED CLINICAL TRIAL [pt]
RANDOMIZED [tiab]
PLACEBO [tiab]
DRUG THERAPY [sh]
RANDOMLY [tiab]
TRIAL [tiab]
GROUPS [tiab]
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
animals [mh] not (humans [mh] and animals [mh])
#9 not #10
IRON [mh]
IRON COMPOUNDS
IRON-DEXTRAN COMPLEX
FERROUS COMPOUNDS
IRON, DIETARY
FERROUS SULFATE
FERROUS GLUCONATE
FERROUS FUMARATE
IRON SUCROSE
FERRIC GLUCONATE
IRON DEXTRAN
#12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
RESTLESS LEGS SYNDROME [mh]
NOCTURNAL MYOCLONUS SYNDROME [mh]
PERIODIC LIMB MOVEMENT*
PERIODIC LEG MOVEMENT*
EKBOM* and SYNDROME
PARASITOSIS
#28 not #29
RESTLESS LEG*
NOCTURNAL MYOCLONUS
PSYCHOMOTOR AGITATION
AKATH*
#24 or #25 or #26 or #27 or #30 or #31 or #32 or #33 or #34
#11 and #23 and #35
Footnotes
Contributions of Authors: Lynn Marie Trotti (LMT) conceived and co-ordinated the review; performed the search; screened the search results for acceptable trials; graded and extracted data from trials; entered and analyzed data; wrote the initial draft of the review; and incorporated feedback from the other authors into the final draft. Srinivas Bhadriraju (SB) screened the search results for acceptable trials; graded and extracted data from trials; and co-wrote the review. Lorne A. Becker (LAB) designed the review; resolved disagreements between LMT and SB regarding quality of trials; analyzed data; and co-wrote the review.
Declarations of Interest: None
Differences between Protocol and Review: A funnel plot was not constructed because we identified fewer than 10 studies for inclusion.
References to studies included in this review
- Allen RP, Butcher A, Du W. Double-blind, placebo controlled multi-center evaluation of restless legs syndrome (RLS) treatment with a 1,000 mg of IV iron (ferric carboxymaltose -- FCM) Sleep. 2009;32(Abstract supplement):A294–A295. [Google Scholar]
- *.Davis BJ, Rajput A, Rajput ML, Aul EA, Eichhorn GR. A randomized, double-blind, placebo-controlled trial of iron in restless legs syndrome. European Neurology. 2000;43:70–75. doi: 10.1159/000008138. [DOI] [PubMed] [Google Scholar]
- *.Earley CJ, Horska A, Mohamed MA, Barker PB, Beard JL, Allen RP. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Medicine. 2009;10:206–11. doi: 10.1016/j.sleep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double-blind, placebo-controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Movement Disorders. 2009;24(10):1445–1452. doi: 10.1002/mds.22562. [DOI] [PubMed] [Google Scholar]
- Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. American Journal of Kidney Diseases. 2004;43(4):663–670. doi: 10.1053/j.ajkd.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Wang J, O'Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Medicine. 2009;10(9):973–975. doi: 10.1016/j.sleep.2008.11.003. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Birgegard G, Schneider K, Ulfberg J. High incidence of iron depletion and restless legs syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron. Vox Sang. 2010;99(4):354–61. doi: 10.1111/j.1423-0410.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- *.Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Medicine. 2004;5:231–235. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- *.Earley CJ, Hecker D, Allen RP. Repeated IV doses of iron provides effective supplemental treatment of restless legs syndrome. Sleep Medicine. 2005;6:301–305. doi: 10.1016/j.sleep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- *.Konofal E, Lecendreux M, Deron J, Marchand M, Cortese S, Zaim M, Mouren MC, Arnulf I. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatric Neurology. 2008;38:20–26. doi: 10.1016/j.pediatrneurol.2007.08.014. [DOI] [PubMed] [Google Scholar]
- *.Mohri I, Kato-Nishimura K, Tachibana N, Ozono K, Taniike M. Restless legs syndrome (RLS): an unrecognized cause for bedtime problems and insomnia in children. Sleep Medicine. 2008;9:701–702. doi: 10.1016/j.sleep.2007.08.005. [DOI] [PubMed] [Google Scholar]
- *.O'Keeffe ST, Gavin K, Lavan JN. Iron status and restless legs syndrome in the elderly. Age and Aging. 1994;23:200–203. doi: 10.1093/ageing/23.3.200. [DOI] [PubMed] [Google Scholar]
- *.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26(6):735–738. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- Zilberman M, Silverberg DS, Schwartz D, Oksenberg A. Restless legs syndrome (RLS) in anemic patients with congestive heart failure and chronic renal failure: lack of effect of anemia treatment. International Journal of Cardiology. 2010;143(2):205–207. doi: 10.1016/j.ijcard.2008.11.177. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Intravenous Iron Metabolism in Restless Legs Syndrome. Ongoing study. 2006 Nov; [Google Scholar]
Additional references
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome. Sleep Medicine. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Allen RP, Adler CH, Du W, Butcher A, Bregman DB, Earley CJ. Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: A multi-centred, placebo-controlled preliminary clinical trial. Sleep Medicine. 2011;12:906–913. doi: 10.1016/j.sleep.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Archives of Internal Medicine. 2004;164:196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Periodic leg movements in sleep and restless legs syndrome: Considerations in geriatrics. Sleep Medicine Clinics. 2006;1:263–71. doi: 10.1016/j.jsmc.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Ondo WG, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–309. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. Journal of Neuroscience Research. 2000;62:623–8. doi: 10.1002/1097-4547(20001201)62:5<623::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. Wiley-Blackwell; Feb, 2008. [Google Scholar]
- Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Medicine Reviews. 2006;10(3):169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome. Movement Disorders. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- Nelson C, Erikson K, Piñero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. Journal of Nutrition. 1997;127:2282–8. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- Scholz H, Trenkwalder C, Kohnen R, Riemann D, Kriston L, Hornyak M. Dopamine agonists for restless legs syndrome. Cochrane Database of Systematic Reviews. 2011 Mar 16;(CD006009) doi: 10.1002/14651858.CD006009.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schormair B, Kemlink D, Roeske D, Eckstein G, Xiong L, Lichtner P, et al. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nature Genetics. 2008;40(8):946–948. doi: 10.1038/ng.190. [DOI] [PubMed] [Google Scholar]
- Silber MH, Ehrenberg BL, Allen RP, Buchfuhrer MJ, Earley CJ, Hening WA, et al. Medical Advisory Board of the Restless Legs Syndrome Foundation. An algorithm for the management of restless legs syndrome. Mayo Clinic Proceedings. 2004;79:916–22. doi: 10.4065/79.7.916. [DOI] [PubMed] [Google Scholar]
- Silverstein SB, Rodgers GM. Parenteral iron therapy options. American Journal of Hematology. 2004;76:74–8. doi: 10.1002/ajh.20056. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, et al. A genetic risk factor for periodic limb movements in sleep. New England Journal of Medicine. 2007;357(7):639–47. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Kohnen R, Allen RP, Benes H, Ferini-Strambi L, Garcia-Borreguero D, Hadjigeorgiou GM, Happe S, Hogl B, Hornyak M, Klein C, Nass A, Montagna P, Oertel WH, O'Keeffe S, Paulus W, Poewe W, Provini F, Pramstaller PP, Sieminski M, Sonka K, Stiasny-Kolster K, de Weerd A, Wetter TC, Winkelmann J, Zucconi M. Clinical trials in restless legs syndrome -- Recommendations of the European RLS Study Group (EURLSSG) Movement Disorders. 2007;22(Suppl. 18):S495–S504. doi: 10.1002/mds.21538. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Hogl B, Benes H, Kohnen R. Augmentation in restless legs syndrome is associated with low ferritin. Sleep Med. 2008;9(5):572–4. doi: 10.1016/j.sleep.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Trotti LM, Bhadriraju S, Rye DB. An update on the pathophysiology and genetics of restless legs syndrome. Current Neurology and Neuroscience Reports. 2008;8:281–7. doi: 10.1007/s11910-008-0044-8. [DOI] [PubMed] [Google Scholar]
- Trotti LM, Bliwise DL, Greer SA, Sigurdsson AP, Gudmundsdottir GB, Wessel T, Organisak LM, Sigthorsson T, Kristjansson K, Sigmundsson T, Rye DB. Correlates of PLMS variability over multiple nights and impact upon RLS diagnosis. Sleep Medicine. 2009;10(6):668–671. doi: 10.1016/j.sleep.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Walters AS. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Movement Disorders. 1995;10(5):634–42. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, Trenkwalder C. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- *.Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nature Genetics. 2007;39(8):1000–6. doi: 10.1038/ng2099. Indicates the major publication for the study. [DOI] [PubMed] [Google Scholar]