Abstract
Objective
Cell–matrix interactions promote cartilage homeostasis. We previously found that Smad1, the transcriptional modulator of the canonical bone morphogenetic protein 7 (BMP-7) pathway, interacted with the cytoplasmic domain of CD44, the principal hyaluronan receptor on chondrocytes. To elucidate the physiologic function of CD44–Smad1 interactions, as well as the role of hyaluronan, we studied the response of chondrocytes isolated from CD44−/− and BALB/c (wild-type [WT]) mice to stimulation with BMP-7.
Methods
In primary murine chondrocytes, CD44 expression was decreased by small interfering RNA (siRNA) transfection or was enhanced by plasmid transfection. Pericellular hyaluronan was removed by hyaluronidase treatment, or its endogenous synthesis was inhibited. Changes in response to BMP-7 stimulation were evaluated by Western blotting of Smad1 phosphorylation and aggrecan messenger RNA (mRNA) expression.
Results
Chondrocytes from CD44−/− mice and WT mice transfected with CD44 siRNA were less responsive than untransfected chondrocytes from WT mice to BMP-7. CD44−/− mouse chondrocytes transfected with pCD44 showed increased sensitivity to BMP-7. Significant increases in aggrecan mRNA were observed in WT mouse chondrocytes in response to 10 ng/ml of BMP-7, whereas at least 100 ng/ml of BMP-7 was required for CD44−/− mouse chondrocytes. However, in chondrocytes from CD44−/− and WT mice, hyaluronidase treatment decreased cellular responses to BMP-7. Treatment of both bovine and murine chondrocytes with 4-methylumbelliferone to reduce the synthesis of endogenous hyaluronan confirmed that hyaluronan promoted BMP-7 signaling.
Conclusion
Taken together, these investigations into the mechanisms underlying BMP-7 signaling in chondrocytes revealed that while hyaluronan-dependent pericellular matrix is critical for BMP-7 signaling, the expression of CD44 promotes the cellular response to lower concentrations of BMP-7.
Changes in the extracellular matrix exert a profound influence on cell behavior mediated via matrix receptors. Often these effects are indirect, such as when matrix components enhance the responsiveness of various tyrosine or serine/threonine kinase receptors to their ligands (1). The interaction of the matrix macromolecule hyaluronan with its primary receptor CD44 is one model of matrix modulation of cell signaling.
CD44 is a single-pass transmembrane glycoprotein receptor for hyaluronan, consisting of distal extracellular domain, membrane-proximal stem domain, transmembrane domain, and cytoplasmic domain (2,3). The distal domain of CD44 is responsible for binding hyaluronan. The cytoplasmic domain lacks inherent kinase activity but has been shown to interact with cytoskeletal adapter proteins (4–6) as well as cortical signaling proteins (7,8). In studies aimed at identifying other possible binding partners for the cytoplasmic domain of CD44, a yeast 2-hybrid system revealed an interaction between CD44 and Smad1 (9), a protein activated in the canonical bone morphogenetic protein (BMP) signaling pathway (10). The β-galactosidase score for the CD44–Smad1 hit was 2.4 SD above background when using the full-length CD44 cytoplasmic domain (amino acids [aa] 292–361) as bait, but no interaction was detected when a truncated CD44 (aa 292–307) was tested. We confirmed the interaction between CD44 and Smad1 by coimmunoprecipitation studies(9). Smad1/Smad4 nuclear translocation in chondrocytes was blocked by transfection of cytoplasmic tail truncation variants of CD44 (9).
In articular chondrocytes, reducing pericellular hyaluronan with hyaluronidase also diminished cellular responses to BMP-7, but not to transforming growth factor β1 (TGFβ1), and the BMP-7 response could be restored by treatment with exogenous hyaluronan or by transfection with phosphorylated hyaluronan synthase 2 (HAS-2) (11), the primary synthase for hyaluronan in chondrocytes (12). The Smad anchor for receptor activation (SARA) protein facilitates the interaction of Smad2 and Smad3 with the activated TGFβ receptor; nevertheless, the expression of SARA is not required for TGFβ signaling (13,14). These studies suggested a physiologic function of the CD44–Smad1 interaction, as well as a mechanism by which extracellular hyaluronan can influence chondrocyte behavior in response to BMP-7.
Many studies of BMP-7, including our own, have used BMP-7 concentrations ≥100 ng/ml to examine cellular responses, whereas the concentration of BMP-7 in human serum is in the range of 0.5–1 ng/ml (15). Nevertheless, we have observed significant increases in the levels of 35S-sulfated proteoglycan per μg of DNA after treatment of human chondrocytes with 10 ng/ml of BMP-7 (16). In a normal adult skeleton, BMP-2 turnover is ~3 ng/day, and in a recent study, implants releasing ~5 ng of BMP-7 per day yielded the formation of the highest bone volumes (17). A recent report suggested that promoting cellular responses to such lower (physiologic range) concentrations of BMP-7 may lessen inflammatory responses and improve the healing of complex fractures (16).
BMP-mediated signaling occurs in many cell types, some of which are devoid of CD44. Thus, the requirement for hyaluronan or CD44 is not absolute (18). One study on the responsiveness of prostate carcinoma cell lines found Smad1 phosphorylation in response to BMP-7 was stronger and occurred at lower concentrations of BMP-7 in PC-3 cells than in LNCaP cells, although in the presence of 100 ng/ml BMP-7, both cell lines were seemingly equivalent (19). While there are several phenotype differences between these 2 prostate carcinoma cell lines, it is noteworthy that PC-3 cells express high levels of CD44 (20), whereas LNCaP cells are CD44 negative due to cytosine methylation of the CD44 promoter (21). These results suggested that the influence of hyaluronan–CD44 interactions on BMP-mediated signaling may be more subtle and therefore more evident at lower concentrations of the ligand.
To determine the physiologic function of CD44–Smad1 interaction and explore the relationship between hyaluronan and CD44 in a more directly comparable model, chondrocytes were isolated from wild-type (WT) mice and CD44−/− mice. CD44−/− mice are born with an appendicular skeleton and cartilage that appear normal and display no overt changes in phenotype (22,23). Chondrocytes represent a cell with behavior highly dependent and sensitive to influences from the extracellular matrix. We investigated murine chondrocytes to compare the findings with those of our previous studies on human and bovine chondrocytes. In this study, we demonstrated that a higher concentration of BMP-7 was required for BMP-mediated responsiveness of chondrocytes from CD44−/− mice as compared to WT mice, and CD44 small interfering RNA (siRNA) knockdown diminished the responsiveness of chondrocytes from WT mice. Enhanced BMP-7 responsiveness could be rescued CD44−/− mouse chondrocytes by pCD44H transfection. However, removal of hyaluronan also exerted a prominent influence on BMP-7 responsiveness of WT and CD44−/− mouse chondrocytes, supporting the idea that pericellular matrix integrity is critical as well.
MATERIALS AND METHODS
Materials
Recombinant human BMP-7 (OP-1) was obtained from Stryker Biotech. Total Smad1 was detected with a polyclonal antibody (Invitrogen), and Smad1 activation by BMP-7 was detected using an antibody that reacts with phospho-Smad1 (Ser463/Ser465)/phospho-Smad5 (Ser463/Ser465)/phospho-Smad8 (Ser426/Ser428) (p-Smad1; Cell Signaling Technology). Cell lysis buffer, anti–activin receptor–like kinase 2 (anti–ALK-2; activin receptor type IA [ACVRIA]) antibody, anti–p-Akt antibody, anti-Akt antibody, anti–p–ERK-1/2 (phospho-p44/42) antibody, and anti–ERK-1/2 (p44/42) antibody were also from Cell Signaling Technology. Anti-mouse CD44 antibody IM7.8.1 (Invitrogen), anti-human CD44 antibody BU-52 (Ancell), anti-GAPDH antibody I-19 (Santa Cruz Biotechnology), and anti–β-actin monoclonal antibody AC-15 (Sigma) were also used.
Cell culture
Primary mouse chondrocytes were isolated from 1-week-old CD44−/− mice (BALB/c background) (24) or BALB/c WT control mice (BALB/cAnNCrl; Charles River) according to an approved protocol of the East Carolina University. Isolated femoral heads and knee joint cartilage samples were subjected to overnight incubation at 37°C in 3 mg/ml of collagenase D (Roche), with stirring. The primary murine chondrocytes were plated at a density of 2 × 105 cells/well in a 48-well plate and cultured for 24 hours in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 gm/liter of glucose, 10% fetal bovine serum (FBS; Hyclone), 2 mM l-glutamine, and 1 μg/ml of ascorbic acid prior to initiation of experimental conditions.
Primary bovine articular chondrocytes were isolated by sequential Pronase/collagenase digestion from full-thickness slices of articular cartilage obtained from the metacarpophalangeal joints of 18–24-month-old adult steers (25). The primary bovine articular chondrocytes were plated as high-density monolayers at a density of 2 × 106 cells/per well in a 6-well plate and were cultured for 24 hours in a 1:1 mixture of DMEM/Ham’s F-12 medium containing 10% FBS, prior to initiation of experimental conditions.
Rat chondrosarcoma cells as described previously (26) were maintained in DMEM containing 10% FBS. The cells were detached from culture flasks with 0.25% trypsin/EDTA and plated as high-density monolayers for analyses.
Treatment of cells
Prior to stimulation, confluent cultures of chondrocytes were grown in serum-free medium for an additional 12 hours. Cells were then stimulated with 0–100 ng/ml of BMP-7 for 15–180 minutes to study Smad1 phosphor-ylation (11) or for 72 hours to study aggrecan mRNA expression (16) or were stimulated with 0–50 ng/ml of epidermal growth factor (EGF) for 5–50 minutes.
To determine the effect of matrix removal on BMP-7 responses, chondrocytes were pretreated for 12 hours with 200 units/ml testicular hyaluronidase to generate “matrix-depleted” cells, rinsed in serum-free medium, and stimulated for 1 hour with 100 ng/ml of BMP-7. To determine the effects of hyaluronan regrowth on BMP-7 responsiveness by matrix-depleted cells, these chondrocytes were cultured in serum-free medium for an additional 24 hours prior to BMP-7 stimulation. For pericellular staining of hyaluronan, murine chondrocytes were incubated with 2 μg/ml of biotinylated hyaluronan binding protein (HABP; Seikagaku USA) and then were incubated with 1 μg/ml of fluorescein isothiocyanate–conjugated Neutr-Avidin, mounted with DAPI, and visualized by fluorescence microscopy.
We next tested the effect of 4-methylumbelliferone (4-MU) on BMP-7 responses. Chondrocytes freshly isolated from cartilage or rat chondrosarcoma cells after trypsin release were plated in the presence of vehicle or 1.0 mM 4-MU, cultured for 48 hours, and then stimulated for 1 hour with 100 ng/ml of BMP-7. Cell viability after 4-MU treatments was determined by coincubation of chondrocytes in 2 μM calcein AM and 4 μM ethidium homodimer 1. Dead cells (red nuclear fluorescence) were evaluated by the uptake of ethidium homodimer 1 (excitation/emission 495 nm/635 nm). Living cells were visualized by the green fluorescence of the calcein (excitation/emission 495 nm/515 nm). The pericellular matrix of living cells was revealed with the particle exclusion assay (27), using calcein AM as a vital stain.
For CD44 inhibition, a CD44 siRNA was constructed as the murine ortholog of a human CD44 siRNA sequence (28). The control siRNA (D-001206-09-05) was as described elsewhere (29). For rescue experiments, CD44−/− mouse chondrocytes were transfected with complementary DNA (cDNA) encoding the full-length standard human isoform of CD44 (p-hCD44FL) (30), and cell surface CD44 was detected using anti-human CD44 antibody BU-52 (9). Murine chondrocytes were released from confluent monolayers with 1 mg/ml of Pronase/collagenase D and resuspended in Amaxa human chondrocyte solution (Lonza) containing either 5 μg of siRNA (Thermo Scientific Dharmacon) or 4 μg of expression vector per 2.0 × 106 cells (29). Chondrocytes were transfected by nucleofection using an Amaxa Nucleofector at setting U-28 and plated for 48 hours prior to BMP-7 stimulation.
Real-time reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from the mouse chondrocyte cultures using TRIzol reagent. Total RNA (0.1 μg) was converted to cDNA with murine leukemia virus reverse transcriptase in the presence of 0.15 μM CD44, aggrecan, type II collagen, or HAS-2–specific reverse primers and amplified at 42°C for 30 minutes using a PTC 100 Thermal Controller (MJ Research). The cDNA was amplified with AmpliTaq DNA polymerase. Primer-specific annealing was performed at 55°C for 1 minute for CD44, at 54°C for 1 minute for aggrecan and type II collagen, and at 60°C for 1 minute for GAPDH and HAS-2. For real-time RT-PCR, PCR products were detected with RT2 Real-Time SYBR Green reagents (SA Biosciences) using a SmartCycler system (Cepheid) (29). Primer-specific amplification was performed at 60°C for 30 seconds. However, fluorescence quantification was performed at 72°C, below the individual melting peak temperature for each PCR product. Real-time RT-PCR efficiency for each primer set was calculated. An increase in the copy numbers of mRNA was determined as the relative ratio of target gene to GAPDH, and the fold stimulation was calculated as previously described (29).
The murine specific primer sequences (Integrated DNA Technologies) were as follows: for aggrecan, 5′-GTTGGTTACTTCGCCTCCAG-3′ (forward) and 5′-GTCCTCCAAGCTCTGTGACC-3′ (reverse) (31); for type II collagen, 5′-CAGGTGAACCTGGACGAGAG-3′ (forward) and 5′-ACCACGATCTCCCTTGACTC-3′ (reverse) (31); for CD44, 5′-TGTCAACCGTGATGGTACTCGC-3′ (forward) and 5′-GTATCCTGATCTCCAGTAGGC-3′ (reverse) (24); for HAS-2, 5′-ATTGTTGGCTACCAGTTTATCCAAAC-3′ (forward) and 5′-TTTCTTTATGGGACTCTTCTGTCTCACC-3′ (reverse; for GAPDH, 5′-CACCATGGAGAAGGCCGGGG-3′ (forward) and 5′-GACGGACACATTGGGGGTAG-3′ (reverse) (32); and for GAPDH (real time), 5′-GTGAAGGTCGGTGTGAACG-3′ (forward) and 5′-CTCGCTCCTGGAAGATGGTG-3′ (reverse) (31).
Western blot analysis
Total protein was extracted using cell lysis buffer containing protease/phosphatase inhibitor cocktails. Twenty micrograms of total protein per sample was loaded and separated on Novex 4–12% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels (Invitrogen). Following electroblot transfer onto nitrocellulose membranes and blocking in 5% nonfat dry milk, membranes were incubated with primary antibodies followed by horseradish peroxidase–conjugated secondary antibodies. Detection was performed using chemiluminescence (Novex ECL; Invitrogen). Some blots were treated with Restore Stripping Buffer (Thermo Scientific) and reprobed with another primary antibody.
Specific antibodies used for analysis were mouse anti-CD44 IM7.8.1 (26), affinity purified anti-CD44 cytoplasmic tail antisera (27), anti–p-Smad1, anti–total Smad1 (11), anti–ALK-2, anti-ERK, anti–p-ERK, anti–p-Akt, anti-Akt antibody (33), anti-GAPDH (27), and anti–β-actin (11). Images were subjected to densitometric analysis using Quantity One 1-D software and the ChemiDoc XRS System (Bio-Rad). The ratio of the pixel intensity for p-Smad1 to total Smad1 was calculated for each condition for replicate experiments. When direct comparisons of chondrocytes from WT and CD44−/− mice were required, these were made using chondrocytes that had been isolated on the same day, grown under the same culture conditions, treated with the same prediluted stock of BMP-7, analyzed by Western blotting with the same stock solution of antibody, and developed as side-by-side blots on the same x-ray film.
Statistical analysis
Data are presented as the mean ± SD of multiple independent experiments. Statistical significance was determined by Student’s t-test and significant differences were noted at P < 0.05.
RESULTS
Characterization of primary chondrocytes isolated from BALB/c WT and CD44−/− mice
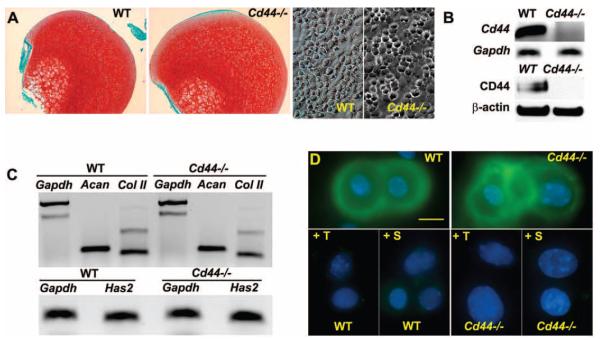
Femoral heads isolated from 7-day-old WT and CD44−/− mice displayed intense Safranin O–fast green staining for matrix proteoglycans, and primary chondrocytes isolated from WT and CD44−/− mice exhibited typical rounded chondrocyte morphology (Figure 1A). Results of RTPCR and Western blotting confirmed the lack of expression of CD44 mRNA and protein in chondrocytes from CD44−/− mice (Figure 1B). No differences were detected in the levels of expression of mRNA for aggrecan, type II collagen, or HAS-2 (12) between chondrocytes from WT mice and those from CD44−/− mice (Figure 1C). WT and CD44−/− mouse chondrocytes also exhibited equivalent pericellular accumulation of hyaluronan (detected using a biotinylated HABP probe) (Figure 1D) and proteoglycan (detected by toluidine blue O staining) (data not shown; see ref. 29). In chondrocytes from WT and CD44−/− mice, pericellular hyaluronan was removed by treatment with testicular hyaluronidase or Streptomyces hyaluronidase (Figure 1D), which in turn, resulted in the loss of stained proteoglycans.
Figure 1.
Characterization of cartilage and primary chondrocytes from BALB/c wild-type (WT) mice and CD44−/− mice. A, Femoral heads isolated from WT and CD44−/− mice were fixed directly and stained with Safranin O–fast green (top). Primary murine chondrocytes isolated from the femoral heads of WT and CD44−/− mice by collagenase digestion, were plated at high density and observed under phase-contrast microscopy (bottom). B, Chondrocytes from WT and CD44−/− mice were characterized for the expression of CD44 by conventional reverse transcription–polymerase chain reaction (RT-PCR) using GAPDH as control or by Western blotting using an anti-mouse CD44 antibody, with β-actin antibody as control. C, The expression of mRNA for aggrecan (Acan), type II collagen (Col II), and hyaluronan synthase 2 (HAS-2) derived from chondrocytes obtained from WT and CD44−/− mice was determined by conventional RT-PCR using GAPDH as control. Results are representative of 2 independent experiments. D, Chondrocytes from WT and CD44−/− mice were stained for hyaluronan (green) either directly or following pretreatment with testicular hyaluronidase (+T) or Streptomyces hyaluronidase (+S). Results are representative of 5 experiments.
CD44 promotion of the cellular response to lower concentrations of BMP-7 in murine chondrocytes
In preliminary studies, chondrocytes from WT and CD44−/− mice exhibited strong Smad1/5 phosphorylation following treatment with the typically used BMP-7 concentration of 100 ng/ml, and 60 minutes provided the optimum time to observe this response (data not shown). Our previous study found a significant increase in proteoglycan synthesis by human chondrocytes in response to 10 ng/ml of BMP-7 (16). Thus, the chondrocytes were stimulated for 1 hour with BMP-7 concentrations of 0–100 ng/ml.
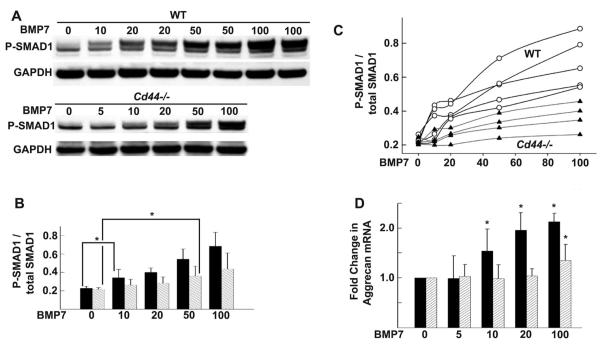
As shown in Figure 2A, sensitivity to BMP-7, as determined by the level of Smad1 phosphorylation, was dependent on the concentration of BMP-7 in cells from both groups of mice. However, the minimum BMP-7 concentration required to initiate a statistically significant increase in Smad1 phosphorylation in WT mouse chondrocytes was 10 ng/ml (Figure 2B). In CD44−/− mouse chondrocytes, the minimum BMP-7 concentration required to initiate a statistically significant increase in Smad1 phosphorylation above that in untreated controls was 50 ng/ml (Figure 2B). Data from individual experiments are shown in Figure 2C. Aggrecan mRNA expression, a downstream product of BMP-7 signaling, showed a statistically significant increase in WT mouse chondrocytes stimulated with 10 ng/ml of BMP-7, whereas in CD44−/− mouse chondrocytes, significant increases were observed only at 100 ng/ml of BMP-7 (Figure 2D).
Figure 2.
Responsiveness of chondrocytes from wild-type (WT) mice and CD44−/− mice to bone morphogenetic protein 7 (BMP-7). Chondrocytes were stimulated with 0–100 ng/ml of BMP-7. A, Representative Western blots of Smad1 phosphorylation (p-Smad1). GAPDH was used as a loading control for protein content. Smad1/5/8 is activated at lower concentrations of BMP-7 in chondrocytes from WT mice (solid bars) than in those from CD44−/− mice (hatched bars). B, Ratio of p-Smad1 to total Smad1, as indicated by the pixel intensities of the quantified protein bands for each BMP-7 concentration. Values are the mean ± SD of 5 separate experiments. * = P < 0.05. C, Ratio p-Smad1 to total Smad1 in chondrocytes from individual WT mice (open circles) and CD44−/− mice (solid triangles), as indicated by pixel intensities of quantified protein bands for each BMP-7 concentration. D, Fold change in aggrecan mRNA copy number following stimulation of chondrocytes from WT mice (solid bars) and CD44−/− mice (hatched bars) with 0–100 ng/ml of BMP-7, as determined by real-time reverse transcription–polymerase chain reaction analysis. Results represent duplicate cultures at each BMP-7 dose, each analyzed in duplicate. Values are the mean ± SD of 4 independent experiments (cell harvests). * = P < 0.05 versus control.
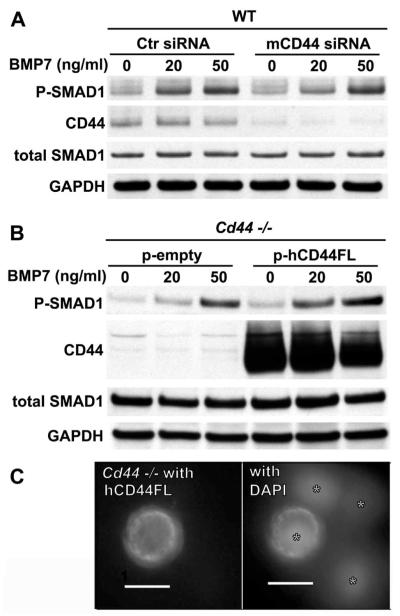
To further examine the participation of CD44 in the BMP-7 signaling pathway, especially at lower concentrations of BMP-7, WT mouse chondrocytes were transfected with mouse CD44 siRNA or a nonspecific control siRNA. With the concomitant decrease of CD44 protein, Smad1 phosphorylation in response to 20 ng/ml BMP-7 was reduced in comparison to control siRNA-transfected chondrocytes (Figure 3A). However, this reduction became less apparent at 50 ng/ml of BMP-7. Next, chondrocytes from CD44−/− mice were transfected with full-length human CD44 (30) and assayed for BMP-7 responsiveness. As shown in Figure 3B, CD44−/− cells transfected with a control empty-vector plasmid exhibited limited Smad1 phosphorylation at 20 ng/ml of BMP-7. However, pCD44-transfected CD44−/− mouse chondrocytes displayed enhanced BMP-7 responsiveness at 20 ng/ml when assayed under identical conditions (Figure 3B). These results suggest that enhanced BMP-7 responsiveness can be rescued in CD44−/− mouse chondrocytes by transfection and cell surface localization (Figure 3C) of full-length human CD44. Again, the differences were less obvious at BMP-7 concentrations >50 ng/ml.
Figure 3.
Altered levels of CD44 expression and sensitivity to bone morphogenetic protein 7 (BMP-7). A and B, To examine the effects of a loss of function in relation to CD44, chondrocytes from wild-type (WT) mice were transfected with either control small interfering RNA (siRNA) or mouse-specific CD44 (mCD44) siRNA (A). To examine the effects of a gain of function in relation to CD44, chondrocytes from CD44−/− mice were transfected with a control empty vector (p-empty) or with cDNA encoding full-length human CD44H (p-hCD44FL) (B). Two days after transfection, the cells were stimulated with 0, 20, or 50 ng/ml of BMP-7 and then analyzed by Western blotting for changes in Smad1 phosphorylation (p-Smad1). CD44 protein expression was probed with an anti–CD44 cytotail antibody. Total Smad1 and GAPDH were used as loading controls for protein content. Each blot is representative of 3 replicate experiments. C, Human CD44FL was expressed at the surface of chondrocytes from CD44−/− mice transfected with hCD44FL (left). Overlay of DAPI staining of nuclei of the same image is also shown (right). Asterisks identify nuclei of cells within the same field. Image is representative of 2 separate experiments. Transduction with a control empty vector yielded no immunofluorescence for human CD44 (data not shown).
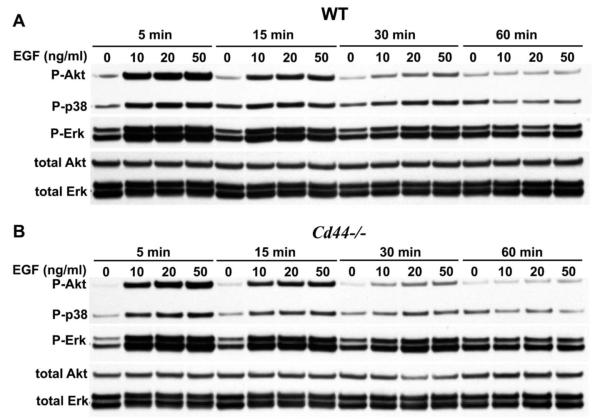
In carcinoma and fibrosis, CD44 has been shown to modulate EGF receptor activity (34,35). To determine whether the promotion of BMP-7 signaling by CD44 was specific to that pathway, chondrocytes from WT and CD44−/− mice were stimulated for 5–50 minutes with 0–50 ng/ml of EGF. Initiation of Akt, p38, and ERK phosphorylation in chondrocytes from WT mice (Figure 4A) and CD44−/− mice (Figure 4B) was observed after 5 minutes of stimulation with 10 ng/ml of EGF, with a decline detected by 30 minutes. However, no differences in activation of Akt, p38, or ERK upon EGF stimulation were detected between WT and CD44−/− mouse chondrocytes. The similarity between WT and CD44−/− mouse chondrocyte responsiveness to EGF further suggests that differences in BMP-7 responsiveness are not due to a more generalized reduction in responsiveness by cells from CD44−/− mice.
Figure 4.
Similar cellular responses of chondrocytes from wild-type (WT) mice and CD44−/− mice to epidermal growth factor (EGF). Chondrocytes from WT mice (A) and CD44−/− mice (B) were stimulated with 0–50 ng/ml of EGF for 5, 15, 30, and 60 minutes and then analyzed by Western blotting for phosphorylation of Akt (p-Akt), p38 (p-p38), and ERK (p-ERK). Total Akt and total ERK were used as loading controls for protein content. Each blot is representative of 4 replicate experiments. Chondrocytes from WT mice and CD44−/− mice responded similarly to a growth factor (EGF) that activates the Akt, p38, and ERK pathways. Thus, the differential responsiveness of chondrocytes from WT and CD44−/− mice to BMP-7 is not due to a generalized reduction in the responsiveness of CD44−/− mouse chondrocytes.
Promotion of the cellular response of WT and CD44−/− mouse chondrocytes to BMP-7 by intact pericellular matrix
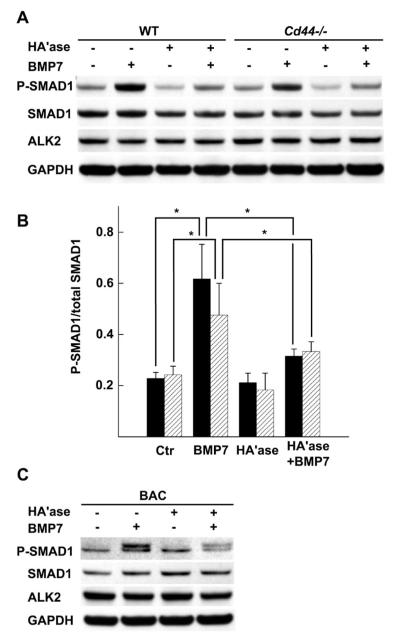
Another approach to assessing the role of CD44 in BMP-7 responsiveness is to modify the principal CD44 ligand, hyaluronan. Chondrocytes were pretreated with hyaluronidase to enzymatically degrade hyaluronan and remove the cell-associated pericellular matrix. Smad1 phosphorylation in control, matrix-intact WT chondrocytes upon stimulation with BMP-7 was reduced following enzymatic depletion of hyaluronan, as determined by Western blotting (Figures 5A and B). Similar effects of hyaluronidase pretreatment on bovine articular chondrocytes was also noted (Figure 5C), as we previously reported (11). Surprisingly, hyaluronidase-treated CD44−/− mouse chondrocytes also exhibited reduced Smad1 phosphorylation upon BMP-7 stimulation (Figures 5A and B).
Figure 5.
Effect of hyaluronidase pretreatment on Smad1 phosphorylation in response to bone morphogenetic protein 7 (BMP-7) in chondrocytes from wild-type (WT) mice and CD44−/− mice. A, WT and CD44−/− murine chondrocytes were pretreated with testicular hyaluronidase (HAase), stimulated with BMP-7, and analyzed by Western blotting for Smad1 phosphorylation (p-Smad1), total Smad, and activin receptor–like kinase 2 (ALK-2). GAPDH was used as a loading control for protein content. B, Ratio of p-Smad1 to total Smad1 in chondrocytes from WT mice (solid bars) and CD44−/− mice (hatched bars), as indicated by the pixel intensities of the quantified protein bands for each experimental condition. Values are the mean ± SD of 5 separate experiments. * = P < 0.05. C, Bovine articular chondrocytes (BACs) from 18–24-month-old adult steers were pretreated with hyaluronidase, stimulated with BMP-7, and analyzed by Western blotting for p-Smad1, total Smad, and ALK-2. GAPDH was used as a loading control for protein content. Results are representative of 2 independent experiments. These results demonstrate that hyaluronan is required for optimal BMP-7 responsiveness in chondrocytes from WT mice, CD44−/− mice, and steers. Additionally, no changes in BMP receptor ALK-2 were observed.
Additional control experiments were performed to determine whether the diminished BMP-7 responsiveness following hyaluronidase pretreatment was due to an unintended off-target alteration of the BMP-7 receptor or total Smad1 expression itself. The robust stimulation of Smad1 phosphorylation in response to BMP-7 was substantially tempered by pretreatment of the chondrocytes with hyaluronidase; however, when the same blots were probed for ALK-2, the principal type I receptor for BMP-7 (36–38), and for total Smad1, there were no changes in ALK-2 or Smad1 protein levels due to hyaluronidase or BMP-7 treatment (Figures 5A and C).
To demonstrate reversibility, hyaluronidasepretreated WT and CD44−/− mouse chondrocytes were allowed to reestablish hyaluronan-rich pericellular matrices. Following 24 hours of recovery, chondrocytes from WT and CD44−/− mice exhibited strong signals for Smad1 phosphorylation upon BMP-7 stimulation, similar to that in control cells that had never been treated with hyaluronidase (data not shown). To determine whether higher concentrations of BMP-7 would compensate for or override the loss of hyaluronan-dependent pericellular matrices, WT and CD44−/− mouse chondrocytes were stimulated with 100–500 ng/ml of BMP-7 with or without hyaluronidase pretreatment. Increasing the BMP-7 concentration above 100 ng/ml resulted in only a modest enhancement of Smad1 phosphorylation; however, even with 500 ng/ml of BMP-7, Smad1 phosphorylation levels in lysates derived from hyaluronidase-pretreated chondrocytes were substantially lower than those in matrix-intact chondrocytes (data not shown). The results suggest that optimal BMP-7 responsiveness in chondrocytes requires an intact hyaluronan-dependent pericellular matrix, a condition that cannot be compensated for by the use of higher BMP-7 concentrations.
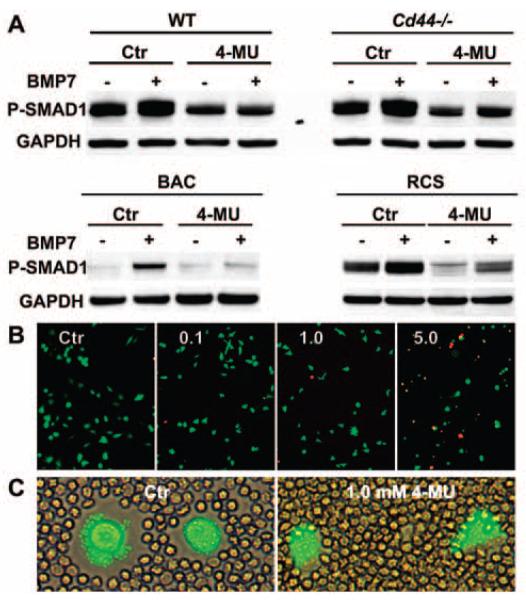
The hyaluronan synthase inhibitor 4-MU (39,40) offers an alternative, nonenzymatic approach to depleting the hyaluronan-dependent pericellular matrix. Freshly isolated WT and CD44−/− murine chondrocytes, bovine articular chondrocytes, and rat chondrosarcoma cells were incubated with 1.0 mM 4-MU for 48 hours prior to stimulation with BMP-7. For all 4 chondrocyte types, pretreatment with 4-MU resulted in reduced Smad1 phosphorylation upon BMP-7 stimulation (Figure 6A). Interestingly, baseline levels of p-Smad1 were also lower in 4-MU–treated murine and rat chondrosarcoma cells. Chondrocyte viability after treatment with 0.1 or 1.0 mM 4-MU was >98%, as determined with calcein AM and ethidium homodimer 1; >85% cell viability was found after treatment with 5.0 mM 4-MU (Figure 6B). Treatment of murine chondrocytes with 1.0 mM 4-MU resulted in a decrease in pericellular matrix retention, as determined by particle-exclusion assay (Figure 6C), but did not result in changes in total Smad1, CD44, or GAPDH protein. These data further suggest that the presence hyaluronan within the pericellular matrix is critical for a robust response by chondrocytes to BMP-7.
Figure 6.
Effect of 4-methylumbelliferone (4-MU), a hyaluronan synthase inhibitor, on bone morphogenetic protein 7 (BMP-7) signaling. A, Freshly isolated chondrocytes from wild-type (WT) mice and CD44−/− mice, as well as bovine articular chondrocytes (BACs) and rat chondrosarcoma (RCS) cells were plated, cultured for 48 hours in the absence or presence of 1.0 mM 4-MU (an intracellular inhibitor of hyaluronan synthesis), stimulated with BMP-7, and analyzed by Western blotting for Smad1 phosphorylation (p-Smad1). GAPDH was used as a loading control for protein content. Results are representative of 2 independent experiments. The results demonstrate that hyaluronan is required for optimal BMP-7 responsiveness in chondrocytes derived from 3 different species. B, Mouse chondrocytes were treated for 48 hours with 0–5 mM 4-MU and then stained for live (red) and dead (green) cells. Images are representative of 2 independent experiments. C, Pericellular matrix retention was examined by a particle-exclusion assay to view the cell coats. Mouse chondrocytes were treated for 48 hours in the absence or presence of 1.0 mM 4-MU and then stained with the viability dye calcein AM. Representative images show parallel sets of chondrocytes exposed to 4-MU at the same time as those depicted in B. Original magnification × ••• in B and ••• in C.
DISCUSSION
The data from this study demonstrate the relationship between CD44 availability and Smad1 phosphorylation in response to BMP-7. Our data on aggrecan suggest that differences in Smad1 phosphorylation observed at 60 minutes of treatment in 3-day cultures translate into even more prominent differences between CD44−/− and WT mouse chondrocytes in response to BMP-7. Previous studies from our laboratory demonstrated direct interactions between CD44 and Smad1 from yeast 2 hybrid and coimmunoprecipitation experiments (9). Thus, one explanation is that CD44 acts as an intracellular scaffold protein, clustering critical signaling intermediaries, as occurs in many receptor signaling systems (41), to enhance Smad1 phosphorylation. We hypothesize that CD44 binds and organizes cytoplasmic Smad1 in such a way that facilitates its phosphorylation/activation, perhaps directing the Smad1 to a unique membrane microdomain, thus functioning as a possible SARA for Smad1.
The function of a SARA as a facilitator is especially important at low concentrations of growth factor, but the requirement for a SARA is not absolute (14). Knockdown of CD44 in WT mouse chondrocytes reduced BMP-7 signaling (Figure 3A), whereas overexpression of human full-length CD44 in CD44−/− mouse chondrocytes partially rescued the BMP-7 response, as these cells exhibited enhanced sensitivity to BMP-7 (Figure 3B). Similarly, the cellular response to BMP-7 by Smad1 phosphorylation in CD44-negative LNCaP cells was less than that in CD44-positive PC-3 cells (19), which is consistent with our BMP-7 dose-response results in murine chondrocytes. The stimulatory effect of CD44 in these mouse chondrocytes appears to be as a facilitator. When cells were assayed in vitro at a conventional concentration of BMP-7 (i.e., 100 ng/ml), little influence of CD44 on BMP-7 signaling could be distinguished. Nonetheless, the BMP-7 concentration in human serum is 0.5–1 ng/ml (15), and based on a BMP-2 turnover rate of ~3 ng/day in the normal adult skeleton, a recent study found that implants releasing ~5 ng of BMP-7 per day yielded the highest bone volumes (17). The hyaluronan pericellular matrix may modulate signaling on chondrocytes that express CD44, promoting repair in response to low levels of BMP-7.
ALK-2 was found to be critical for BMP-7 signaling. Down-regulation of ALK-2 alone resulted in ~76% reduction in signaling for BMP-7, whereas down-regulation of ALK-3 alone resulted in only ~18% reduction in signaling for BMP-7 (39). BMP-4 was found to bind to ALK-3 and/or ALK-6, whereas BMP-6 and BMP-7 preferentially bound to ALK-2 (38). The CD44 effects we observed do not appear to affect the EGF pathway and do not promote crosstalk (such as p-p38) that might participate in noncanonical BMP signaling pathways. As ALK-2 expression in WT and CD44−/− mouse chondrocytes did not differ, the mechanism that CD44 directly regulates ALK-2 is unlikely.
An unexpected but exciting outcome of this study was the effects of hyaluronidase on BMP-7 signaling in CD44−/− chondrocytes. Hyaluronidase pretreatment significantly diminished the BMP-7–induced activation of Smad1 in both CD44−/− and WT mouse chondrocytes (Figure 5), as well as bovine chondrocytes, and the effect was reversible with time for pericellular matrix recovery. Chondrocytes were also less responsive to BMP-7 when 4-MU was used as an alternative, nonenzymatic approach to depletion of hyaluronan from the pericellular matrix (Figure 6). CD44 clustering has been shown to be induced by exogenous hyaluronan on COS-7–transfected cells expressing CD44; moreover, CD44 clustering on the surface of HK-2 and BT-549 cells was found to be reduced by treatment with 0.5 mM 4-MU or testicular hyaluronidase, confirming that endogenous hyaluronan is a critical factor in the formation of CD44 clusters (42). Such CD44 clusters may enhance Smad1 presentation to the BMP signaling receptors, and this would be reduced by treatment with testicular hyaluronidase or 4-MU. Smad1 is present in the cytoplasm and can be phosphorylated in CD44−/− cells, but the reduced response may be rescued by transfection with CD44. Another possibility is that the presence of hyaluronan facilitates BMP-7 responsiveness by way of an extracellular mechanism that is independent of CD44.
Other studies have described the complex contribution of the extracellular matrix in the regulation of BMP signaling (43). Proteoglycans can bind BMP inhibitory binding proteins such as Noggin and Chordin (1), or BMPs can be trapped within the matrix (44); thus, altered signaling is likely to be dependent on the cellular context. For chondrocytes, removal of the hyaluronan-dependent pericellular matrix diminishes BMP-7 responsiveness.
Several possibilities may explain the mechanism of the hyaluronidase effect. It is possible that the hyaluronan pericellular coat promotes the functional organization of molecules within the plasma membrane, and this may include lipid rafts, since the HAS enzymes are usually found in lipid rafts (45). Hyaluronan may serve as a binding scaffold for BMP-7 itself, facilitating presentation to the signaling BMP receptor; alternatively, hyaluronan removal could release an as-yet-unknown hyaluronan-bound coactivator or could allow an inhibitor of the initiation of the BMP signaling pathway to be more effective. Yet another possibility is that hyaluronan removal may stimulate cell surface–associated proteolytic activity, affecting the degradation of a critical BMP signaling coactivator. We and other investigators have demonstrated that the loss of a hyaluronan-rich pericellular matrix induces membrane-bound membrane-type matrix metalloproteinase or ADAM-10/17–mediated cleavage of CD44 (27,46). It also remains possible that the removal of hyaluronan affects BMP-7 responsiveness by modulating the activity of a hyaluronan receptor, including CD44, but in the case of the CD44−/− cells, crosstalk by another compensating receptor. All of these possibilities open new avenues to explore in the future.
Hyaluronidase pretreatment had no effect on Smad2/4 nuclear translocation following stimulation with TGFβ1 in bovine chondrocytes, whereas in the same cell system, hyaluronidase pretreatment diminished Smad1 phosphorylation as well as Smad1 or Smad4 nuclear translocation in response to BMP-7; this inhibition was rescued by overexpression of pIRES2-eGFP-HAS-2 (11). Taken together, our data suggest that in chondrocytes, alteration of the hyaluronan matrix and CD44 affects a more select influence over BMP-7 responsiveness than does EGF- or TGFβ1-mediated signaling. Thus, our hypothesis that CD44 functions in a manner similar to a SARA in BMP-7 signaling by sequestering Smad1 for presentation to the BMP receptors for activation (9,11,43) takes the twist that the extracellular matrix component hyaluronan mediated the function of CD44. However, the presence of pericellular hyaluronan is clearly important for BMP signaling in chondrocytes.
Many reports discuss the need for meticulous regulation and redundancy in BMP signaling as a requirement for the fine-tuning of cell fates during development, the establishment of morphogenetic gradients, and the maintenance of tissue homeostasis (43,47). The question remains as to whether there is clinical relevance for this kind of regulation in adult tissues. During osteoarthritis, the loss of cell surface hyaluronan/proteoglycan aggregates is a conspicuous event. Our previous studies have shown that chondrocytes “sense” these changes in part via the hyaluronan receptor CD44. When the loss of hyaluronan is induced experimentally, as in this study and others, BMP-7 responsiveness is reduced. During early osteoarthritis, the resident chondrocytes are thought to mount a reparative response that includes the synthesis and secretion of endogenous BMP-7 (48,49). However, if there is damaged extracellular matrix and increasing proteolytic cleavage of CD44 (27,50), a diminished BMP response would coincide with the time frame for endogenous BMP-7 secretion, limiting the attempted repair. The therapeutic combination of enhanced HAS-2 synthetic activity in the presence of CD44 may enhance cartilage reparative responses to endogenous and/or exogenous BMP-7.
ACKNOWLEDGMENTS
The authors thank Drs. Tibor T. Glant and Katalin Mikecz (Rush University Medical Center, Chicago, IL) for graciously providing the CD44−/− mice (BALB/c background).
Supported in part by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01-AR-043384 to Dr. W. Knudson and R01-AR-039507 to Dr. C. B. Knudson).
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
AUTHOR CONTRIBUTIONS All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. C. B. Knudson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Luo, W. Knudson, Askew, Veluci, C. B. Knudson.
Acquisition of data. Luo, W. Knudson, Askew, Veluci, C. B. Knudson.
Analysis and interpretation of data. Luo, W. Knudson, Askew, Veluci, C. B. Knudson.
REFERENCES
- 1.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–28. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 3.Ponta H, Sherman L, Herrlich P. CD44: From adhesion molecules to signaling regulators. Nature Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 4.Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol. 1994;126:1099–109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–80. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohara Y, Ishiguro N, Machida K, Kurata H, Thant AA, Senga T, et al. Hyaluronan activates cell motility of v-Src-transformed cells via Ras-mitogen-activated protein kinase and phosphoinositide 3-kinase-Akt in a tumor-specific manner. Mol Biol Cell. 2001;12:1859–68. doi: 10.1091/mbc.12.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–92. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 9.Peterson RS, Andhare RA, Rousche KT, Knudson W, Wang W, Grossfield JB, et al. CD44 modulates Smad1 activation in the BMP-7 signaling pathway. J Cell Biol. 2004;166:1081–91. doi: 10.1083/jcb.200402138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddi A. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotech. 1998;16:247–52. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 11.Andhare RA, Takahashi N, Knudson W, Knudson CB. Hyaluronan promotes the chondrocyte response to BMP-7. Osteoarthritis Cartilage. 2009;17:892–902. doi: 10.1016/j.joca.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida Y, Knudson CB, Nietfeld JJ, Margulis A, Knudson W. Antisense inhibition of hyaluronan synthase-2 in human articular chondrocytes inhibits proteoglycan retention and matrix assembly. J Biol Chem. 1999;274:21893–9. doi: 10.1074/jbc.274.31.21893. [DOI] [PubMed] [Google Scholar]
- 13.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell. 1998;95:779–91. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 14.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 15.Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 16.Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W. Osteogenic protein 1 stimulates cell-associated matrix assembly by normal human articular chondrocytes: up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum. 2000;43:206–14. doi: 10.1002/1529-0131(200001)43:1<206::AID-ANR25>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Wu G, Hunziker EB, Zheng Y, Wismeijer D, Liu Y. Functionalization of deproteinized bovine bone with a coating-incorporated depot of BMP-2 renders the material efficiently osteoinductive and suppresses foreign-body reactivity. Bone. 2011;49:1323–30. doi: 10.1016/j.bone.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Z, Watson N, Rodriguez C, Lodish HF. Nucleocytoplasmic shuttling of Smad1 conferred by its nuclear localization and nuclear export signals. J Biol Chem. 2001;276:39404–10. doi: 10.1074/jbc.M103117200. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Zhong C, Frenkel B, Reddi AH, Roy-Burman P. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65:5769–77. doi: 10.1158/0008-5472.CAN-05-0289. [DOI] [PubMed] [Google Scholar]
- 20.Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64:5702–11. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 21.Lou W, Krill D, Dhir R, Becich MJ, Dong JT, Frierson HF, et al. Methylation of the CD44 metastasis suppressor gene in human prostate cancer. Cancer Res. 1999;59:2329–31. [PubMed] [Google Scholar]
- 22.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–33. [PubMed] [Google Scholar]
- 23.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–23. [PubMed] [Google Scholar]
- 24.Szanto S, Gal I, Gonda A, Glant TT, Mikecz K. Expression of L-selectin, but not CD44, is required for early neutrophil extravasation in antigen-induced arthritis. J Immunol. 2004;172:6723–34. doi: 10.4049/jimmunol.172.11.6723. [DOI] [PubMed] [Google Scholar]
- 25.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFκB and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2006;281:17952–60. doi: 10.1074/jbc.M602750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudson W, Aguiar DJ, Hua Q, Knudson CB. CD44-anchored hyaluronan-rich pericellular matrices: an ultrastructural and biochemical analysis. Exp Cell Res. 1996;228:216–28. doi: 10.1006/excr.1996.0320. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Knudson CB, Thankamony S, Ariyoshi W, Mellor L, Im HJ, et al. Induction of CD44 cleavage in articular chondrocytes. Arthritis Rheum. 2010;62:1338–48. doi: 10.1002/art.27410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–20. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 29.Ariyoshi W, Knudson CB, Luo N, Fosang AJ, Knudson W. Internalization of aggrecan G1 domain neoepitope ITEGE in chondrocytes requires CD44. J Biol Chem. 2010;285:36216–24. doi: 10.1074/jbc.M110.129270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H, Peterson RS, Wang W, Bartnik E, Knudson CB, Knudson W. A requirement for the CD44 cytoplasmic domain for hyaluronan binding, pericellular matrix assembly and receptor mediated endocytosis in COS-7 cells. J Biol Chem. 2002;277:10531–8. doi: 10.1074/jbc.M108654200. [DOI] [PubMed] [Google Scholar]
- 31.Salvat C, Pigenet A, Humbert L, Berenbaum F, Thirion S. Immature murine articular chondrocytes in primary culture: a new tool for investigating cartilage. Osteoarthritis Cartilage. 2005;13:243–9. doi: 10.1016/j.joca.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–78. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz I, Ariyoshi W, Takahashi N, Knudson CB, Knudson W. Hyaluronan oligosaccharide treatment of chondrocytes stimulates expression of both HAS-2 and MMP-3, but by different signaling pathways. Osteoarthritis Cartilage. 2010;18:447–54. doi: 10.1016/j.joca.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meran S, Luo D, Simpson R, Martin J, Wells A, Steadman, et al. Hyaluronan facilitates transforming growth factor-β1–dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem. 2011;286:17618–30. doi: 10.1074/jbc.M111.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase Cε-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–40. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- 36.Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–36. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- 37.Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, et al. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci. 2001;114:1483–9. doi: 10.1242/jcs.114.8.1483. [DOI] [PubMed] [Google Scholar]
- 38.Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–58. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kakizaki I, Kojima K, Takagaki K, Endo M, Kannagi R, Ito M, et al. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem. 2004;279:33281–9. doi: 10.1074/jbc.M405918200. [DOI] [PubMed] [Google Scholar]
- 40.Kultti A, Pasonen-Seppanen S, Jauhiainen M, Rilla KJ, Karna R, Pyoria E, et al. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–23. doi: 10.1016/j.yexcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–6. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C, Cao M, Liu H, He Y, Xu J, Du Y, et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 2012;287:43094–107. doi: 10.1074/jbc.M112.349209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 44.Fisher MC, Li Y, Seghatoleslami M, Dealy CN, Kosher RA. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol. 2006;25:27–39. doi: 10.1016/j.matbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Rilla K, Pasonen-Seppnen S, Deen AJ, Koistinen VV, Wojciechowski S, Oikari S, et al. Hyaluronan production enhances shedding of plasma membrane-derived microvesicles. Exp Cell Res. 2013;319:2006–18. doi: 10.1016/j.yexcr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Sugahara KN, Murai T, Nishinakamura H, Kawashima H, Saya H, Miyasaka M. Hyaluronan oligosaccharides induce CD44 cleavage and promote cell migration in CD44-expressing tumor cells. J Biol Chem. 2003;278:32259–65. doi: 10.1074/jbc.M300347200. [DOI] [PubMed] [Google Scholar]
- 47.Kuo W, Digman M, Lander AD. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell. 2010;21:4028–41. doi: 10.1091/mbc.E10-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002;1588:126–34. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 49.Lories RJU, Luyten FP. Bone morphogenetic protein signaling in joint homeostasis and disease. Cytokine Growth Factor Rev. 2005;16:287–98. doi: 10.1016/j.cytogfr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]