Abstract
Aims
Dabigatran is largely cleared by renal excretion. Renal function is thus a major determinant of trough dabigatran concentrations, which correlate with the risk of thromboembolic and haemorrhagic outcomes. Current dabigatran dosing guidelines use the Cockcroft–Gault (CG) equation to gauge renal function, instead of contemporary equations including the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations employing creatinine (CKD-EPI_Cr), cystatin C (CKD-EPI_Cys) and both renal biomarkers (CKD-EPI_CrCys).
Methods
A linear regression model including the dabigatran etexilate maintenance dose rate, relevant interacting drugs and genetic polymorphisms (including CES1), was used to analyse the relationship between the values from each renal function equation and trough steady-state plasma dabigatran concentrations.
Results
The median dose-corrected trough steady-state plasma dabigatran concentration in 52 patients (38–94 years) taking dabigatran etexilate was 60 µg/L (range 9–279). The dose-corrected trough concentration in a patient on phenytoin and phenobarbitone was >3 standard deviations below the cohort mean. The CG, CKD-EPI_Cr, CKD-EPI_Cys and CKD-EPI_CrCys equations explained (R 2, 95 % CI) 32 % (9–55), 37 % (12–60), 41 % (16–64) and 47 % (20–69) of the variability in dabigatran concentrations between patients, respectively. One-way analysis of variance (ANOVA) comparing the R 2 values for each equation was not statistically significant (p = 0.74).
Discussion
Estimates of renal function using the four equations accounted for 32–47 % of the variability in dabigatran concentrations between patients. We are the first to provide evidence that co-administration of phenytoin/phenobarbitone with dabigatran etexilate is associated with significantly reduced dabigatran exposure.
Electronic supplementary material
The online version of this article (doi:10.1007/s40268-014-0045-9) contains supplementary material, which is available to authorized users.
Key Points
| Estimated GFR using the Cockcroft–Gault equation, and modern creatinine- and cystatin C-based equations, was found to explain 32–47 % of the variability in trough steady-state dabigatran plasma concentrations between patients. |
| We are the first to show that co-administration of dabigatran etexilate with phenytoin and/or phenobarbitone is associated with markedly reduced dabigatran exposure. |
Introduction
Dabigatran, a thrombin inhibitor, is an oral anticoagulant that is used especially for thromboprophylaxis in the setting of atrial fibrillation (AF) [1–3]. It is administered orally as the prodrug dabigatran etexilate. Higher plasma dabigatran concentrations have been shown to be associated with a decreased risk of thromboembolism and an increased risk of haemorrhage [4]. There are several factors that may determine differences in dabigatran concentrations between individuals (Table 1) [5–14]. For example, the oral availability of dabigatran etexilate is affected by stomach pH, and consequently, drugs that increase gastric pH (e.g., proton-pump inhibitors) have been found to reduce the dabigatran concentrations [11, 12]. Dabigatran etexilate is also a substrate for the efflux transporter P-glycoprotein (P-gp) in the intestinal wall [10]. Drugs that alter P-gp function (e.g., amiodarone), and genetic polymorphisms in the ABCB1 gene, which encodes P-gp, are associated with altered oral availability [5, 13]. Following entry into the circulation, hepatic carboxylesterase-1 (CES1) is responsible for the metabolism of dabigatran etexilate to dabigatran, via two parallel intermediate metabolites, BIBR 951 and BIBR 1087 [13]. Genetic polymorphisms in the CES1 gene have been found to alter dabigatran concentrations [13].
Table 1.
Covariates of dabigatran plasma concentrations
| Covariate | Mean exposure ratio (90 % CI)a |
|---|---|
| Proton-pump inhibitor [12] | 0.80 (0.67–0.95) |
| Intestinal P-gp function | |
| Ketoconazole [5] | 2.50 (NA) |
| Dronedarone [6] | 1.99 (1.79–2.21) |
| Verapamil [8] | 1.71 (1.34–2.15) |
| Amiodarone [5] | 1.60 (NA) |
| Quinidine [5] | 1.50 (NA) |
| Clarithromycin [9] | 1.49 (NA) |
| Ticagrelor [59] | 1.46 (NA) |
| Clopidogrel, loading doseb [7] | 1.35 (1.07–1.69) |
| rs4148738 [13] | 1.12 (1.08–1.17) |
| rs1045642 [14] | 1.08 (NA) |
| Rifampicin [10] | 0.33 (0.27–0.41) |
| Carbamazepine, phenytoin, phenobarbitone [10] | NAc |
| Hepatic CES1 function | |
| rs2244613 [13] | 0.85 (0.81–0.90) |
| rs4122238 [13] | 0.86 (0.81–0.91) |
| rs8192935 [13] | 0.89 (0.85–0.93) |
| Renal impairment [16] | |
| Mild | 1.50 (0.78–2.90) |
| Moderate | 3.15 (1.63–6.08) |
| Severe | 6.31 (3.54–11.25) |
AUC 0–∞ area under the concentration-time curve from zero to infinity, CES1 carboxylesterase-1, NA not available, P-gp P-glycoprotein
aThis represents the mean ratio of the AUC0–∞ of individuals with the covariate to healthy controls without the covariate, or, for genetic polymorphisms, the mean ratio (95 % CI) of either peak (P-gp) or trough (CES1) concentrations of single allele carriers to wildtype
bSteady-state dosing of clopidogrel has not been shown to significantly alter dabigatran AUC0–∞ [7]
cMay be associated with decreased dabigatran AUC0–∞ [10]
As dabigatran is mainly cleared by the kidneys (fraction excreted unchanged in urine of 0.8), renal function is a major determinant of dabigatran concentrations [15, 16]. Glucuronidation is responsible for the remaining 20 % of dabigatran clearance [15, 17]. The dabigatran glucuronides are equipotent to dabigatran against thrombin, and appear to be primarily renally cleared [15, 17]. Hence, it has been recommended that maintenance dose rates of dabigatran etexilate should be adjusted to take renal function into account [5, 18].
The standard representation of renal function is the glomerular filtration rate (GFR) [19, 20]. The gold standard methods for determining GFR are based on the clearance of renally eliminated exogenous compounds [21]. However, as these are inconvenient for routine clinical use, several equations for estimating GFR based on the measurement of endogenous compounds are currently recommended [19, 20]. The Cockcroft–Gault (CG) equation [22], which uses the endogenous renal biomarker, creatinine, has been used for many years to gauge renal function in relation to drug dosing [23]. More recently, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation [24] was developed using creatinine assays standardised against the isotope dilution mass spectrometry (IDMS) method, and has become one of the most commonly used GFR equations [25, 26].
Cystatin C is an alternative renal function biomarker that has received considerable attention [27]. Whereas creatinine assay standardisation was introduced in 2006, the first certified reference material (ERM-DA471/IFCC) for standardising cystatin C assays has only been available since 2010 [28]. Hence, while a multitude of cystatin C-based GFR equations have been developed over the years [29], only a few have employed assays that are traceable to ERM-DA471/IFCC [30, 31]. These include the CKD-EPI equations that feature cystatin C [30].
All GFR equations are expected to explain some of the variance in dabigatran concentrations. However, to our knowledge, the abilities of the existing renal function equations to describe differences in dabigatran concentrations have not been assessed and compared [32]. The potential finding that one of the CKD-EPI equations is superior to the CG equation could lead to changes to the current guidelines, which currently stipulate that the CG equation is used to guide dabigatran etexilate dosing [5]. Further, the impact of the different GFR equations on the dose selection of dabigatran etexilate has not been examined. The aims of the current study were to evaluate the correlation of trough concentrations of dabigatran at steady-state with four contemporary renal function equations, and to simulate the differences in dosing resulting from the use of these equations (Table 2).
Table 2.
GFR equations
| Equation (units) | Description |
|---|---|
| CG (mL/min) | |
| CKD-EPI_Cr a (mL/min per 1.73 m2) | |
| CKD-EPI_Cys (mL/min per 1.73 m2) | |
| CKD-EPI_CrCysb (mL/min per 1.73 m2) |
CG Cockcroft–Gault equation, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration equation, Cr creatinine, Cys cystatin C, GFR glomerular filtration rate, TBW total body weight
a α is 0.7 for females and 0.9 for males, β is −0.329 for females and −0.411 for males
bWhere k is 0.7 for females and 0.9 for males, α is −0.248 for females and −0.207 for males
Methods
Study Design
This observational study was carried out in Christchurch, New Zealand, between July 2012 and May 2013. The Upper South B Regional Ethics Committee, New Zealand provided ethical approval for this study (URB/12/02/009 and URB/12/02/009 AM01). Each participant in the study provided written consent.
Participants
Patients treated with dabigatran etexilate for non-valvular AF and aged ≥18 years were included if they had been on the same dose rate for at least 7 days and had not missed any doses in the 7 days prior to the study day (self-reported). Patients were assumed to have steady-state dabigatran concentrations after dosing for 7 days as the half-life of dabigatran has been reported to range from 14–28 hours for patients with a GFR of 21–110 mL/min [16]. Patients were excluded if, on the study day, they required hospitalisation for an acute illness. Patients were otherwise eligible if they were outpatients in the community, electively admitted for diagnostic tests or were inpatients for physical rehabilitation. Age, sex, weight, height, dabigatran etexilate dose rates, co-prescribed medications and comorbidities were recorded. Using these data, we calculated each individual’s CHA2DS2-VASc (1 point for each of Congestive heart failure, Hypertension, Diabetes mellitus, Vascular disease, Age 65–74 years, Female sex, 2 points for each of Age ≥75 years, Previous stroke) and HAS-BLED (1 point for each of Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly) scores, which estimate thromboembolic and haemorrhagic risks, respectively [33, 34].
GFR was estimated for each individual using the four equations listed in Table 2. The results from the various CKD-EPI equations were converted from units of mL/min per 1.73 m2 to mL/min according to Eq. 1:
| 1 |
where the body surface area of the individual (BSA) was calculated using Mosteller’s equation [35–39].
Sample Collection and Laboratory Analysis
Each patient provided a set of venous blood samples 10–16 hours post-dose for measuring plasma creatinine and cystatin C concentrations, plasma free thyroxine and thyroid-stimulating hormone (TSH) concentrations (BD Vacutainer® lithium heparin tubes); Hemoclot® Thrombin Inhibitor times (HTI, Hyphen BioMed, Neuville-sur-Oise, France) (BD Vacutainer® citrate tubes); plasma dabigatran concentrations (BD Vacutainer® K2 ethylene diamine tetraacetic acid [EDTA] tubes). Blood cells from the EDTA tubes were used for genotyping. Serum creatinine and cystatin C concentrations were only measured at a single point in time for each participant, as intra-individual variance (coefficient of variation, CV) of these biomarker concentrations has been reported to be around 7 % in clinically stable individuals [40].
Serum creatinine was measured using an Abbott® Aeroset analyser (Abbott Park, IL, USA) by the modified Jaffe reaction. This was IDMS-aligned for the period of this study and had an inter-day CV of <4.0 %. Serum cystatin C was measured using a particle-enhanced nephelometric immunoassay on a Behring Nephelometer II analyser (Siemens Diagnostics, Marburg, Germany), with a CV <4.5 % [41]. The use of a contemporary Siemens assay for cystatin C is consistent with the recommendations by Shlipak et al. [42]. Siemens calibrators standardised to the international certified cystatin C reference material (ERM-DA471/IFCC) were employed [28]. Serum free thyroxine (CV <5.8 %) and TSH (CV <6.4 %) were measured using an Abbott® Architect analyser (Abbott Park, IL, USA) by a chemiluminescent microparticle immunoassay (CMIA). The HTI assay was performed on an ACL TOP 700 instrument (Instrumentation Laboratory, Bedford, MA, USA) and had an inter-day CV of <11 %.
Plasma Dabigatran Assay
Plasma dabigatran concentrations were measured using a validated liquid chromatography–mass spectrometry (LC–MS/MS) method, based on a previously published method [43]. Briefly, 50 µL plasma was added to 450 µL of internal standard. Internal standard consisted of 10 µg/L of [13C6]-dabigatran in methanol and 0.1 mmol/L aqueous HCl (9:1, v/v). This was vortexed and then centrifuged at 15,000 g for 5 minutes for protein precipitation. A 50 µL aliquot of clear supernatant was added to 500 µL of water, and transferred to an autosampler vial. A 10 µL volume was injected into the LC–MS system (Agilent 1290 Infinity Series High Performance Liquid Chromatograph connected to an Agilent 6460 Series Triple Quadrupole Mass Spectrometer, Agilent Technologies, Santa Clara, CA, USA). For the range of 5–1,000 µg/L, the intra- and inter-day precision (CV) values were ≤11.8 % and bias was ≤8.3 %.
ABCB1 and CES1 Genotyping
DNA was collected from white blood cells using guanidine isothiocyanate extraction [44]. Genotyping for ABCB1 single nucleotide polymorphisms (SNPs) rs1045642, rs1128503 and rs4148738 was performed using the pre-designed SNP TaqMan® assays C_7586657_20, C_7586662_10 and C_1253813_10, respectively. ABCB1 rs2032582 is a tri-allelic SNP, and therefore separate pre-designed assays, C_11711720D_40 and C_11711720C_30, were needed in order to identify the two minor alleles ABCB1 2677A and ABCB1 2677T. Results of each ABCB1 rs2032582 assay were analysed separately and then combined to determine the overall minor allele frequency for this SNP. Genotyping for CES1 SNPs rs8192935, rs2244613 and rs412223 was performed using custom-designed SNP TaqMan® assays. All genotyping assays were sourced from Applied Biosystems (Applied Biosystems, Carlsbad, CA, USA). Each reaction was performed in a total volume of 5 µL following the recommendations of the manufacturer and run on a Roche LightCycler® 480 Real-Time PCR System (Roche Diagnostics Corporation, IN, USA) in 384-well format. Briefly, the thermal cycling conditions comprised an activation step of 10 minutes at 95 °C, followed by 40 cycles of denaturation (15 s at 92 °C) and annealing/extension (1 min at 63 °C). Genotypes were assigned using endpoint genotyping analysis software (Roche Diagnostics Corporation, IN, USA). The accuracy of the TaqMan® assays was confirmed by repeat analysis of 10 % of samples. Concordance between original and repeat genotype calls was 100 % for the two assays. PLINK software was used to test for deviations in Hardy–Weinberg Equilibrium (HWE) [45].
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (version 6.03, GraphPad Software, La Jolla, CA, USA) and SPSS (IBM SPSS Statistics for Windows, version 20.0.0.2, IBM Corporation, Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
Correlation Between Glomerular Filtration Rate (GFR) Equations and Dabigatran Concentrations
The primary aim of the correlation analysis was to assess the correlations of the estimated GFR values with dabigatran concentrations normalised for all other known covariates. This analysis was conducted in two stages, as follows.
Dose-corrected trough plasma dabigatran concentrations (dabigatrantrough, with units of µg/L per mg/day) were regressed against non-renal clinical factors (covariates) known to alter dabigatran exposure (Table 1), as well as the time period between the last dose of dabigatran etexilate and the trough sample. Other than the time period, which was treated as a continuous variable, all of the non-renal covariates were treated as nominal variables. The dabigatrantrough values were log-transformed, and were tested for normality using the D’Agostino–Pearson omnibus test (with p > 0.05 indicating that the data passed the normality test). If these data were judged to be normally distributed, the log-transformed dabigatrantrough values were then converted to z-scores (standardised values). Covariates were entered simultaneously into a multiple linear regression model based on biological plausibility rather than statistical criteria. These covariates included those that have been found in the literature to significantly correlate with either dabigatran area under the concentration–time curve (AUC) or trough plasma concentrations. Using this model, standardised residuals were generated for each individual.
The estimates of GFR (in units of mL/min) from each of the four equations were standardised (z-scores) and then correlated (R 2), in turn, with the standardised residuals from the regression model described above. The R 2 values from each of the four renal function equations were compared on the basis of the 95 % CI of each R 2 value. Further, the unstandardised residuals, from the correlation between each renal function equation and the standardised residuals of the multiple linear regression model, were compared using repeated measures one-way analysis of variance (ANOVA). Finally, the equation with the highest R 2 was included in the multiple linear regression model, and the R 2 of this model for the z-scores of the log-transformed dabigatrantrough calculated.
These analyses were repeated after excluding patients on corticosteroids and/or with abnormal thyroid function tests. Corticosteroid therapy and abnormal thyroid function tests have been demonstrated to substantially affect plasma cystatin C concentrations [46], and therefore would be expected to impact on cystatin C-based renal function equations.
As we did not measure the renally cleared active metabolites of dabigatran, the dabigatran glucuronides, we correlated the HTI times with the measured dabigatrantrough. The HTI assay is a metric for assessing the total concentration of all thrombin inhibitors, comprising dabigatran and its glucuronides, present in the plasma sample [47]. A high R 2 suggests that measured plasma dabigatran concentrations reflect the concentrations of all thrombin inhibitors.
As we were not aware of any previous comparison between the correlations of estimated GFR from renal function equations with measured dabigatran concentrations, the data in the literature were considered to be inadequate to inform an a priori power analysis to calculate sample size.
Comparison of Simulated Dabigatran Etexilate Dosing Recommendations According to GFR Equations
Dosing recommendations for dabigatran etexilate in relation to renal function are available from the manufacturer [48]. For thromboprophylaxis in the setting of non-valvular AF, these guidelines recommend dose rates of 150 mg twice daily and 110 twice daily, for estimated GFR of >50 mL/min and 30–50 mL/min, respectively, with GFR <30 mL/min being a contraindication to dabigatran therapy. These guidelines were used to determine recommended dose rates based on the estimated GFR values from the four equations (Table 2) in the study participants. Each participant, having four estimates of GFR, would thus have four recommended dose rates. The percentage of agreement in recommended dose rates was calculated per pair of GFR equations.
Results
The characteristics of the 52 recruited patients are provided in Table 3. All patients had been on a stable dabigatran etexilate dose rate for at least 10 days. The mean (SD) of the dabigatrantrough values was 0.32 (0.26) µg/L per mg/day. The ABCB1 and CES1 genotype and allele frequencies of the patients are shown in Table 4.
Table 3.
Patient characteristics (n = 52)
| Characteristic | Median (range)a |
|---|---|
| Age, years | 67 (38–94) |
| Male, n (%) | 41 (79) |
| Weight, kg | 95 (56–187) |
| Height, m | 1.75 (1.55–1.93) |
| BMI, kg/m2 | 31.6 (18.4–55.8) |
| BSA, m2 | 2.16 (1.61–3.08) |
| CHA2DS2-VASc | 3 (0–7) |
| HAS-BLED | 1 (0–4) |
| Duration on dabigatran etexilate, weeks | 6.0 (1.5–52.0) |
| Dabigatran etexilate dose rate | |
| 75 mg twice daily, n (%) | 3 (6) |
| 110 mg twice daily, n (%) | 24 (46) |
| 150 mg twice daily, n (%) | 25 (48) |
| GFR equations | |
| CG, mL/min | 90 (41–246) |
| CKD-EPI_Cr, mL/min | 87 (38–168) |
| CKD-EPI_Cys, mL/min | 93 (26–149) |
| CKD-EPI_CrCys, mL/min | 88 (40–142) |
| Proton-pump inhibitor, n (%) | 11 (21) |
| Drugs affecting P-gp functionb | |
| Amiodarone and/or verapamil, n (%) | 9 (17) |
| Phenytoin and phenobarbitone, n (%) | 1 (2) |
| Trough plasma dabigatran concentration, µg/L | 60 (9–279)c |
| Dabigatrantrough, µg/L per mg/day | 0.23 (0.04–1.06) |
CHA2DS2-VASc and HAS-BLED are scoring systems for assessing thromboembolic and haemorrhagic risk, respectively, in the setting of atrial fibrillation [33, 34]. See Table 2 for details of renal function equations
BMI body mass index, BSA body surface area, CG Cockcroft–Gault equation, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration equation, Cr creatinine, Cys cystatin C, GFR glomerular filtration rate, P-gp P-glycoprotein, dabigatran trough dose-corrected trough plasma dabigatran concentration
aUnless stated otherwise
bNo patient was on any of the other drugs listed in Table 1
cSee Supplementary Fig. 1 (ESM) for a histogram of measured concentrations
Table 4.
Comparison of ABCB1 and CES1 genotype and allele frequencies of 52 patients on dabigatran etexilate with Caucasians included in the CEUa dataset
| Gene (SNP) | Allele change | Genotype, n (frequency) | Minor allele | MAF, n (%) | HWE, p value | MAF (CEU), p value | ||
|---|---|---|---|---|---|---|---|---|
| ABCB1 (rs4148738) | T>C |
T/T 13 (0.250) |
C/T 31 (0.596) |
C/C 8 (0.154) |
C | 0.45 | 0.14 | 0.48 |
| ABCB1 (rs1045642) | C>T |
T/T 16 (0.308) |
C/T 26 (0.500) |
C/C 10 (0.192) |
C | 0.44 | 0.92 | 0.43 |
| CES1 (rs2244613) | T>G |
T/T 38 (0.731) |
G/T 12 (0.231) |
G/G 2 (0.038) |
G | 0.15 | 0.41 | 0.15 |
| CES1 (rs4122238) | C>T |
C/C 40 (0.769) |
C/T 12 (0.231) |
T/T 0 |
T | 0.12 | 0.35 | 0.12 |
| CES1 (rs8192935) | A>G |
G/G 27 (0.519) |
A/G 23 (0.442) |
A/A 2 (0.038) |
A | 0.26 | 0.28 | 0.31 |
HWE Hardy–Weinberg equilibrium, MAF minor allele frequency, SNP single nucleotide polymorphism
aUtah residents with ancestry from northern and western Europe (CEU) (http://snp.cshl.org/citinghapmap.html.en)
Correlation Between GFR Equations and Dabigatran Concentrations
The log-transformed dabigatrantrough values were found to be normally distributed (p = 0.98). Of the published non-renal covariates (Table 1), only the concomitant use of the P-gp inducers phenytoin and phenobarbitone explained a significant portion of the variability in dabigatrantrough values between the 52 patients (p = 0.012, Supplementary Table 1, electronic supplementary material [ESM]). Administration of phenytoin and phenobarbitone occurred in a single individual prescribed dabigatran etexilate 110 mg twice daily who had a low trough plasma dabigatran concentration of 9 µg/L (dabigatrantrough = 0.04 µg/L per mg/day, z-score of the log-transformed dabigatrantrough = −3.25). This individual had been electively admitted for sleep studies, and the blood samples were taken on the fourth day of his stay as an inpatient. His hospital prescription chart revealed that dabigatran etexilate was administered to him throughout the admission (total of 6 doses) as per his aforementioned prescribed dose rate. A multiple linear regression model was constructed consisting of this covariate, as well as the presence of concomitant proton-pump inhibitors [11, 12], concomitant P-gp inhibitors (verapamil and amiodarone) [5, 7] and three CES1 SNPs (rs8192935, rs2244613 and rs4122238) [13]. The multiple linear regression model that included these covariates had an unadjusted R 2 of 0.29 for the z-scores of the log-transformed dabigatrantrough.
The R 2 values of the four renal function equations for the standardised residuals of the multiple linear regression model are presented in Table 5. All the 95 % CI of the correlation coefficients overlapped (p = 0.74). Numerically, the highest R 2 value (0.47) was associated with the CKD-EPI_CrCys equation.
Table 5.
Correlation of renal function equations with standardised residuals from the multiple linear regression model for dabigatrantrough (n = 52)a
| Renal function equation | R (95 % CI) | p Value | R 2 (95 % CI) |
|---|---|---|---|
| CG | −0.56 (−0.74 to −0.31) | <0.001 | 0.32 (0.09–0.55) |
| CKD-EPI_Cr | −0.61 (−0.77 to −0.35) | <0.001 | 0.37 (0.12–0.60) |
| CKD-EPI_Cys | −0.64 (−0.80 to −0.40) | <0.001 | 0.41 (0.16–0.64) |
| CKD-EPI_CrCys | −0.69 (−0.83 to −0.45) | <0.001 | 0.47 (0.20–0.69) |
CG Cockcroft–Gault equation, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration equation, Cr creatinine, Cys cystatin C
aMultiple linear regression model for the z-scores of the log-transformed dabigatrantrough, details in Sect. 2.4.1
When the estimates of GFR from this equation were added into the multiple linear regression model, the unadjusted R 2 was 0.69 for the z-scores of the log-transformed dabigatrantrough (Table 6).
Table 6.
Final multiple linear regression model for z-scores of log-transformed dabigatrantrough (n = 52)
| Predictora | B | SE (B) | p Value |
|---|---|---|---|
| Constant | 3.99 | 1.08 | 0.001 |
| CKD-EPI_CrCysb | −0.69 | 0.09 | <0.001 |
| Time between last dose and sample | −0.09 | 0.06 | 0.11 |
| Phenytoin and phenobarbitone | −2.62 | 0.65 | <0.001 |
| Proton-pump inhibitor | −0.55 | 0.22 | 0.017 |
| Amiodarone and/or verapamil | 0.35 | 0.23 | 0.13 |
| rs2244613 | 0.18 | 0.47 | 0.70 |
| rs4122228 | −0.13 | 0.47 | 0.79 |
| rs8192935 | 0.03 | 0.22 | 0.91 |
Unadjusted R 2 = 0.69
B unstandardised coefficients, SE standard error, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, Cr creatinine, Cys cystatin C
aFor all drugs, a value of 1 was assigned to those without the drug, and a value of 2 assigned to those on the drug. A value of 1 was assigned to patients who had a wildtype genotype. Patients who were heterozygous or homozygous for the single nucleotide polymorphism of interest were assigned a value of 2
bThe z-scores of the log-transformed CKD-EPI_CrCys values
No patients were treated with corticosteroids at the time of the study. Four had abnormal thyroid function test results, characterised by plasma TSH concentrations (0.28, 4.19, 5.16, 5.61 mU/L) outside the local reference range (0.40–4.00 mU/L), but with free plasma thyroxine concentrations (19, 11, 14, 14 pmol/L, respectively for the TSH values) that were within the local reference range (10–24 pmol/L). One of these four patients was the patient treated with phenytoin and phenobarbitone. Excluding these patients from the analyses did not significantly change the results (48 patients, Supplementary Tables 2 and 3 [ESM]).
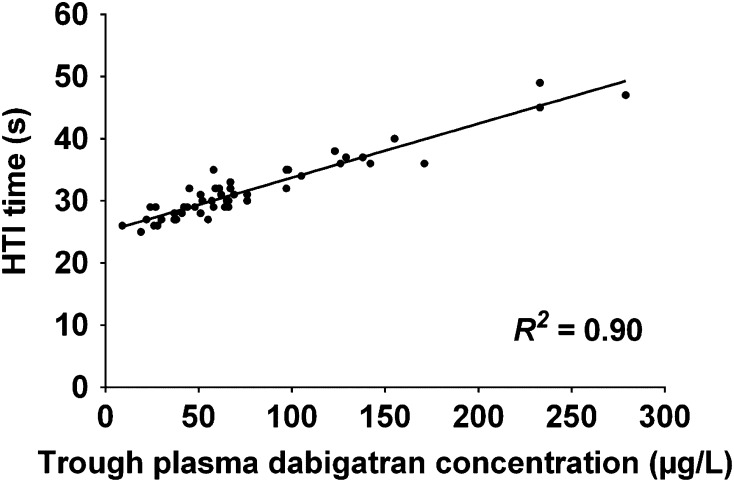
There was a high correlation (R 2 = 0.90) between the plasma dabigatran concentrations and HTI times, as shown in Fig. 1.
Fig. 1.
Correlation plot for Hemoclot® Thrombin Inhibitor (HTI) times against trough plasma dabigatran concentrations (n = 52). R 2 value is for the line of best fit
Comparison of Simulated Dabigatran Etexilate Dosing Recommendations According to GFR Equations
Most of the 52 patients had estimated GFR of >50 mL/min (92–98 %, depending on the GFR equation). The comparisons in dabigatran etexilate dosing recommendations between pairs of equations are detailed in Table 7, and show that there was agreement in 94–98 % of comparisons.
Table 7.
Comparison of dabigatran dosing recommendations between GFR equations (n = 52)
| GFR equation | Estimated GFR (mL/min)a | Agreement in dosing recommendation between GFR equations | |||
|---|---|---|---|---|---|
| 30–50 | >50 | CKD-EPI_Cr | CKD-EPI_Cys | CKD-EPI_CrCys | |
| CG | 3 (6) | 49 (94) | 50 (96) | 49 (94) | 50 (96) |
| CKD-EPI_Cr | 1 (2) | 51 (98) | 49 (94) | 50 (96) | |
| CKD-EPI_Cys | 4 (8) | 48 (92) | 51 (98) | ||
| CKD-EPI_CrCys | 3 (6) | 49 (94) | |||
See Table 2 for details of GFR equations. All results are in n (%). Empty cells represent redundant comparisons
CG Cockcroft–Gault equation, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, Cr creatinine, Cys cystatin C, GFR glomerular filtration rate
aNo patient had an estimated GFR of <30 mL/min for any of the four GFR equations
Discussion
The dosing of renally cleared drugs can be guided by the use of equations that estimate renal function in the individual [23, 49]. The choices of dabigatran etexilate dose rates, resulting from differences in estimates of GFR between various renal function equations, have been compared using simulated data [50, 51]. However, the correlations of estimated GFR from renal function equations with measured dabigatran concentrations have not been compared previously [32]. To our knowledge, the present study is the first to address this, using trough plasma dabigatran concentrations at steady-state as the reference. We demonstrated a clear association between the estimates of GFR from the renal function equations and trough plasma dabigatran concentrations at steady-state, after accounting for non-renal covariates. We did not find any significant differences between the equations in the ability to describe inter-individual differences in trough dabigatran concentrations.
Given that dabigatran is largely cleared by the kidneys unchanged, it is important to assess and compare the performances of the renal function equations in patients treated with dabigatran etexilate for the following reasons. Firstly, as the renal function equations were primarily developed to gauge GFR, rather than drug clearance, using these to guide dosing represents a secondary use by extrapolation [23]. Secondly, given the absence of a validated method for monitoring the clinical efficacy of dabigatran, dose adjustment according to estimated GFR represents a logical approach to the dose individualisation of dabigatran etexilate [18, 52]. Finally, while the CG equation has been recommended for guiding dabigatran etexilate dosing [5], a previous survey of clinicians revealed that the majority use the creatinine-only CKD-EPI equation instead [26].
Hijazi et al. [53] recently compared the thromboembolic and haemorrhagic outcomes observed in a trial comparing dabigatran with warfarin according to estimated GFR, using various renal function equations. In patients with a CKD-EPI ≥80 mL/min/1.73 m2, dabigatran was associated with a lower major bleeding rate in comparison with warfarin (p ≤ 0.005), whereas this was not demonstrable in patients with CG ≥80 mL/min (p ≥ 0.061) [53]. Further, they reported that around 50 % of the dabigatran patients who were classified as having a creatinine clearance ≥80 mL/min according to the CG equation had a GFR ≤80 mL/min/1.73 m2 according to the CKD-EPI equation. Hijazi et al. [53] thus propose that the CKD-EPI equation is better than the CG equation at identifying patients with normal or ‘enhanced’ renal function, in whom the risk of major bleeding is lower for a given dose rate of dabigatran etexilate. In our study we also observed a greater, albeit non-significant, correlation with the creatinine-only CKD-EPI equation compared with the CG equation for trough dabigatran concentrations (Table 5).
Contemporary renal function equations featuring cystatin C have demonstrated similar or superior performance to equations employing creatinine [30, 31]. We therefore sought to examine those cystatin C-based GFR equations that had been developed using an internationally standardised cystatin C assay [28]. These include two cystatin C-based equations developed by the CKD-EPI group [30]. We did not assess the Berlin Initiative Study (BIS) equation because it was specifically designed for individuals aged ≥70 years, of which we had few patients [31]. While the 95 % CI of the R 2 of the four equations overlapped (Table 5), the CKD-EPI equation featuring both creatinine and cystatin C was numerically associated with the highest R 2. This is in agreement with the findings of the CKD-EPI and BIS groups, who found that the equations that employed both renal biomarkers were superior to those using either biomarker alone for estimating GFR [30, 31].
Two of the non-renal covariates that appear to have the largest impact on plasma cystatin C concentrations are glucocorticoid therapy and thyroid dysfunction [46]. None of our study population received glucocorticoid therapy. When patients with thyroid test abnormalities were excluded, there was no significant change in the results. This may reflect the mild nature of the test abnormalities, as evidenced by free thyroxine concentrations within the ‘normal’ reference range.
The agreement in simulated dabigatran etexilate dosing recommendations between the four GFR equations was high for our cohort (94–98 %, Table 7). This finding is predictable given that ≥92 % of our study participants had estimated GFR >50 mL/min, with a median GFR of around 90 mL/min (Table 3). The majority of differences in estimated GFR between the four equations were thus away from the 50 mL/min threshold for dose reduction, and would not be expected to contribute to discordance in dosing recommendations. We plan to repeat this simulation in a larger group of patients with moderate to severe chronic renal impairment who have had creatinine and cystatin C measured.
The dose-corrected steady-state trough dabigatran concentration of the single individual treated with phenytoin and phenobarbitone (0.04 µg/L per mg/day, in the individual with a trough concentration of 9 µg/L on dabigatran etexilate 110 mg twice daily) was notable as it was more than 3 SD below the mean dose-corrected trough concentration of our study population (0.32 µg/L per mg/day, which is equivalent to 70 µg/L on 110 mg twice daily). Further, it is well below target trough dabigatran concentrations that have been suggested in the literature; for example, Chin et al. [54] have proposed 30–130 µg/L. While phenytoin and phenobarbitone are known P-gp inducers, the impact of concomitant use on the pharmacokinetics of dabigatran has not previously been reported [55]. Rifampicin, another P-gp inducer, has been demonstrated to reduce dabigatran concentrations by around 67 % [10]. To our knowledge, these are the first data to support the notion that phenytoin and/or phenobarbitone have a significant effect on dabigatran concentrations.
Limitations
Our study has several limitations. Firstly, the primary aim, to assess and compare the correlations of the renal function equations with trough plasma dabigatran concentrations, may have been better addressed by gathering data from individuals given intravenous dabigatran. From such data, true dabigatran clearance could have been calculated, without the need to consider oral availability, which is affected by many covariates (see Table 1). The bias and imprecision of the renal function equations against dabigatran clearance could then have been compared. However, this approach would also have been more challenging logistically. By comparison, trough concentrations are a convenient and useful representation of apparent oral clearance with which to compare the equations, as these have been correlated with the risk of thromboembolic and haemorrhagic outcomes in the setting of AF [4].
Secondly, there could be a statistical power problem since we had a dataset of only 52 individuals. By comparing the equations with the lowest and highest R 2 for the multiple linear regression model for trough plasma dabigatran concentrations (CG and CKD-EPI_CrCys, respectively), we calculate that, for future studies, around 680 subjects are needed to have 80 % power (α = 0.05) to detect a difference between these two equations. This is valuable data to inform the conduct of future studies.
Thirdly, we did not measure the active precursor of dabigatran, BIBR 951, or the active metabolites of dabigatran, its glucuronides [15]. While BIBR 951 is thought to have concentrations <0.4 % of those of dabigatran [15], the dabigatran glucuronides have been reported to make up 10–35 % of the total active drug concentrations following ingestion of dabigatran etexilate [7, 12, 15, 16, 56, 57]. Given this 3-fold variation in contribution of the glucuronides to the total active drug concentrations, the measurement of dabigatran concentrations alone may not be representative of total active drug concentrations. The glucuronides are thought to be cleared renally unchanged, and are thus relevant when considering the impact of renal function on total active drug exposure following the administration of dabigatran etexilate [15]. We chose to evaluate total active drug concentrations by using the HTI time. Alternative methods of such evaluation include the indirect measurement of the dabigatran glucuronides by alkalinisation of plasma samples to hydrolyse the glucuronides from dabigatran [7, 12, 15, 16, 56, 57], or using a calibrated HTI assay that determines total dabigatran concentrations [47]. However, concerns have been expressed in the literature regarding the validity of the alkalinisation method, and a detailed description of this method is yet to be published [54]. Further, the accuracy of the calibrated HTI assay exceeds FDA bioanalytical quality limits at total dabigatran concentrations ≤50 µg/L [47, 58]. As the 10th to 90th percentile of trough total dabigatran concentrations have been reported to be around 40–220 µg/L in patients given dabigatran etexilate 150 mg twice daily, we considered the calibrated HTI assay to be unsuitable for this study [14]. Instead, we used the HTI time as a gauge of total dabigatran concentrations for comparison with our measured dabigatran concentrations. The high R 2 of 0.90 between the trough HTI times and our measured trough plasma dabigatran concentrations is consistent with the notion that the latter were highly representative of the total concentration of thrombin inhibitors. Therefore, we expect that the results of the correlation analyses performed in this study would be similar if the dabigatran glucuronide concentrations were included in the models. To this end, we repeated the analyses of the four renal function equations, using the trough HTI times instead of the dabigatrantrough. A multiple linear regression model for the z-scores of the log-transformed trough HTI times was constructed. This included the same covariates as those used in the dabigatrantrough model, with the addition of dabigatran etexilate maintenance dose rates as a scalar covariate. This regression model had an unadjusted R 2 of 0.17 for the z-scores of the log-transformed trough HTI times. The R 2 values of the four renal function equations for the standardised residuals of the regression model are presented in Supplementary Table 4 (ESM). All the 95 % CI of the correlation coefficients overlapped (p = 0.49), with the highest R 2 value being associated with the CKD-EPI_CrCys equation. When this equation was added into the multiple linear regression model, the unadjusted R 2 was 0.53 for the z-scores of the log-transformed trough HTI times (Supplementary Table 5, ESM).
In conclusion, we have demonstrated that the estimates of renal function using the four renal function equations explained 32–47 % of the variability in trough plasma dabigatran concentrations, after other relevant covariates have been considered. Numerically, the CKD-EPI equation employing both creatinine and cystatin C had the highest correlation for trough dabigatran concentrations. In the setting of a drug for which there is no currently validated method for monitoring its clinical efficacy, it is useful to know that all of the tested renal function equations have a similar capacity to guide adjustment of dabigatran etexilate dose rates. Further research to determine the impact of each GFR equation on dabigatran dosing requirements using simulations from a non-linear mixed model is underway.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Stephanie Rose, Amjad Hamid, Amr BinSadiq and Lorraine Skelton (Christchurch Hospital) for assistance with patient recruitment; Mark Lewis (Canterbury Health Laboratories) for assistance with the dabigatran assay; Lesney Stuart and the staff at Core Biochemistry (Canterbury Health Laboratories) for the creatinine and thyroid-related assays; Charles Hawes (Canterbury Health Laboratories) for the cystatin C assays; and Chris Frampton for advice with the statistical analyses. Paul K. L. Chin is a recipient of the Health Research Council of New Zealand Clinical Research Training Fellowship (2012–2014).
References
- 1.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 2.Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28(2):125–136. doi: 10.1016/j.cjca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S–e88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al. The Effect of Dabigatran Plasma Concentrations and Patient Characteristics on the Frequency of Ischemic Stroke and Major Bleeding in Atrial Fibrillation Patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy) J Am Coll Cardiol. 2014;63(4):321–328. doi: 10.1016/j.jacc.2013.07.104. [DOI] [PubMed] [Google Scholar]
- 5.Huisman MV, Lip GY, Diener HC, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107(5):838–847. doi: 10.1160/TH11-10-0718. [DOI] [PubMed] [Google Scholar]
- 6.Brunet A, Hermabessiere S, Benain X. Pharmacokinetic and pharmacodynamic interaction of dronedarone and dabigatran in healthy subjects. Eur Heart J. 2011;32(Suppl. 1):313–631. doi:10.1093/eurheartj/ehr323.
- 7.Hartter S, Sennewald R, Schepers C, Baumann S, Fritsch H, Friedman J. Pharmacokinetic and pharmacodynamic effects of comedication of clopidogrel and dabigatran etexilate in healthy male volunteers. Eur J Clin Pharmacol. 2013;69(3):327–339. doi: 10.1007/s00228-012-1304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa((R))) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75(4):1053–1062. doi: 10.1111/j.1365-2125.2012.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delavenne X, Ollier E, Basset T, Bertoletti L, Accassat S, Garcin A, et al. A semi-mechanistic absorption model to evaluate drug-drug interaction with dabigatran: application with clarithromycin. Br J Clin Pharmacol. 2013;76(1):107–113. doi: 10.1111/bcp.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartter S, Koenen-Bergmann M, Sharma A, Nehmiz G, Lemke U, Timmer W, et al. Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin. Br J Clin Pharmacol. 2012;74(3):490–500. doi: 10.1111/j.1365-2125.2012.04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stahle H, et al. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45(5):555–563. doi: 10.1177/0091270005274550. [DOI] [PubMed] [Google Scholar]
- 12.Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47(1):47–59. doi: 10.2165/00003088-200847010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Pare G, Eriksson N, Lehr T, Connolly S, Eikelboom J, Ezekowitz MD, et al. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation. 2013;127(13):1404–1412. doi: 10.1161/CIRCULATIONAHA.112.001233. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Briefing information for the September 20, 2010, meeting of the cardiovascular and renal drugs advisory committee; 2010. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM247244.pdf. Accessed 9 Sep 2013.
- 15.Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36(2):386–399. doi: 10.1124/dmd.107.019083. [DOI] [PubMed] [Google Scholar]
- 16.Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49(4):259–268. doi: 10.2165/11318170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Ebner T, Wagner K, Wienen W. Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010;38(9):1567–1575. doi: 10.1124/dmd.110.033696. [DOI] [PubMed] [Google Scholar]
- 18.Chin PK, Vella-Brincat JW, Barclay ML, Begg EJ. Perspective on dabigatran etexilate dosing: why not follow standard pharmacological principles? Br J Clin Pharmacol. 2012;74(5):734–740. doi: 10.1111/j.1365-2125.2012.04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KDIGO KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 20.KDIGO KDIGO clinical practice guideline for chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 21.Florkowski CM, Chew-Harris JS. Methods of estimating GFR—different equations including CKD-EPI. Clin Biochem Rev. 2011;32(2):75–79. [PMC free article] [PubMed] [Google Scholar]
- 22.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 23.Matzke GR, Aronoff GR, Atkinson AJ, Jr, Bennett WM, Decker BS, Eckardt KU, et al. Drug dosing consideration in patients with acute and chronic kidney disease—a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80(11):1122–1137. doi: 10.1038/ki.2011.322. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156(11):785–795. doi: 10.7326/0003-4819-156-11-201203200-00391. [DOI] [PubMed] [Google Scholar]
- 26.Howey OK, Chin PK. Usage of renal function equations to guide prescribing in general medicine. N Z Med J. 2013;126(1383):97–99. [PubMed] [Google Scholar]
- 27.Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H. Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand. 1985;218(5):499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 28.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 29.Chew JS, Saleem M, Florkowski CM, George PM. Cystatin C—a paradigm of evidence based laboratory medicine. Clin Biochem Rev. 2008;29(2):47–62. [PMC free article] [PubMed] [Google Scholar]
- 30.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 32.Chin PK, Vella-Brincat JW, Walker SL, Barclay ML, Begg EJ. Dosing of dabigatran etexilate in relation to renal function and drug interactions at a tertiary hospital. Intern Med J. 2013;43(7):778–783. doi: 10.1111/imj.12170. [DOI] [PubMed] [Google Scholar]
- 33.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 34.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 35.Mathew TH. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust. 2005;183(3):138–141. doi: 10.5694/j.1326-5377.2005.tb06958.x. [DOI] [PubMed] [Google Scholar]
- 36.Stevens LA, Levey AS. Use of the MDRD study equation to estimate kidney function for drug dosing. Clin Pharmacol Ther. 2009;86(5):465–467. doi: 10.1038/clpt.2009.124. [DOI] [PubMed] [Google Scholar]
- 37.Spruill WJ, Wade WE, Cobb HH., 3rd Continuing the use of the Cockcroft–Gault equation for drug dosing in patients with impaired renal function. Clin Pharmacol Ther. 2009;86(5):468–470. doi: 10.1038/clpt.2009.187. [DOI] [PubMed] [Google Scholar]
- 38.Chin PK, Florkowski CM, Begg EJ. The performances of the Cockcroft–Gault, modification of diet in renal disease study and chronic kidney disease epidemiology collaboration equations in predicting gentamicin clearance. Ann Clin Biochem. 2013;50(Pt 6):546–557. doi: 10.1177/0004563213492320. [DOI] [PubMed] [Google Scholar]
- 39.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 40.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Within-person variability in kidney measures. Am J Kidney Dis. 2013;61(5):716–722. doi: 10.1053/j.ajkd.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chew-Harris JS, Florkowski CM, George PM, Elmslie JL, Endre ZH. The relative effects of fat versus muscle mass on cystatin C and estimates of renal function in healthy young men. Ann Clin Biochem. 2013;50(Pt 1):39–46. doi: 10.1258/acb.2012.011241. [DOI] [PubMed] [Google Scholar]
- 42.Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62(3):595–603. doi: 10.1053/j.ajkd.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delavenne X, Moracchini J, Laporte S, Mismetti P, Basset T. UPLC MS/MS assay for routine quantification of dabigatran—a direct thrombin inhibitor—in human plasma. J Pharm Biomed Anal. 2012;58:152–156. doi: 10.1016/j.jpba.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Ciulla TA, Sklar RM, Hauser SL. A simple method for DNA purification from peripheral blood. Anal Biochem. 1988;174(2):485–488. doi: 10.1016/0003-2697(88)90047-4. [DOI] [PubMed] [Google Scholar]
- 45.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem. 2005;38(1):1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23(2):138–143. doi: 10.1097/MBC.0b013e32834f1b0c. [DOI] [PubMed] [Google Scholar]
- 48.Boehringer Ingelheim Pharma GmbH & Co. KG. Pradaxa. Summary of Product Characteristics. European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf. Accessed 5 Jan 2014.
- 49.Begg EJ, Chin PK. A unified pharmacokinetic approach to individualized drug dosing. Br J Clin Pharmacol. 2012;73(3):335–339. doi: 10.1111/j.1365-2125.2011.04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellden A, Odar-Cederlof I, Nilsson G, Sjoviker S, Soderstrom A, Euler M et al. Renal function estimations and dose recommendations for dabigatran, gabapentin and valaciclovir: a data simulation study focused on the elderly. BMJ Open. 2013;3(4). doi:10.1136/bmjopen-2013-002686. [DOI] [PMC free article] [PubMed]
- 51.MacCallum PK, Mathur R, Hull SA, Saja K, Green L, Morris JK, et al. Patient safety and estimation of renal function in patients prescribed new oral anticoagulants for stroke prevention in atrial fibrillation: a cross-sectional study. BMJ Open. 2013;3(9):e003343. doi: 10.1136/bmjopen-2013-003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffull SB, Wright DF, Al-Sallami HS, Zufferey PJ, Faed JM. Dabigatran: rational dose individualisation and monitoring guidance is needed. N Z Med J. 2012;125(1357):148–154. [PubMed] [Google Scholar]
- 53.Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129(9):961–970. doi: 10.1161/CIRCULATIONAHA.113.003628. [DOI] [PubMed] [Google Scholar]
- 54.Chin PK, Wright DF, Patterson DM, Doogue MP, Begg EJ. A proposal for dose-adjustment of dabigatran etexilate in atrial fibrillation guided by thrombin time. Br J Clin Pharmacol. 2014. doi:10.1111/bcp.12364. [DOI] [PMC free article] [PubMed]
- 55.Schuetz EG, Beck WT, Schuetz JD. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996;49(2):311–318. [PubMed] [Google Scholar]
- 56.Stangier J, Stahle H, Rathgen K, Roth W, Shakeri-Nejad K. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol. 2008;48(12):1411–1419. doi: 10.1177/0091270008324179. [DOI] [PubMed] [Google Scholar]
- 57.Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.US Food and Drug Administration. Guidance for industry: bioanalytical method validation; 2001. http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf. Accessed 6 April 2013.
- 59.Boehringer Ingelheim (N.Z.) Limited. Pradaxa: New Zealand Datasheet. Medsafe; 2013. http://www.medsafe.govt.nz/profs/Datasheet/p/Pradaxacap.pdf. Accessed 28 Oct 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.