Highlights
-
•
Description of recent advances in plant lipid metabolism.
-
•
Description of break-through achievements in plant metabolic engineering.
-
•
Insights into the practical applications of plant synthetic biology.
Abstract
The manipulation of plant seed oil composition so as to deliver enhanced fatty acid compositions suitable for feed or fuel has long been a goal of metabolic engineers. Recent advances in our understanding of the flux of acyl-changes through different key metabolic pools such as phosphatidylcholine and diacylglycerol have allowed for more targeted interventions. When combined in iterative fashion with further lipidomic analyses, significant breakthroughs in our capacity to generate plants with novel oils have been achieved. Collectively these studies, working at the interface between metabolic engineering and synthetic biology, demonstrate the positive fundamental and applied outcomes derived from such research.
Current Opinion in Plant Biology 2014, 19:68–75
This review comes from a themed issue on Physiology and metabolism
Edited by Sarah E O’Connor and Thomas P Brutnell
For a complete overview see the Issue and the Editorial
Available online 15th April 2014
http://dx.doi.org/10.1016/j.pbi.2014.04.001
1369-5266/© 2014 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Introduction
Plant seeds play a vital role in human life, providing multiple sources of food and fuel. This is predominantly derived from the storage compounds (oil, protein and carbohydrate) that the developing seed accumulates as energy reserves for catabolism during germination. The ability to harness and use these storage compounds has historically underpinned the transition from hunter-gather to agricultural-based society, and now continues to feed the ever-increasing global population. The predominant storage oil in seeds are neutral lipids such as triacylglycerol and given their significance for nutrition and industry, considerable effort has focussed on the desire to improve both the composition and yield of vegetable oils. However, the apparent simplicity of a seed (as the inert container of useful storage reserves) is misleading, since there is still much to learn about how these compounds are co-ordinately synthesized and compartmentalised. On the other hand, our ability to manipulate and tailor the composition of these reserves is steadily increasing, driven forward by advanced plant metabolic engineering/synthetic biology and informed by detailed metabolite analyses. The adoption of such multidisciplinary approaches has extended our understanding of seed lipid metabolism and, as will be discussed below, generated new specialized platforms for oil production. Indeed, progress in vegetable oil production is now extending beyond the seed and exciting insights are emerging about the possibility of oil production in vegetative tissue. Given the pressures on the carbon economy, exemplified by an increased demand and a declining supply of conventional fossil oil, progress in meeting the requirements for vegetable oil is timely. In this article, we will focus on the synthesis and manipulation of one specific type of storage reserve — triacylglycerol – since this represents one of the best examples of complex metabolic engineering in transgenic plants.
Development of crop metabolic engineering platforms for translation of specialty oil traits
Over the last two decades, the challenge for researchers has been the accumulation of novel fatty acids, which have beneficial functional groups or properties, into oilseeds with good agronomical traits. Although, metabolic engineering of oil-related traits has largely relied principally upon Arabidopsis as a host to test individual genes and gene combinations for modifying seed oils and more recently for engineering of oil production in vegetative tissues as described below. As proof of principal has been established in Arabidopsis, interest has grown in translation of these oil traits in established oilseed crops. For specialty oils, including those enriched in fish oil-type long chain polyunsaturated fatty acids (LC-PUFAs) and industrially valuable unusual fatty acid structures, attention has centred on non-food oilseed crops to mitigate the unintended mixing of food and specialized oil traits. Camelina (Camelina sativa) has emerged as a particularly attractive metabolic engineering host because it can be readily transformed using an Agrobacterium-based floral infiltration method [1]. With a relatively short-life cycle, complex metabolic engineering involving the stacking of numerous pathway genes is therefore feasible in Camelina [2•], as described below for LC-PUFA engineering. To facilitate genetic improvement of Camelina, a developing seed transcriptome was recently generated, and its utility was demonstrated by its use for engineering a high oleic acid oil trait for improved oil oxidative stability [2•]. This was achieved by the seed-specific RNAi suppression of FAD2 (Fatty Acid Desaturase 2) that controls Δ12 desaturation of oleic acid and FAE1 (fatty acid elongase 1) that mediates oleic acid elongation to C20 and C22 chain lengths [2•]. In addition to the use of Camelina, interest has arisen in Crambe (Crambe abyssinica) as a dedicated industrial oil crop, and the recent development of an Agrobacterium-mediated transformation system has enabled its use for metabolic engineering of seed oil traits [3]. As proof-of-principal efforts progress to engineer oil production in Arabidopsis leaves, the need for transformable biomass crops, such as sweet sorghum, will be required for translation of this trait.
Nutritional enhancement of seed lipids — omega-3 polyunsaturated fatty acids
Virtually all plant seeds contain storage lipids in the form of triacylglycerol (TAG). As the terminal point in seed oil biosynthesis, TAG is comprised of a glycerol backbone onto which three fatty acids are sequentially esterified. Plant oils are rich in C18 fatty acids, including the essential fatty acids linoleic acid (18:2Δ9,12,n−6; LA) and α-linolenic acid (18:3Δ9,12,15,n−3; ALA), but are devoid of LC-PUFAs, such as arachidonic acid (20:4Δ5,8,11,14, n−6; ARA), eicosapentaenoic acid (20:5Δ5,8,11,14,17,n−3; EPA) and docosahexaenoic acid (22:6Δ4,7,10,13,16,19,n−3; DHA), which typically only enter the human diet as oily fish. The health benefits of the omega-3 LC-PUFAs EPA and DHA are now well-established [4], and the omega-6 ARA is important for infant nutrition [5]. Given the desire for a sustainable supply of LC-PUFA, efforts have focussed on enhancing the composition of vegetable oils to include the essential LC-PUFAs. The omega-3 forms, specifically EPA and DHA, have been targeted with the ultimate goal of producing a terrestrial plant-based source of these so-called fish oils. Although historically considerable effort has been expended towards this goal (e.g. [6–10], reviewed in [11,12•]), efficient modification of seed oil profiles to include these non-native fatty acids has until recently met with limited success. This is despite the early functional characterisation of all the genes required for the primary biosynthesis of EPA and DHA from a range of lower eukaryotes such as algae, diatoms and oomycetes [12•,13]. Latterly, two different approaches have shown important advances, both focussed on overcoming the inherent metabolic bottlenecks previously identified as rate-limiting in the heterologous reconstitution of this pathway in transgenic plants [12•]. Petrie et al. [14] first developed a leaf-based transient expression system to identify a set of omega-3 LC-PUFA biosynthetic genes with high enzyme activities and desired substrate (acyl-CoA) preference. They also co-expressed the master seed regulator WRI1, resulting in the ectopic expression of seed-specific metabolic pathways and the synthesis of seed storage reserves such as TAG, but also facilitating the expression of these omega-3 LC-PUFA transgenes under the control of seed-specific promoters. The utility of this approach allowed for the rapid validation of seed-specific constructs, which would otherwise be dependent on stable transformation [14]. With this knowledge the authors were then able to assemble a large T-DNA construct for stable seed-specific transformation of Arabidopsis, and reported a high level of DHA (but not EPA) in seed oil [15•]; a similar approach yielded lines accumulating significant ARA [16]. In an alternative approach to the identification of optimal enzyme activities, Sayanova et al. [17] used heterologous yeast expression combined with acyl-CoA profiling to select efficient activities, which were then validated by stable expression in Arabidopsis and camelina. A systematic study was then carried out to identify preferential combinations of biosynthetic enzymes (desaturases and elongases), resulting in the evaluation of 12 different constructs (of 3–7 transgenes) in Arabidopsis [18•]. The efficacy of each enzyme combination was validated using lipidomic analysis to inform each subsequent iteration. Using this approach, the authors were able to show a 10-fold increase in the accumulation of EPA [15•]. Collectively, these recent studies demonstrate that in the case of the model Arabidopsis, accumulation of significant (meaning similar to that found in fish oils) levels of EPA or DHA is now achievable. Recently, Camelina seed oil was engineered to accumulate EPA and DHA [19••] — in this study, the authors report the highest levels of C20+ omega-3 LC-PUFAs in a recognised oilseed crop — 31% EPA or 25% EPA plus DHA. This represents not only a new source of fish oils, but a significant demonstration of the power of plant metabolic engineering to overwrite endogenous lipid metabolism.
Making industrial oils in seeds
A long-term goal of oilseed metabolic engineering has been the generation of fatty acid traits targeted for industrial applications. A particular focus has been the transfer of biosynthetic and metabolic pathways for unusual fatty acids, such as hydroxy and epoxy fatty acids (used for lubricants, nylon precursors, and plasticizers) from non-agronomic plant species to existing oilseed crops. After more than a decade of gene discovery efforts and numerous basic and translational breakthroughs [20], many challenges remain for achieving levels of unusual fatty acid accumulation in engineered oilseeds that approach the high levels typically found in seeds of non-agronomic gene source species. This is particularly true for metabolic pathways involving the production of unusual fatty acids from functionally divergent Δ12 desaturases (or, FAD2). The most studied of these is the pathway for production of ricinoleic acid (12 OH-18:1Δ9) and related C18-C22 omega-6 hydroxylated fatty acids. These fatty acids are generated by variant FAD2 hydroxylases that principally use oleic acid (18:1Δ9) bound to phosphatidylcholine as a substrate. Castor bean (Ricinus communis), which has limited commercial cultivation because of the high content of ricin toxins in it seeds, accumulates ricinoleic acid to 90% of the fatty acids of its seed oil through this pathway. To date, the transfer of the castor FAD2-related hydroxylase together with specialized castor acyltransferases, including the castor diacylglycerol acyltransferase 2 (DGAT2) and phospholipid-diacylglycerol acyltransferase 1 (PDAT1), have yielded only 20–30% ricinoleic acid and other derivative hydroxy fatty acids in transgenic Arabidopsis seeds [21,22]. Results from recent labeling studies of Arabidopsis seeds engineered to express the castor bean hydroxylase indicated an inefficiency in diacylglycerol (DAG) flux through phosphatidylcholine (PC) following oleate hydroxylation for the formation triacylglycerol containing hydroxylated fatty acids (summarised in Figure 1) [23•]. Consistent with this, the Arabidopsis rod1 mutant defective in phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT)-mediated flux of DAG through PC, displayed reduced hydroxy fatty acid synthesis in seeds engineered for castor bean hydroxylase expression [24]. The substitution of Arabidopsis PDCT with the castor bean PDCT in this background yielded increased hydroxy fatty acid accumulation, demonstrating that a specialized castor bean PDCT activity is necessary for high level hydroxy fatty acid accumulation [24].
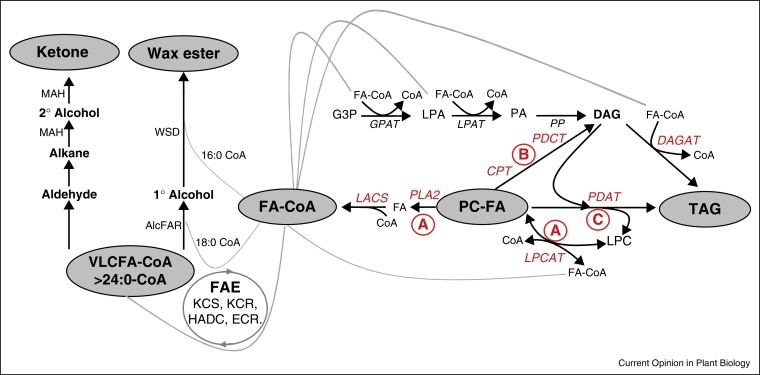
Figure 1.
Schematic diagram of the main lipid classes and biochemical pathways involved in the production of TAG and other lipids in developing seeds. There are three mechanisms for the removal of LC-PUFA from PC to make it then available for incorporation into TAG (mechanisms A, B, and C) — please see [23•] for full description. For mechanism A, FAs esterified to phosphatidylcholine (PC) (such as FAD2-like products) are under a constant dynamic exchange with the acyl-CoA pool in a process described as acyl editing. Removal of FAs from PC can proceed by the reverse action of acyl-CoA:lysophosphatidylcholine acyltransferase (LPCAT) or the combined action of phospholipase A2 PLA2 and long-chain acyl-CoA synthetase, LACS. Once in the acyl-CoA pool, acyl-CoAs and glycerol-3-phosphate (G3P) can be converted into TAG by the consecutive action of acyl-CoA:glycerol 3-phosphate acyltransferase (GPAT), acyl-CoA:lysophosphatidic acid acyltransferase (LPAT), phosphatidic acid phosphatase (PAP), and acyl-CoA:diacylglycerol acyltransferase (DGAT). For mechanism B, the PC head group can be removed, producing a DAG molecule containing the same FAs. This reaction can proceed by four enzymatic mechanisms: phospholipase C, phospholipase D along with PAP, the reverse action of CDP-choline:diacylglycerol cholinephosphotransferase (CPT), or the recently identified phosphatidylcholine:diacyglycerol cholinephosphotransferase, (PDCT). The DAG produced by these mechanisms can then be utilized to produce TAG. For mechanism C, direct transfer of the sn-2 FA of PC to the sn-3 of DAG produces TAG via a phospholipid:diacylglycerol acyltransferase (PDAT). In addition, the acyl-CoA pool generated by mechanism A or resulting from direct export from the plastid can be accessed by additional enzymes such as the endogenous FAE elongase system to generate very long chain fatty acids (VLCFAs) or heterologous activities such as those involved in wax ester or ketone biosynthesis.
Defective flux of acyl chains from PC to TAG in engineered seeds appears to be a common bottleneck for the accumulation of unusual fatty acids. This was previously noted for conjugated fatty acid accumulation in Arabidopsis and soybean seeds engineered to express FAD2-related fatty acid conjugases that convert either the Δ9 or Δ12 double bond of linoleic acid linked to PC into two conjugated double bonds, which enhances the drying properties of vegetable oils [25]. More recently, studies with the Escherichia coli cyclopropane synthase illustrated how the transgenic expression of this enzyme in Arabidopsis seeds effectively converted the Δ9 double bond of oleic acid into a cyclopropane ring, a result which confers vegetable oils with a wide range of industrial functionalities [26]. This reaction uses oleic acid bound primarily to the sn-1 position of PC as a substrate [26]. The seed-specific co-expression of the E. coli cyclopropane synthase with a lysophosphatidic acid acyltransferase (LPAT) from Sterculia foetida seeds, which naturally accumulate high levels of cyclopropane fatty acids, resulted in small increases in cyclopropane fatty acid accumulation. LPAT catalyzes the acylation of the sn-2 position of the glycerol backbone in the TAG biosynthetic pathway. Consistent with this activity, increased amounts of cyclopropane fatty acids in TAG in the engineered Arabidopsis seeds was due primarily to enhanced accumulation at the TAG sn-2 position [26]. Despite this, cyclopropane fatty acid levels were disproportionately high in PC, accounting for >40% of the PC fatty acids compared to ∼9% of the TAG fatty acids [26]. These results underscore the significance of the metabolic bottleneck for flux of unusual fatty acids into TAG following their synthesis on PC as a major limitation for producing industrial fatty acids in engineered oilseeds.
Recent efforts to produce oils with industrial functionality have also targeted pathways that use only acyl-CoA substrates to produce novel oils, bypassing the intricacies of unusual fatty acid biosynthetic pathways involving PC-linked substrates. One such pathway is that for wax ester biosynthesis. Wax esters lack diacylglycerol backbones and consist of a fatty acid linked through an ester bond to a fatty alcohol. These molecules are desirable for use as high temperature lubricants and are synthesized in a two-step biosynthetic pathway involving conversion of a fatty acyl-CoA to a fatty alcohol via a fatty alcohol reductase (FAR) and condensation of the fatty alcohol with an acyl-CoA via a wax synthase (WS). Recently, Heilmann et al. demonstrated the feasibility of co-expressing an endoplasmic reticulum (ER)-localized mouse WS with a mouse peroxisomal FAR retargeted for ER localization to generate wax esters in Arabidopsis seeds with principally C18 and C20 fatty acid and fatty alcohol components [27]. By linking theses enzymes at their amino-termini to oleosin, an oil body structural protein, and fluorescent protein tags, wax ester contents as high as 45 μg/mg seed weight or ∼15% of the total seed oil were achieved [27]. In addition, wax esters highly enriched in oleoyl alcohol and oleic acid moieties were obtained by expression of the mouse enzymes in an Arabidopsis fad2/fae1 mutant that has high levels of oleic acid in its seeds [27].
Metabolic engineering of very long-chain fatty acid production also offers an opportunity for generating industrial oils through acyl-CoA reactions that bypass PC-linked biosynthetic pathways. Crambe seed oil is naturally enriched in erucic acid (22:1; ∼60% of the total oil), a C22 monounsaturated fatty acid [28•]. This fatty acid is a precursor of erucamides, which are slip agents in polyethylene film. To address this need for high-erucic acid vegetable oils, a newly developed transformation protocol was used for introduction of three transgenes with seed-specific promoters: FAD2 RNAi transgene to increase oleic acid content; Brassica napus FAE1 to enhance elongation of oleic acid to erucic acid; and a specialized Limnanthes douglasii LPAT to increase erucic acid incorporation into the sn-2 position of TAG [12•]. The result of this multi-gene engineering effort was an increase in erucic acid of up to 73% of the oil in the top performing lines [28•]. Additional analyses of these seeds using radiolabeling indicated that compared to other oilseeds, including safflower (Carthamus tictorius) seeds, Crambe seeds are particularly effective at producing high levels of erucic acid through acyl-CoA reactions, due to a low PDCT activity that effectively precludes exchange of fatty acids between DAG and PC [29•]. Labeling studies of the engineered crambe seeds at different developmental stages revealed that the majority of erucic acid is synthesized at later stages of seed development. Based on this finding, enhanced erucic acid production could be achieved by engineering initiation of biosynthetic and metabolic pathways for erucic acid at earlier seed development stages [29•].
Oil production in green biomass: metabolic engineering of high oilseed-like triacylglycerol accumulation in vegetative tissues
The pressing need to produce more energy from plant biomass has encouraged attempts to produce oil in vegetative tissues. Although seeds and some fruit pericarps (e.g. oil palm, olive and avocado) are by far the largest source of plant produced oils, many other tissues are capable of synthesizing triacylglycerols and a number of studies have reported the presence of cytosolic lipid droplets in leaf mesophyll cells [30]. TAGs notably accumulate during senescence in leaves, under stress and in Arabidopsis mutants disrupted in ER to chloroplast lipid trafficking. Nevertheless, the oil content of vegetative tissues is typically very low in the majority of plant species [31••,32].
The possibility of producing TAGs for biodiesel in leaves and other vegetative tissues has recently attracted considerable interest [33]. A number of studies have demonstrated that TAG accumulation can be increased by ectopic expression of individual biosynthetic enzymes such as acyl CoA:diacylglycerol acyltransferase (DGAT) or monoacyglycerol acyltransferases MGAT [34,35•], transcription factors such as LEAFY COTYLEDON1 (LEC1), LEC2 or WRINKLED1 (WRI1) [36,37,38] that control seed development and maturation, or by mutating genes involved in TAG and fatty acid turnover such as COMATOSE (CTS2), SUGAR DEPENDENT1 (SPD1) or COMPARATIVE GENE IDENTIFICATION-58 (CGI58) [31••,39,40]. However, in most of these studies increases in TAG leaf content was only very modest and/or dependent on the supply of carbohydrates. Since key enzymes for both oil synthesis and breakdown are expressed in vegetative tissue it was suggested that achieving substantial levels of storage lipid in leaf biomass required the re-orientation of carbon flux into TAG, as indicated by the additional effect observed when overexpressing LEC2 in the cts2 β-oxidation mutant [39,41••]. Recently, several groups have reported improved oil accumulation in leaves by modifying the expression of gene pairs i.e. combinations of either WRI1 or LEC2 [32,34] or an engineered oleosin [42] with DGAT1 or PDAT [43]. However, dramatically increased TAG levels (exceeding 15% of dry weight in vegetative tissue) have only been achieved via integrated metabolic approaches (so-called ‘Push, Pull and Protect’) enhancing fatty acid and TAG synthesis while preventing lipolysis [31••,41••]. Latterly, the identification of non-seed proteins involved in the binding and stabilization of lipid-rich particles in the cytosol of plant cells [44] has identified a new aspect of the cellular machinery regulating the packaging of triacylglycerol's in plant vegetative tissue.
It will be interesting to investigate whether oil accumulation in green biomass can be further improved without severely impacting photosynthesis and plant development. One possibility for achieving this could be the use of senescence induced promoters to engineer plants in which TAG accumulation is initiated only after leaves have reached their maximum size [33]. Another might directly connect carbon fixation to fatty acid biosynthesis; introducing a functional glycolytic pathway converting 3-phosphoglycerate to phosphenolpyruvate. Whichever possibility is adopted, the goal of using photosynthetic cells to accumulate very high levels of oil is attractive. However, matching the accumulations seen in seeds able to accumulate more than 35% TAG (% of dry weight) remains a formidable metabolic engineering challenge.
Conclusions
The engineering of economically viable levels of LC-PUFAs in camelina seeds and ‘ultra-high’ levels of erucic acid in crambe seeds represent recent successes in the translation of specialty fatty acid traits to oilseed crops. The ability to achieve high amounts of unusual fatty acid production by transfer of PC-linked biosynthetic and metabolic pathways from seeds of non-agronomic species to seeds of either the Arabidopsis model or existing oilseed crops remains elusive to metabolic engineers. Solving bottlenecks that limit the synthesis and accumulation of these fatty acids will require more in-depth understanding of fatty acid metabolic pathways in seeds that naturally accumulate high levels of unusual fatty acids. It will also be necessary to determine the relative contributions of different enzymes specialized for these pathways in the native species and to possibly down-regulate non-productive, competing pathways in seeds of host oilseeds- this is summarised in Figure 2. The integrated approach of engineering transcription factors that up regulate fatty acid synthesis and overexpression of TAG biosynthetic enzymes to sequester the enhanced fatty acid production coupled with downregulation of TAG catabolic enzymes is proving to be an effective strategy for generating substantial levels of oil in leaves of model plants. Successful translation of these strategies in existing biomass crops such as sweet sorghum will likely also require the selection of promoters for transgenes that allow the persistence of accumulated oil through leaf senescence. Future success of metabolic engineering of specialty oil traits will likely rely on more predictability of genetic modifications on fatty acid and oil metabolism in seeds and other target tissues of crop hosts by use of techniques, such as mass spectrometry-based lipidomics that was essential for optimizing LC-PUFA engineering in camelina seeds, as described by Ruiz et al. [19••]. Similarly, emerging techniques such as matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI) as applied recently to engineering of oil pathways in Camelina seeds [45•] and tobacco leaves [32] are providing insights into spatial heterogeneity of fatty acid compositions in specific lipid classes among cell types in target tissues to enable for more informed metabolic engineering. Ultimately, the task of integrating a small number of transgene-derived activities with a much greater number of endogenous metabolic processes still remains an exciting challenge.
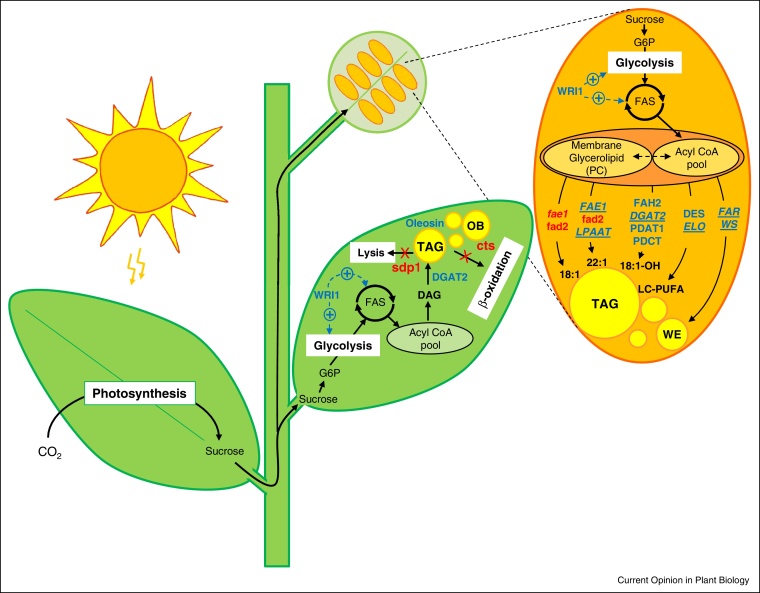
Figure 2.
Schematic representation of metabolic engineering strategies for manipulation of oil content and composition in vegetative and seed tissues. Different approaches described in this article are highlighted. Blue: target genes suitable for overexpression; Red: target genes for inactivation by mutation or RNAi constructs. Genes encoding enzymes using acyl-CoA substrates are underlined. FAS = plastid localised fatty acid synthase
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Rothamsted Research receives grant-aided support from the BBSRC (grant no.BBS/E/C/00005207 and BB/J00166X/1) (UK).
References
- 1.Lu C., Kang J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 2008;27:273–278. doi: 10.1007/s00299-007-0454-0. [DOI] [PubMed] [Google Scholar]
- 2•.Nguyen H.T., Silva J.E., Podicheti R., Macrander J., Yang W., Nazarenus T.J., Nam J.W., Jaworski J.G., Lu C., Scheffler B.E. Camelina seed transcriptome: a tool for meal and oil improvement and translational research. Plant Biotechnol J. 2013;11:759–769. doi: 10.1111/pbi.12068. [DOI] [PubMed] [Google Scholar]; This report provides a deep transcriptome of developing Camelina seeds and seed storage protein and lipid gene databases to facilitate genetic improvement of Camelina seeds. The utility of the transcriptome was demonstrated by its use for metabolic engineering of high oleic acid and 2S seed storage protein-null lines of Camelina.
- 3.Li X., Fan J., Gruber J., Guan R., Frentzen M., Zhu L.H. Efficient selection and evaluation of transgenic lines of Crambe abyssinica. Front Plant Sci. 2013;4:162–166. doi: 10.3389/fpls.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saravanan P., Davidson N.C., Schmidt E.B., Calder P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540–550. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 5.Janssen C.I., Kiliaan A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2013;53C:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Abbadi A., Domergue F., Bauer J., Napier J.A., Welti R., Zähringer U., Cirpus P., Heinz E. Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell. 2004;16:2734–2748. doi: 10.1105/tpc.104.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G., Truksa M., Datla N., Vrinten P., Bauer J., Zank T., Cirpus P., Heinz E., Qiu X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat Biotechnol. 2005;23:1013–1017. doi: 10.1038/nbt1107. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann M., Wagner M., Abbadi A., Fulda M., Feussner I. Metabolic engineering of ω-3 very long chain polyunsaturated fatty acid production by an exclusively acyl-CoA-dependent pathway. J Biol Chem. 2008;283:22352–22362. doi: 10.1074/jbc.M802377200. [DOI] [PubMed] [Google Scholar]
- 9.Cheng B., Wu G., Vrinten P., Falk K., Bauer J., Qiu X. Towards the production of high levels of eicosapentaenoic acid in transgenic plants: the effects of different host species, genes and promoters. Transgenic Res. 2010;19:221–229. doi: 10.1007/s11248-009-9302-z. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-López N., Haslam R.P., Venegas-Calerón M., Li T., Bauer J., Napier J.A., Sayanova O. Enhancing the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Arabidopsis thaliana via iterative metabolic engineering and genetic crossing. Transgenic Res. 2012;21:1233–1243. doi: 10.1007/s11248-012-9596-0. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-López N., Sayanova O., Napier J.A., Haslam R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J Exp Bot. 2012;63:2397–2410. doi: 10.1093/jxb/err454. [DOI] [PubMed] [Google Scholar]
- 12•.Haslam R.P., Ruiz-Lopez N., Eastmond P., Moloney M., Sayanova O., Napier J.A. The modification of plant oil composition via metabolic engineering — better nutrition by design. Plant Biotechnol J. 2013;11:157–168. doi: 10.1111/pbi.12012. [DOI] [PubMed] [Google Scholar]; This is a comprehensive review which covers in some detail the metabolic bottlenecks which constrain the accumulation of non-native fatty acids, and also strategies to overcome these blockades.
- 13.Walsh T.A., Metz J.G. Producing the omega-3 fatty acids DHA and EPA in oilseed crops. Lipid Technol. 2013;25:103–105. [Google Scholar]
- 14.Petrie J.R., Shrestha P., Liu Q., Mansour M.P., Wood C.C., Zhou X.R., Nichols P.D., Green A.G., Singh S.P. Rapid expression of transgenes driven by seed-specific constructs in leaf tissue: DHA production. Plant Methods. 2010;6:8–12. doi: 10.1186/1746-4811-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Petrie J.R., Shrestha P., Zhou X.R., Mansour M.P., Liu Q., Belide S., Nichols P.D., Singh S.P. Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS ONE. 2012;7:e49165. doi: 10.1371/journal.pone.0049165. [DOI] [PMC free article] [PubMed] [Google Scholar]; This important study reports the highest level yet achieved of the important omega-3 LC-PUFA DHA in the model system Arabidopsis. The authors also describe the assembly of a single large T-DNA insert in which seven independent expression cassettes are used to direct the synthesis of DHA. The accumulation of DHA in TAG is also demonstrated.
- 16.Petrie J.R., Shrestha P., Belide S., Mansour M.P., Liu Q., Horne J., Nichols P.D., Singh S.P. Transgenic production of arachidonic acid in oilseeds. Transgenic Res. 2012;21:139–147. doi: 10.1007/s11248-011-9517-7. [DOI] [PubMed] [Google Scholar]
- 17.Sayanova O., Ruiz-Lopez N., Haslam R.P., Napier J.A. The role of Δ6-desaturase acyl-carrier specificity in the efficient synthesis of long-chain polyunsaturated fatty acids in transgenic plants. Plant Biotechnol J. 2012;10:195–206. doi: 10.1111/j.1467-7652.2011.00653.x. [DOI] [PubMed] [Google Scholar]
- 18•.Ruiz-Lopez N., Haslam R.P., Usher S.L., Napier J.A., Sayanova O. Reconstitution of EPA and DHA biosynthesis in Arabidopsis: iterative metabolic engineering for the synthesis of n−3 LC-PUFAs in transgenic plants. Metab Eng. 2013;17:30–41. doi: 10.1016/j.ymben.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the systematic evaluation of multiple combinations of genes for the transgenic synthesis of EPA. Detailed lipidomic analysis underpinned these iterations and allowed for a ten-fold increase in the achieved levels.
- 19••.Ruiz-Lopez N., Haslam R.P., Napier J.A., Sayanova O. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J. 2013 doi: 10.1111/tpj.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]; This significant advance reports the accumulation of EPA and DHA in the crop Camelina sativa to levels equivalent to found in fish oils. These omega-3 LC-PUFAs were shown to accumulate in TAG to a level of ∼24% of total fatty acids. Thus, this demonstrates the power of plant metabolic engineering/applied synthetic biology to deliver on previously made promises.
- 20.Bates P.D., Stymne S., Ohlrogge J. Biochemical pathways in seed oil synthesis. Curr Opin Plant Biol. 2013;16:358–364. doi: 10.1016/j.pbi.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Burgal J., Shockey J., Lu C., Dyer J., Larson T., Graham I., Browse J. Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J. 2008;6:819–831. doi: 10.1111/j.1467-7652.2008.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Erp H., Bates P.D., Burgal J., Shockey J., Browse J. Castor phospholipid:diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol. 2011;155:683–693. doi: 10.1104/pp.110.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Bates P.D., Browse J. The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 2011;68:387–399. doi: 10.1111/j.1365-313X.2011.04693.x. [DOI] [PubMed] [Google Scholar]; The study provides evidence for defective flux of hydroxy fatty acids from their site of synthesis on phosphatidylcholine to storage in triacylglycerol via diacylglycerol as a major limitation for hydroxy fatty acid accumulation in engineered Arabidopsis seeds. Combined with previous findings from other researchers, this may be a general bottleneck for metabolic engineering of high levels of production of unusual fatty acids derived from phosphatidylcholine-linked biosynthetic reactions.
- 24.Hu Z., Ren Z., Lu C. The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol. 2012;158:1944–1954. doi: 10.1104/pp.111.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahoon E.B., Dietrich C.R., Meyer K., Damude H.G., Dyer J.M., Kinney A.J. Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds. Phytochemistry. 2006;67:1166–1176. doi: 10.1016/j.phytochem.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Yu X.H., Prekash R., Sweet M., Shanklin J. Coexpressing E. coli cyclopropane synthase with Sterculia foetida lysophosphatidic acid acyltransferase enhances cyclopropane fatty acid accumulation. Plant Physiol. 2014;164:455–465. doi: 10.1104/pp.113.230953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heilmann M., Iven T., Ahmann K., Hornung E., Stymne S., Feussner I. Production of wax esters in plant seed oils by oleosomal cotargeting of biosynthetic enzymes. J Lipid Res. 2012;53:2153–2161. doi: 10.1194/jlr.M029512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Li X., van Loo E.N., Gruber J., Fan J., Guan R., Frentzen M., Stymne S., Zhu L.H. Development of ultra-high erucic acid oil in the industrial oil crop Crambe abyssinica. Plant Biotechnol J. 2012;10:862–870. doi: 10.1111/j.1467-7652.2012.00709.x. [DOI] [PubMed] [Google Scholar]
- 29•.Guan R., Lager I., Li X., Stymne S., Zhu L.H. Bottlenecks in erucic acid accumulation in genetically engineered ultrahigh erucic acid Crambe abyssinica. Plant Biotechnol J. 2013 doi: 10.1111/pbi.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]; These two papers describe the successful use of crambe for metabolic engineering of a high value industrial oil trait. Seeds from wild type and engineered crambe lines were compared with other oilseeds in detailed biochemical studies to identify metabolic constraints that limit the engineering of high levels of erucic acid production.
- 30.Lin W., Oliver D.J. Role of triacylglycerols in leaves. Plant Sci. 2008;175:233–237. [Google Scholar]
- 31••.Kelly A.A., van Erp H., Quettier A.L., Shaw E., Menard G., Kurup S., Eastmond P.J. The SUGAR-DEPENDENT1 lipase limits triacylglycerols accumulation in vegetative tissues of Arabidopsis. Plant Physiol. 2013;162:1282–1289. doi: 10.1104/pp.113.219840. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that combined manipulation of carbohydrate supply, fatty acid synthesis, TAG synthesis and TAG breakdown can lead to substantial oil accumulation in vegetative tissue in Arabidopsis, particularly in heterotrophic tissue (i.e. roots and stems). This article also stimulates an interesting discussion about the physiological role TAG in vegetative tissue.
- 32.Vanhercke T., El Tahchy A., Shrestha P., Zhou X.R., Singh S.P., Petrie J.R. Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett. 2013;587:364–369. doi: 10.1016/j.febslet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Durrett T.P., Benning C., Ohlrogge J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008;54:593–607. doi: 10.1111/j.1365-313X.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- 34.Andrianov V., Borisjuk N., Pogrebnyak N., Brinker A., Dixon J., Spitsin S., Flynn J., Matyszczuk P., Andryszak K., Laurelli M. Tobacco as a production platform for biofuel:overexpression of Arabidopsis DGAT and LEC2genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol J. 2010;8:277–287. doi: 10.1111/j.1467-7652.2009.00458.x. [DOI] [PubMed] [Google Scholar]
- 35•.Petrie J.R., Vanhercke T., Shrestha P., El Tahchy A., White A., Zhou X.R., Liu Q., Mansour M.P., Nichols P.D., Singh S.P. Recruiting a new substrate for triacylglycerol synthesis in plants: the monoacylglycerol acyltransferase pathway. PLoS ONE. 2012;7:e35214. doi: 10.1371/journal.pone.0035214. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the concept of a new method of increasing oil content in vegetative tissues by using MAG as a substrate for TAG biosynthesis via a route that is independent and complementary to the endogenous Kennedy pathway and other TAG synthesis routes.
- 36.Cernac A., Benning C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 2004;40:575–585. doi: 10.1111/j.1365-313X.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- 37.Santos Mendoza M., Dubreucq B., Miquel M., Caboche M., Lepiniec L. L EAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Lett. 2005;579:4666–4670. doi: 10.1016/j.febslet.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 38.Sanjaya, Miller R., Durrett T.P., Kosma D.K., Lydic T.A., Muthan B., Koo A.J., Bukhman Y.V., Reid G.E., Howe G.A. Altered lipid composition and enhanced nutritional value of Arabidopsis leaves following introduction of an algal diacylglycerol acyltransferase 2. Plant Cell. 2013;25:677–693. doi: 10.1105/tpc.112.104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slocombe S.P., Cornah J., Pinfield-Wells H., Soady K., Zhang Q., Gilday A., Dyer J.M., Graham I.A. Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol J. 2009;7:694–703. doi: 10.1111/j.1467-7652.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 40.James C.N., Horn P.J., Case C.R., Gidda S.K., Zhang D., Mullen R.T., Dyer J.M., Anderson R.G., Chapman K.D. Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants. Proc Natl Acad Sci U S A. 2010;107:17833–17838. doi: 10.1073/pnas.0911359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Vanhercke T., El Tahchy A., Liu Q., Zhou X.R., Shrestha P., Divi U.K., Ral J.P., Mansour M.P., Nichols P.D., James C.N., Horn P.J. Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol J. 2013 doi: 10.1111/pbi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article report accumulation of high levels of TAG in green vegetative tissue (15% by dry weight) in tobacco without severely impacting plant development. This demonstrates the technical feasibility of developing a vegetative TAG production platform in a biomass crop resulting in oil yields comparable to current oilseed crops.
- 42.Winichayakul S., Scott R.W., Roldan M., Hatier J.H., Livingston S., Cookson R., Curran A.C., Roberts N.J. In vivo packaging of triacylglycerols enhances Arabidopsis leaf biomass and energy density. Plant Physiol. 2013;162:626–639. doi: 10.1104/pp.113.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J., Yan C., Zhang X., Xu C. Dual role for phospholipid:diacylglycerol acyltransferase: enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves. Plant Cell. 2013;25:3506–3518. doi: 10.1105/tpc.113.117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horn P.J., James C.N., Gidda S.K., Kilaru A., Dyer J.M., Mullen R.T., Ohlrogge J.B., Chapman K.D. Identification of a new class of droplet associated proteins in plants. Plant Physiol. 2013;162:1926–1936. doi: 10.1104/pp.113.222455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Horn P.J., Silva J.E., Anderson D., Fuchs J., Borisjuk L., Nazarenus T.J., Shulaev V., Cahoon E.B., Chapman K.D. Imaging heterogeneity of membrane and storage lipids in transgenic Camelina sativa seeds with altered fatty acid profiles. Plant J. 2013;76:138–150. doi: 10.1111/tpj.12278. [DOI] [PubMed] [Google Scholar]; This report demonstrates the use of matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI) for imaging specific molecular species of triacylglycerol and phosphatidylcholine in camelina seeds engineered for several altered fatty acid profiles. The technique showed marked differences in triacylglycerol and phosphatidylcholine molecular species between cotyledons and embryonic axes of engineered seeds. These findings provided predictive metabolic engineering strategies for improvement of the desired traits.