Abstract
Emotions can color our attitudes toward unrelated objects in the environment. Prior evidence suggests that such emotional coloring is particularly strong when emotion-triggering information escapes conscious awareness. But, is emotional reactivity stronger following non-conscious versus conscious emotional provocation? Or does conscious processing specifically change the association between emotional reactivity and evaluations of unrelated objects? In this study, we independently indexed emotional reactivity and coloring as a function of emotional-stimulus awareness to disentangle these accounts. Specifically, we recorded skin conductance responses (SCRs) to spiders and fearful faces, along with subsequent preferences for novel neutral faces during visually aware and unaware states. Fearful faces increased SCRs comparably in both aware and unaware conditions. Yet, only when visual awareness was precluded did SCRs to fearful faces predict decreased likeability of neutral faces. These findings suggest a regulatory role for conscious awareness in breaking otherwise automatic associations between physiological reactivity and evaluative emotional responses.
Emotional events rapidly mobilize our central and peripheral nervous systems toward adaptive action. As affective changes inform decision making regarding the people and contexts we want to approach or avoid, it is important they do so accurately. Here, we report that conscious awareness changes the relationship between objectively measured emotional responses and subsequent appraisal of unrelated objects in the environment, effectively rescuing neutral stimuli from otherwise tainted first impressions.
Conscious awareness to a sensory stimulus refers to processing accompanied by subjective perceptual experience, about which one can report under normal circumstances [also called conscious access; (Block, 2005; Dehaene & Changeux, 2011; Lau & Rosenthal, 2011)]. Biologically relevant emotional stimuli do not require conscious awareness to mobilize early-appraisal by central structures like the amygdala (Jiang & He, 2006), or to activate facial expressive (Dimberg, Thunberg, & Elmehed, 2000) and autonomic nervous systems (Ohman & Soares, 1994). For example, the amygdala responds to facial expressions even when their visibility is prevented in experiments adopting backward masking [for a quantitative meta analysis, see (Brooks et al., 2012)] or interocular suppression (Jiang & He, 2006; Pasley, Mayes, & Schultz, 2004; Williams et al., 2004), coupled with rigorous procedures to verify stimulus unawareness (e.g., via 2-alternative-forced-choice reporting paradigms) (e.g., Jiang & He, 2006). With regard to the type of functional processing enabled in such reduced-awareness states, the amygdala can promote rapid mobilization of the organism via its downstream projections to neuromodulator-releasing brainstem nuclei and the hypothalamic-pituitary-adrenal axis [reviewed in (Ledoux, 2012)]. Accordingly, the magnitude of skin-conductance responses, a marker of sympathetic nervous system activity under the excitatory influence of the amygdala (Mangina & Beuzeron-Mangina, 1996), increases following the presentation of masked fear-relevant facial stimuli [e.g., (Ohman & Soares, 1994; Olsson & Phelps, 2004)]. Taken together, relevant studies suggest that conscious awareness of an affective stimulus is not a pre-requisite for it to engender peripheral-physiological responses indicative of initial emotional reactivity, and that the amygdala may support the central representations that drive such responses in states of reduced visual awareness. What then, if any, is the role of conscious awareness in the processing of emotional stimuli?
Conscious awareness of an emotional stimulus has been suggested to render affective processing qualitatively different: In the “affect-as-information” theory, Schwarz & Clore (1983) proposed that affect from a non-identified source is particularly prone to influence one’s evaluations of unrelated objects, as we are motivated to seek explanations for our affective states (Schwarz & Clore, 1983; Wyer & Carlston, 1979). If affect elicited by a stimulus outside of awareness is more likely to be misattributed than when its source is available and correctly identified, binding of affect to incidental stimuli should be greater when conscious awareness of emotional stimuli is prevented. Accordingly, in the work of Murphy & Zajonc (1993), only when happy and angry faces were presented below the threshold for visual awareness did their affective content color preferences for subsequently presented, otherwise neutral Chinese ideographs [replicated in (Rotteveel, De Groot, Geutskens, & Phaf, 2001)]. Similarly, the finding that facial expressions of which we are unaware can color our subsequent evaluations of neutral stimuli has been confirmed with additional awareness-manipulation techniques including attentional crowding (Kouider, Berthet, & Faivre, 2011) and continuous flash suppression [(Anderson, Siegel, White, & Barrett, 2012; Almeida, Pajtas, Mahon, Nakayama, & Caramazza, 2012), but also see (Faivre, Berthet, & Kouider, 2012)]. Moreover, greater affective coloring during subliminal, relative to supraliminal, processing of emotional information was also reported when using valenced odors as the emotional stimuli (Li, Moallem, Paller, & Gottfried, 2007). In summary, the perceived “positivity” of a neutral stimulus can be modulated according to the affective content of a temporally preceding, non-perceived stimulus, with conscious awareness weakening such effects.
However, how conscious awareness impacts affective coloring following an emotional provocation remains ambiguous: Does conscious awareness of emotional stimuli reduce (a) the magnitude of emotional reactivity per se, thereby reducing the affective information that can bind to unrelated stimuli [as suggested by increased physiological reactivity to emotional stimuli presented to the subjectively blind visual field of individuals with affective blindsight; (Tamietto et al., 2009)]? Or, does conscious awareness instead attenuate (b) the relationship between emotional reactions and subsequent appraisals of the environment, whereby emotional responses preferentially color evaluations of unrelated stimuli in unaware states [as predicted by the affect-as-information theory (Schwarz & Clore, 1983)]? If the latter is true, finding that the extent of affective coloring reflects the magnitude of the provoked emotional reactivity only when individuals are unaware of the emotional stimulus would constitute a strong demonstration of such a mechanism. Because studies to date have not examined how conscious awareness of an emotional stimulus impacts emotional reactivity (e.g., via peripheral-physiological channels), subsequent coloring, and their association simultaneously, these alternative accounts remain unaddressed.
We sought to adjudicate between these accounts by simultaneously measuring physiological responses to neutral stimuli (flowers) and two categories of emotional stimuli (fearful faces and spiders) presented in blocks (see Fig. 1), as well as by collecting evaluative judgments of misattribution of affect to novel neutral faces. We manipulated conscious awareness in a within-subjects fashion by employing continuous flash suppression [CFS; (Tsuchiya & Koch, 2005)], a powerful method that capitalizes on the phenomenon of binocular rivalry. In CFS, a continuously flashing colorful pattern is presented to one eye (usually at ~10Hz), and a low-contrast static stimulus is presented to the other. In general, the static stimulus can remain reliably suppressed from visual awareness for relatively long durations (typically ~1000ms), a dramatic increase in suppression compared to backward masking (typically ~16–33ms). Such increase in stimulus-presentation time facilitates the measurement of affective changes via objective peripheral-physiological indices, such as skin conductance responses (SCRs). Accordingly, we quantified SCRs to the emotional (relative to neutral) stimuli to index emotional reactivity following the affective provocation.
Fig. 1. Schematic representation of the experimental trial structure.
Emotional (fearful faces or spiders) and neutral (flowers) stimuli were shown for ~1 sec in a blocked fashion. Conscious awareness of these stimuli was manipulated within-subjects. In stimulus-unaware blocks, the presentation of high-contrast, flashing Mondrian patterns to participants’ dominant eye precluded visibility of stimuli shown to their non-dominant eye. In aware blocks, stimuli were presented to both dominant and non-dominant eyes. In half the trials, participants were asked to rate the likeability of novel neutral faces using their immediate first impression, thus providing an index of affect coloring. Skin conductance data were recorded continuously and were used to index emotional reactivity following the affective provocation.
To index affective coloring, individuals rated the likeability of novel neutral faces shown during emotional and neutral blocks [e.g., (Anderson et al., 2012; Li et al., 2007)]. To examine whether emotional responses per se were modulated by conscious awareness, we compared the extent of emotional reactivity between stimulus-aware and unaware conditions following presentation of each category of emotional stimuli (fearful faces vs. flowers & spiders vs. flowers) as reflected by stimulus-locked changes in SCRs. Next, we adopted an individual-differences approach to examine an account compatible with the affect-as-information theory; namely, that conscious awareness changed the association between emotional reactivity following affective provocation (indexed by SCR magnitude) and the extent of affective coloring (indexed by likeability ratings of neutral faces). Specifically, from such an account we predicted that larger SCRs to negative stimuli rendered non-conscious by CFS should be associated with decreased likeability for subsequently presented neutral faces, an association that should be attenuated when the emotional stimuli were fully visible (and thus the source-of-affect was available for conscious processing).
Methods
Participants
A total of 67 right-handed individuals from a pool of Introduction to Psychology students (43 females, 24 males) participated in this study. All volunteers provided written consent, and study procedures were approved by the University of Wisconsin-Madison Institutional Review Board.
Apparatus
We used an adjus mirror stereoscope mounted on a chin rest to present images displayed on an LCD monitor (75Hz) at a 50cm viewing distance.
Stimuli
CFS stimuli consisted of 80 Mondrian-patterned images created by drawing rectangles of random colors at random locations in a 3.2° × 3.2° square. Affective-provocation images consisted of 8 fearful faces (half female), 8 pictures of spiders, and 8 pictures of flowers subtending 3.2° × 3.2° and matched on average luminance and Root Mean Square (RMS) contrast. To facilitate binocular fusion, a .28° × .28° black border surrounded the stimuli. To assess affective coloring, 24 neutral faces (half female) were chosen, and resized to 4.5° × 5.7° rectangles surrounded by a .28° × .28° border. (See Supplemental Material available online for details on stimulus characteristics, including databases used for stimulus selection.)
Procedure
The first part of the experiment consisted of the Emotion Processing Task. Next, a 2-alternative forced choice (2AFC) stimulus detection task took place to ensure the effectiveness of stimulus visibility suppression via the CFS manipulation.
Emotion Processing Task
Stimuli were presented stereoscopically in a 3 (Stimulus Category: Fearful Faces, Spiders, Flowers) × 2 (Awareness: Aware, Unaware) within-subjects design. To accentuate emotion-elicitation effects, Stimulus Category and Awareness were manipulated in blocks. Block order and stimulus assignment to Aware (i.e., stimulus presented to both eyes) vs. Unaware (i.e., stimulus presented during CFS) conditions was counterbalanced across participants (see Supplemental Material available online). Each neutral face used to examine affective coloring was presented only once, and randomly assigned to Stimulus Category and Awareness conditions.
In Unaware blocks, we implemented CFS (Tsuchiya & Koch, 2005): For 1488ms, Mondrian-patterned images flashed to participants’ dominant eye at 10.71Hz, while a static, low-contrast (emotional or neutral) stimulus was presented to their non-dominant eye during the first 1023ms. Eye dominance was determined using the hole-in-the-card test (Durand & Gould, 1910). In Aware blocks, the low contrast stimuli were presented for 1023ms to both dominant and non-dominant eyes, and were thus fully visible.
Before the start of the experiment, we attached Ag-AgCl sensors on participants’ left hand (see “Skin Conductance Recording and Processing” for details in Supplemental Material). Participants were asked to remain still and maintain central fixation throughout the experiment. Prior to Unaware blocks, they were told that another image may be presented simultaneously with the moving squares, and asked to indicate with a button press if they ever thought they saw an image in addition to the squares.
In each block, 4 unique stimuli were presented twice as described above (see Fig. 1). Trials started with a 2000ms .5° × .5° fixation cross, followed by the affective-provocation stimulus, which was in turn followed by a 6000ms–8000ms (7000ms average) inter-trial interval (ITI; thus allowing for stimulus-evoked SCRs to unfold). In half the trials, the ITI was followed by a neutral-face likeability rating task, whereby individuals were asked to rate how much they liked a novel neutral face, which was presented for 4000ms (“How much do you like this person?”) with a button box using a 1–4 scale, where “1”= “not at all”; and “4” = “quite a bit”. Participants were instructed to report on their immediate impression of the faces based on their gut feeling. Responses were only accepted within the 4000ms. Two individuals failed to provide sufficient neutral-face likeability data (i.e., did not press a button within 4000ms); one across Aware and Unaware blocks, and one during Aware blocks only.
To examine whether the affective provocation resulted in changes in subjective experience, at the end of each block participants were asked to report on their mood (“How do you feel right now?”) with a button box using a 1–4 scale, where “1”= “very negative”; and “4” = “very positive”.
2AFC Task
Since it is critical to ensure that participants experienced robust suppression of stimulus visibility during CFS, in addition to requesting that they press a button in the event of image breakthrough in the Emotion Processing Task, we also examined the effectiveness of CFS following the Emotion Processing Task by testing stimulus-identification performance in a 2AFC procedure [cf. (Jiang & He, 2006); see Supplemental Material].
Skin Conductance Recordings and Processing
Statistical Analysis
We verified that observers included in the analysis were unaware of stimuli in CFS blocks by using both 2AFC performance and subjective reports. In addition, we ensured that participants had detectable stimulus-evoked SCRs (i.e., greater than 0.02 μS) in a minimum of 5% of trials (for details, see Supplemental Material). Fifteen individuals (out of 67) performed significantly above chance in the 2AFC stimulus identification task, and were thus excluded from all analysis reported below (for details, see “Stimulus-Awareness Testing” under the Supplemental Material available online). Of the remaining 52 individuals, 6 did not show SC responses greater than 0.02 μS in at least 5% of the trials, and were thus considered non-responders. Within the remaining sample (N = 46), occasional trials where observers indicated they saw an image in addition to the colorful squares were excluded from the analysis of both SCRs and likeability ratings data (0.3% of trials).
We conducted a 2 × 3 multivariate analyses of variance (MANOVA) with Awareness (Unaware, Aware) and Stimulus Category (Fearful Faces, Spiders, Flowers) on SCRs to verify the efficacy of the affective provocation, and to examine whether emotional reactivity differed in magnitude as a function of conscious awareness. Likewise, 2 × 3 MANOVAs on neutral-face-likeability ratings (presented in the Supplemental Material available online) and on mood ratings assessed how Awareness and Stimulus Category modulated affective coloring and the subjective experience of affect, respectively.
To examine if conscious awareness modulated the association between emotional reactivity and affect coloring, we first computed pairwise correlations between changes in SCRs by emotion (e.g., fear – flower) in trials preceding neutral face likeability ratings and the corresponding change in neutral-face likeability ratings (e.g., fear – flower) across individuals separately for Aware and Unaware conditions. We used Spearman’s rank coefficients to estimate these associations given the increased robustness of this method to mild outliers (observed in the Aware condition). Next, we tested for the difference between correlation coefficients obtained in Aware and Unaware conditions (i.e., Aware – Unaware). To that end, we used Zou’s (2007) method, which yields a confidence interval for the difference of dependent correlation coefficients (Zou, 2007); thus, statistical significance at p < .05 is indicated by the 95% confidence interval not including zero.
Results
Emotion Processing Task
Fearful faces increase sympathetic nervous system activation comparably across aware and unaware conditions
The affective provocation employed here successfully provoked emotional reactivity as indicated by a main effect of Stimulus Category on SCR magnitudes, F(2,44) = 6.46, p = .003, ηρ2 = .14. Specifically, fearful faces and spiders elicited significantly greater SCRs than did flowers, (fearful faces vs. flowers: p = .001, d = 0.56; spiders vs. flowers: p = .038, d = 0.34). In addition, a main effect of Awareness indicated that the magnitude of SCRs across stimuli was on average larger during the presentation of colorful Mondrian patterns, F(1,45) = 7.74, p = .008, ηρ2 = .11.
Suggesting that sympathetic nervous system responding does not rely on conscious awareness in the face of biologically relevant stimuli, the Awareness * Stimulus Category interaction did not approach significance, F(2,44) = .57, p = .56, ηρ2 = .007. However, examination of the paired contrasts, shown in Fig. 2, revealed that the similarity in reliability of emotional activation across stimulus-Aware and Unaware conditions was found in response to fearful faces (relative to flowers), but not to spiders (relative to flowers): During Unaware stimulus presentations, fearful faces produced significantly larger skin conductance responses than flowers, p = .023, d = .32. Likewise, when visible in the Aware condition, fearful faces also elicited significantly larger SCRs than flowers, p = .003; d = .53. In contrast, when rendered invisible via CFS, spiders did not reliably increase SCRs relative to flowers, p = .22; d = .19. Only when spiders were visible did they elicit greater SCRs than flowers, p = .04, d = .3. In summary, fearful faces provoked reliable emotional reactivity as indexed by SCRs regardless of stimulus awareness, contrary to the possibility that conscious awareness decreases the magnitude of emotional responses per se.
Fig. 2. Increased sympathetic nervous system activation following the presentation of emotional stimuli.
Skin conductance responses (in √μS*s) are plotted as a function of Stimulus Category and Stimulus Awareness. Regardless of their visibility, fearful faces elicited significantly greater skin conductance responses than flowers. Paired contrasts significant at p < .05 are marked with an asterisk. Error bars represent the within-subjects 95% confidence intervals per condition computed based on Morey’s (2008) method (Morey, 2008).
Conscious awareness prevents the association between emotional reactivity and likeability of novel neutral faces
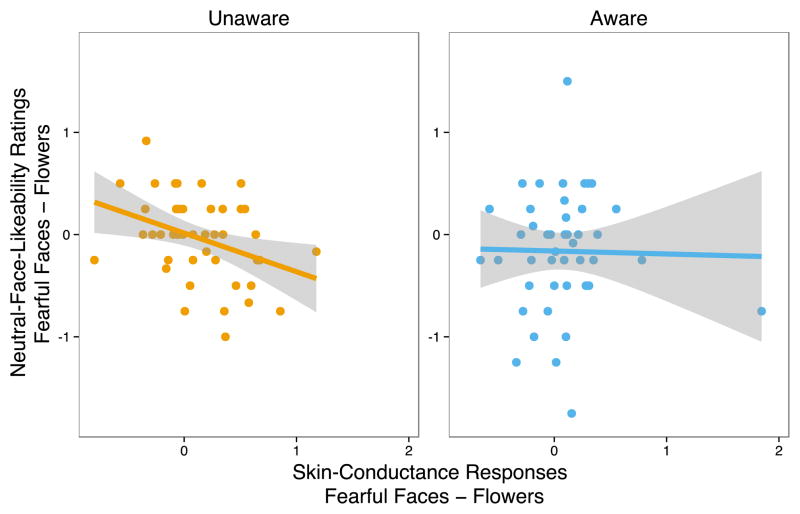
Because fearful faces provoked robust physiological responses regardless of stimulus awareness, we analyzed the consequences of such emotional reactivity as it pertains to subsequent affective coloring. As predicted from an account whereby affect from a non-identified source is prone to bind to other non-related objects [“affect-as information” theory; (Schwarz & Clore, 2003, 1983)], only during stimulus unawareness did larger SCRs to fear faces (relative to flowers) predict decreased likeability of neutral faces that followed them, Spearman’s ρ = −.38, p = .01 (see Fig. 3). No such association was found during stimulus awareness, Spearman’s ρ = .09, p > .54. Critically, awareness significantly attenuated the strength of the emotional reactivity and affective coloring relationship as indicated by the confidence interval on the difference between these two correlation coefficients, 95% CI = −.05, −.83, p < .05.
Fig. 3. Increased emotional reactivity to negative stimuli is associated with decreased liking of novel neutral faces, but only when participants are unaware of the emotional provocation.
Correlation of extent of sympathetic nervous system activation to [fearful faces – flowers] as indexed by skin conductance responses shown as a function of changes in neutral face likeability ratings [fearful faces – flowers], plotted separately for Unaware and Aware stimulus presentation conditions. The association between emotional activation and affective coloring of novel neutral faces was significantly greater during Unaware compared to Aware emotional provocation, p < .05.
Controlling for mood
Participants’ self reported mood was not significantly modulated by Stimulus Category or Awareness, Fs < 1.38, ps > .26, ηρ2s > .06.
Importantly, the modulation of emotional reactivity and affective coloring by conscious awareness remained significant when controlling for corresponding changes in mood: Changes in emotional reactivity continued not to predict neutral-face likeability during Aware processing when changes in mood were included in the regression model, B = .16, SE = .23, t = .72, p > .4. During Unaware processing, increases in SCRs continued to predict decreased likeability of novel neutral faces, even after changes in mood were entered as a covariate in the model, B = −.48, SE = .2, t = −2.37, p = .022. Therefore, when affect is used as information during unaware stimulus processing, it does not depend on affective changes themselves to reach conscious awareness and be subjectively experienced. Instead, the results are consistent with the idea that, in the absence of stimulus awareness, emotional activation diffuses into preferences for incidental stimuli in the environment without it being translated into a reportable mood [akin to effects reported in (Winkielman, Berridge, & Wilbarger, 2005)].
Discussion
In this experiment, we examined how conscious awareness of emotional stimuli impacts physiological and evaluative responses, as well as their association. In half the trials, we manipulated visual awareness via a robust interocular suppression technique, CFS. We measured SCRs to the emotional stimuli to index emotional reactivity, and subsequent likeability ratings of novel neutral faces to index emotional coloring of evaluations. We found that even though fearful faces increased SCRs similarly across aware and unaware conditions, only in the unaware condition did SCR magnitudes predict subsequent decreases in likeability of novel neutral faces. In contrast, when stimuli were presented binocularly and were consciously accessible, there was no relationship between peripheral-physiological responding to fearful faces and subsequent appraisal of neutral faces. Thus, this experiment highlights that even though conscious awareness does not reliably impact physiological responses to certain emotional stimuli, awareness can prevent emotional responses from diffusing to unrelated targets in the environment — such as a novel neutral face.
An emotional stimulus processed without conscious awareness, especially if biologically prepared, can modulate emotion response systems such as sympathetic (Ohman & Soares, 1994) and facial-expressive (Dimberg et al., 2000) via rapid valence and arousal computations in structures like the amygdala, which can directly influence peripheral-physiological responses (Mangina & Beuzeron-Mangina, 1996). Accordingly, fearful faces modulate amygdalar activity in the absence of visual awareness across a variety of masking techniques (Jiang & He, 2006; Pasley et al., 2004; Whalen et al., 1998; Williams et al., 2004). Relatedly, a fear-relevant stimulus (an angry face previously paired with a shock) has been recently reported to increase SCRs, an index of sympathetic nervous system activation, even when visually suppressed via CFS (Raio, Carmel, Carrasco, & Phelps, 2012). Here, we extend this finding by showing reliable increases in SCRs to fearful faces rendered invisible via CFS, which were comparable in magnitude to SCRs observed when fearful faces were fully visible.
Furthermore, these results suggest that mobilization of autonomic nervous system influences affective judgments of neutral stimuli when unchecked by conscious awareness. Indeed, if affect is used as information during source-of-affect unawareness (Schwarz & Clore, 2003, 1983), the extent of affective coloring should scale accordingly to the magnitude of the provoked affect, an idea that had not been previously tested. Here, by independently indexing the magnitude of sympathetic nervous system activation by an affective stimulus, and the subsequent misattribution of affect toward neutral faces, we confirmed this prediction: Only during stimulus unawareness did the extent of affective coloring vary as a function of the magnitude of the previously provoked emotional response. Thus, this study underscores the relevance of central (especially amygdalar) responding to non-perceived facial expressions [e.g., (Brooks et al., 2012; Jiang & He, 2006)] by suggesting that, to the extent that such central processing is translated into peripheral-physiological changes, an individual may find himself/herself prejudiced against other, temporally adjacent external stimuli, a biasing that can be prevented via awareness of the source of emotion. Given the ubiquity of subtle facial behavior in our environment, examining whether the effects reported here also manifest in naturalistic experimental contexts, such as in response to micro expressions, is an important direction for future research.
In our study, pictures of spiders did not modulate skin conductance responses in the absence of visual awareness, whereas fearful faces did (Fig. 2). It is possible that fearful faces hold a greater unconditioned value than spiders do (Ohman, 2009). Indeed, a recent report examining subjective and physiological responses to spiders revealed that heart rate increases by spiders do depend on stimulus visibility (Peira, Ohman, & Anders, 2012). On the other hand, it is also possible that other venomous animals such as snakes, which are believed to have exerted central survival pressure on primates for an efficient perceptual decoding machinery, would modulate autonomic nervous system activity independent of visual awareness in humans (Ohman, 2009). Accordingly, relative to spiders, snakes drive visual attention in a more efficient manner (Soares, Esteves, Lundqvist, & Ohman, 2009). Thus, future research exploring the limits of unaware emotional-information processing should include stimuli for which humans have more “biologically prepared” fear than they do for spiders, such as snakes.
At least two important questions raised by this experiment warrant further research: First, given the important role of individual differences in determining the magnitude of affective-coloring effects, what are the neurobiological features that distinguish individuals who are more prone to responding physiologically to non-perceived emotional stimuli from individuals whose autonomic nervous system is minimally affected during non-conscious processing? Second, given that physiological responding to emotional stimuli was only associated with affective misattribution during visually unaware states, what aspect of the affective computing circuitry explains the reduced affective coloring when emotional stimuli are consciously processed? The search for the neural bases of conscious processing of visual information has consistently implicated the fronto-parietal network, including medial and dorsolateral regions of the prefrontal cortex (PFC) [for a review, see (Dehaene & Changeux, 2011)]. Given the well-described amygdala-inhibitory role of ventro-medial PFC (vmPFC) (Kim et al., 2011), as well as the vmPFC participation in amygdalar down-regulation in contexts where dorso-lateral (DL) PFC regions may initiate emotional regulation [such as in cognitive reappraisal (Delgado, Nearing, Ledoux, & Phelps, 2008)], is it plausible that the mere engagement of DLPFC and vmPFC structures resulting from visual information processing reaching the fronto-parietal network could inhibit central-amygdalar activity—which in turn could prevent affective misattribution? Such proposal is speculative, and should be tested in future studies conducting functional connectivity analysis of neuroimaging data coupled with assessments of physiological responding and affective coloring during aware and unaware emotional processing states.
In conclusion, this study highlights our ability to respond to emotional facial signals even when we are not consciously aware of them, and underscores an emotion-regulatory role for conscious awareness in decoupling physiological responses from evaluations of unrelated (and otherwise neutral) stimuli in the environment.
Supplementary Material
Acknowledgments
We would like to thank Daniel B. Levinson and Andrew N. DeClercq for providing helpful feedback on the manuscript, Danielle Fenner for her assistance with data collection, and Isa Dolski and John Koger for administrative support. We thank Michelle E. Davis for her generous gift to the Psychology Department at the University of Wisconsin-Madison. We also gratefully acknowledge the contribution of the late Lawrence L. Greischar for his assistance with skin conductance data processing. This study was supported by National Institute of Mental Health grants R01-MH43454 and P50-MH069315 to R.J.D. and by the James L. Davis Fellowship for Affective Neuroscience (R.C. Lapate).
References
- Almeida J, Pajtas PE, Mahon BZ, Nakayama K, Caramazza A. Affect of the unconscious: Visually suppressed angry faces modulate our decisions. Cognitive, Affective & Behavioral Neuroscience. 2012;13(1):94–101. doi: 10.3758/s13415-012-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Siegel E, White D, Barrett LF. Out of sight but not out of mind: Unseen affective faces influence evaluations and social impressions. Emotion. 2012;12:1210–21. doi: 10.1037/a0027514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block N. Two neural correlates of consciousness. Trends in Cognitive Sciences. 2005;9(2):46–52. doi: 10.1016/j.tics.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Savov V, Allzén E, Benedict C, Fredriksson R, Schiöth HB. Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. Neuroimage. 2012;59(3):2962–73. doi: 10.1016/j.neuroimage.2011.09.077. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70(2):200–27. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11(1):86–9. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Durand AC, Gould GM. A method of determining ocular dominance. JAMA. 1910;55:369–370. [Google Scholar]
- Faivre N, Berthet V, Kouider S. Nonconscious influences from emotional faces: a comparison of visual crowding, masking, and continuous flash suppression. Frontiers in Psychology. 2012;3:129. doi: 10.3389/fpsyg.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, He S. Cortical responses to invisible faces: dissociating subsystems for facial-information processing. Current Biology. 2006;16(20):2023–9. doi: 10.1016/j.cub.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks Ra, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223(2):403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouider S, Berthet V, Faivre N. Preference is biased by crowded facial expressions. Psychological Science. 2011;22(2):184–9. doi: 10.1177/0956797610396226. [DOI] [PubMed] [Google Scholar]
- Lau H, Rosenthal D. Empirical support for higher-order theories of conscious awareness. Trends in Cognitive Sciences. 2011;15(8):365–73. doi: 10.1016/j.tics.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Ledoux J. Perspective Rethinking the Emotional Brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moallem I, Paller KA, Gottfried JA. Subliminal smells can guide social preferences. Psychological Science. 2007;18(12):1044–1049. doi: 10.1111/j.1467-9280.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- Mangina Ca, Beuzeron-Mangina JH. Direct electrical stimulation of specific human brain structures and bilateral electrodermal activity. International Journal of Psychophysiology. 1996;22(1–2):1–8. doi: 10.1016/0167-8760(96)00022-0. [DOI] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: A correction to Cousineau (2005) Tutorial in Quantitative Methods for Psychology. 2008;4(2):61–64. [Google Scholar]
- Murphy ST, Zajonc RB. Affect, cognition, and awareness: Affective priming with optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology. 1993;64:723–739. doi: 10.1037//0022-3514.64.5.723. [DOI] [PubMed] [Google Scholar]
- Ohman A, Soares JJ. “Unconscious anxiety”: phobic responses to masked stimuli. Journal of Abnormal Psychology. 1994;103(2):231–240. doi: 10.1037//0021-843x.103.2.231. [DOI] [PubMed] [Google Scholar]
- Ohman Arne. Of snakes and faces: an evolutionary perspective on the psychology of fear. Scandinavian Journal of Psychology. 2009;50(6):543–52. doi: 10.1111/j.1467-9450.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Learned Fear of “Unseen” Faces after Pavlovian, Observational, and Instructed Fear. Psychological Science. 2004;15(12):822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Mayes LC, Schultz RT. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42(1):163–72. doi: 10.1016/s0896-6273(04)00155-2. [DOI] [PubMed] [Google Scholar]
- Peira N, Öhman A, Anders S. Emotional responses in spider fear are closely related to picture awareness Emotional responses in spider fear are closely related to picture awareness. Cognition & Emotion. 2012;26:37–41. doi: 10.1080/02699931.2011.579087. [DOI] [PubMed] [Google Scholar]
- Raio CM, Carmel D, Carrasco M, Phelps Ea. Nonconscious fear is quickly acquired but swiftly forgotten. Current Biology. 2012;22(12):R477–9. doi: 10.1016/j.cub.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotteveel M, De Groot P, Geutskens A, Phaf RH. Stronger suboptimal than optimal affective priming? Emotion. 2001;1(4):348–364. doi: 10.1037/1528-3542.1.4.348. [DOI] [PubMed] [Google Scholar]
- Schwarz N, Clore GL. Mood as information: 20 years later. Psychological Inquiry. 2003;14(3):296–303. [Google Scholar]
- Schwarz N, Clore GL. Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology. 1983;45(3):513–523. [Google Scholar]
- Soares SC, Esteves F, Lundqvist D, Ohman A. Some animal specific fears are more specific than others: Evidence from attention and emotion measures. Behaviour Research and Therapy. 2009;47(12):1032–42. doi: 10.1016/j.brat.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Tamietto M, Castelli L, Vighetti S, Perozzo P, Geminiani G, Weiskrantz L, De Gelder B. Unseen facial and bodily expressions trigger fast emotional reactions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(42):17661–17666. doi: 10.1073/pnas.0908994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nature Neuroscience. 2005;8(8):1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike Ma. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Ma, Morris AP, McGlone F, Abbott DF, Mattingley JB. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. Journal of Neuroscience. 2004;24(12):2898–904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Personality & Social Psychology Bulletin. 2005;31(1):121–135. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]
- Wyer RS, Carlston DE. Social Cognition. Hillsdale, NJ: Psychology Press; 1979. [Google Scholar]
- Zou GY. Toward using confidence intervals to compare correlations. Psychological Methods. 2007;12:399–413. doi: 10.1037/1082-989X.12.4.399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.