Abstract
We examined the influence of regulatory dendritic cells (DCreg), generated from cytokine-mobilized donor blood monocytes in vitamin D3 and IL-10, on renal allograft survival in a clinically-relevant rhesus macaque model. DCreg expressed low MHC class II and costimulatory molecules, but comparatively high levels of programmed death ligand-1 (B7-H1), and were resistant to pro-inflammatory cytokine-induced maturation. They were infused intravenously (3.5–10×106/kg), together with the B7-CD28 costimulation blocking agent CTLA4Ig, 7 days before renal transplantation. CTLA4Ig was given for up to 8 weeks and rapamycin, started on day −2, was maintained with tapering of blood levels until full withdrawal at 6 months. Median graft survival time was 39.5 days in control monkeys (no DC infusion; n=6) and 113.5 days (p< 0.05) in DCreg-treated animals (n=6). No adverse events were associated with DCreg infusion, and there was no evidence of induction of host sensitization based on circulating donor-specific alloantibody levels. Immunologic monitoring also revealed regulation of donor-reactive memory CD95+ T cells and reduced memory/regulatory T cell ratios in DCreg-treated monkeys compared with controls. Termination allograft histology showed moderate combined T cell- and Ab-mediated rejection in both groups. These findings justify further pre-clinical evaluation of DCreg therapy and their therapeutic potential in organ transplantation.
Keywords: dendritic cells, costimulation blockade, rapamycin, renal transplant, memory T cells, rhesus macaques
Introduction
There is increasing interest in the potential of regulatory immune cell therapy for the control of allograft rejection and reducing dependence on/elimination of immunosuppressive drugs (1–4). Bone marrow-derived dendritic cells (DC) are uniquely well-equipped antigen (Ag)-presenting cells (APC), with inherent tolerogenic properties (5–7), that play crucial roles in regulating innate and adaptive immune responses (8). In rodents and humans, DC promote central or peripheral tolerance through various mechanisms, that include clonal deletion, inhibition of T effector cells and the expansion or induction of regulatory T cells (Treg) (2, 6, 9). Moreover, several studies have documented the ability of DC to inhibit mouse or human memory T cell responses (10–12), an important barrier to the induction of transplantation tolerance (13–15).
In rodents, infusion of donor- or recipient-derived DC with tolerogenic properties, either alone or in combination with immunosuppressive agents, prolongs organ allograft survival indefinitely (16–21), in association with regulation of donor-specific T cell responses. Accordingly, regulatory DC (DCreg) are considered promising cellular therapeutic agents to promote organ transplant tolerance (1, 2, 22–24). Non-human primates (NHP) provide important pre-clinical models for testing such strategies (25, 26); NHP DCs have been well-characterized (27, 28) and shown to modulate alloimmune reactivity in vitro (29, 30) and in vivo (31). However, to date, no testing of the influence of DCreg on NHP organ transplant survival has been reported.
An important consideration regarding cell therapy with DCreg is to ensure that any potential risk of host sensitization is minimized. Multiple studies have shown that conventional rodent or human DC propagated in or exposed to anti-inflammatory or immunosuppressive agents, either in vitro or in vivo, exhibit phenotypic and functional immaturity, resist maturation in response to pro-inflammatory stimuli, and induce alloAg-specific T cell hyporesponsiveness (32). These agents include the vitamin D3 (VitD3) metabolite 1α,25 dihydroxyvitamin D3 (1α25(OH)2D3) and its analogues (33), IL-10 (34), glucocorticoids (35), cyclosporine (36, 37), tacrolimus (37), sirolimus (38), and mycophenolate mofetil (39). In vivo administration of such immature, donor-derived DC, particularly those that are maturation-resistant, promotes long-term or indefinite rodent organ allograft survival, particularly in combination with the costimulation blocking agents cytotoxic T lymphocyte Ag 4 (CTLA4)Ig (18, 40) or anti-CD154 mAb (16, 41, 42).
We have shown previously (31) that rhesus macaque monocyte-derived DC propagated in VitD3 and IL-10 are stably immature, resistant to maturation following potent pro-inflammatory cytokine stimulation, and can induce T cell hyporesponsiveness to alloAg in vitro. When administered systemically to normal rhesus macaques, in combination with CTLA4Ig, these DCreg modulate alloimmune reactivity, with resulting T cell hyporesponsiveness to donor alloAg, and no detectable circulating IgM or IgG anti-donor alloAb (31). We have therefore examined the influence of DCreg generated from CD14+ blood monocytes of allogeneic donors on the survival of subsequent renal transplants from the same donor monkeys. The DCreg were infused together with CTLA4Ig and the mammalian target of rapamycin (mTOR) serine threonine kinase inhibitor rapamycin, an immunosuppressive agent that inhibits DC maturation and effector T cell function, and that has purported ‘tolerance-sparing’ properties (43, 44). Our data reveal that DCreg inhibit acute allograft rejection in this clinically-relevant NHP model.
Materials and Methods
Experimental animals and donor-recipient selection
Captive-bred, simian immunodeficiency virus-negative, herpes B virus-negative, male Indian juvenile rhesus macaques (Macacca mulatta, n=18; 5–7 kg), obtained from the NIAID-sponsored rhesus macaque colony (Yemasse, S.C.) were maintained in the NHP Research Facility of the Department of Laboratory Animal Resources at the University of Pittsburgh School of Medicine. Donor-recipient pairs were selected based on anti-donor reactivity that was determined in vitro by carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR)-labeled mixed leukocyte reaction (MLR). All procedures and medications were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Experiments were conducted according to the guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Specific environment enrichment was provided.
Donor leukapheresis
To mobilize blood monocytes, prospective transplant donors underwent cytokine treatment comprising GM-CSF (Leukine; Genzyme, Cambridge, MA; 10µg/kg/day for 4 days), followed by G-CSF (Neupogen; Amgen, Thousand Oaks, CA; 10µg/kg/day for 4 days). Leukapheresis was then performed using a dedicated COBE® Spectra Apheresis System (Lakewood, CO). Leukapheresis products were processed, and peripheral blood mononuclear cells (PBMC) isolated, then stored in liquid N2 until needed for DCreg generation. A minimum interval of 28 days was implemented before donor nephrectomy, to allow restoration of normal leukocyte levels.
DCreg generation
DCreg were generated as described (31) from purified CD14+ cells in recombinant human (rhu) GM-CSF + rhu IL-4 over 7 days, with the addition of VitD3 on days 1 and 5, and rhu IL-10 on day 5. Details of the yields of DCreg from single leukapheresis products of donor monkeys are shown in Supplemental Table 1.
Renal transplantation
Renal transplantation was performed as described (45). Bilateral nephrectomy of native kidneys was performed before graft insertion and recipient pairs, i.e., control (no DC infusion) and experimental (DCreg infusion), received kidney grafts from the same donor. The experimental end-point was determined as the first day that serum creatinine reached >2.5 mg/dL (with persistent rise/no subsequent decline), blood urea nitrogen >100 mg/dL and/or weight loss of >20%, in addition to histological evidence of rejection on biopsy or terminal graft analysis.
DCreg infusion and immunosuppression
The immunosuppressive protocol used in each graft recipient is shown in Table 1 and Figure 3A. In the DCreg group, DCreg (3.5–10×106/kg) were infused intravenously, one week before transplantation (day −7). All recipients in the control and DCreg groups received CTLA4Ig (abatacept; Bristol-Myers Squibb; Princeton, NJ; 12.5 mg/kg i.v.) on day −7 and day −4, to further minimize risk of host sensitization. Eight recipients (four in each group) received further, short-term costimulation blockade (CTLA4Ig; 20 mg/kg on days −1, 0, 2, 4, 7 and 10), while four recipients (two in each group) received long-term costimulation blockade (CTLA4Ig; 20 mg/kg on days −1, 0, 3, 7, 10, 14, 21 and 28, then 10 mg/kg on days 35, 42, 49, and 56). Intramuscular rapamycin (LC laboratories, Woburn, MA) was given daily, starting on day −2 for six months. Whole blood trough levels were measured twice weekly and maintained between 10 and 15 ng/ml for the first month, between 5 and 10 ng/ml for the subsequent 4 months, and between 1 and 5 ng/ml for the sixth month. Immunosuppressive therapy was withdrawn completely at 6 months.
Table 1.
Immunosuppressive regimens and graft survival in control and DCreg-treated rhesus monkeys
| Group | Recipient | Immunosuppression |
DCreg infused (×106/kg body weight)1 |
Experiment end point (days)2,3 |
|
|---|---|---|---|---|---|
| CTLA4-Ig | Rapamycin | ||||
| Control (No DC infusion; n=6) | M50 | Short-term | Trough level of 10–15 ng/ml maintained for 1 month, then 5–10 ng/ml for 4 months, and 1–5 ng/ml for the 6th month | N/A | 32 |
| M49 | 75 | ||||
| M111 | 40 | ||||
| M112 | 39 | ||||
| M143 | Long-term | 28 | |||
| M145 | 57 | ||||
| DCreg infusion; n=6)* | M114 | Short-term | 4.6 | 50 | |
| M113 | 5.2 | 300 | |||
| M45 | 10 | 118 | |||
| M46 | 5.2 | 54 | |||
| M148 | Long-term | 4.0 | 109 | ||
| M147 | 3.5 | 160 | |||
Donor-derived DCreg infused on d −7
Experiment end-point for both groups was determined based on 3 criteria; serum creatinine level, blood urea nitrogen, and/or >20% body weight loss.
Numerous reports in the literature indicate a median graft survival time of 7 days in untreated rhesus renal allograft recipients.
Figure 3. DCreg infusion prolongs MHC-mismatched renal allograft survival in rhesus monkeys.
(A), Costimulation blockade/rapamycin-based immunosuppression protocol. Monkeys received DCreg (3–10×106/kg i.v.) (n=6) or no cells (n=6) on day −7 relative to kidney transplantation on day 0. Each group of monkeys was given CTLA4Ig (25 mg/kg i.v.), either from day −7 to day 10 post-transplant (n=4 per group) or from day −7 to day 56 (n=2 per group). Intramuscular rapamycin was commenced on day −2 and whole blood trough levels maintained at 10–15 ng/ml (day 0-day 28); 5–10 ng/ml (day 29-day 150) and 1–5 ng/ml (day 151-day 180). Immunosuppressive treatment was stopped at day 180. (B), serum creatinine levels in control (n=6) and DCreg-treated animals (n=6) at various times post transplant; (C), body weight loss in each group at various times post-transplant (D), urinary protein/creatinine ratios in the same groups of monkeys, 6–8 weeks post-transplant and (E), actuarial graft survival data.
Measurement of donor-specific alloantibody (Ab) levels
Serum samples were stored at −20°C and alloAb binding to donor or third party CD3+ T cells determined by flow cytometric analysis, as described (31). For CD3+ T cells, a mean fluorescence intensity (MFI) shift >2-fold above baseline (pre-transplant) values for IgM and IgG was considered positive for donor alloAb (46).
Monitoring of anti-donor T cell alloreactivity by CFSE-MLR
PBMC were isolated before and at various times after transplantation. CD2+T cells purified by positive selection, and anti-donor and anti-third party proliferative activity determined in 5-day CFSE-MLR, as described (31). Data acquired using an LSR II flow cytometer (Becton Dickinson, Franklin Lakes, NJ) were analyzed with FlowJo software (Tree Star, San Carlos, CA).
Phenotypic analysis of rhesus T cells
The following fluorochrome-labeled mAbs were used for cell surface or intra-cellular staining of rhesus T cells: CD2 FITC (Biolegend), CD3 PerCP-Cy5.5 (BD Biosciences), CD4 Pacific Blue, CD8α AF700 and CD95 PE-Cy7 (all Biolegend), PD-1/CD279 PE and CD25 PE-Cy7 (each eBioscience), CD127 (IL-3Rα) PE and CTLA4/CD152 (each BD Biosciences), FoxP3 APC (Miltenyi Biotec), and Granzyme-B Alexa Fluor 647 (BD Biosciences). Absolute numbers of CD4+CD95+ and CD8+CD95+ memory T cells (Tmem) were calculated based on conventional total white blood cell counts and percentages of these populations determined by flow cytometric analysis
Graft histology and immunohistological analysis
Graft tissue obtained at necropsy and needle biopsies performed on day 28 post-transplant and on clinical suspicion of rejection, were fixed in 10% formalin and processed in paraffin blocks for H&E staining and immunohistochemical analysis. Sections (5μm) were labeled with primary Abs specific for C4d (ALPCO Diagnostics), IgM, IgG, and CD3 (all DAKO), and staining visualized using the LSAB + labeled streptavidin-biotin kit (DAKO). H & E-stained sections were scored for rejection based on Banff renal transplant pathology criteria (47), by a transplant pathologist (AJD) ‘blinded’ to the identity of the graft recipients.
Statistical analyses
Survival graphs were obtained by Kaplan-Meier’s analysis. The significance of differences between means was determined using Mantel Cox and Wilcoxon tests, or Student’s ‘t’ test, as appropriate. Significance was defined as p < 0.05.
Results
Propagation of DCreg from leukapheresis products of prospective renal allograft donors
Supplemental Table 1 shows the numbers of DCreg that we propagated from each of the 6 prospective transplant donors. An average of 38 ± 13×106 purified DCreg was obtained, permitting an infusion cell dose ranging from 3.5–10 (average 5.4 ± 2.3)×106/kg body weight.
Characterization of DCreg
We examined the cell surface phenotype of all batches of rhesus monocyte-derived DCreg in comparison to control DC preparations. Figure 1A shows the expression MHC class II (HLA-DR), the myeloid DC marker CD11c, the monocyte marker CD14, costimulatory molecules (CD80 and CD86), the DC maturation marker CD83, and the chemokine receptor CCR7 on DCreg compared with control DC, the latter cultured identically without VitD3 and IL-10. Unstimulated rhesus DCreg expressed higher levels of CD14, but much lower levels of CD80, CD86 and CD83 than unstimulated control DC, indicative of an immature myeloid APC. The DCreg retained low levels of CCR7 expression, an important pre-requisite for (in vivo) homing to secondary lymphoid tissue. Significantly, the rhesus DCreg expressed comparatively high levels of the coregulatory molecule, programmed death ligand-1 (PD-L1=B7-H1; CD274), and the PD-L1:CD86 ratio was consistently higher on DCreg than on control DC. When DCreg were stimulated in vitro for 24hr with a potent pro-inflammatory cytokine cocktail (ICC) (31) comprising TNFα, IL-1β, IL-6 and prostaglandin E2, they demonstrated maturation resistance by failing to upregulate CD80, CD86 and CD83. By contrast, ICC-stimulated control DC showed markedly enhanced expression of these molecules. Compared with unstimulated control DC, and consistent with their comparatively low levels of costimulatory molecules, the DCreg were weak stimulators of allogeneic CD4+ and CD8+ T cell proliferation in 5-day CFSE-MLR (Figure 1B). As we reported previously (31), the rhesus T cells stimulated initially with DCreg failed to proliferate following re-stimulation with donor alloAg in secondary MLR, indicating that functional unresponsiveness to donor alloAgs (anergy) was induced (data not shown). Thus, VitD3/IL-10-conditioned rhesus monocyte-derived DC met stringent phenotypic and functional criteria (2, 24) for the generation of stably-immature DCreg for in vivo testing in allograft recipients.
Figure 1. Phenotypic and functional characteristics of control DC and DCreg propagated from CD14+ blood monocytes mobilized in rhesus monkey leukapheresis products.
(A), DC were propagated in GM-CSF + IL-4 from CD14+ monocytes, in the absence (control DC) or presence of VitD3/IL-10 (DCreg), as described in the Materials and Methods. Phenotypic data obtained using flow cytometry are shown for unstimulated control DC and DCreg, and also for both populations stimulated for 24 h with a potent, pro-inflammatory cytokine cocktail. (B), DCreg induce minimal allogeneic CD4+ and CD8+ T cell proliferation compared with control DC. Left: Proliferative responses of normal allogeneic CD4+ and CD8+ T cells in response to control DC or DCreg were evaluated. CFSE-labeled rhesus PBMC (0.2×106) were cocultured with either control DC or DCreg (0.01×106) at 20:1 ratio for 5 days. After gating on CD4+ and CD8+ T cells, proliferation was evaluated by CFSE dilution analysis. Right: Mean CFSE proliferation of allogeneic CD4+ and CD8+ T cells was significantly less in response to DCreg compared to control DC. Data are representative of 5 independent experiments using different monkeys.
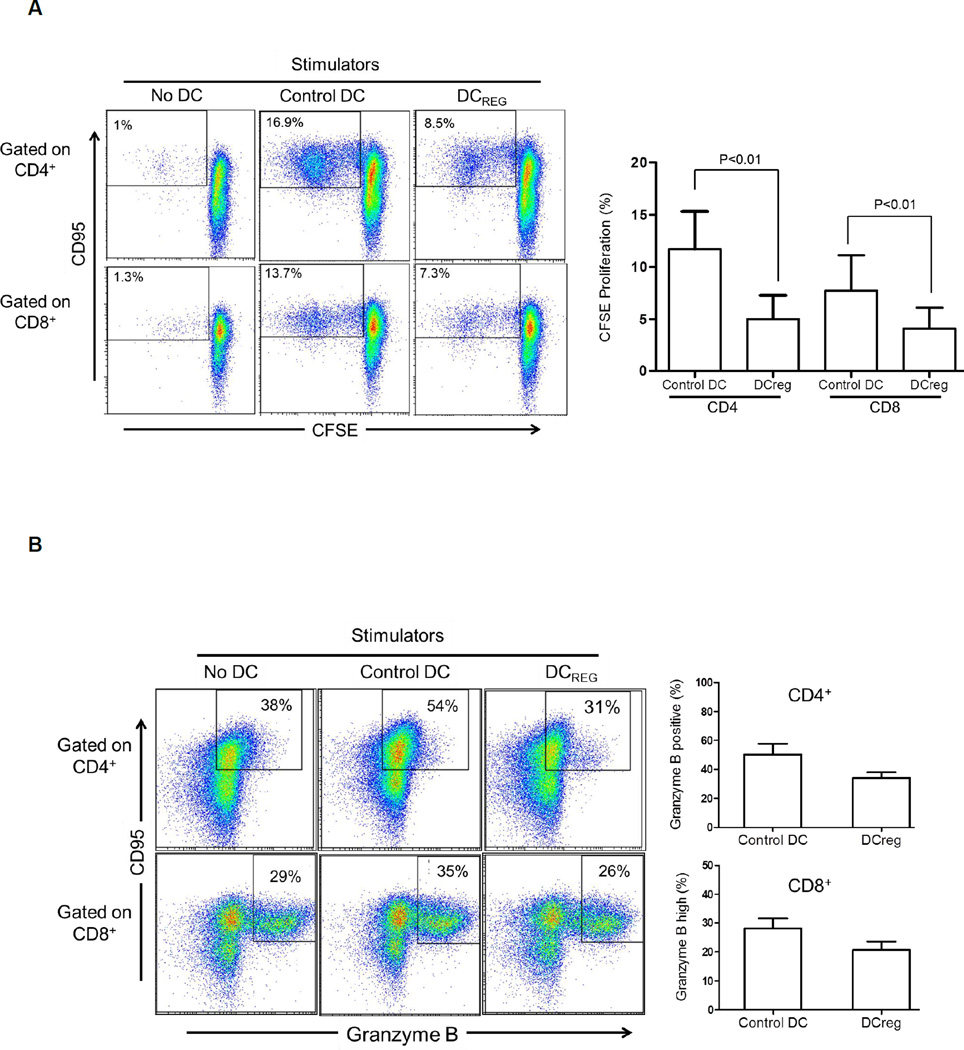
DCreg are weak stimulators of allogeneic T cells and enhance apoptosis of CD95+ memory T cells
While most allogeneic T cells that proliferated in response to control DC or DCreg stimulation in 5-day CFSE-MLR were CD95+ Tmem, DCreg induced much lower levels of both CD4+ and CD8+ CD95+ Tmem proliferation (Figure 2A). Moreover, a higher incidence of CD95+ granzyme B+ effector memory cells was observed after coculture with control DC than with DCreg. Indeed, in the latter instance, the incidence of granzyme B+ memory CD95+ T cells was similar to that in cultures without DC (Figure 2B). Notably, there was no increase in the incidence of Treg (CD4+CD25+Foxp3+CD127−) after coculture with either control DC or DCreg (data not shown). We next evaluated the incidence of apoptosis in allogeneic CD4+ and CD8+ T cells after coculture with DC in 5-day CFSE-MLR. As shown in Figure 2C, the weak T cell proliferative response induced by DCreg compared with control DC was associated with a higher incidence of Annexin V+, non-proliferating CD4+ and CD8+ T cells. Consistent with this finding, the incidence of CD95+ Annexin V+ CD4+ and CD8+ T cells after 5-day co-culture with DCreg was higher than with control DC (Figure 2D). Taken together, these findings indicate that DCreg, that are PD-L1hi, are not only weak stimulators of alloreactive T cells, but promote the apoptosis of CD95+CD4+ and CD95+CD8+ Tmem,- the predominant T cell population responding to allostimulation.
Figure 2. Rhesus DCreg enhance apoptosis of alloreactive T memory cells.
As in Figure 1, CFSE-labeled rhesus PBMC were cocultured with control DC or DCreg at 20:1 ratio for 5 days, followed by evaluation of T cell proliferation by CFSE dilution analysis. (A) Left: DCreg elicit less CD4+/CD8+ CD95+ T memory cell proliferation. Right: Mean of 5 experiments showing significantly less proliferation of CD95+ T cells in response to DCreg. (B) Left: DCreg elicit less granzyme B+ CD95+ effector memory T cells in comparison to control DC in 5-day MLR. Right: Mean of 3 independent experiments using different donor:responder pairs, showing less induction of granzyme B+ T cells after coculture with DCreg. Gating for CD4+ T cells was based on isotype controls. As the vast majority of CD8+ T cells were Granzyme B+ under all culture conditions, gating was based on granzyme Bhi populations. (C) Rhesus DCreg induce less allogeneic CD4+ and CD8+ T cell proliferation in 5-day CFSE-MLR, but enhance apoptosis of non-proliferating T cells compared with control DC. (D), DCreg induce higher incidences of CD4+/CD8+ CD95+ Annexin+ memory T cells than control DC after 5-day MLR culture. Data in C and D are representative of 2 independent experiments using different stimulator-responder pairs.
Infusion of donor-derived DCreg prolongs kidney allograft survival
DCreg phenotype and function were verified for each donor monkey. They were infused i.v. 7 days before kidney transplantation from the same donor, together with CTLA4Ig (Figure 3A). The target dose of approx. 5×106 DCreg/kg was achieved or exceeded in 3/6 (50%) of cases (range 3.5–10 × 106/kg) (Supplemental Table 1). Creatinine levels in the control and DCreg groups are shown in Figure 3B. Notably, creatinine levels were not uniformly high in the graft recipients in the control group (Figure 3B), where, in 3/6 recipients, serum creatinine levels remained < 2mg/dl (despite proven rejection by histology). However, all graft recipients in the control group underwent body weight loss of >20% at the time of rejection (Figure 3C), in contrast to the DCreg group, in which body weight loss was significantly less (p<0.01),- a difference that became more marked with increased time post-transplant. Furthermore, consistent with these findings, there was significantly more severe urinary protein loss in the control group, in which urinary protein/creatinine ratios were significantly (7–8 fold) higher (p<0.05) than in the DCreg group (Figure 3D). A significant, negative correlation was observed between the urinary protein/creatinine ratio and renal graft survival (r2=−0.0692). Survival of grafts in monkeys that received DCreg (range 50 – 300 days; median=113.5 days) was prolonged significantly (Figure 3E) compared with controls (range 28–75 days; median=39.5 days) (p<0.05). There was no significant difference in graft survival between monkeys that received the short or long course of CTLA4Ig, whether or not they were given DCreg.
Histological analysis of the allografts
Termination graft histology for both groups of monkeys showed evidence of combined T cell-and Ab-mediated rejection (Table 2 and Supplemental Figure 1). There was evidence of either focal or diffuse IgM and IgG deposition, diffuse peritubular capillaritis, focal lymphocytic arteritis, but only minimal complement (C4d) deposition- an indicator of Ab-mediated rejection (48) in both groups in the formalin-fixed, paraffin-embedded tissue samples. Moderate to severe T cell infiltration was seen in all grafts. Moderate plasma cell infiltration was detected in all but one kidney in each group. Examination of the biopsy obtained 100 days after withdrawal of immunosuppression from a monkey in the DCreg group revealed moderate to severe interstitial inflammation with active tubulitis (Supplemental Figure 1).
Table 2.
Summary of the pathologic findings in rejected kidney allografts of control and DCreg-treated rhesus monkeys
| Abs | Cell infiltration | Tubulitis | Glomerulitis | Diagnosis | ||||
|---|---|---|---|---|---|---|---|---|
| Recipient | IgM | IgG | T cells | Plasma cells | ||||
| Control | ||||||||
| M50 | Focal | Focal | Yes | No | Yes | No | Moderate combined T cell and ab-mediated rejection | BANFF 1B |
| M49 | Focal | Focal | Yes | Yes | Yes | No | Severe combined T cell and ab-mediated rejection | BANFF 1B |
| M111 | Diffuse | Diffuse | Yes | Yes | Yes | Yes | Moderate combined T cell and ab-mediated rejection | BANFF 1B |
| M112 | Diffuse | Diffuse | Yes | Yes | Yes | Yes | Moderate combined T cell and ab-mediated rejection | BANFF 1B |
| M143 | Focal | Focal | Yes | Yes | Yes | No | Moderate T cell-mediated rejection | BANFF 1B |
| M145 | Focal | Focal | Yes | Yes | Yes | No | Moderate T cell-mediated rejection | BANFF 1B |
| DCreg | ||||||||
| M114 | Diffuse | Diffuse | Yes | Yes | Yes | Yes | Moderate combined T cell and ab-mediated rejection | BANFF 1B |
| M113 | Diffuse/Focal | Diffuse/Focal | Yes | Yes | Yes | Yes | Moderate combined T cell and ab-mediated rejection | BANFF 1B |
| M45 | Focal | Focal | Yes | No | Yes | Yes | Severe combined T cell and ab-mediated rejection | BANFF 2A |
| M46 | Diffuse | Diffuse | Yes | Yes | Yes | No | Moderate combined T cell and ab-mediated rejection | BANFF 1B |
| M148 | Focal | Focal | Yes | Yes | Yes | No | Severe T cell-mediated rejection | BANFF 2B |
| M147 | Focal | Focal | Yes | Yes | Yes | No | Moderate T cell-mediated rejection | BANFF 1B |
DCreg do not induce circulating donor-specific alloAb
Serum IgM and IgG anti-donor alloAb levels were measured sequentially after transplantation and compared to normal, pre-transplant levels. As indicated in Supplemental Figure 2, circulating anti-donor Abs were not detected in any of the graft recipients from either the control or DCreg group (in which there was longer follow-up).
Host CD4+ and CD8+T cell responses to donor and third party alloAg
Graft recipient CD4+ and CD8+ T cell proliferative responses to donor or third party alloAg stimulation were evaluated in both control and DCreg-infused groups before and after transplantation (6–8 weeks) by CFSE-MLR. As shown in Figure 4A, there was no consistent pattern in the T cell proliferative responses of the transplanted monkeys to either donor or third-party alloAg after transplantation, although similar trends towards depression of CD4+ T cell reactivity to donor (compared with pre-treatment values) were observed in both groups. One DCreg-treated recipient (M113) with markedly prolonged graft survival, exhibited sustained, donor-specific T cell hyporesponsiveness on day 180 (with minimal rapamycin levels) and at day 240 (2 months after immunosuppression had been withdrawn completely), although this hyporesponsiveness was eventually lost at the time of graft rejection (experimental endpoint; day 300) (Figure 4B).
Figure 4. DCreg infusion exerts no consistent effect on anti-donor T cell proliferative responses.
(A), Ex vivo anti-donor and anti-third party proliferative responses of circulating CD4+ and CD8+ T cells from control or DCreg-treated rhesus renal allograft recipients before, and 4–6 and 8–12 weeks post-transplant. Each graph represents the response of one recipient from the DCreg group and its concurrent recipient from the control group against the same donor cells or 3rd party cells. CFSE-MLRs were performed as described in the Materials and Methods. PBMC from each recipient were cocultured with T cell-depleted PBMC from either donor or third party at 1:1 ratio for 5 days. (B), proliferative responses of T cells from a DCreg-treated monkey (M113) whose transplant survived 300 days, showing initial depression (day 180) of anti-donor and anti-third party responses, but later recovery of anti-donor reactivity (day 240) after withdrawal of all immunosuppression, followed by enhanced anti-donor reactivity at the time of rejection (day 300).
Circulating levels of CD4+CD25+CD127loFoxp3hi Treg post-transplant are not affected by DCreg infusion
There is evidence from rodent and human studies (40, 49), that DCreg can expand or induce Treg. We therefore evaluated the incidence of circulating CD4+CD25+CD127loFoxp3hi Treg before and at various times after renal transplantation. As shown in Supplemental Figure 3A, there was no significant change in the incidence of Treg (%CD4+ T cells) after transplantation, in either the DCreg or control group. In the one DCreg-treated graft recipient (M113) whose renal transplant survived well over 6 months, there were reduced ratios of both memory CD4+CD95+ and CD8+CD95+ T cells to Treg that persisted after immunosuppressive drug withdrawn at 6 months. However, these ratios had increased to above pre-treatment levels by the time of graft rejection (Supplemental Figure 3B), coinciding with loss of donor-specific T cell hyporesponsiveness (Figure 4B).
DCreg infusion suppresses circulating CD4+ and CD8+ Tmem post transplant
Rhesus monkey CD95 (Fas)+ T cells are considered Tmem (50), that correlate with allograft rejection (46, 51, 52) and also appear to be rapamycin-resistant (53). We determined absolute numbers of CD4+CD95+ and CD8+CD95+ T cells sequentially in 4 of the graft recipients (2 each from the control and DCreg groups). As shown in Figure 5A, both Tmem populations continued to rise in controls until the time of rejection, whereas no increases were observed in DCreg-treated monkeys over the corresponding time-period, until they rejected their grafts at later time-points.
Figure 5. DCreg infusion suppresses CD4+ and CD8+ Tmem in renal-allografted monkeys and enhances PD-1 and CTLA4 expression on donor-reactive Tmem.
(A), Absolute numbers of circulating CD4+ and CD8+ CD95+ Tmem in control and DCreg-treated renal allograft recipients at various times pre- and post-transplant. (B, C) Incidences of PD1+CTLA4+ Tmem in ex-vivo-stimulated CD4+ and CD8+ T cell populations from (B) M112 and M143 (control) monkeys, and (C) M147 and M148 (DCreg-treated) monkeys. Recipient PBMC obtained 4 weeks (and 8 weeks in the DCreg group monkeys) post transplant, were cocultured with either donor or third party stimulators for 5 days as in Figure 4. CD4+ and CD8+ CD95+ T cells were then analyzed for cell surface PD-1 and CTLA4 expression. POD = post-operative day.
CTLA4 and PD-1 are upregulated on Tmem of DCreg-infused hosts in response to donor, but not third party, stimulation
The B7:CD28 family members PD-1 and CTLA4 negatively regulate T cell activation (54) and survival (55–57) and promote T cell exhaustion in macaques (58). Four weeks post transplant, we stimulated graft recipient T cells with donor or third party alloAg in 5-day MLR. CD4+CD95+ and CD8+CD95+ T cells were then evaluated for cell surface expression of PD1 and CTLA4. In the control group, there was no change in the incidence of PD1+CTLA4+ memory CD4+ or CD8+ T cells, when host cells were stimulated with either donor or third party cells (Figure 5B). By contrast, T cells from the DCreg-treated group showed enhanced incidences of PD1+CTLA4+ memory cells in response to donor, but not third party stimulation, suggesting attenuation of donor-reactive Tmem.
Discussion
We have used a rigorous, pre-clinical NHP model to evaluate the impact of DCreg infusion on renal allograft survival. Our data show that administration of these regulatory innate immune cells before transplantation, together with a calcineurin inhibitor-free, steroid-sparing regimen of CTLA4Ig (abatacept) and tapered rapamycin (sirolimus) (i.e., CD28 + mTOR blockade), suppresses rejection and significantly prolongs graft survival. Similar to the control group, there was no evidence of induction of circulating alloantibodies after DCreg infusion. The extent of graft prolongation we observed with DCreg (MST 114 days) is superior to that reported (59) using donor-specific transfusion (MST 33 days) in combination with CTLA4Ig and rapamycin in the same rhesus renal allograft model. Our analysis of the immune signature that accompanied this therapeutic effect of DCreg infusion provides evidence of a shift towards depression of the CD95+ Tmem phenotype, with expression by these cells of markers (CTLA4 and PD1) indicative of selective impairment of anti-donor reactivity. By contrast, a recent report (60) indicates that, when combined with B7-CD28 and mTOR blockade, non-specific elimination of Tmem (using the CD2-specific fusion protein LFA3-Ig) does not improve renal allograft survival in rhesus monkeys and increases cytomegalovirus reactivation. In the context of the present findings, it is noteworthy that “steady-state” immature DC, similar phenotypically to those that we generated from rhesus monkeys and bearing cognate Ag, can terminate Tmem responses in rodents (61). Although rhesus DCreg infusion combined with targeting of both the B7:CD28 (using CTLA4Ig) and mTOR pathways did not induce tolerance, our data nevertheless suggest that the use of DCreg, that was not associated with any adverse effects, is a promising means to impact costimulation blockade-resistant host T cell responses, especially the role of donor-reactive Tmem (62, 63).
In addressing whether DCreg could inhibit renal allograft rejection, we chose to evaluate DCreg of donor origin, based on compelling evidence in rodents, that such donor-derived DCreg, able to induce alloAg-specific T cell hyporesponsiveness/anergy in vitro, promote long-term organ allograft survival (2, 17, 23, 64). Rodent studies have also shown that the therapeutic effect of DCreg, and their ability to promote transplant tolerance, is potentiated by either B7-CD28 (18, 40) or CD40-CD40L co-stimulatory pathway blockade (16). In addition, our earlier studies in rhesus monkeys (31) indicated that DCreg infusion could downmodulate allogeneic T cell responses in normal recipients when combined with CTLA4Ig. Moreover, when combined with rapamycin, an agent that inhibits DC maturation and enhances their tolerogenicity (21, 38), CTLA4Ig promotes allograft tolerance in rodents (65). As in the present report, in each of the rodent transplant studies that have demonstrated tolerogenic properties of donor-derived DCreg, the cells have been infused systemically into immunologically-quiescent, prospective graft recipients, usually one week before surgery. Earlier or later infusion of DCreg diminishes or abolishes the effect (17) that is also lost in mice when syngeneic or third-party DCreg or fresh donor BM cells are infused (64). In rats however, autologous DC have been reported to promote transplant tolerance (66). In addition to using rhesus donor DCreg that are robustly resistant to maturation, together with concomitant CTLA4Ig administration to further minimize risk of host sensitization, our pre-emptive approach appears to favor immune regulatory effects of DCreg infusion. In the DCreg-treated recipient (M113) with markedly prolonged graft survival, sustained donor-specific T cell hyporesponsiveness beyond day 180 (when rapamycin monotherapy was withdrawn completely), was followed by the recovery of donor alloreactivity at the time of graft rejection (day 300). This points to the possible additive effects of both mTOR inhibition (rapamycin) and DCreg in promoting hyporesponsiveness of donor-reactive T cells.
Due to the constraints of NHP transplantation and the need to repeat leukapheresis in individual monkeys, we did not evaluate the impact of DCreg infusion in excess of 10.106/kg (the maximum number of monocyte-derived DCreg that could be propagated from a single pheresis product) or the influence of multiple cell infusions. Whether such changes in regimen could achieve a greater therapeutic effect clearly requires further investigation. Whether recipient-derived DCreg can modulate graft outcome in NHP has also not been addressed in the present study, but is currently under investigation in our laboratory. Several groups have reported the ability of ex vivo-propagated host DC (unloaded or loaded with donor Ag) to prolong allograft survival in rodents (20, 67–69) an approach that, if proven safe and effective in humans, could allow generalization of DCreg therapy to both live and deceased donor transplantation. However, such studies, including pre-clinical testing in NHP, are likely to require lengthy evaluation of different sources of donor Ag, including donor cell lysate, apoptotic cells and exosomes. Moreover, since deceased donors are not identified until shortly before transplant and DC propagation takes approximately 7 days, autologous DCreg pulsed with donor Ag could not be infused until the day of transplantation surgery and/or later, when an inflammatory environment would be encountered.
In rodents, DCreg promote allograft survival through several mechanisms, including the development of donor-specific Treg (2, 23). On the other hand, in patients receiving HLA-mismatched allografts, prolonged graft survival without ongoing immunosuppression has been attributed to the deletion or anergy of donor-reactive T cells, while Treg may play a role only during the early phase after transplantation (70). In our NHP model, we observed no increase in the incidence of circulating Treg after transplantation with or without DCreg infusion. An underlying factor may be the use of costimulation blockade, which may be detrimental to Treg survival (71) and can prevent IL-2-driven Treg expansion and restore rejection of mis-matched allografts (72). While we detected Treg in the grafts at the time of rejection in both groups, no significant differences were observed (data not shown).
Tmem are believed to require less costimulation for activation and to acquire effector functions (73), that may lead to development of costimulation-blockade-resistant T cells after transplantation (63). In rhesus macaques, CD95+ T cells are considered Tmem (50), and include central (CD95+ CD28+) and effector memory (CD95+ CD28−) T cells. Skewing of the host T cell compartment towards a CD95+ phenotype is associated with allograft rejection in this species (46, 51, 52). Notably, rhesus CD95+ Tmem can resist inhibition by rapamycin in vitro (53). In the present study, we observed early post-transplant increases in CD95+ T cells associated with early rejection in control animals given co-stimulation blockade and rapamycin, whereas elevations in Tmem did not occur until later in DCreg-infused monkeys, suggesting a causative relationship between DCreg infusion, attenuation of the Tmem response, and prolongation of graft survival. Several reagents have been used to target Tmem and to promote kidney allograft survival in NHP. However, these agents (such as anti-LFA-1 Ab) target both donor-specific and non-specific Tmem. Significantly, in the present study, infusion of DCreg and consequent prolongation of graft survival was associated with enhanced expression of markers of CTLA4 and PD-1. Both PD-1 and CTLA4 are considered markers of exhaustion, and are expressed on rhesus CD95+T cells in vivo. However, we found no difference in the expression of these molecules between CD95+T cells in the blood of the control and DCreg groups. Only when CD95+T cells from DCreg recipients were stimulated with donor (but not 3rd party) cells, were PD-1 and CTLA4 upregulated, suggesting that when these memory T cells encounter donor antigen following infiltration of the graft, they may upregulate PD-1 and CTLA4, which in turn are likely to control CD95+T cell activation. The enhanced expression of these regulatory molecules on CD8+ cells is considered relevant to improved graft survival in the DCreg group, owing to memory CD8+T cell resistance to immunosuppression and their role in kidney allograft rejection
While we have not established the in vivo longevity of the donor DCreg we infused in this NHP study, donor DC that migrate from rodent organ allografts can survive for several days in secondary lymphoid tissue of non-immunosuppressed recipients (74) or for weeks in immunosuppressed individuals (75). Additional in vivo studies using labeled cells could provide critical information regarding the migration pattern, fate and longevity of the infused DCreg in monkeys. The precise mechanisms that underlie the immune regulatory effects of donor DCreg infusion remain unclear. Many studies in rodents have implicated direct pathway DCreg-host T cell interactions leading to deletion or anergy/regulation of donor-reactive T cells. An additional/alternative explanation may be that killing of infused donor DCreg by NK cells (76) or CD8+ T cells (77) can suppress indirect alloimmune responses, since donor Ag can be transferred to quiescent conventional host APCs that may be critical for regulation of donor-specific reactivity and prolongation of graft survival (78).
In summary, our studies demonstrate, for the first time, that a single, systemic, pre-transplant infusion of DCreg of donor origin, results in prolongation of renal allograft survival in a robust pre-clinical NHP model. No evidence of host sensitization was observed. Moreover, DCreg infusion was associated with selective attenuation of donor-reactive Tmem. The data support further testing of DCreg together with co-stimulation blockade and rapamycin in a clinically-translatable, calcineurin inhibitor-free and steroid-sparing regimen to reduce dependence on immunosuppressive reagents and promote rejection-free renal transplant survival.
Supplementary Material
Acknowledgments
The study was supported by National Institutes of Health grant U01 AI051698. ME is in receipt of a Starzl Transplantation Institute Joseph A. Patrick Fellowship. We thank Drs. Brian Hermann and Kyle Orwig for advice on apheresis in monkeys, Drs. Burcin Ekser, Hayato Iwase and Eefje Dons for surgical assistance, and Ms. Miriam Freeman for skillful administrative support.
Abbreviations
- CTLA4Ig
cytotoxic T lymphocyte antigen 4
- DCreg
regulatory dendritic cells
- ICC
inflammatory cytokine cocktail
- NHP
non-human primate
- PD-1
programmed death-1
- PD-L1
programmed death ligand-1
- Tmem
memory T cells
- Treg
regulatory T cells
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by The American Journal of Transplantation. AWT and AJD are inventors of US patents for generation of dendritic cells to promote organ transplant survival.
References
- 1.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 2.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi G, Sagoo P, Scotta C, Fazekasova H, Smyth L, Tsang J, et al. Cell therapy to promote transplantation tolerance: a winning strategy? Immunotherapy. 2011;3(4 Suppl):28–31. doi: 10.2217/imt.11.42. [DOI] [PubMed] [Google Scholar]
- 4.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119(22):5088–5103. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steptoe RJ, Thomson AW. Dendritic cells and tolerance induction. Clin Exp Immunol. 1996;105:397–402. doi: 10.1046/j.1365-2249.1996.d01-779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 7.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206(3):549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasreen M, Waldie TM, Dixon CM, Steptoe RJ. Steady-state antigen-expressing dendritic cells terminate CD4+ memory T-cell responses. Eur J Immunol. 2010;40(7):2016–2025. doi: 10.1002/eji.200940085. [DOI] [PubMed] [Google Scholar]
- 11.Kenna TJ, Waldie T, McNally A, Thomson M, Yagita H, Thomas R, et al. Targeting antigen to diverse APCs inactivates memory CD8+ T cells without eliciting tissue-destructive effector function. J Immunol. 2010;184(2):598–606. doi: 10.4049/jimmunol.0900032. [DOI] [PubMed] [Google Scholar]
- 12.Anderson AE, Sayers BL, Haniffa MA, Swan DJ, Diboll J, Wang XN, et al. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol. 2008;84(1):124–133. doi: 10.1189/jlb.1107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6(4):647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 14.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14(9):2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 15.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15(4):405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu L, Li W, Fu F, Chambers FG, Qian S, Fung JJ, et al. Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation. 1997;64(12):1808–1815. doi: 10.1097/00007890-199712270-00031. [DOI] [PubMed] [Google Scholar]
- 17.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30(7):1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Mirenda V, Berton I, Read J, Cook T, Smith J, Dorling A, et al. Modified dendritic cells coexpressing self and allogeneic major histocompatibility complex molecules: an efficient way to induce indirect pathway regulation. J Am Soc Nephrol. 2004;15(4):987–997. doi: 10.1097/01.asn.0000119575.98696.1d. [DOI] [PubMed] [Google Scholar]
- 19.Beriou G, Peche H, Guillonneau C, Merieau E, Cuturi MC. Donor-specific allograft tolerance by administration of recipient-derived immature dendritic cells and suboptimal immunosuppression. Transplantation. 2005;79(8):969–972. doi: 10.1097/01.tp.0000158277.50073.35. [DOI] [PubMed] [Google Scholar]
- 20.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5(2):228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 21.Turnquist H, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 22.van Kooten C, Lombardi G, G KA, Sagoo P, Buckland M, Lechler RI, et al. Dendritic cells as a tool to induce transplantation tolerance: obstacles and opportunities. Transplantation. 2011;91:2–7. doi: 10.1097/tp.0b013e31820263b3. [DOI] [PubMed] [Google Scholar]
- 23.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23(4):252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naranjo-Gomez M, Raich-Regue D, Onate C, Grau-Lopez L, Ramo-Tello C, Pujol-Borrell R, et al. Comparative study of clinical grade human tolerogenic dendritic cells. J Transl Med. 2011;9(1):89. doi: 10.1186/1479-5876-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev. 2003;196:176–196. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 26.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: progress, current challenges and unmet needs. Am J Transplant. 2006;6(5 Pt 1):884–893. doi: 10.1111/j.1600-6143.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 27.Coates PT, Barratt-Boyes SM, Zhang L, Donnenberg VS, O'Connell PJ, Logar AJ, et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102(7):2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 28.Jesudason S, Collins MG, Rogers NM, Kireta S, Coates PT. Non-human primate dendritic cells. J Leukoc Biol. 2012;91(2):217–228. doi: 10.1189/jlb.0711355. [DOI] [PubMed] [Google Scholar]
- 29.Moreau A, Chiffoleau E, Beriou G, Deschamps JY, Heslan M, Ashton-Chess J, et al. Superiority of bone marrow-derived dendritic cells over monocyte-derived ones for the expansion of regulatory T cells in the macaque. Transplantation. 2008;85(9):1351–1356. doi: 10.1097/TP.0b013e31816f22d6. [DOI] [PubMed] [Google Scholar]
- 30.Zahorchak AF, Raimondi G, Thomson AW. Rhesus monkey immature monocyte-derived dendritic cells generate alloantigen-specific regulatory T cells from circulating CD4+CD127-/lo T cells. Transplantation. 2009;88(9):1057–1064. doi: 10.1097/TP.0b013e3181ba6b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zahorchak AF, Kean LS, Tokita D, Turnquist HR, Abe M, Finke J, et al. Infusion of stably immature monocyte-derived dendritic cells plus CTLA4Ig modulates alloimmune reactivity in rhesus macaques. Transplantation. 2007;84(2):196–206. doi: 10.1097/01.tp.0000268582.21168.f6. [DOI] [PubMed] [Google Scholar]
- 32.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4(1):24–35. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 33.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 34.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159(10):4772–4780. [PubMed] [Google Scholar]
- 35.Moser M, De Smedt T, Sornasse T, Tielemans F, Chentoufi AA, Muraille E, et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25(10):2818–2824. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 36.Lee JI, Ganster RW, Geller DA, Burckart GJ, Thomson AW, Lu L. Cyclosporine A inhibits the expression of costimulatory molecules on in vitro-generated dendritic cells: association with reduced nuclear translocation of nuclear factor kappa B. Transplantation. 1999;68(9):1255–1263. doi: 10.1097/00007890-199911150-00007. [DOI] [PubMed] [Google Scholar]
- 37.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000;30(7):1807–1812. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 38.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 39.Mehling A, Grabbe S, Voskort M, Schwarz T, Luger TA, Beissert S. Mycophenolate mofetil impairs the maturation and function of murine dendritic cells. J Immunol. 2000;165(5):2374–2381. doi: 10.4049/jimmunol.165.5.2374. [DOI] [PubMed] [Google Scholar]
- 40.Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, DeCreus A, et al. 'Alternatively-activated’ dendritic cells preferentially secrete IL-10, expand Foxp3+ CD4+ T cells and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177(9):5868–5877. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Morelli AE, Hackstein H, Kaneko K, Thomson AW. Marked inhibition of transplant vascular sclerosis by in vivo-mobilized donor dendritic cells and anti-CD154 mAb. Transplantation. 2003;76(3):562–571. doi: 10.1097/01.TP.0000068901.11693.C3. [DOI] [PubMed] [Google Scholar]
- 42.Bjorck P, Coates PT, Wang Z, Duncan FJ, Thomson AW. Promotion of long-term heart allograft survival by combination of mobilized donor plasmacytoid dendritic cells and anti-CD154 monoclonal antibody. J Heart Lung Transplant. 2005;24(8):1118–1120. doi: 10.1016/j.healun.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuhaus P, Neuhaus R, Wiersema HD, Borleffs JC, Balner H. The technique of kidney transplantation in rhesus monkeys. J Med Primatol. 1982;11(3):155–162. [PubMed] [Google Scholar]
- 46.Page A, Srinivasan S, Singh K, Russell M, Hamby K, Deane T, et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am J Transplant. 2012;12(1):115–125. doi: 10.1111/j.1600-6143.2011.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 48.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Immunol. 2012;8(11):670–678. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 49.Thomson AW, Turnquist HR, Zahorchak AF, Raimondi G. Tolerogenic dendritic cell-regulatory T-cell interaction and the promotion of transplant tolerance. Transplantation. 2009;87(9 Suppl):S86–S90. doi: 10.1097/TP.0b013e3181a2dcec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 51.Kean LS, Adams AB, Strobert E, Hendrix R, Gangappa S, Jones TR, et al. Induction of chimerism in rhesus macaques through stem cell transplant and costimulation blockade-based immunosuppression. Am J Transplant. 2007;7(2):320–335. doi: 10.1111/j.1600-6143.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- 52.Larsen CP, Page A, Linzie KH, Russell M, Deane T, Stempora L, et al. An MHC-defined primate model reveals significant rejection of bone marrow after mixed chimerism induction despite full MHC matching. Am J Transplant. 2010;10(11):2396–2409. doi: 10.1111/j.1600-6143.2010.03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88(12):1349–1359. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 54.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 55.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203(10):2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177(9):5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 58.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182(10):5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15(7):746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo DJ, Anderson DJ, Weaver TA, Leopardi F, Song M, Farris AB, et al. Belatacept and Sirolimus Prolong Nonhuman Primate Renal Allograft Survival Without a Requirement for Memory T Cell Depletion. Am J Transplant. 2013 doi: 10.1111/j.1600-6143.2012.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kenna TJ, Thomas R, Steptoe RJ. Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood. 2008;111(4):2091–2100. doi: 10.1182/blood-2007-07-103200. [DOI] [PubMed] [Google Scholar]
- 62.Ford ML, Kirk AD, Larsen CP. Donor-reactive T-cell stimulation history and precursor frequency: barriers to tolerance induction. Transplantation. 2009;87(9 Suppl):S69–S74. doi: 10.1097/TP.0b013e3181a2a701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farber DL. Identification and targeting of costimulation-resistant T cells in renal transplantation. Am J Transplant. 2011;11(1):8–9. doi: 10.1111/j.1600-6143.2010.03361.x. [DOI] [PubMed] [Google Scholar]
- 64.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62(5):659–665. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5(11):1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 66.Hill M, Thebault P, Segovia M, Louvet C, Beriou G, Tilly G. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through IFN- and EB13. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03651.x. In Press. [DOI] [PubMed] [Google Scholar]
- 67.Garrovillo M, Ali A, Depaz HA, Gopinathan R, Oluwole OO, Hardy MA, et al. Induction of transplant tolerance with immunodominant allopeptide-pulsed host lymphoid and myeloid dendritic cells. Am J Transplant. 2001;1(2):129–137. [PubMed] [Google Scholar]
- 68.Ikeguchi R, Sacks JM, Unadkat JV, Solari M, Horibe EK, Thomson AW, et al. Long-term survival of limb allografts induced by pharmacologically conditioned, donor alloantigen-pulsed dendritic cells without maintenance immunosuppression. Transplantation. 2008;85(2):237–246. doi: 10.1097/TP.0b013e31815e870e. [DOI] [PubMed] [Google Scholar]
- 69.Peche H, Trinite B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5(2):255–267. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 70.Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11(6):1236–1247. doi: 10.1111/j.1600-6143.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229(1):41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Charbonnier LM, Vokaer B, Lemaitre PH, Field KA, Leo O, Le Moine A. CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am J Transplant. 2012;12(9):2313–2321. doi: 10.1111/j.1600-6143.2012.04184.x. [DOI] [PubMed] [Google Scholar]
- 73.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229(1):271–293. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 74.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Demetris AJ, Murase N, Starzl TE. Donor dendritic cells after liver and heart allotransplantation under short-term immunosuppression. Lancet. 1992;339(8809):1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203(8):1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laffont S, Coudert JD, Garidou L, Delpy L, Wiedemann A, Demur C, et al. CD8+ T-cell-mediated killing of donor dendritic cells prevents alloreactive T helper type-2 responses in vivo. Blood. 2006;108(7):2257–2264. doi: 10.1182/blood-2005-10-4059. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z, Divito SJ, Shufesky WJ, Sumpter T, Wang H, Tkacheva OA, et al. Dendritic cell therapies in transplantation revisited: deletion of recipient DCs deters the effect of therapeutic DCs. Am J Transplant. 2012;12(6):1398–1408. doi: 10.1111/j.1600-6143.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.