Abstract
A potent fibrinolytic enzyme-producing Bacillus cereus IND1 was isolated from the Indian food, rice. Solid-state fermentation was carried out using agroresidues for the production of fibrinolytic enzyme. Among the substrates, wheat bran supported more enzyme production and has been used for the optimized enzyme production by statistical approach. Two-level full-factorial design demonstrated that moisture, supplementation of beef extract, and sodium dihydrogen phosphate have significantly influenced enzyme production (P < 0.05). A central composite design resulted in the production of 3699 U/mL of enzyme in the presence of 0.3% (w/w) beef extract and 0.05% (w/w) sodium dihydrogen phosphate, at 100% (v/w) moisture after 72 h of fermentation. The enzyme production increased fourfold compared to the original medium. This enzyme was purified to homogeneity by ammonium sulfate precipitation, diethylaminoethyl-cellulose ion-exchange chromatography, Sephadex G-75 gel filtration chromatography, and casein-agarose affinity chromatography and had an apparent molecular mass of 29.5 kDa. The optimum pH and temperature for the activity of fibrinolytic enzyme were found to be 8.0 and 60°C, respectively. This enzyme was highly stable at wide pH range (7.0–9.0) and showed 27% ± 6% enzyme activity after initial denaturation at 60°C for 1 h. In vitro assays revealed that the enzyme could activate plasminogen and significantly degraded the fibrin net of blood clot, which suggests its potential as an effective thrombolytic agent.
1. Introduction
Cardiovascular diseases (CVDs) including acute myocardial infarction, ischemic heart disease, peripheral vascular disease, high blood pressure, and stroke are the leading causes of death worldwide [1]. According to the World Health Organization, it is estimated that 17.3 million people died from CVDs in 2008 and that it would be more than 23 million people by 2030 [2]. A variety of thrombolytic agents such as tissue plasminogen activator (t-PA), urokinase plasminogen activator (u-PA), and streptokinase were used to treat CVDs. In the cases of t-PA and u-PA, they are generally safe but very expensive. In contrast, bacterial streptokinase is a cheap thrombolytic agent but it is life-threatening [3]. Hence, the search for a novel fibrinolytic agent to treat CVDs continues. Nattokinase, a fibrinolytic enzyme isolated from Bacillus natto, had potent thrombolytic activity. These bacterial fibrinolytic enzymes are generally safe, and the oral administration of the nattokinase enzyme could increase the fibrinolytic activity in human plasma [4]. Fibrinolytic enzymes can be found in a variety of foods, such as Japanese natto, tofuyo, Korean cheonggukjang soy sauce, edible honey mushroom [1], Chinese douche [5], Indonesian tempeh [6], Taiwanese fermented red bean [7], Japanese shiokara [8], and Asian fermented shrimp paste [9]. These fibrinolytic enzymes possess three antithrombotic activities, which included the conversion of plasminogen to plasmin, the activation of t-PA, and finally the degradation of fibrin by fibrinolytic activity of plasmin in conjunction with nattokinase [10].
Solid-state fermentation (SSF) has emerged as a potential technology for the production of pharmaceutically significant products. Many agroindustrial residues were used for the production of these products using SSF. The agroindustrial residues such as pigeon pea, green gram husk, potato peel, and wheat bran were widely used for the production of proteases [11–14]. Utilization of these agroindustrial residues as substrates in SSF processes provides an alternate avenue and value addition. Product's yield is mostly higher using SSF when compared with submerged fermentation. Selection of an appropriate substrate is another key aspect of SSF [14]. Although the fibrinolytic enzyme production was found to be high while using SSF, no much work on the use of SSF and the statistical approach of optimization of fibrinolytic enzyme production has been carried out. SSF was employed for the production of fibrinolytic enzymes with few solid substrates (rice chaff and Fusarium oxysporum [15] and Bacillus firmus NA-1 and soybean grits [16]). Response surface methodology (RSM) has been used widely for the production of various enzymes, including polygalacturonase [17], arginine deiminase [18], α-amylase [19], acid protease [20], and β-galactosidase [21]. However, there are few studies on the optimization of medium components for microbes to produce fibrinolytic enzymes via two-level full-factorial design and RSM [22].
The aim of this work was to optimize the fermentation medium by RSM to increase fibrinolytic enzyme production in SSF. The optimization procedure included the following: a two-level full-factorial design, a central composite design (CCD), and an RSM. The extracted fibrinolytic enzyme was purified by chromatographic methods and was used to study its blood clot lytic properties in vitro.
2. Materials and Methods
2.1. Screening of Fibrinolytic Enzyme-Producing Organisms from the Indian Rice
Approximately 1.0 g of fermented rice was transferred to an Erlenmeyer flask (250 mL) with 50 mL of sterile double-distilled water, shaken vigorously for 10 min, and 1 mL of this solution was resuspended in sterile double-distilled water and aliquots were then spread on nutrient agar plates composed of the following (g/L): peptic digest of animal tissue, 5.0; beef extract, 1.5; yeast extract, 1.5; sodium chloride, 5.0; and 10 skimmed milk (pH 7.0). Colonies, indicated by clear zones corresponding to protease activity, were selected and cultured in SSF using wheat bran as the substrate. SSF was performed by inoculating 10% (v/w) precultured Bacillus cereus and incubated at 37°C for 72 h. The pH of the fermenting medium was maintained as 8.0 using Tris buffer (0.1 M) and 100% (v/w) moisture. Fibrinolytic activity of the crude enzyme was tested in a fibrin plate composed of 0.1 M sodium phosphate buffer (pH 7.4), 1% (w/v) agarose, 1.2% (v/v) fibrinogen, and thrombin (100 NIH units/mL) [23]. The fibrin plate was allowed to stand for 1 h at room temperature to form a fibrin clot layer. About 10 μL of crude enzyme was dropped into holes and incubated at 37°C for 5 h. The fibrinolytic enzyme exhibited a clear zone of degradation of fibrin around the well, thus indicating its fibrinolytic activity.
2.2. Identification of the Fibrinolytic Enzyme-Secreting Organism
The isolated strain IND1 with highest activity was identified on the basis of the biochemical properties, the phenotypical characteristics [24], and the 16S rRNA gene sequencing. The genomic DNA was extracted from the cells of an 18-h cultured IND1 strain by using a QIAGEN DNA purification kit (Germany) according to the manufacturer's instructions. The 16S rRNA gene of the isolate was amplified by polymerase chain reaction (PCR) using the upstream primer P1: 5′-AGAGTTTGATCMTGGCTAG-3′ and the downstream primer P2: 5′-ACGGGCGG TGTGTRC-3′ (Sigma-Aldrich). Amplification of DNA was carried out using the research gradient Peltier Thermal cycler machine PTC-225 and DNA polymerase (Sigma) under the following conditions: denaturation at 95°C for 3 min followed by 30 cycles at 95°C for 1 min, 55°C for 30 s, and 72°C for 1 min and 50 s. The amplified product was sequenced at Scigenome Laboratories, India. Sequence comparison with databases was performed using BLAST through the NCBI server [25]. The isolate IND1 was identified as B. cereus IND1. The 910 bp sequences were submitted to the GenBank database, and an accession number was assigned to those sequences. The GenBank accession number of the sequence reported in this paper is KF250417.
2.3. Evaluation of Agroindustrial Residues for Fibrinolytic Enzyme Production
The substrates such as banana peel, tapioca peel, rice bran, wheat bran, and green gram husk were collected locally and dried for several days and powdered. About 2.0 g of substrates was taken in an Erlenmeyer flask, and the moisture content was maintained as 100% level. The contents were mixed thoroughly and inoculated with 0.2 mL of 18 h grown culture (OD 600 nm = 1.08). To the fermented medium, 20 mL of double-distilled water was added and placed in an orbital shaker at 150 rpm for 30 min. After this, it was centrifuged at 10,000 ×g for 10 min, and the supernatant was used as the crude enzyme.
2.4. Assay of Fibrinolytic Activity
Fibrinolytic activity of the sample was measured by the hydrolysis of fibrin [26]. The reaction mixture contained 2.5 mL of 1.2% (w/v) fibrin (pH 7.8), 2.5 mL of 0.1 M Tris-HCl buffer (containing 0.01 M CaCl2, pH 7.8), and 0.05 mL of enzyme solution. After 30 min at 37°C, the reaction was stopped by adding 5 mL of 0.11 M trichloroacetic acid containing 0.22 M sodium acetate and 0.33 M acetic acid. The reaction mixture was centrifuged at 10,000 ×g for 10 min, and the absorbency of the sample was read at 275 nm against a sample blank. A fibrinolytic unit was defined as the amount of enzyme that gave an increase in absorbency at 275 nm equivalent to 1 μg of tyrosine/min at 37°C. The total protein content determination was performed as described by Lowry et al. [27].
2.5. Evaluation of Significant Factors with 25 Factorial Design
The main factors that significantly influence fibrinolytic enzyme production were screened using 25 factorial design and the RSM. With the results obtained from the one-at-a-time strategy, three nutrients were selected for further optimization. Important nutrient factors such as maltose, beef extract, and NaH2PO4 were added to the solid substrate according to 25 full-factorial design. From an SSF point of view, moisture content is one of the significant factors. Hence, the optimum moisture content and pH were evaluated with respect to the production of the fibrinolytic enzyme. Based on the two-level full-factorial design, each factor was examined at two levels: −1 for low level and +1 for high level. Table 1 lists the variables and levels (high and low) in detail. Fibrinolytic activity assay was carried out in duplicates, and the average value was taken as response Y (Table 2). Analysis of variance (ANOVA) was used to estimate the statistical parameters, and the values of “Prob >F” < 0.05 indicate that the model terms are significant (Table 3). Statistical software (Design-Expert 8.0.7.1; StatEase Inc., Minneapolis, Minnesota) was used to design and analyze the experiment. The significant main effect can be calculated using (1) after neglecting the insignificant main effects.
Table 1.
Independent variables and their levels for the 25 factorial experimental design.
| Factor | Name | Units | Coded levels | |
|---|---|---|---|---|
| −1 | +1 | |||
| A | Maltose | % | 0.1 | 0.5 |
| B | NaH2PO4 | % | 0.01 | 0.1 |
| C | Beef extract | % | 0.05 | 0.25 |
| D | pH | 7.0 | 8.0 | |
| E | Moisture | % | 60 | 100 |
Table 2.
Results of the 25 factorial design.
| Run | Factor: A | Factor: B | Factor: C | Factor: D | Factor: E | Response (Y) |
|---|---|---|---|---|---|---|
| 1 | 1 | −1 | 1 | 1 | −1 | 1473 |
| 2 | −1 | 1 | −1 | −1 | −1 | 1116 |
| 3 | −1 | −1 | 1 | −1 | −1 | 2040 |
| 4 | 1 | −1 | −1 | −1 | 1 | 2946 |
| 5 | −1 | 1 | −1 | 1 | 1 | 521 |
| 6 | 1 | −1 | −1 | 1 | 1 | 1200 |
| 7 | −1 | −1 | 1 | −1 | 1 | 1189 |
| 8 | −1 | −1 | −1 | 1 | 1 | 1912 |
| 9 | −1 | 1 | 1 | −1 | −1 | 2352 |
| 10 | 1 | −1 | −1 | −1 | −1 | 1894 |
| 11 | −1 | −1 | −1 | −1 | 1 | 2029 |
| 12 | 1 | 1 | 1 | −1 | −1 | 1903 |
| 13 | −1 | 1 | −1 | −1 | 1 | 1281 |
| 14 | 1 | 1 | −1 | 1 | 1 | 1491 |
| 15 | −1 | 1 | 1 | 1 | −1 | 274 |
| 16 | −1 | 1 | 1 | 1 | 1 | 3267 |
| 17 | 1 | 1 | 1 | 1 | −1 | 1500 |
| 18 | 1 | −1 | 1 | −1 | 1 | 1555 |
| 19 | 1 | 1 | 1 | −1 | 1 | 1638 |
| 20 | 1 | −1 | 1 | 1 | 1 | 1766 |
| 21 | 1 | −1 | −1 | 1 | −1 | 1985 |
| 22 | −1 | −1 | 1 | 1 | −1 | 2379 |
| 23 | 1 | −1 | 1 | −1 | −1 | 1262 |
| 24 | −1 | 1 | 1 | −1 | 1 | 1446 |
| 25 | −1 | 1 | −1 | 1 | −1 | 732 |
| 26 | 1 | 1 | −1 | −1 | −1 | 750 |
| 27 | −1 | −1 | −1 | −1 | −1 | 878 |
| 28 | 1 | 1 | −1 | 1 | −1 | 1345 |
| 29 | 1 | 1 | −1 | −1 | 1 | 2498 |
| 30 | −1 | −1 | 1 | 1 | 1 | 2406 |
| 31 | 1 | 1 | 1 | 1 | 1 | 2480 |
| 32 | −1 | −1 | −1 | 1 | −1 | 1262 |
Table 3.
ANOVA table for 25 factorial experimental design.
| Source | Sum of squares | df | Mean square | F value | P value | |
|---|---|---|---|---|---|---|
| Model | 1.442E + 007 | 26 | 5.547E + 005 | 10.42 | 0.0080 | Significant |
| A—maltose | 2.116E + 005 | 1 | 2.116E + 005 | 3.97 | 0.1028 | |
| B—NaH2PO4 | 4.010E + 005 | 1 | 4.010E + 005 | 7.53 | 0.0406 | |
| C—beef extract | 8.096E + 005 | 1 | 8.096E + 005 | 15.21 | 0.0114 | |
| D—pH | 19208.00 | 1 | 19208.00 | 0.36 | 0.5742 | |
| E—moisture | 1.312E + 006 | 1 | 8.327E + 005 | 15.64 | 0.0108 | |
| Residual | 2.662E + 005 | 5 | 53237.08 | |||
| Cor total | 1.469E + 007 | 31 | ||||
Consider the following
| (1) |
where α ij and α ijk are the ijth and ij kth interaction coefficients, respectively, α i is the ith linear coefficient, and α 0 is an intercept.
2.6. Optimization of Enzyme Production by RSM
RSM and the CCD were employed to optimize the most significant factors (moisture, beef extract, and NaH2PO4) regarding the fibrinolytic enzyme production. Each of the variables used was analyzed at five coded levels (−α, −1, 0, +1, +α) as listed in Table 4. According to the Design-Expert 8.0.7.1, a CCD design of three factors consists of 20 runs (eight factorial, six axial, and six center points). Central point of the CCD is the actual level of variables designed on the basis of initial experiments (one factor at a time and the 25 factorial design). About 2.0 g of wheat bran was taken in an Erlenmeyer flask and mixed with a predetermined quantity of Tris buffer (0.1 M, pH 8.0), and a calculated amount of beef extract and NaH2PO4 was also added to the flask. The substrate and nutrients were mixed carefully and sterilized at 121°C for 20 min. All the Erlenmeyer flasks were inoculated with a 10% (v/w) inoculum and incubated at 37°C for 72 h. Twenty milliliters of sterilized double-distilled water was added to extract the fibrinolytic enzyme unless otherwise stated. The enzyme activity assay was carried out in duplicates, and the average of these experimental values was taken as response Y (Table 5). Values of “Prob >F” < 0.05 indicate that the model terms are significant. In this model, the P value was <0.05; hence this model was significant (Table 6). The experimental results of the CCD were fitted with a second-order polynomial equation as shown in (2).
Table 4.
Independent variables selected for CCD and RSM.
| Factors (%) | Symbols | Coded values | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Moisture | A | 46.36 | 60 | 80 | 100 | 113.64 |
| Beef extract | B | 0.059 | 0.1 | 0.2 | 0.3 | 0.341 |
| NaH2PO4 | C | 0.018 | 0.025 | 0.0375 | 0.05 | 0.0568 |
Table 5.
Experimental design and results of the CCD.
| Run | Type | Moisture | Beef extract | NaH2PO4 | Response (Y) (units/mL) |
|---|---|---|---|---|---|
| 1 | Factorial | −1 (60) | −1 (0.1) | 1 (0.05) | 2175 |
| 2 | Center | 0 (80) | 0 (0.2) | 0 (0.0375) | 2553 |
| 3 | Center | 0 (80) | 0 (0.2) | 0 (0.0375) | 3150 |
| 4 | Center | 0 (80) | 0 (0.2) | 0 (0.0375) | 2868 |
| 5 | Factorial | −1 (60) | 1 (0.3) | 1 (0.05) | 2592 |
| 6 | Factorial | 1 (100) | −1 (0.1) | 1 (0.05) | 2916 |
| 7 | Axial | 1.682 (113.64) | 0 (0.2) | 0 (0.0375) | 1522 |
| 8 | Axial | 0 (80) | 0 (0.2) | −1.662 (0.0182) | 2731 |
| 9 | Factorial | 1 (100) | 1 (0.3) | 1 (0.05) | 3699 |
| 10 | Axial | 0 (80) | −1.682 (0.34) | 0 (0.0375) | 2463 |
| 11 | Center | 0 (80) | 0 (0.2) | 0 (0.0375) | 3147 |
| 12 | Factorial | −1 (60) | −1 (0.1) | −1 (0.025) | 1372 |
| 13 | Factorial | −1 (60) | 1 (0.3) | −1 (0.025) | 2689 |
| 14 | Axial | 0 (80) | −1.682 (0.06) | 0 (0.0375) | 3012 |
| 15 | Factorial | 1 (100) | 1 (0.3) | −1 (0.025) | 2182 |
| 16 | Factorial | 1 (100) | −1 (0.1) | −1 (0.025) | 2209 |
| 17 | Center | 0 (80) | 0 (0.2) | 0 (0.0375) | 3048 |
| 18 | Axial | 0 (80) | 0 (0.2) | 1.682 (0.0568) | 3334 |
| 19 | Center | 0 (80) | 0 (0.2) | 0 (0.0375) | 2779 |
| 20 | Axial | −1.682 (46.36) | 0 (0.2) | 0 (0.0375) | 86 |
Table 6.
Results of the regression analysis of the CCD.
| Source | Sum of squares | df | Mean square | F value | P value |
|---|---|---|---|---|---|
| Model | 1.108E + 007 | 9 | 1.231E + 006 | 9.76 | 0.0007 |
| A—moisture | 1.541E + 006 | 1 | 1.541E + 006 | 12.21 | 0.0058 |
| B—beef extract | 8.531E + 005 | 1 | 8.531E + 005 | 6.76 | 0.0265 |
| C—NaH2PO4 | 1.139E + 006 | 1 | 1.139E + 006 | 9.03 | 0.0132 |
| AB | 1.196E + 005 | 1 | 1.196E + 005 | 0.95 | 0.3533 |
| AC | 2.880E + 005 | 1 | 2.880E + 005 | 2.28 | 0.1618 |
| BC | 1012.50 | 1 | 1012.50 | 8.023E − 003 | 0.9304 |
| A 2 | 6.645E + 006 | 1 | 6.645E + 006 | 52.65 | <0.0001 |
| B 2 | 234.01 | 1 | 234.01 | 1.854E − 004 | 0.9665 |
| C 2 | 1.691E + 005 | 1 | 1.691E + 005 | 1.34 | 0.2739 |
| Residual | 1.262E + 006 | 10 | 1.262E + 005 | ||
| Lack of fit | 9.840E + 005 | 5 | 9.840E + 005 | 3.54 | 0.0958 |
| Pure error | 2.780E + 005 | 5 | 55596.57 | ||
| Cor total | 1.234E + 007 | 19 |
Consider the following:
| (2) |
where Y is the fibrinolytic activity (units/mL); A is the coded value of moisture; B is the coded value of the beef extract; C is the coded value of NaH2PO4; α 1, α 2, and α 3 are the linear coefficients; α 1 α 2, α 1 α 3, and α 2 α 3 are the interactive coefficients; and α 1 α 1, α 2 α 2, and α 3 α 3 are the quadratic coefficients.
Response surface graphs were plotted to determine the optimum fibrinolytic enzyme production. The fitted polynomial equation was expressed as three-dimensional (3D) surface plots to visualize the relation between responses and the experimental levels of each factor used in the design. Validation of the model was performed under the conditions predicted by the model. SSF was carried out, and fibrinolytic activity was assayed as described earlier.
2.7. Purification of Fibrinolytic Enzyme
The crude extract was precipitated by the addition of solid ammonium sulfate at 30%–70% saturation. The precipitate was allowed to form at 4°C overnight and was collected by centrifugation at 10,000 ×g in a refrigerated centrifuge for 15 min. The precipitate was dissolved in 5 mL of buffer A (0.025 M sodium phosphate buffer, pH 7.0). It was dialysed against buffer A overnight and loaded on DEAE-cellulose (Merck, Bangalore) column, which was preequilibrated with buffer A. The column was washed with buffer A to remove all unbound proteins, and a linear gradient of 0–0.75 M NaCl-added buffer A was used to elute the bound proteins. Fractions exhibiting fibrinolytic activity were pooled and concentrated with ammonium sulfate. The precipitate was collected by centrifugation at 10,000 ×g in a refrigerated centrifuge at 4°C for 15 min, dissolved in buffer A, and dialysed against the same buffer overnight. This sample was loaded on Sephadex G-75 (Amersham Biosciences, Sweden) gel filtration column (0.7 × 45 cm) which was preequilibrated with buffer A. The eluate was monitored for protein concentration at 280 nm and was assayed for fibrinolytic activity. Fractions with high fibrinolytic enzyme activity were pooled and loaded on casein-agarose affinity column (Sigma) and washed with buffer A. The fibrinolytic enzyme was eluted with buffer A containing 0.1, 0.2, 0.3, 0.4, 0.5, 0.7, and 0.8 M NaCl. All fractions were subjected to fibrinolytic activity assay. The purified enzyme was stored at −20°C and used for characterization studies.
2.8. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Molecular Mass Determination
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 12%) was performed to determine the molecular mass of the fibrinolytic enzyme following the methods of Laemmli [28]. The molecular weight of the fibrinolytic enzyme was estimated with phosphorylase b (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), soybean trypsin inhibitor (20.1 kDa), and lysozyme (14.3 kDa) markers.
2.9. Properties of Fibrinolytic Enzyme
The optimum pH for the activity of an enzyme was determined using the following buffers (0.1 M): citrate buffer (pH 3.0 and 4.0), succinate buffer (pH 5.0), sodium phosphate buffer (pH 6.0 and 7.0), Tris buffer (pH 8.0), and glycine-NaOH buffer (pH 9.0 and 10.0). The stability of fibrinolytic enzyme activity in response to pH was evaluated by incubating the enzyme with the above buffers at 37°C for 1 h prior to incubation with substrate. The effect of temperature on enzyme activity was determined by performing the reactions at various temperatures: 30, 40, 50, 60, and 70°C. To determine the thermal stability, the fibrinolytic enzyme was incubated (without substrate at various temperatures, 30–70°C) for 1 h. Enzyme activity was assayed as described earlier. To study the effect of divalent ions on enzyme activity, the enzyme sample was incubated with various divalent ions, namely, Ca2+, Co2+, Cu2+, Mg2+, Mn2+, Hg2+, Fe2+, and Zn2+. The enzyme activity was determined as described earlier.
2.10. Analysis of Fibrinolysis on Plasminogen-Rich and Plasminogen-Free Fibrin Plates
The plasminogen-rich and plasminogen-free plates were prepared to evaluate the efficacy of fibrinolytic enzyme on plasminogen and direct fibrin clot lysis. The fibrin plate was heated at 80°C for 30 min to inactivate plasminogen present with the commercially available fibrinogen. Twenty microliters of fibrinolytic enzyme was placed on the plasminogen-free and plasminogen-rich plates and incubated at room temperature for 4 h. The enzyme exhibited a clear zone of degradation of fibrin on plasminogen-rich plate and plasminogen-free plate was observed.
2.11. Blood Clot Lysis of Fibrinolytic Enzymes on Human Blood
The clot lytic effect of fibrinolytic enzyme was studied with an artificial clot in vitro. The blood was collected from healthy male volunteer with written informed consents. Artificial blood clot was made by spontaneous coagulation in the centrifuge vials. Then, different doses (100, 200, and 300 units) of the fibrinolytic enzyme were added. Streptokinase (250 units) was used as the positive control; buffered saline solution was used as the negative control. These were incubated at room temperature for 60 min and analyzed.
3. Results and Discussion
3.1. Screening of a Potent Fibrinolytic Enzyme-Producing Strain
The bacterium B. cereus IND1 displayed more activity on skimmed milk agar plates and fibrin plates. It produced approximately an 11 mm zone on the fibrin plate, which was higher than the other isolates. The isolated strain was Gram positive, rod shaped, catalase positive, and oxidase positive and had tested negative for indole formation, citrate utilization, and the hydrolysis of urea. It was able to hydrolyze starch and casein and also tested negative for gelatin hydrolysis.
3.2. Utilization of Agroresidues for the Production of Fibrinolytic Enzyme
The results showed that fibrinolytic enzyme production by B. cereus IND1 varied with the type of substrates. Enzyme production was found to be high in the wheat bran medium (840 ± 32 units/mL; Figure 1). This organism effectively utilized wheat bran for its growth and fibrinolytic enzyme production. The selection of suitable agroresidues for enzyme production in a SSF process depends on several factors including cost and availability [14].
Figure 1.
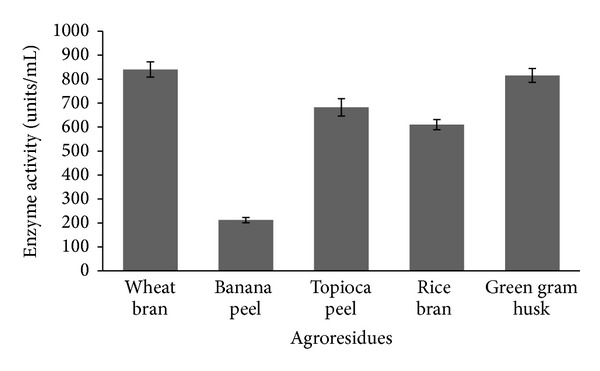
Screening of different agroresidues for the production of fibrinolytic enzyme.
3.3. Evaluation of Medium Components Affecting Fibrinolytic Enzyme Production Using RSM and 25 Factorial Design
The 25 factorial experimental design proved to be a valuable tool for the evaluation of the main effects of fibrinolytic enzyme production. The results of the 25 factorial design have been listed in Table 2. From Table 2, the fibrinolytic activity obtained during production using the 25 factorial design varied between 274 and 3267 units/mL. ANOVA was performed to verify the validity of the models, and the results have been listed in Table 3. Based on ANOVA, the “F-value” for the overall regression model (10.42) is significant at the 5% level. There is only a 0.80% chance that a “model F-value” this large could occur due to noise. In this model, B, C, E, AC, BC, CD, ABD, ADE, BDE, CD E, A BC E, and BC D E are significant. Maltose significantly increased the fibrinolytic enzyme production. These results were in accordance with reported fibrinolytic enzyme production in the presence of maltose for Bacillus natto [22]. The medium pH and the NaH2PO4 concentration affected the fibrinolytic enzyme production significantly. Enzyme production was greatly affected by moisture (P < 0.01), beef extract (P < 0.01), and NaH2PO4 (P < 0.05) concentrations with the medium. The equation in terms of the coded factors is given as follows:
| (3) |
On the basis of calculated t values, the moisture content, beef extract, and NaH2PO4 were selected for further optimization using RSM.
3.4. Response Surface Methodology
RSM is a powerful technique for testing multiple process variables, because fewer experimental trials are required when compared with the study of one variable at a time. Also, interactions between variables can be identified and quantified by this technique [29]. The significant factors (moisture, beef extract, and NaH2PO4) were investigated further using CCD and RSM. The CCD model helps to study the interactions between the various variables, and RSM helps to explore the optimum concentrations of each of the variables. RSM had been successfully used for the enhancement of fibrinolytic enzyme production by the Bacillus natto [22], Bacillus subtilis [30], and Bacillus sp. strain AS-S20-1 [31].
The second-order polynomial model was used to correct the independent variables with fibrinolytic activity. The highest activity of the fibrinolytic protease that was observed was at 3699 units/mL at run 9 (Table 5). The model F-value of 9.76 implied that the model was significant (Table 6). There is only a 0.07% chance that a “model F-value” of this magnitude could occur due to noise. The data obtained best fitted into a quadratic model. The regression analysis of the experimental design showed that the linear model terms (A, B, and C) and the quadratic model term (A 2) were significant. However, the interactive model terms (AB, AC, and BC) and quadratic model terms (B 2 and C 2) were found to be insignificant (P > 0.05). Yuguo et al. [32] stated that the coefficient (R 2) could be at least 0.8 for a good fit of the model. The multiple correlation coefficient (R 2) of this model is 0.89 (a value >0.75 indicates aptness of the model), which means that the model can explain 89% of the variation in the response. The adjusted coefficient (R 2) obtained for the model was 0.805. According to ANOVA, the lack of fit is insignificant, indicating that the second-order model with interaction is very adequate in approximating the response surface of the experimental design. The lack of fit's F-value of 3.4 implies that there is a 9.58% chance that a large lack of fit F-value could occur due to noise. A nonsignificant lack of fit is good. Applying multiple regression analysis, the results were fitted into a second-order polynomial (2).
Three-dimensional response surfaces were plotted on the basis of the model equation to investigate the interaction among the various variables and to determine the optimum concentration of each factor for the maximum fibrinolytic enzyme production (Figures 2(a)–2(c)). The fibrinolytic enzyme production varied significantly upon changing the initial moisture content and concentrations of the beef extract. The 3D plots depicted that there was an increased enzyme production up to 100% moisture content of the medium and then depleted thereafter. The increase of enzyme activity is attributed to a higher production of enzyme in the presence of an optimal level of moisture. This result was in agreement with Pandey et al. [14] who stated that moisture content is one of the critical factors for enzyme production in SSF. Increasing concentrations of the beef extract and NaH2PO4 resulted in the fibrinolytic enzyme production up to the optimum level. The optimal concentrations for the production of enzyme were 100% (v/w) moisture, 0.3% (w/w) beef extract, and 0.05% (w/w) NaH2PO4. The final equation in terms of coded factors for the CCD model is expressed in (2). To understand the effect of all the three factors, a perturbation plot was generated from this experiment (Figure 2(d)). A low value (14.06%) of the coefficient of variance indicates a high degree of precision and good reliability of experimental values. The RSM and the perturbation graph clearly show that sodium dihydrogen phosphate and moisture content greatly affect the production of the fibrinolytic enzyme. The “adequate precision” is a measure of signal-to-noise ratio, and a value greater than four is desirable. In this model, a ratio of 13.048 indicates an adequate signal, and this model can be used to navigate the design space. The regression equation coefficient was calculated, and the data were fitted into a second-order polynomial equation as given below:
| (4) |
where A is moisture, B is beef extract, and C is NaH2PO4.
Figure 2.
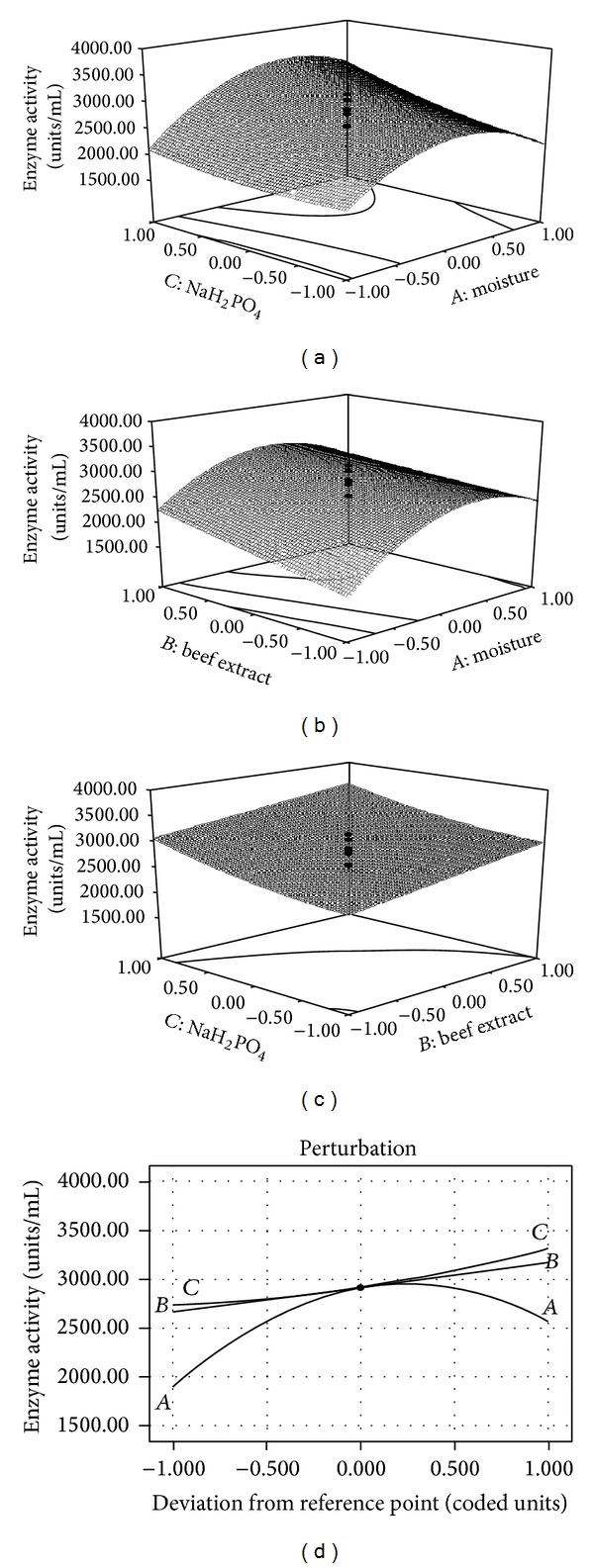
Response surface plot for fibrinolytic enzyme production by B. cereus IND1. (a) The interactive effects of moisture and NaH2PO4; (b) the interactive effects of moisture and beef extract; (c) the interactive effects of beef extract and NaH2PO4; (d) perturbation graph shows the effect of moisture, beef extract, and NaH2PO4 on fibrinolytic enzyme production.
Results revealed that the RSM-optimized medium increased fourfold of fibrinolytic enzyme production compared to the unoptimized medium. The enzyme production was comparatively higher than the earlier report of RSM on B. subtilis [30] and on Bacillus sp. strain AS-S20-1 [31].
3.5. Validation of the Optimized Condition
Based on the RSM result, the quadratic model predicted that the maximum production of fibrinolytic enzyme was 3705 units/mL, while the A, B, and C code levels were 0, 0, and 1.682 corresponding to 80% (v/w) moisture, 0.2% (w/w) beef extract, and 0.0568% sodium dihydrogen phosphate. To verify the predicted result, a validation experiment was performed in triplicates. Under optimized conditions, the observed average experimental fibrinolytic enzyme production was 3750 ± 118 units/mL, indicating that the experimental and predicted values (3670 ± 48 units/mL) were in good agreement. The fibrinolytic activity obtained from the experiments was very close to the actual response predicted by the model, which proved the validity of the model.
3.6. Purification of Fibrinolytic Enzyme
The enzyme was purified through four steps including ammonium sulfate precipitation, DEAE cellulose, gel filtration, and affinity chromatography. In the present study, the 30%–70% of saturated ammonium sulfate was found to be optimum for fibrinolytic enzyme precipitation. The recovery and purification were 4.57% and 12.8-fold, respectively, after casein-agarose affinity chromatography. The specific activity of the purified fibrinolytic enzyme was 960 units/mg protein. The purification summary is listed in Table 7. The purity of the casein-agarose affinity fractions was tested using SDS-PAGE. The SDS-PAGE shows a single band with an apparent molecular mass of 29.5 kDa, indicating the homogeneity of an enzyme (Figure 3). The Bacillus cereus IND1 fibrinolytic enzyme had a molecular mass comparable with that of the serine protease of Bacillus amyloliquefaciens DC-4 (28 kDa) [33], Bacillus sp. DJ-4 (29 kDa) [34], and B. subtilis LD-8547 (30 kDa) [35].
Table 7.
Summary of purification of fibrinolytic enzyme from B. cereus IND1.
| Procedure | Total activity (units) | Total protein (mg) | Specific (units/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude enzyme | 27300 | 364 | 75 | 1 | 100 |
| Ammonium sulfate fraction (30%–70% saturation) | 16505 | 128 | 129 | 1.72 | 60.5 |
| DEAE cellulose | 5418 | 23 | 235 | 3.1 | 19.9 |
| Sephadex G-75 | 3241 | 11.5 | 281 | 3.8 | 11.8 |
| Casein-agarose | 1248 | 1.3 | 960 | 12.8 | 4.57 |
Figure 3.
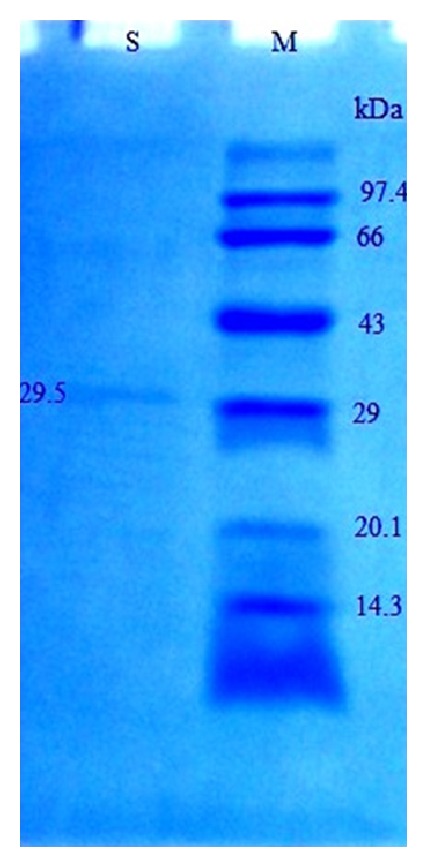
SDS-PAGE of purified fibrinolytic enzyme from B. cereus IND1 (S: purified enzyme after casein-agarose affinity chromatography and M: marker).
3.7. Properties of Fibrinolytic Enzymes
The results of optimum pH on enzyme activity and pH stability are shown in Figure 4(a). The fibrinolytic enzyme was activated at neutral and alkaline pH values, and optimal reaction for fibrinolytic enzyme was obtained at pH 8.0. The optimized pH value of the fibrinolytic enzyme was higher than many fibrinolytic enzymes, such as pH 7.0 of Bacillus sp. KA38 metalloprotease [36] and B. subtilis strain A1 nattokinase [37]. The enzyme activity was optimum at 60°C, which was higher than the B. subtilis natto B-12 [38]. The optimum temperature and thermostability of the enzyme are shown in Figure 4(b). As the denaturation time was increased to 1 h at 50°C, the enzyme activity decreased approximately to 40%.
Figure 4.
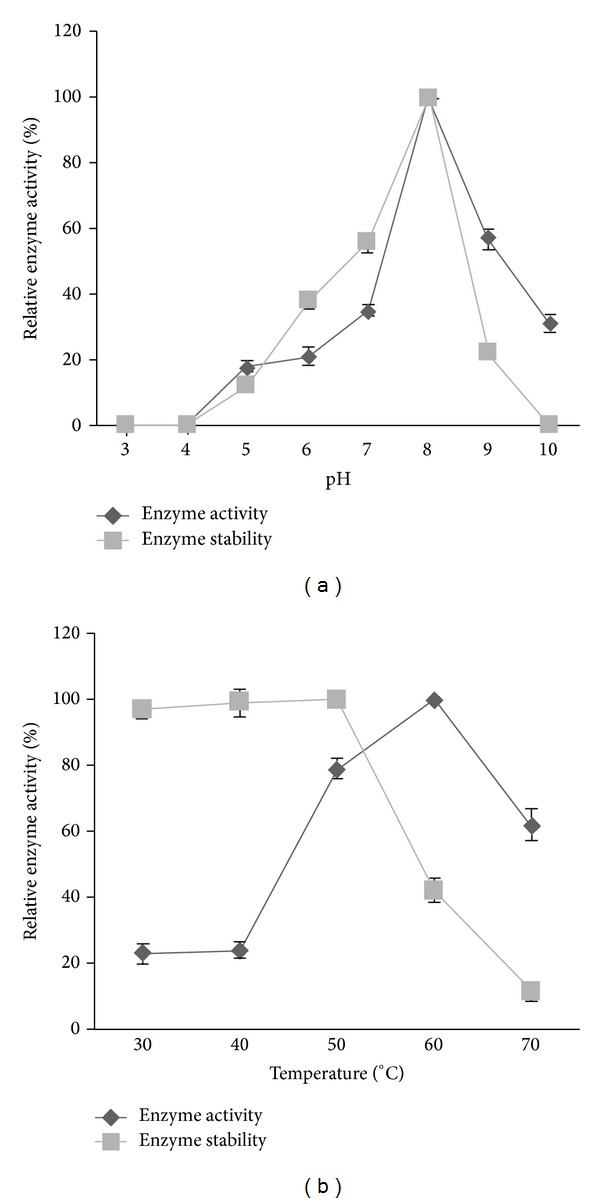
Effect of (a) pH and (b) temperature on enzyme activity and stability.
3.8. PA Activity and Direct Clot Lytic Effect of Fibrinolytic Enzyme
The plasminogen-rich plate showed 1.2-fold activity compared to plasminogen-free plate (Figure 5(a)). These results demonstrate that the fibrinolytic enzyme activates plasminogen and also has plasmin-like activity. The clot lysis was studied by incubating the human blood clot with fibrinolytic enzyme. The fibrinolytic enzyme digested the fibrin net completely within 60 min of incubation at room temperature (30 ± 2°C). At higher fibrinolytic enzyme concentration (300 units), it digested fibrin net within 40 min (Figure 5(b)). In the control tube, clot degradation was not observed. These results suggested that fibrinolytic enzymes had obvious effect on dissolving blood clot, and the effect was enhanced as the concentration of fibrinolytic enzyme increased. This kind of dose-dependent clot lysis was reported with B. subtilis LD-8547 fibrinolytic enzymes [39]. The degradation of fibrin suggested predominant secretion of fibrinolytic protease from this strain using wheat bran as a substrate, which may have great application in the treatment of CVDs.
Figure 5.
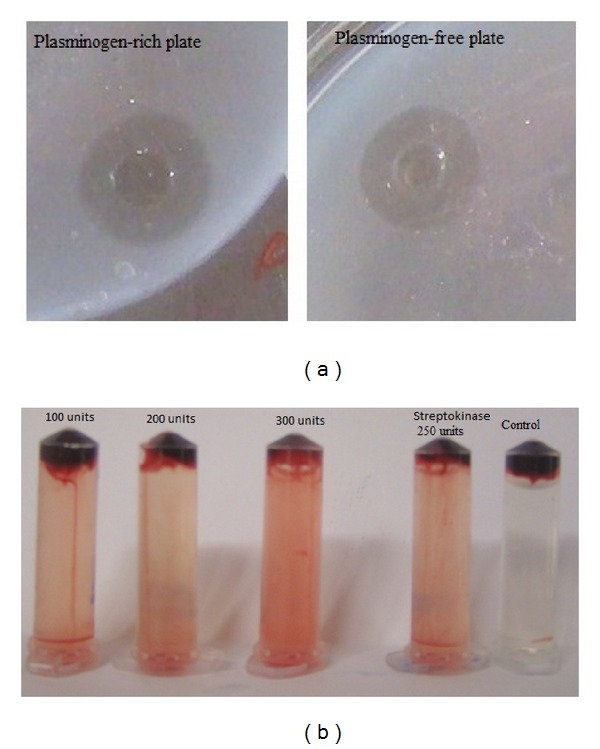
Analysis of fibrinolysis by fibrinolytic enzyme on plasminogen-rich fibrin plate and plasminogen-free plate (a). In vitro digestion of fibrin net of human blood clot (b). Streptokinase was used as the standard, and phosphate buffer (pH 7.4) was used as the blank.
4. Conclusion
A potent fibrinolytic enzyme was produced using agroresidues by B. cereus IND1. This organism effectively utilized wheat bran for the fibrinolytic enzyme production, and the process parameters were optimized by two-level full-factorial design and the RSM. This statistical approach proved to be a powerful tool for the optimization of fibrinolytic enzyme production. The optimal medium showed fourfold enzyme production compared to the unoptimized medium. Among the nutrient sources, sodium dihydrogen phosphate significantly increased enzyme production. The purified enzyme was active at higher temperatures and at wide pH ranges. It digested human blood clot in vitro. This study explores new sources of fibrinolytic enzymes to treat and prevent CVDs.
Acknowledgment
P. Vijayaraghavan gratefully acknowledges the Council for Scientific and Industrial Research (CSIR), India, for providing a Senior Research Fellowship (Ref. 09/652(0024)/2012 EMR-1).
Conflict of Interests
The authors have declared no conflict of interests.
References
- 1.Mine Y, Kwan Wong AH, Jiang B. Fibrinolytic enzymes in Asian traditional fermented foods. Food Research International. 2005;38(3):243–250. [Google Scholar]
- 2.World Health Organization. The World Health Report-Cardiovascular diseases (CVDs) 2013.
- 3.Banerjee A, Chisti Y, Banerjee UC. Streptokinase—a clinically useful thrombolytic agent. Biotechnology Advances. 2004;22(4):287–307. doi: 10.1016/j.biotechadv.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematologica. 1990;84(3):139–143. doi: 10.1159/000205051. [DOI] [PubMed] [Google Scholar]
- 5.Wang CT, Ji BP, Li B, et al. Purification and characterization of a fibrinolytic enzyme of Bacillus subtilis DC33, isolated from Chinese traditional Douchi. Journal of Industrial Microbiology and Biotechnology. 2006;33(9):750–758. doi: 10.1007/s10295-006-0111-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim S-B, Lee D-W, Cheigh C-I, et al. Purification and characterization of a fibrinolytic subtilisin-like protease of Bacillus subtilis TP-6 from an Indonesian fermented soybean, Tempeh. Journal of Industrial Microbiology and Biotechnology. 2006;33(6):436–444. doi: 10.1007/s10295-006-0085-4. [DOI] [PubMed] [Google Scholar]
- 7.Chang C-T, Wang P-M, Hung Y-F, Chung Y-C. Purification and biochemical properties of a fibrinolytic enzyme from Bacillus subtilis-fermented red bean. Food Chemistry. 2012;133(4):1611–1617. [Google Scholar]
- 8.Sumi H, Nakajima N, Yatagai C. A unique strong fibrinolytic enzyme (katsuwokinase) in skipjack “Shiokara”, a Japanese traditional fermented food. Comparative Biochemistry and Physiology B. 1995;112(3):543–547. doi: 10.1016/0305-0491(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 9.Wong AHK, Mine Y. Novel fibrinolytic enzyme in fermented shrimp paste, a traditional Asian fermented seasoning. Journal of Agricultural and Food Chemistry. 2004;52(4):980–986. doi: 10.1021/jf034535y. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya S, Hagimori M, Ogasawara M, Arakawa M. In vivo evaluation method of the effect of nattokinase on carrageenan-induced tail thrombosis in a rat model. Acta Haematologica. 2010;124(4):218–224. doi: 10.1159/000321518. [DOI] [PubMed] [Google Scholar]
- 11.Johnvesly B, Manjunath BR, Naik GR. Pigeon pea waste as a novel, inexpensive, substrate for production of a thermostable alkaline protease from thermoalkalophilic Bacillus sp. JB-99. Bioresource Technology. 2002;82(1):61–64. doi: 10.1016/s0960-8524(01)00147-x. [DOI] [PubMed] [Google Scholar]
- 12.Prakasham RS, Rao CS, Sarma PN. Green gram husk-an inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresource Technology. 2006;97(13):1449–1454. doi: 10.1016/j.biortech.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee AK, Adhikari H, Rai SK. Production of alkaline protease by a thermophilic Bacillus subtilis under solid-state fermentation (SSF) condition using Imperata cylindrica grass and potato peel as low-cost medium: characterization and application of enzyme in detergent formulation. Biochemical Engineering Journal. 2008;39(2):353–361. [Google Scholar]
- 14.Pandey A, Soccol CR, Nigam P, Brand D, Mohan R, Roussos S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochemical Engineering Journal. 2000;6(2):153–162. doi: 10.1016/s1369-703x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 15.Tao S, Peng L, Beihui L, Deming L, Zuohu L. Solid state fermentation of rice chaff for fibrinolytic enzyme production by Fusarium oxysporum . Biotechnology Letters. 1997;19(5):465–467. [Google Scholar]
- 16.Seo J-H, Lee S-P. Production of fibrinolytic enzyme from soybean grits fermented by Bacillus firmus NA-1. Journal of Medicinal Food. 2004;7(4):442–449. doi: 10.1089/jmf.2004.7.442. [DOI] [PubMed] [Google Scholar]
- 17.Rekha VPB, Ghosh M, Adapa V, Oh SJ, Pulicherla KK, Sambasiva Rao KRS. Optimization of polygalacturonase production from a newly isolated Thalassospira frigidphilosprofundus to use in pectin hydrolysis: statistical approach. BioMed Research International. 2013;2013:12 pages. doi: 10.1155/2013/750187.750187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur B, Kaur R. Application of response surface methodology for optimizing arginine deiminase production medium for Enterococcus faecium sp. GR7. The Scientific World Journal. 2013;2013:12 pages. doi: 10.1155/2013/892587.892587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Fattah YR, Soliman NA, El-Toukhy NM, El-Gendi H, Ahmed RS. Production, purification, and characterization of thermostable α-amylase produced by Bacillus licheniformis isolate AI20. Journal of Chemistry. 2013;2013:11 pages.673173 [Google Scholar]
- 20.Siala R, Frikha F, Mhamdi S, Nasri M, Sellami Kamoun A. Optimization of acid protease production by Aspergillus niger I1 on shrimp peptone using statistical experimental design. The Scientific World Journal. 2012;2012:11 pages. doi: 10.1100/2012/564932.564932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil KA, Mustafa S, Mohammad R. Optimization of milk-based medium for efficient cultivation of Bifidobacterium pseudocatenulatum G4 using face-centered central composite-response surface methodology. BioMed Research International. 2014;2014:10 pages. doi: 10.1155/2014/787989.787989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Xing J, Chang T, Ma Z, Liu H. Optimization of nutritional conditions for nattokinase production by Bacillus natto NLSSE using statistical experimental methods. Process Biochemistry. 2005;40(8):2757–2762. [Google Scholar]
- 23.Astrup T, Müllertz S. The fibrin plate method for estimating fibrinolytic activity. Archives of Biochemistry and Biophysics. 1952;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- 24.Holt JG, Krieg NR, Sneath PH, Stanley JJ, Williams ST. Bergey's Manual of Determinative Bacteriology. Baltimore, Md, USA: Williams and Wilkins; 1994. [Google Scholar]
- 25.Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansen ML. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. Journal of General Physiology. 1939;22:78–79. doi: 10.1085/jgp.22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry OH, Rasebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 28.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Dean A, Vass D. Design and Analysis of Experiments. New York, NY, USA: Springer; 1999. Response surface methodology; pp. 483–529. [Google Scholar]
- 30.Deepak V, Kalishwaralal K, Ramkumarpandian S, Babu SV, Senthilkumar SR, Sangiliyandi G. Optimization of media composition for Nattokinase production by Bacillus subtilis using response surface methodology. Bioresource Technology. 2008;99(17):8170–8174. doi: 10.1016/j.biortech.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee AK, Rai SK. A statistical approach for the enhanced production of alkaline protease showing fibrinolytic activity from a newly isolated Gram-negative Bacillus sp. strain AS-S20-I. New Biotechnology. 2011;28(2):182–189. doi: 10.1016/j.nbt.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Yuguo Z, Zhao W, Xiaolong C. Citric acid production from the mash of dried sweet potato with its dregs by Aspergillus niger in an external-loop airlift bioreactor. Process Biochemistry. 1999;35(3-4):237–242. [Google Scholar]
- 33.Peng Y, Huang Q, Zhang R-H, Zhang Y-Z. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional Chinese soybean food. Comparative Biochemistry and Physiology B. 2003;134(1):45–52. doi: 10.1016/s1096-4959(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 34.Kim S-H, Choi N-S. Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp. strain DJ-4 screened from Doen-Jang. Bioscience, Biotechnology and Biochemistry. 2000;64(8):1722–1725. doi: 10.1271/bbb.64.1722. [DOI] [PubMed] [Google Scholar]
- 35.Wang SH, Zhang C, Yang YL, Diao M, Bai MF. Screening of a high fibrinolytic enzyme producing strain and characterization of the fibrinolytic enzyme produced from Bacillus subtilis LD-8547. World Journal of Microbiology and Biotechnology. 2008;24(4):475–482. [Google Scholar]
- 36.Lee A, Si-Kyung A, Bae D-H, et al. Purification and characterization of a fibrinolytic enzyme from Bacillus sp. KDO-13 isolated from soybean paste. Journal of Microbiology and Biotechnology. 2001;11(5):845–852. [Google Scholar]
- 37.Jeong Y-K, Kim JH, Gal S-W, et al. Molecular cloning and characterization of the gene encoding a fibrinolytic enzyme from Bacillus subtilis Strain A1. World Journal of Microbiology and Biotechnology. 2004;20(7):711–717. [Google Scholar]
- 38.Wang C, Ming DU, Zheng D, Kong F, Zu G, Feng Y. Purification and characterization of nattokinase from Bacillus subtilis natto B-12. Journal of Agricultural and Food Chemistry. 2009;57(20):9722–9729. doi: 10.1021/jf901861v. [DOI] [PubMed] [Google Scholar]
- 39.Yuan J, Yang J, Zhuang Z, Yang Y, Lin L, Wang S. Thrombolytic effects of Douchi Fibrinolytic enzyme from Bacillus subtilis LD-8547 in vitro and in vivo. BMC Biotechnology. 2012;12, article 36 doi: 10.1186/1472-6750-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]


