Abstract
Background
Fixed-dose combinations of hypertensive drugs have been advocated as a suitable option for hypertensive patients who require two or more drugs to achieve blood pressure (BP) targets.
Objectives
Our objective was to assess the efficacy and safety of lercanidipine/enalapril in clinical practice.
Methods
This observational study collected data for patients with hypertension treated by 46 specialists at clinics across Portugal with lercanidipine/enalapril (10/20 mg). The primary outcome measure was the reduction from baseline in systolic BP (SBP) and diastolic BP (DBP).
Results
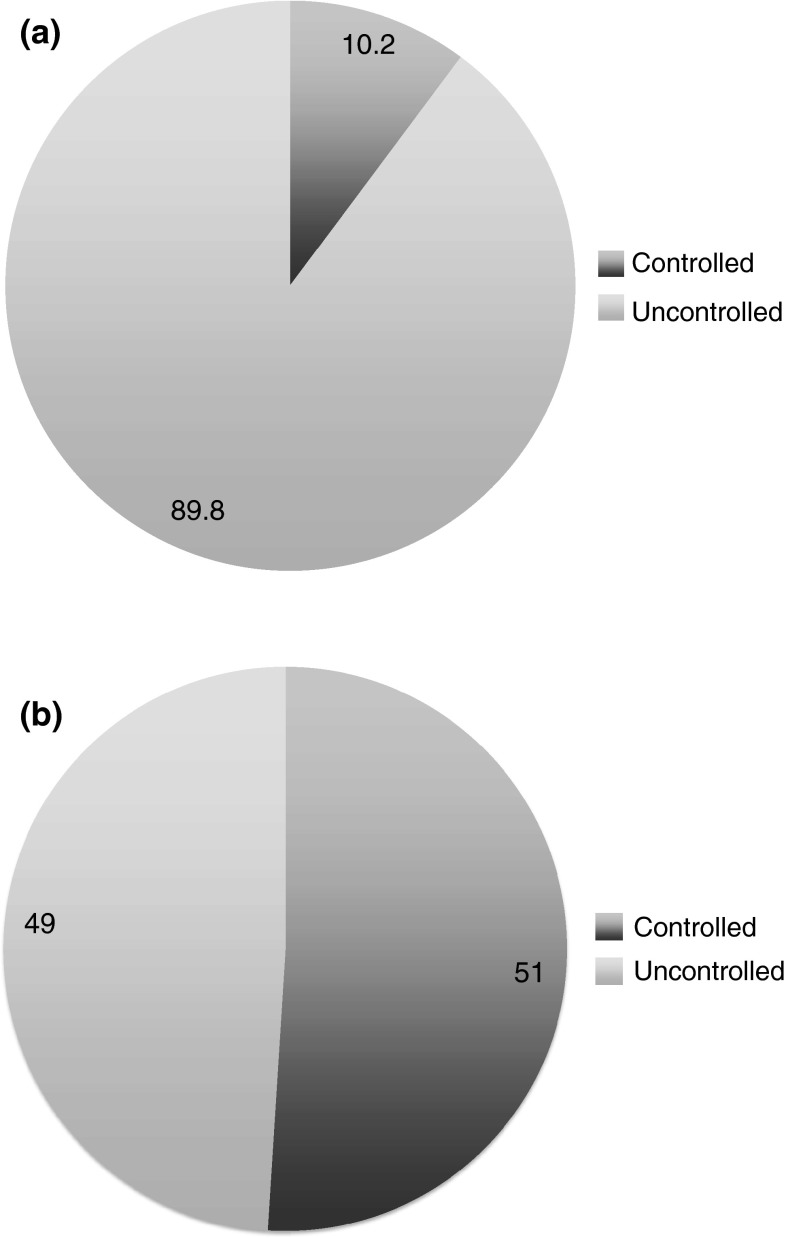
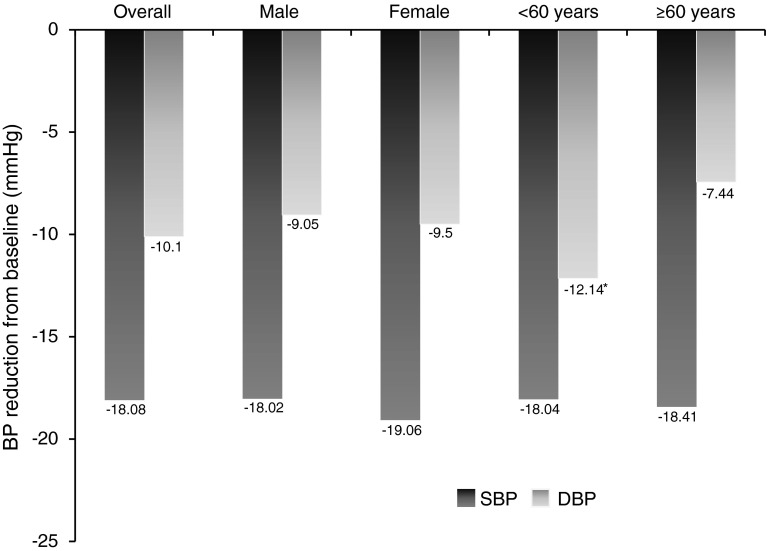
The registry enrolled 315 patients (59.1 % females; mean age 64.84 ± 12.18 years). Baseline SBP and DBP were 159.11 ± 16.93 and 88.32 ± 12.35 mmHg, respectively. At a mean 2.88 ± 1.75 months after starting lercanidipine/enalapril, the mean change from baseline in SBP and DBP were −18.08 ± 15.91 and −10.10 ± 11.46 mmHg, respectively (both p < 0.001). This corresponded to reductions of 11.4 and 11.3 % in SBP and DBP, respectively. SBP was reduced independently of sex and age, and DBP was reduced independently of sex. The BP control (<140/90 mmHg) rate significantly increased from 10.2 % at baseline to 51.0 % after a mean of 2.88 months of treatment with lercanidipine/enalapril (p < 0.001). Adverse effects were seen in only one patient (0.3 %), who developed a persistent dry cough.
Conclusions
Treatment with the fixed-dose combination lercanidipine/enalapril was associated with significant reductions in SBP and DBP, and a significant increase in the BP control rate. This fixed-dose combination has been shown to effectively reduce BP, generally independently of age and sex, and with an excellent safety profile.
Key Points
| This study was an observational registry enrolling 315 patients treated by 46 specialists in hypertension clinics across Portugal. Patients received lercanidipine/enalapril (10/20 mg) fixed-dose combination (FDC) for ~2 months, and efficacy and safety of the treatment were assessed. |
| Treatment with lercanidipine/enalapril FDC was associated with significant reductions from baseline in systolic and diastolic blood pressure (BP), and increases in the rate of BP control (<140/90 mmHg). |
| The lercanidipine/enalapril FDC had an excellent safety profile in this population, with treatment-emergent adverse events reported in only one patient. |
| These results suggest that lercanidipine/enalapril (10/20mg) FDC is an effective and safe treatment for the general hypertensive population in Portugal. |
Introduction
It is well recognized that arterial hypertension is a leading cause of death and disability worldwide [1]. Hypertension is a significant risk factor for cardiovascular disease, stroke, peripheral vascular disease, and end-stage renal disease [2]. The disease prevalence is impressive, with more than one-quarter of the world’s adult population having hypertension at present, and it is expected to increase in future [3].
Reducing blood pressure (BP) has been shown to reduce the risk of hypertension-associated morbidity and mortality [4–6]. However, despite the progressive improvements observed in many countries [7], BP control rates remain suboptimal [8]. Reasons for not achieving BP targets include a lack of adherence to or persistence with antihypertensive therapy, often due to the occurrence of adverse events, the use of drugs that do not target the mechanism(s) of BP elevation in that patient, and monotherapy being insufficient to control BP [9].
Because there are multiple possible mechanisms of BP elevation, and the response to a drug may be attenuated by counter-regulatory responses, two or more antihypertensive drugs of different classes are often required to achieve BP control [9, 10]. It has been shown that combination therapy using antihypertensive drugs with complementary mechanisms of action has additive BP-lowering effects and is more effective than high-dose monotherapy with the same drugs [11, 12]. Furthermore, because it allows the use of lower doses of each drug than monotherapy, and because in some cases one drug class can attenuate the adverse events that occur with another, combination therapy is likely to be better tolerated [9, 11].
A potential disadvantage of combination therapy is the additional pill burden, particularly in patients taking multiple medications for comorbidities. Increasing complexity of dosing has been shown to reduce adherence and persistence with therapy [10, 12, 13]. A strategy to address this problem is the use of fixed-dose combinations (FDCs), which simplifies dosing by allowing two or more drugs to be administered as a single pill. The use of FDCs has been shown to improve adherence to antihypertensive therapy and increase BP control rates [6, 12, 14]. In fact, in some countries, a parallel increase has been noted in BP control rates and the use of combination therapy for the treatment of hypertension [15, 16].
There are numerous possible combinations of antihypertensive drugs available as FDCs. The combination of a calcium channel blocker (CCB) and a modulator of the renin-angiotensin system (RAS) appears to be a primary option [6, 17–19]. One such combination is the third-generation vasoselective dihydropyridine CCB lercanidipine plus the angiotensin-converting enzyme inhibitor (ACEI) enalapril, which is available as an FDC. This combination has been shown to be effective and well tolerated in clinical trials [20–22]. However, there is a lack of data on its efficacy and tolerability in real-world clinical practice, where patients’ characteristics are likely to differ from those included in controlled clinical trials. In this context, the CONCEPT Collaborative Group (CCG) aimed to evaluate the efficacy and tolerability of a lercanidipine 10 mg plus enalapril 20 mg FDC in patients with hypertension treated in the non-hospital setting.
Methods
Study Design
The CCG consists of 46 specialists with a particular interest in cardiovascular diseases (internal medicine and cardiologists) practicing in private clinics in Portugal who decided to perform a critical analysis of their clinical management of private out-of-hospital patients. The CCG established an observational registry to assess the efficacy and safety of lercanidipine/enalapril for the treatment of hypertension. Patient recruitment and assessment took place during a 6-month period.
Patients
All patients with hypertension presenting to a CCG member’s clinic who were prescribed lercanidipine/enalapril (10/20 mg) were included in the registry. Patients were required to be aged 18 years or older and to have been prescribed the lercanidipine/enalapril FDC as either initial therapy or after previous antihypertensive treatment due to issues of efficacy or tolerability with their existing therapy or because the specialist considered the lercanidipine/enalapril to be a more suitable treatment than that prescribed by the patient’s general practitioner. Patients were initially given lercanidipine/enalapril 10/10 mg, with the dose increased to 10/20 mg from the second clinic visit. Lercanidipine/enalapril 10/20 mg was given either alone or in combination with other antihypertensive drugs in order to achieve a BP target of <140/90 mmHg.
Assessments
Data were collected at baseline and after approximately 2 months of treatment with lercanidipine/enalapril 10/20 mg. At both consultations, the patients’ weight and height were measured, and body mass index (BMI) was calculated in kg/m2. BP was also measured at baseline and 2 months after the patient started treatment with lercanidipine/enalapril 10/20 mg. BP measurements were taken in a supine position and after a 10-min resting period by an experienced operator using an oscilometric automatic sphygmomanometer (clinically validated—class A), with appropriate cuff. Before their appointment, patients were advised to avoid coffee or tobacco consumption. Three measurements were taken at each assessment, with a 2-min interval between each measurement, and the arithmetic mean was used in the analysis. Adverse events were collected by the specialists who were instructed to report all situations of interest. For all assessments, a quality check was performed on a regular basis to ensure adequate compliance with all the necessary conditions to warrant the validation of the study.
Objectives
The primary outcome measure was the reduction in systolic and diastolic BP (SBP and DBP, respectively) from baseline after 2 months of treatment with lercanidipine/enalapril 10/20 mg. Secondary endpoints included the proportion of patients achieving BP control, defined as 140/90 mmHg, the number and classes of concomitant antihypertensive medications at baseline and endpoint (therapeutic profile), and the incidence of treatment-emergent adverse events after starting treatment with lercanidipine/enalapril.
Data Management
All data were codified and personally delivered to the study coordinator (João Maldonado), blinding the name and other means of identifying individual patients. Electronic medical records for individual patients were not obtained by the registry coordinating team. A quality analysis of the data was then performed by the registry coordinators, and all registries with incoherent or incomplete data were excluded.
Ethical Considerations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients included in the registry.
Statistical Analysis
The data were entered into a central database and analyzed using SPSS for Windows, version 17.0. The distribution of the variables was tested for normality using the Shapiro–Wilk test and for homogeneity of variance by Levene’s test. Simple descriptive statistics were used to characterize the sample and the distribution of variables.
Within-group comparisons were made using the chi-squared test with Fisher’s correction, for categorical variables, the Student’s t-test for pairwise samples, or the Wilcoxon test for quantitative variables with or without normal distribution.
The criterion for statistical significance used was p ≤ 0.05 for a confidence interval of 95 %.
Results
Baseline Characteristics
The registry included 315 patients (59.1 % females) who were treated with lercanidipine/enalapril as first-line therapy or after previous antihypertensive therapy due to lack of efficacy (n = 283), adverse events (n = 21), or because their physician considered the FDC to be a more suitable treatment than that previously prescribed by the patient’s general practitioner (n = 59). Many patients switched therapy for more than one reason.
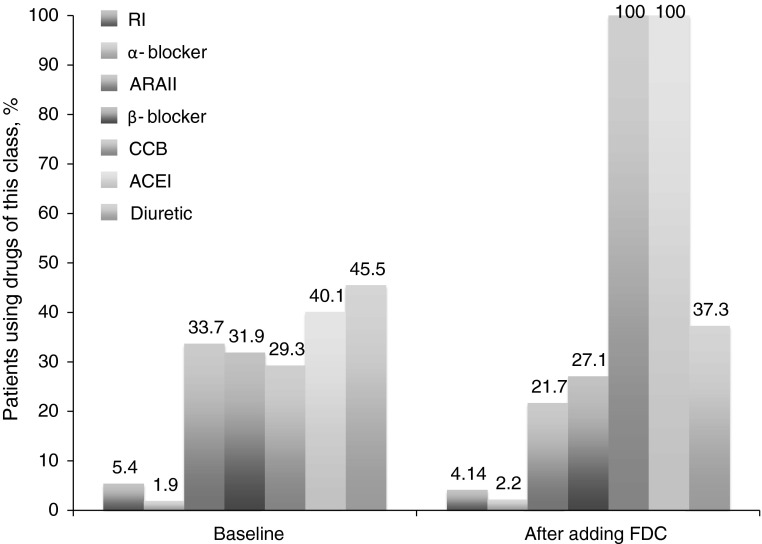
Baseline characteristics are presented in Table 1. The mean age was 64.84 ± 12.18 years (range 35–93), and the mean time since the diagnosis of hypertension was 12.28 ± 13.54 years. Baseline SBP and DBP were 159.11 ± 16.93 and 88.32 ± 12.35 mmHg, respectively. BP was controlled (<140/90 mmHg) in 10.2 % of patients. Antihypertensive treatments at baseline are shown in Table 1. The mean number of antihypertensive drugs per patient at baseline was 2.1 ± 1.3. The most commonly used antihypertensive classes were diuretics (45.5 % of patients), ACEIs (40.1 %), angiotensin II receptor antagonists (33.7 %), β-blockers (31.9 %), and CCBs (29.3 %). Free combinations were used in 32.2 % of the patients and FDCs in 33.4 %.
Table 1.
Baseline clinical and therapeutic profile of the study population
| Total (n = 315) | Females (n = 186) | Males (n = 129) | p value | |
|---|---|---|---|---|
| Age, years | 64.84 ± 12.18 | 65.27 ± 11.82 | 64.22 ± 12.75 | 0.48 |
| SBP, mmHg | 159.11 ± 16.93 | 159.64 ± 16.57 | 161.18 ± 16.94 | 0.45 |
| DBP, mmHg | 88.32 ± 12.35 | 88.23 ± 11.79 | 90.19 ± 11.58 | 0.17 |
| BP <140/90 mmHg | 10.2 | 7.9 | 7.0 | 0.82 |
| α-blocker | 1.9 | 2.1 | 1.6 | 0.52 |
| ARAII | 33.7 | 35.4 | 27.1 | 0.06 |
| β-blocker | 31.9 | 30.8 | 32.9 | 0.38 |
| CCB | 29.3 | 30.9 | 28.7 | 0.42 |
| ACEI | 40.1 | 42.1 | 39.7 | 0.50 |
| Diuretic | 45.5 | 49.4 | 31.8 | 0.01 |
| Renin inhibitor | 5.4 | 5.9 | 4.6 | 0.40 |
| Free combination | 32.2 | 34.6 | 20.2 | 0.23 |
| Fixed-dose combination | 33.4 | 34.5 | 25.6 | 0.05 |
| Number of antihypertensive drugs | 2.1 ± 1.3 | 2.09 ± 1.24 | 1.71 ± 1.26 | 0.06 |
All values are mean ± SD or % of patients, unless otherwise stated
ACEI angiotensin-converting enzyme inhibitor, ARAII angiotensin II receptor antagonist, BP blood pressure, CCB calcium-channel blocker, DBP diastolic blood pressure, pts patients, SBP systolic blood pressure, SD standard deviation
Blood Pressure (BP) Reduction and Control Rates
BP was measured at a mean of 2.88 ± 1.75 months after initiating treatment with lercanidipine/enalapril. Mean changes from baseline for SBP and DBP were −18.08 ± 15.91 and −10.10 ± 11.46 mmHg (Fig. 1; Table 2; p < 0.0001 for both). This corresponded to mean reductions in SBP and DBP of 11.4 and 11.3 %, respectively, compared with baseline. The BP control rate significantly increased from 10.2 % at baseline to 51.0 % after treatment with lercanidipine/enalapril (p < 0.001) (Fig. 2). SBP was reduced from baseline, independently of sex and age (Fig. 1), while DBP was reduced independently of sex; patients aged <60 years had a significantly greater reduction from baseline in DBP than patients aged ≥60 years (p = 0.001; Fig. 1). BP control rates in the analysis by age were similar to those of the overall population; control rates before and after treatment in patients aged <60 years were 4.3 and 51.1 %, while those in patients aged ≥61 years were 8.7 and 50 %.
Table 2.
Blood pressure levels before and after adding lercanidipine/enalapril fixed-dose combination
| Baseline | After adding FDC | Mean difference (95 % CI) | p value | |
|---|---|---|---|---|
| Mean SBP, mmHg | 159.11 ± 16.93 | 141.04 ± 14.60 | −18.08 ± 15.91 (−19.84, −16.31) | <0.0001 |
| Mean DBP, mmHg | 88.32 ± 12.35 | 78.22 ± 11.86 | −10.10 ± 11.46 (−11.37, −8.83) | <0.0001 |
All values are mean ± SD unless otherwise stated
CI confidence interval, DBP diastolic blood pressure, FDC fixed-dose combination, SBP systolic blood pressure, SD standard deviation
Fig. 2.
Blood pressure control rate (a) before (baseline) and (b) after adding lercanidipine/enalapril 10/20 mg fixed-dose combination
Fig. 1.
Blood pressure reduction after adding lercanidipine/enalapril 10/20 mg fixed-dose combination; overall population, and stratified according to sex and age. *p = 0.001 versus DBP reduction in patients aged ≥60 years. BP blood pressure, DBP diastolic blood pressure, SBP systolic blood pressure
This effect was observed irrespective of whether or not patients were receiving concomitant antihypertensive treatment; however, the magnitude of the BP reduction observed was greater in patients receiving lercanidipine/enalapril alone compared with patients receiving the FDC with other antihypertensive drugs (Table 3). These differences may arise from the fact that patients who received the FDC alone had higher baseline BP and lower baseline BP control rates (despite the fact that all patients who received FDC alone were not antihypertensive treatment naïve) than those who received the FDC with other antihypertensive drugs (1.9 vs. 11.8 %, respectively; p = 0.033). By ~2 months of treatment with lercanidipine/enalapril, the BP levels were similar between patients receiving the FDC alone and patients receiving the FDC with other antihypertensive drugs (141.16 ± 15.06 vs. 140.38 ± 12.10 for SBP; 78.03 ± 12.45 vs. 79.15 ± 8.31 for DBP), as were the control rates (51.5 and 48.1 %).
Table 3.
Change in blood pressure levels in patients who received lercanidipine/enalapril fixed-dose combination alone and those who received the lercanidipine/enalapril in combination with other antihypertensive drugs
| Change from baseline | Lercanidipine/enalapril alone (n = 52) | Lercanidipine/enalapril + antihypertensives (n = 262) | p value |
|---|---|---|---|
| Mean SBP, mmHg | −28.52 ± 15.00 | −16.00 ± 15.28 | <0.0001 |
| Mean DBP, mmHg | −9.36 ± 11.89 | −13.79 ± 8.05 | 0.01 |
All values are mean ± SD unless otherwise stated
DBP diastolic blood pressure, SBP systolic blood pressure
The magnitude of the BP response was slightly greater in patients not previously treated with ACEIs and/or CCBs, as expected, although BP significantly reduced in both conditions (Table 4). Baseline and post-lercanidipine/enalapril BP levels were similar in both cases.
Table 4.
Change in blood pressure levels with lercanidipine/enalapril fixed-dose combination treatment in patients who were receiving angiotensin-converting enzyme inhibitor and/or calcium-channel blocker treatment at baseline compared with patients who were not
| Change from baseline with lercanidipine/enalapril treatment | Previous ACEI and/or CCB | No previous ACEI/CCB | p value |
|---|---|---|---|
| Mean SBP, mmHg | −16.33 ± 15.73 | −20.11 ± 15.93 | 0.036 |
| Mean DBP, mmHg | −8.41 ± 10.73 | −12.06 ± 11.99 | 0.005 |
All values are mean ± SD unless otherwise stated
ACEI angiotensin-converting enzyme inhibitor, CCB calcium-channel blocker, DBP diastolic blood pressure, SBP systolic blood pressure, SD standard deviation
Finally, there were no significant differences between the number of concomitant drugs received between the age groups, although a trend for a lower number was seen in the younger group (1.7 vs. 2.0, p = not significant).
Therapeutic Profile
The use of most other classes of antihypertensive medication decreased slightly from baseline after starting treatment with lercanidipine/enalapril; only the proportion of patients receiving an α-blocker (2.2 %) was higher than at baseline (Fig. 3). All patients were given lercanidipine/enalapril, and 23.3 % were taking a free combination regimen; none of the patients received an FDC other than lercanidipine/enalapril. No patients switched to lercanidipine + enalapril as a free combination. The mean number of antihypertensive drugs per patient increased to 2.8 ± 0.9 at a mean of 2.88 months after addition of lercanidipine/enalapril, although the difference from baseline was not statistically significant (p = 0.321).
Fig. 3.
Therapeutic profile before (baseline) and after adding lercanidipine/enalapril 10/20 mg fixed-dose combination. ACEI angiotensin-converting enzyme inhibitor, ARAII angiotensin II receptor antagonist, CCB calcium channel blocker, FDC fixed-dose combination, RI renin inhibitor
Tolerability
Treatment with lercanidipine/enalapril was well tolerated. Treatment-emergent adverse effects occurred in only one patient (0.3 %), who developed a persistent dry cough after the initiation of lercanidipine/enalapril treatment. This cough was considered to be possibly related to treatment with enalapril. None of the patients developed edema.
Discussion
This observational registry study showed that treatment with a lercanidipine/enalapril FDC was associated with significant reductions in SBP and DBP and a significant increase in the proportion of patients achieving BP control compared with baseline.
The reduction in BP observed in our study was as expected with combinations of two or more antihypertensive drugs. A meta-analysis by Law et al. [11] found that the use of two antihypertensive drugs at half-standard doses produced reductions in SBP and DBP of 13.3 and 7.3 mmHg, respectively; corresponding values for three drugs at half-standard doses were 19.9 and 10.7 mmHg, respectively11. Our results are also in agreement with the well known efficacy of an FDC of a CCB with a modulator of the RAS [20], even if we consider the relatively old population evaluated, and the extended period of treatment between diagnosis and inclusion in this study. In this context, the rate of BP control was also impressive, being observed in 51 % of patients with BP <140/90 mmHg after a mean of 2.88 months of treatment with the fixed-dose regimen.
In randomized, controlled phase III trials of lercanidipine/enalapril FDC, reductions in SBP and DBP of 7.7–9.8 and 7.1–9.2 mmHg, respectively, were observed after 12 weeks of treatment [21]. The reductions in SBP and DBP observed in our study were greater than this (18.08 and 10.10 mmHg, respectively). In these two studies, the proportion of patients with normalized SBP and DBP was 22–24 % [21]. It should be noted that these studies included only patients who had not achieved BP control with either lercanidipine or enalapril as monotherapy, and this could have contributed to the smaller reductions in BP and lower BP control rates compared with our study. Furthermore, one of these studies used a lower dose of enalapril (10 mg) than in our study and produced smaller reductions in SBP and DBP than seen with lercanidipine/enalapril 10/20 mg in the second study. It should also be noted that the patients included in our registry had been receiving antihypertensive regimens prescribed by general practitioners rather than specialists. It is therefore possible that even where their initial therapy had shown BP-lowering activity it may have been suboptimal, and thus further reduction in BP could be obtained by switching to a more suitable therapy, in this case the lercanidipine/enalapril FDC.
The population of our registry was relatively old (mean age approximately 65 years). The age of the study population may have meant that there was a higher proportion of patients with isolated systolic hypertension (ISH) than would have been seen for a study with a younger population. However, baseline BP measurements were averaged, so it was not possible to determine the proportion of patients with ISH. Patients with ISH have marked arterial stiffening, which makes BP control more difficult. In light of the possibility that a significant proportion of patients in our study could have had ISH, the BP-lowering and BP control rates observed are even more impressive. Our results are comparable to those seen in a study in elderly patients (age 60–85 years), in which treatment with the combination of lercanidipine 10 mg plus enalapril 20 mg for 4 weeks was associated with a reduction in SBP of 16.9 mmHg compared with baseline, and a BP control rate of 45 % [20].
In this study, the BP-reducing effect of lercanidipine/enalapril was greater in patients receiving lercanidipine/enalapril alone compared with patients receiving the FDC with other antihypertensive drugs. However, at the end of the study period, the mean BP values and BP control rates in both patient groups were similar. This can best be explained by the fact that the magnitude of the therapeutic benefit is generally correlated with baseline BP values [22]. As the patients who received lercanidipine/enalapril alone had significantly greater baseline BP values and lower BP control rates than those who received lercanidipine/enalapril with other antihypertensive drugs, the greater magnitude of improvement at the end of the study in patients who received lercanidipine/enalapril alone was expected.
The introduction of this FDC, in addition to the noted efficacy, did not significantly increase the number of drugs required to achieve BP control. These results may be particularly interesting from an economic perspective, as a reduction in the number of concomitant medications has the potential to produce cost savings, particularly for a high-prevalence disease such as hypertension.
The primary limitation of this study was that it was an open-label pharmaco-epidemiological registry, with all the inherent limitations and advantages of such a design. Other limitations were the relatively small number of patients and the short follow-up duration. The size of the study was necessarily limited by the number of patients presenting to CCG members’ clinics during the study period for whom the lercanidipine/enalapril (10/20 mg) FDC was considered the most appropriate treatment.
Finally, the extremely low incidence of adverse effects noted after initiating treatment with the lercanidipine/enalapril FDC was especially interesting. Despite the excellent tolerability attributed to the new dihydropyridines, namely with respect to the incidence of ankle edema [23, 24], it may be surprising that none of the patients developed edema with lercanidipine in this study. However, the combination of a CCB with a modulator of the RAS has been shown to reduce the incidence of such events, through a well established mechanism [21, 25]. Only a single case of cough was reported in our study, and this was considered to be possibly related to enalapril as cough is a known adverse effect of ACEIs [26]. Cough was the most common adverse event observed in clinical trials of lercanidipine/enalapril FDC [21]. The incidence of peripheral edema with the FDC also appears to be low, with only 1.5 % of patients treated with lercanidipine/enalapril 10/20 mg for up to 52 weeks in clinical trials experiencing this adverse event [21].
Conclusion
Treatment with an FDC of lercanidipine/enalapril (10/20 mg) for a mean of 2.88 months was associated with a significant reduction of SBP and DBP and an increase in the BP control rate from 10.2 to 51.0 %, relative to baseline, a result achieved with a reduction in the number of drugs used. The lercanidipine/enalapril FDC was shown to effectively reduce BP, generally independently of age and sex, and with an excellent safety profile.
Acknowledgments
This registry was funded by an operational grant from Jaba Recordati S.A., Portugal.
Medical writing assistance was provided by Raewyn Poole, on behalf of inScience Communications, Springer Healthcare. This assistance was funded by Jaba Recordati S.A., Portugal.
Authors’ conflict of interests
João Maldonado declares that he has no conflict of interest. Telmo Pereira declares that he has no conflict of interest. Alfredo Tavares is an employee of Jaba Recordati S.A.
Appendix: Participants in the CONCEPT Collaborative Group
This registry is the result of the commitment and dedication of a group of 46 specialists with a particular interest in cardiovascular diseases, listed below. Paula Gago, Idalécio Bernardo, Pedro Miguel Balza, Sanjiva Cadocar, Nuno Jorge Fonseca, Filipe Seixo, Lurdes Almeida, Marco Aurélio Castro, Pedro Silva Cunha, Hugo Filipe Pego, Fátima Veiga, Luís Filipe Pereira, Susana Castela, Carvalho Rodrigues, João Maria Abecassis, Susana Martins, Graça Almeida, Omar Zalueta Pereira, Paulo Ramos, João Madeira Lopes, Sílvio Leal, Carlos Aguiar, Pedro Von Haffe, Maria José Ferreira, Cristina Rodrigues, Isabel Maria Vilaça, Emília Barbosa, Abílio Ribeiro, Gonçalo Rocha, Sérgio Miguel Silva, Manuel Pinto Monteiro, Fernando Santos Reis, José Bernardes Correia, João Porto, Ana Sofia Teixeira, Rui Providência, José Alexandre Antunes, Rui Pires, António Antunes, Leonel Pinto, João Miguel Santos, João Maldonado, André Paupério, Meireles Brandão, Mário Almeida, Pedro Semedo.
Footnotes
On behalf of the CONCEPT Collaborative Group.
The participants in the group are given in the Appendix.
References
- 1.World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. 2009. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. Accessed 31 May 2013.
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 7.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22:11–19. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 9.Gradman AH, Basile JN, Carter BL, Bakris GL, Materson BJ, Black HR, et al. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4:90–98. doi: 10.1016/j.jash.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Dusing R. Optimizing blood pressure control through the use of fixed combinations. Vasc Health Risk Manag. 2010;6:321–325. doi: 10.2147/VHRM.S9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- 13.Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–719. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 15.Coca A. Evolucion del control de la hipertension arterial en atencion primaria en Espana. Resultados del estudio Controlpress 2003. Hipertension. 2005;22:5–14. doi: 10.1016/S0212-8241(05)74809-X. [DOI] [Google Scholar]
- 16.Sociedade Portuguesa de Hipertensao Prevalencia da hipertensao arterial e consumo de sal em Portugal. Rev Port Hipertensao e Risco Cardiovascular. 2013;34:8–9. [Google Scholar]
- 17.Bakris G, Molitch M, Hewkin A, Kipnes M, Sarafidis P, Fakouhi K, et al. Differences in glucose tolerance between fixed-dose antihypertensive drug combinations in people with metabolic syndrome. Diabetes Care. 2006;29:2592–2597. doi: 10.2337/dc06-1373. [DOI] [PubMed] [Google Scholar]
- 18.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 19.Matsui Y, Eguchi K, O’Rourke MF, Ishikawa J, Miyashita H, Shimada K, et al. Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension. 2009;54:716–723. doi: 10.1161/HYPERTENSIONAHA.109.131466. [DOI] [PubMed] [Google Scholar]
- 20.Puig JG, Calvo C, Luurila O, Luurila H, Sulosaari S, Strandberg A, et al. Lercanidipine, enalapril and their combination in the treatment of elderly hypertensive patients: placebo-controlled, randomized, crossover study with four ABPM. J Hum Hypertens. 2007;21:917–924. doi: 10.1038/sj.jhh.1002248. [DOI] [PubMed] [Google Scholar]
- 21.Hair PI, Scott LJ, Perry CM. Fixed-dose combination lercanidipine/enalapril. Drugs. 2007;67:95–106. doi: 10.2165/00003495-200767010-00007. [DOI] [PubMed] [Google Scholar]
- 22.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 23.Makani H, Bangalore S, Romero J, Htyte N, Berrios RS, Makwana H, et al. Peripheral edema associated with calcium channel blockers: incidence and withdrawal rate–a meta-analysis of randomized trials. J Hypertens. 2011;29:1270–1280. doi: 10.1097/HJH.0b013e3283472643. [DOI] [PubMed] [Google Scholar]
- 24.Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med. 2011;124:128–135. doi: 10.1016/j.amjmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Messerli FH, Oparil S, Feng Z. Comparison of efficacy and side effects of combination therapy of angiotensin-converting enzyme inhibitor (benazepril) with calcium antagonist (either nifedipine or amlodipine) versus high-dose calcium antagonist monotherapy for systemic hypertension. Am J Cardiol. 2000;86:1182–1187. doi: 10.1016/S0002-9149(00)01199-1. [DOI] [PubMed] [Google Scholar]
- 26.Izzo JL, Jr, Weir MR. Angiotensin-converting enzyme inhibitors. J Clin Hypertens. 2011;13:667–675. doi: 10.1111/j.1751-7176.2011.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]