Abstract
The goal of regenerative endodontics is to restore the functions of the dental pulp–dentin complex. Two approaches are being applied toward dental pulp–dentin regeneration: cell transplantation and cell homing. The majority of previous approaches are based on cell transplantation by delivering ex vivo cultivated cells toward dental pulp or dentin regeneration. Many hurdles limit the clinical translation of cell transplantation such as the difficulty of acquiring and isolating viable cells, uncertainty of what cells or what fractions of cells to use, excessive cost of cell manipulation and transportation, and the risk of immune rejection, pathogen transmission, and tumorigenesis in associated with ex vivo cell manipulation. In contrast, cell homing relies on induced chemotaxis of endogenous cells and therefore circumvents many of the difficulties that are associated with cell transplantation. An array of proteins, peptides, and chemical compounds that are yet to be identified may orchestrate endogenous cells to regenerate dental pulp–dentin complex. Both cell transplantation and cell homing are scientifically valid approaches; however, cell homing offers a number of advantages that are compatible with the development of clinical therapies for dental pulp–dentin regeneration.
Introduction
Dental pulp, along with other dental structures such as dentin and cementum, originates from neural crest-derived mesenchyme. Dental pulp is the only vascularized connective tissue in the tooth and provides vital functions of homeostasis (1). Dentin and pulp work as a physiological and functional unit. Dental pulp consists of heterogeneous cell populations, among which stem/progenitor cells are anticipated to replenish odontoblasts upon infection or trauma in adulthood. The pulp is richly vascularized and innervated, which are important for homeostasis and pathology. Interestingly, there has apparently been no report of neoplasm in dental pulp.
The American Dental Association (ADA) recently adopted a clinical procedure with a code for “pulpal regeneration” (2). This procedure produces increased apical formation and induction of periapical bleeding into the root canal, with a goal of restoring the vitality of dental pulp in immature, young permanent teeth. However, clinical literature dating back 30–40 years suggests that the outcome of this procedure can positively re-establish partial vitality of the pulp but is highly variable amongst individuals (3). Thus, this ADA-approved procedure may or may not lead to dental pulp regeneration in patients.
Significant progress has been made in our understanding of the biology of dental pulp at the cellular and molecular levels, and more recently, approaches toward dental pulp–dentin regeneration (1). However, a limited number of existing in vivo animal studies on dental pulp–dentin regeneration have not illustrated a valid pathway toward clinical applications for reasons that are outlined below. In this review, the authors will discuss the challenges and opportunities in dental pulp–dentin regeneration by contrasting cell transplantation and cell homing approaches.
Cell transplantation for dental pulp regeneration
Stem/progenitor cells in dental pulp derive from dental papilla during tooth development. Hypothetically, mesenchymal stem/progenitor cells in the pulp replenish all pulp cells including odontoblasts in post-natal life. It is, therefore, intuitive to transplant mesenchymal stem/progenitor cells from dental pulp directly into endodontically treated root canals in order to regenerate the dental pulp–dentin complex. However, cell transplantation for the purpose of dental pulp–dentin regeneration has encountered numerous hurdles in clinical application.
Cell transplantation has been a major in vivo preclinical pulp tissue regeneration approach using stem/progenitor cells not only from dental pulp but also of other origins. The transplanted stem/progenitor cells, irrespective of their origins, are thought to participate in the regeneration/repair process not only by supplying cells per se but also by providing growth factors or signaling molecules released from transplanted cells as trophic factors to enhance the cellular activity of endogenous as well as transplanted cells (1,4-7). In a tooth slice model, Cordeiro et al. showed that vascular pulp-like tissue was regenerated when a gel seeded with stem cells from human exfoliated deciduous teeth was implanted into the dorsum of immunodeficient mice (8). A similar study using an ectopic tooth fragment model by Huang et al. demonstrated the formation of pulp-like tissue with deposition of dentin-like tissue upon implantation of stem/progenitor cells from the apical papilla and dental pulp stem cells (DPSC) in mice (9). In an effort to maximize the efficiency of cell transplantation, Iohara et al. used cell fractions such as CD31−/CD146− and CD105+ side population (SP) cells from dental pulp for pulp regeneration; they possessed higher self-renewal capacity and differentiation potential compared to unfractionated cells (10,11). These SP cells were angiogenic and neurogenic, and therefore considered a promising cell source for pulp regeneration (12). In an orthotopic canine model, Iohara et al. further showed that pulp-like tissue with blood vessels and innervation was formed upon transplantation of CD105+ SP cells in a collagen scaffold with stromal derived factor-1 after pulpectomy (13). Using a similar orthotopic canine model, Ishizaka et al. demonstrated that CD31− SP cells from dental pulp, bone marrow, and adipose tissue yielded pulp-like tissue upon cell implantation into emptied root canals (14). The adipose-derived SP cells showed higher matrix formation and odontoblastic differentiation than those from other sources (14). This suggests that CD31− SP cells from adipose tissue may be an alternative to pulp-derived SP cells, similar to the potential use of adipose stem cells in other tissue engineering applications (15,16).
What are the scientific values of cell transplantation?
Cell transplantation has scientific value in preclinical research to answer the following critical questions, although few studies on this have been undertaken (Table 1).
How do transplanted stem/progenitor cells participate in pulp regeneration? Cells can be tagged with specific markers such as green fluorescent protein and transplanted into the root canal space. In in vivo cell tracing studies, the proportions of the transplanted cells in regenerated pulp tissues can be investigated relative to host cells. Furthermore, in vivo cell lineage tracing may reveal the cellular mechanisms of pulp regeneration and fate of transplanted cells in newly regenerated tissues (e.g. how the cells give rise to specialized cells such as collagen-forming cells, endothelial cells, and odontoblasts).
Will some of the transplanted stem/progenitor cells maintain “stemness” in the newly regenerated tissues? The transplanted cells can be harvested again from the regenerated tissues and implanted in other animals to test their regeneration potential. Serial transplantation may demonstrate if cells with stemness (including self-renewal, potency, and clonogenicity) are truly present and participate in in vivo pulp regeneration. This involves the development of orthotopic animal models to reproducibly regenerate pulp tissues, and molecular assays to capture the specific cell-cycle stages with signals dictating self-renewal and differentiation.
How do systemic and local pathological conditions affect the transplanted cells in the process of pulp regeneration? Systemic diseases such as congenital or inherited disorders (e.g. sickle cell anemia, dentinogenesis imperfecta) and immunocompromised conditions (e.g. chronic granulomatous disease, severe combined immunodeficiency) may alter the intrinsic host mechanisms of wound healing and change the behaviors of transplanted cells in pulp regeneration. Local pathology such as the presence of microorganisms in root canals and/or periapical lesions may also play a significant role in the regeneration process with the involvement of neurogenic inflammatory reactions and immune responses.
Table 1.
Goals and methods to study stem cells in dentin—pulp regeneration
| Fates of transplanted cells |
In vivo cell tracing In vivo cell lineage tracing |
| Stemness | Serial transplantation Cell cycle analysis Evaluation in orthotopic animal models |
| Effects of local and systemic pathology on regeneration |
Development of disease models Molecular assays Immune epitope analysis |
What are clinically valid cell sources for dental pulp–dentin regeneration?
Suppose that a patient sits in a dental chair for pulp regeneration therapy; where can we obtain cells to transplant into clinically prepared root canals? If the patient has a wisdom tooth or a healthy tooth that needs to be extracted for orthodontic reasons, stem/progenitor cells can be harvested from the extracted tooth. However, these patients are rarely found. Other cell sources that have been used for dental pulp regeneration include postnatal stem/progenitor cells such as stem cells from exfoliated deciduous teeth (17), stem cells from apical papilla (9,18), dental follicle precursor cells (19-21), periodontal ligament stem cells (22,23), and bone marrow mesenchymal stem/stromal cells (24,25). Invariably, complex cell harvest procedures need to be followed, including tooth extraction, pulp extirpation, in vitro cell culture, selection of stem/progenitor cell populations, ex vivo cell expansion, and storage and shipping to the practitioner. Also, there are other hurdles such as potential contamination during cell manipulation, storage, and transplantation, as well as the potential risk of the development of tumorigenesis during ex vivo cell manipulation (26,27).
Would induced pluripotent stem (iPS) cells be an ideal cell source for dental pulp regeneration? Oda et al. demonstrated that mesenchymal stromal cells from pulp tissues of third molars could be reprogrammed into iPS cells by retroviral transduction of Oct3/4, Sox2, and Klf4 (28). Importantly, Oda et al. showed that iPS cells with a high yield could be acquired without Myc (a universal amplifier of expressed genes in lymphocytes and embryonic stem cells) when stromal cells from pulp tissues were used (28). Despite high iPS generation efficiency, non-viral reprogramming methods such as plasmid, protein, and microRNA need to be utilized in order to avoid genomic mutations before iPS cell transplantation for therapeutic applications. Oh et al. demonstrated that epithelial-mesenchymal transition (EMT) can be used to reprogram human epidermal keratinocytes by ΔNp63α transduction to generate mesenchymal stem cells (MSCs) (29). The ΔNp63α-transduced cells showed morphological and functional similarity to MSCs with upregulation of pluripotent stem cell markers such as Nanog and Lin28. However, iPS cells also suffer from excessive cost and safety concerns.
Therapeutic models
Pulp regeneration by cell transplantation dictates a point-of-care (POC) therapy. Unless the clinician is trained to isolate and culture cells, one must rely on commercial stem cell products. POC refers to one doctor treating one patient using the patient’s own cells from the point of cell harvesting to transplantation. The use of patient-specific cells is clinically beneficial because the cells carry the same genetic information as the host and thereby will efficiently engraft without immune rejection upon transplantation. When allogeneic cells are used, donor-associated variation such as age, gender, and method of isolation may substantially influence the characteristics of harvested cells and will likely cause variable treatment outcomes. Phinney et al. showed large variation in the in vitro growth and differentiation capacity of donor cells within and among the donors (30). Notably, MSCs simultaneously isolated at different locations from the same donor also showed a large growth rate difference (30). Therefore, the heterogeneity in cell characteristics associated with donor variability can be a limitation in POC therapy. The lack of practitioner training in cell harvesting and handling procedures will be another challenge in this therapeutic approach.
Currently, there are no stem cells approved for cell therapy of any disease. When approved, commercial stem cells may be an alternative to the patient’s own cells. Due to the limited source of patient-specific cells, human allogeneic cells will likely be used for commercialized stem cell products with the premise that the allogeneic donor cells do not mount an immune response against the tissues of the recipient (immune rejection) (31-33). Regardless of the therapeutic model, pulp regeneration therapy using cell transplantation is likely to suffer from a lack of clinical viability, difficulty with regulatory approval, and the high cost associated with storage (cell cryopreservation/banking system) and packaging, not to mention the risk of immunorejection, pathogen transmission, and tumorigenesis during engraftment.
Most stem cells remain in a quiescent state in vivo. For example, the osteoblastic niche harbors hematopoietic stem cells in a quiescent state, while the vascular niche plays a role in the mobilization, proliferation, and differentiation of stem cells (34,35). Quiescent stem cells may be activated by microenvironment changes such as tissue injury or disease (36). Cell quiescence is critical in maintaining stem cell populations in a tissue without exhausting their proliferative potentials (37,38). However, in clinical regenerative therapy, stem cells must enter the cell cycle and be activated from their quiescent form. A recent study by Feng et al. showed that, in response to pulp tissue damage, MSCs can be activated and migrate from their distinct niche, perivascular area, or cervical loop end toward the site of injury, proliferate, and differentiate into odontoblasts (39). But it was noted that few cells could be mobilized to replenish damaged odontoblasts.
Cell transplantation often requires in vitro cell expansion before cells are transplanted into a tissue (6,40). Stem cells for pulp regeneration therapy also need to be expanded in in vitro culture before transplantation but there are certain risks associated with this process. During in vitro expansion, cells can lose their potency and experience cell transformation (41-43), although a mechanical method of scale-up with minimal risk of potency loss has been elaborated in a recent study using DPSC (44). Nonetheless, some findings have yielded positive results (45-47). Sadri-Ardekani et al. (45) demonstrated that human spermatogonial cells can be subcultured from testicular cell cultures and propagated for as long as 28 weeks. The expression of spermatogonial stem cell markers was found to be maintained in the subcultured cells throughout the entire culture period. Yu et al. (46) showed that the proliferation potential of STRO1+ DPSC was maintained for up to 9 passages. In an attempt to optimize a cell culture condition for stem cells, Liu et al. (47) added BMP-4 to cell culture media in order for periodontal ligament stem cells to maintain stemness potentials. With the addition of BMP-4, the expression of stem cell markers including SOX2, Oct-4, and c-Myc was maintained in periodontal ligament stem cells for as long as 7 passages. However, it is still a challenge to achieve safe and reliable in vitro cell expansion that obviates the risks of cell transformation and potential loss of stemness during long-term cell expansion (48,49). As well, the potency of cells observed in in vitro conditions may not be adequately expressed in the transplanted site, resulting in the formation of tissues far from their original architectures (50). Additional potential risks such as immunorejection and pathogen transmission are the other concerns related to cell transplantation (26).
Cell homing for dental pulp regeneration
Cell homing has been underexplored in the field of tissue engineering. From a therapeutic perspective, cell homing may circumvent many of the challenges associated with cell transplantation as outlined in Table 1. Cell homing is defined as the migration of cells, including stem/progenitor cells, to tissue defects. In tissue regeneration, cell homing should be followed by tissue synthesis by homed and/or locally resident cells (1,51-53). Cell homing describes the migration and mobilization of cells, including stem/progenitor cells, to the site of regeneration and/or injury, the process of which is induced by biological signaling molecules (53,54). In order to harness the innate healing potentials of endogenous cells, different types and forms of signaling molecules with scaffolds have been added to the tissues to engineer in vivo. For example, the articular surface of the synovial joint was regenerated in vivo with a cell-free approach using an anatomically correct bioscaffold embedded with a growth factor, TGFβ3 (53). The regenerated avascular cartilage was integrated with regenerated subchondral bone. In a recent study by Kim et al. (55), regeneration of tooth-like structures was demonstrated using a cell homing approach. Anatomically shaped tooth scaffolds mimicking a human mandibular first molar were fabricated by three-dimensional (3D) bioprinting with 80% (m/m) polycaprolactone (PCL) and 20% (m/m) of hydroxyapatite (HA). A porous structure with 3D microstrands and 200-μm diameter interconnecting microchannels allows for cell migration and proliferation. A cocktail of stromal cell-derived factor 1 (SDF1) and bone morphogenetic protein 7 (BMP7) was added to a 2 mg/mL neutralized type 1 collagen solution. The scaffolds embedded with the mixture of SDF1, BMP7, and collagen solution were implanted in subcutaneous tissues. At nine weeks post-surgery, human-shaped mandibular first molar scaffolds retrieved from the dorsum of rats showed that endogenous cells were recruited into microchannels of the scaffold with isolated mineralization. Notably, significantly more cells and blood vessels were observed in the microchannels of the scaffolds with SDF1 and BMP7 delivery than without the growth factor delivery. In addition, regeneration of periodontal ligament-like tissues and newly formed alveolar bone were also observed in an orthotopic model using rat incisor scaffolds after nine weeks.
Cell homing has been utilized to induce the regeneration of dental pulp-like tissues. Kim et al. (56) showed that several growth factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), and BMP7 served as signaling molecules for pulp regeneration. Human extracted canines and incisors were endodontically treated without root canal filling materials. The emptied root canals were filled with collagen scaffolds impregnated with growth factors. The teeth were implanted into the dorsum of mice for 3 weeks. Upon retrieval of the implanted teeth, vascular pulp-like tissues with innervation and odontoblast layers were observed along the entire length of the root canals. With the delivery of bFGF and/or VEGF, newly formed recellularized and revascularized connective tissues were integrated into native dentinal wall in root canals. With the combined delivery of bFGF, VEGF, or PDGF with a basal set of NGF and BMP7, cellularized and vascularized tissues positive of VEGF antibody staining were regenerated with new dentin formation over the native dentin in some endodontically treated teeth. Newly regenerated dental pulp tissues were dense with cells and extracellular matrix. This study in an ectopic model is the first to demonstrate that host endogenous cells can migrate, proliferate, and differentiate into cells that constitute pulp tissue. This complicated regeneration process is thought to be guided by growth factors that filled the emptied root canals of the extracted teeth. For example, bFGF and PDGF were biological cues for chemotaxis; PDGF and VEGF for vasculogenesis/angiogenesis; NGF for neuronal growth and survival; and BMP-7 for odontoblast differentiation and mineralization. It is anticipated in the foreseeable future that a diseased dental pulp could be treated with less invasive root canal therapy including minimal mechanical instrumentation for pulp regeneration. Sufficient disinfection followed by delivery of injectable bioactive cues will provide a microenvironment for the recruited cells to overcome the residual infection, if any, in the root canals with native defense mechanisms and proliferate and differentiate into cells that contribute to pulp regeneration. Some of the advantages of cell homing for dental pulp–dentin regeneration in comparison to cell transplantation are summarized below.
Cell homing circumvents some of the safety issues associated with cell transplantation such as potential immune rejection, pathogen transmission and tumorigenesis, the manufacturing issues such as high costs associated with storage (cell cryopreservation/banking system), packaging, and shipping, the technical issues related to lack of training of handling cells for practitioners, and the regulatory issues for FDA approval.
Clinicians do not require special training for the delivery of cell homing products (signaling molecules) due to their ease of delivery into root canals, whereas clinicians need to learn how to properly handle and apply stem cell products via special training.
Commercialized cell homing products that are approved by the FDA already exist in the market. FDA approval of other signaling molecules is expected to be relatively easier than that of stem cell products.
Since the earlier work by Nygaard-Østby et al. (57), there have been a number of clinical studies investigating the regeneration of pulp tissue. However, most of the studies were case reports or case series in young patients (58-68). With few exceptions, the following clinical scenarios were stated in these studies. Root canals were disinfected with antimicrobial solutions followed by intracanal medicaments such as calcium hydroxide or triple/double antibiotics. After the symptoms disappeared, bleeding was induced in the root canals, which were then sealed with permanent restorations with or without mineral trioxide aggregates. A recent study by Shimizu et al. (67) showed that this clinical pulp revacularization/regeneration procedure could regenerate the pulp tissues in a human immature permanent tooth with irreversible pulpitis. It was demonstrated in this case report that pulp-like connective tissues were regenerated in the root canal space with odontoblast-like cell layers. From the perspective of the cell-free approach, however, there were no growth factors involved in these studies, although some growth factors might be released from the dentin matrix. A recent case report by Torabinejad & Turman used platelet-rich plasma (PRP) in a replanted, immature, necrotic maxillary premolar following accidental extraction (68). PRP, although not a pure form of growth factors, can be considered a combination of biological signaling molecules such as PDGF, transforming growth factor beta (TGFβ), FGF, VEGF, and connective tissue growth factor (69,70). In this study, PRP from the patient’s blood was injected into the root canals after disinfection. At about the 5-month recall, continued root maturation was observed radiographically, suggesting that vital tissue regeneration in the root canal space was achieved. Pulp regeneration using PRP is a novel approach but suffers from disadvantages such as drawing blood from the patient and additional centrifuge and purification processes.
Growth factors
An understanding of the functions of growth factors is fundamental in the cell homing approach (71). Among many growth factors, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), and bone morphogenetic protein-7 (BMP7) may be important in pulp regeneration as shown by Kim et al. (56).
VEGF is a heparin-binding protein and plays an essential role in vasculogenesis/angiogenesis (72). VEGF induces the proliferation of endothelial cells and increases their survival while stimulating new blood vessel formation at the site of the injury (73). It was shown in an in vivo study by Mullane et al. that the density of microvessels in tooth slices with severed dental pulp significantly increased when the teeth were treated with VEGF and implanted into subcutaneous tissues of severe combined immunodeficiency (SCID) mice (74). Marchionni et al. showed the differentiation of DPSC into endothelial cells based on the immunophenotypical profile, increased expression of VEGF receptors, and microvessel formation after VEGF treatment (75). The osteogenic differentiation of DPSC under osteogenic conditions may be enhanced by the addition of VEGF (76).
Basic fibroblast growth factor (bFGF), also known as FGF2, may play an important role in pulp regeneration due to its potent angiogenic potential in the dental pulp (77-80), along with PDGF (81,82) and VEGF (74). bFGF is also potent in recruiting DPSCs. In a study by Suzuki et al. using a transwell migration assay, significantly more DPSCs were mobilized in the bFGF group compared with the growth factor-free group and the BMP7 group (83). bFGF is also potent in stimulating the proliferation of DPSCs without differentiation (84).
PDGF, released by platelets, is of significance in cell proliferation and angiogenesis (81,82). However, PDGF has little potency in mineralization because it inhibits alkaline phosphatase (ALP) activity in dental pulp cells in cultures (85-87). Four isoforms of PDGF are present as homodimers (PDGF-AA, -BB, -CC, and -DD) whilePDGF-AB is a heterodimer (88). The expression of dentin sialoprotein (DSP) is stimulated by PDGF-AB and PDGF-BB but is inhibited by PDGF-AA, suggesting that the effects of PDGF on odontoblast differentiation depend on its dimeric form (89). PDGF-BB also enhances the expression of VEGF in osteoblasts and promotes angiogenesis (82).
NGF promotes the survival and maintenance of sympathetic and sensory neurons as well as non-neuronal cells (90,91). The expression of NGF and its receptors was shown to increase in dental pulp cells at the injury site (92) and during tooth development (93). NGF has been shown to induce differentiation of immortalized dental papilla cells into odontoblasts in vitro, suggesting its role as a stimulant for mineralization (94).
BMP7, also known as osteogenic protein-1 (OP-1), is effective in dentinogenesis as shown in several animal models such as rats (95), ferrets (96), and miniature swine (97). This dentinogenic effect relies on the odontoblast differentiation induced by BMPs including BMP2 (98-104), BMP4 (101,102), BMP7 (103,104), and BMP11 (105,106).
Remaining tasks
The use of biological signaling molecules for the cell homing approach makes pulp regeneration more approachable by practitioners because the delivery of growth factors is not nearly as complicated and costly as cell transplantation. Immune rejection, potential contamination during cell manipulation, and unintended differentiating patterns of transplanted cells leading to tumorigenesis are minimized (Table 2). Regulatory approval and commercialization of signaling molecules are not as complicated as with stem cell transplantation. Several existing FDA-approved growth factor products are being used in patients. For example, GEM-21S® (Osteohealth, Shirley, NY) is β-tricalcium phosphate loaded with PDGF-BB for periodontal tissue regeneration. INFUSE® bone graft (Medtronic, Minneapolis, MN) has human recombinant BMP-2 as an active ingredient to promote bone regeneration. However, there are many other growth factors that play key roles in pulp regeneration and also need to be approved by the FDA and commercialized for therapeutic use.
Table 2.
Comparison of cell transplantation and cell homing
| Cell transplantation | Cell homing | |
|---|---|---|
| Cell source | Transplanted cells | Host endogenous cells |
| Risks | Immune rejection Pathogen transmission Tumorigenesis |
Few biological concerns except for shortage of endogenous cells in defect sites |
| Shortcomings | High cost for commercialization and manufacturing stem cell products Special training required for cell handling No FDA pathways |
Existing FDA approval pathways (growth factors) |
| Advantages | Ability to control cell number Ability to control cell type (subpopulation of stem cells) |
Harness the patient’s innate healing potential No special training for use of growth factor product No immune rejection |
Until now, no randomized controlled trials have been performed to investigate the outcome of dental pulp-dentin regeneration using either cell transplantation or cell homing approaches. Without a reasonable rate of cure, pulp regeneration will not be an alternative to conventional root canal therapy. Therefore, the outcome of clinical pulp regeneration needs to be reported as a result of controlled studies and must be reasonably high in order to allow clinicians to implement this treatment as an alternative therapy. As a means to meet this clinical demand, the American Association of Endodontists (AAE) Foundation has sought proposals for clinical research on regenerative endodontic therapy with up to $2.5 million grants (107).
Concluding Remarks
Dental pulp–dentin regeneration using cell transplantation has encountered significant hurdles and, to date, has not illustrated a clinically viable pathway. Cell homing is a clinically translatable approach for dental pulp–dentin regeneration and circumvents some of the key challenges associated with cell transplantation. Whereas new knowledge about dental pulp–dentin regeneration will result from both cell transplantation and cell homing studies, cell homing presents clear advantages in terms of clinical application.
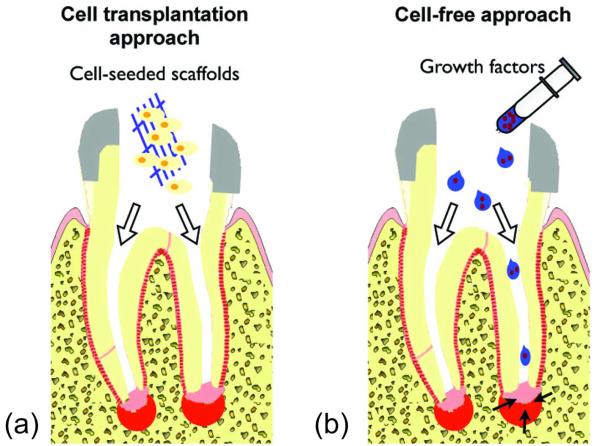
Fig 1.
Diagrams of the cell transplantation and cell-free approaches. (a) In the cell transplantation approach, stem/progenitor cells are placed into root canals in the form of cell-seeded scaffolds. The transplanted cells are thought to participate in the regeneration process not only by supplying cells per se but also by providing growth factors released from the transplanted cells as trophic factors. (b) In the cell-free approach, scaffolds impregnated with growth factors are injected into root canals to induce the migration, proliferation, and differentiation of endogenous stem/progenitor cells residing around the apex of a root.
Acknowledgements
The authors wish to thank F. Guo and J. Melendez for technical and administrative assistance. The effort for the composition of this article is supported by NIH grants R01DE018248, R01EB009663, and RC2DE020767 to J.J. Mao.
References
- 1.Mao JJ, Prockop DJ. Stem cells in the face: tooth regeneration and beyond. Cell Stem Cell. 2012;11:291–301. doi: 10.1016/j.stem.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Dental Association . CDT 2011-2012: The ADA Practical Guide to Dental Procedure Codes. American Dental Association; Chicago: 2010. [Google Scholar]
- 3.Nygaard-Østby B. Tissue formation in the root canal following pulp. Scand J Dental Res. 1971;79:333–349. doi: 10.1111/j.1600-0722.1971.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 4.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 5.Barzilay R, Melamed E, Offen D. Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells. 2009;27:2509–2515. doi: 10.1002/stem.172. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greco N, Laughlin MJ. Umbilical cord blood stem cells for myocardial repair and regeneration. Methods Mol Biol. 2010;660:29–52. doi: 10.1007/978-1-60761-705-1_3. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–615. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 11.Iohara K, Zheng L, Ito M, Ishizaka R, Nakamura H, Into T, Matsushita K, Nakashima M. Regeneration of dental pulp after pulpotomy by transplantation of CD31(−)/CD146(−) side population cells from a canine tooth. Regen Med. 2009;4:377–385. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20:435–440. doi: 10.1016/j.cytogfr.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17:1911–1920. doi: 10.1089/ten.TEA.2010.0615. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33:2109–2118. doi: 10.1016/j.biomaterials.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 16.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35:1536–1542. doi: 10.1016/j.joen.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–65. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43:406–408. doi: 10.1080/03008200290001023. [DOI] [PubMed] [Google Scholar]
- 21.Kémoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F, Briand-Mesange F, Gadelorge M, Arzate H, Narayanan AS, Brunel G, Salles JP. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283–294. doi: 10.1007/s00441-007-0397-3. [DOI] [PubMed] [Google Scholar]
- 22.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 23.Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84:907–912. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- 24.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 25.Miranda SC, Silva GA, Mendes RM, Abreu FA, Caliari MV, Alves JB, Goes AM. Mesenchymal stem cells associated with porous chitosan-gelatin scaffold: a potential strategy for alveolar bone regeneration. J Biomed Mater Res A. 2012;100:2775–2786. doi: 10.1002/jbm.a.34214. [DOI] [PubMed] [Google Scholar]
- 26.Mao JJ, Kim SG, Zhou J, Ye L, Cho S, Suzuki T, Fu SY, Yang R, Zhou X. Regenerative endodontics: barriers and strategies for clinical translation. Dent Clin North Am. 2012;56:639–649. doi: 10.1016/j.cden.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildirim S, Fu SY, Kim K, Zhou H, Lee CH, Li A, Kim SG, Wang S, Mao JJ. Tooth regeneration: a revolution in stomatology and evolution in regenerative medicine. Int J Oral Sci. 2011;3:107–116. doi: 10.4248/IJOS11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda Y, Yoshimura Y, Ohnishi H, Tadokoro M, Katsube Y, Sasao M, Kubo Y, Hattori K, Saito S, Horimoto K, Yuba S, Ohgushi H. Induction of pluripotent stem cells from human third molar mesenchymal stromal cells. J Biol Chem. 2010;285:29270–29278. doi: 10.1074/jbc.M109.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh JE, Kim RH, Shin KH, Park NH, Kang MK. DeltaNp63α protein triggers epithelial-mesenchymal transition and confers stem cell properties in normal human keratinocytes. J Biol Chem. 2011;286:38757–38767. doi: 10.1074/jbc.M111.244939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–436. [PubMed] [Google Scholar]
- 31.Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667–676. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 32.Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1:5. doi: 10.1186/scrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding G, Wang W, Liu Y, An Y, Zhang C, Shi S, Wang S. Effect of cryopreservation on biological and immunological properties of stem cells from apical papilla. J Cell Physiol. 2010;223:415–422. doi: 10.1002/jcp.22050. [DOI] [PubMed] [Google Scholar]
- 34.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann NY Acad Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- 36.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008;321:1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viatour P, Somervaille TC, Venkatasubrahmanyam S, Kogan S, McLaughlin ME, Weissman IL, Butte AJ, Passegué E, Sage J. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell. 2008;3:416–428. doi: 10.1016/j.stem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA. 2011;108:6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 42.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 43.Pal R, Hanwate M, Totey SM. Effect of holding time, temperature and different parenteral solutions on viability and functionality of adult bone marrow-derived mesenchymal stem cells before transplantation. J Tissue Eng Regen Med. 2008;2:436–444. doi: 10.1002/term.109. [DOI] [PubMed] [Google Scholar]
- 44.Lizier NF, Kerkis A, Gomes CM, Hebling J, Oliveira CF, Caplan AI, Kerkis I. Scaling-up of dental pulp stem cells isolated from multiple niches. PLoS One. 2012;7:e39885. doi: 10.1371/journal.pone.0039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F, de Rooij DG, Repping S, van Pelt AM. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 46.Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010;11:32. doi: 10.1186/1471-2121-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L, Wei X, Huang R, Ling J, Wu L, Xiao Y. Effect of bone morphogenetic protein-4 (BMP-4) on the expression of Sox2, Oct-4 and c-Myc in human periodontal ligament cells during long-term culture. Stem Cells Dev. 2013 Mar 1; doi: 10.1089/scd.2012.0548. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, McNiece IK. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 50.Worthley DL, Ruszkiewicz A, Davies R, Moore S, Nivison-Smith I, Bik To L, Browett P, Western R, Durrant S, So J, Young GP, Mullighan CG, Bardy PG, Michael MZ. Human gastrointestinal neoplasia-associated myofibroblasts can develop from bone marrow-derived cells following allogeneic stem cell transplantation. Stem Cells. 2009;27:1463–1468. doi: 10.1002/stem.63. [DOI] [PubMed] [Google Scholar]
- 51.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quesenberry PJ, Becker PS. Stem cell homing: rolling, crawling, and nesting. Proc Natl Acad Sci USA. 1998;95:15155–15157. doi: 10.1073/pnas.95.26.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 55.Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89:842–847. doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A. 2010;16:3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nygaard-Østby B. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol Scand. 1961;19:322–353. [PubMed] [Google Scholar]
- 58.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–187. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 59.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34:876–887. doi: 10.1016/j.joen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009;35:160–164. doi: 10.1016/j.joen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod. 2009;35:745–749. doi: 10.1016/j.joen.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343–1349. doi: 10.1016/j.joen.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds K, Johnson JD, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J. 2009;42:84–92. doi: 10.1111/j.1365-2591.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 65.Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: a case series. J Endod. 2010;36:536–541. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod. 2011;37:562–567. doi: 10.1016/j.joen.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu E, Jong G, Partridge N, Rosenberg PA, Lin LM. Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J Endod. 2012;38:1293–1297. doi: 10.1016/j.joen.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. J Endod. 2011;37:265–268. doi: 10.1016/j.joen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 70.Yu W, Wang J, Yin J. Platelet-rich plasma: a promising product for treatment of peripheral nerve regeneration after nerve injury. Int J Neurosci. 2011;121:176–180. doi: 10.3109/00207454.2010.544432. [DOI] [PubMed] [Google Scholar]
- 71.Kim SG, Zhou J, Solomon C, Zheng Y, Suzuki T, Chen M, Song S, Jiang N, Cho S, Mao JJ. Effects of growth factors on dental stem/progenitor cells. Dent Clin North Am. 2012;56:563–575. doi: 10.1016/j.cden.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A. 2010;16:3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 74.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mullane EM, Dong Z, Sedgley CM, Hu JC, Botero TM, Holland GR, Nör JE. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J Dent Res. 2008;87:1144–1148. doi: 10.1177/154405910808701204. [DOI] [PubMed] [Google Scholar]
- 76.Marchionni C, Bonsi L, Alviano F, Lanzoni G, Di Tullio A, Costa R, Montanari M, Tazzari PL, Ricci F, Pasquinelli G, Orrico C, Grossi A, Prati C, Bagnara GP. Angiogenic potential of human dental pulp stromal (stem) cells. Int J Immunopathol Pharmacol. 2009;22:699–706. doi: 10.1177/039463200902200315. [DOI] [PubMed] [Google Scholar]
- 77.D’ Alimonte I, Nargi E, Mastrangelo F, Falco G, Lanuti P, Marchisio M, Miscia S, Robuffo I, Capogreco M, Buccella S, Caputi S, Caciagli F, Tetè S, Ciccarelli R. Vascular endothelial growth factor enhances in vitro proliferation and osteogenic differentiation of human dental pulp stem cells. J Biol Regul Homeost Agents. 2011;25:57–69. [PubMed] [Google Scholar]
- 78.Tran-Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. J Dent Res. 2006;85:819–823. doi: 10.1177/154405910608500908. [DOI] [PubMed] [Google Scholar]
- 79.Kitamura C, Nishihara T, Terashita M, Tabata Y, Washio A. Local regeneration of dental pulp–dentin complex using controlled release of fgf-2 and naturally derived sponge-like scaffolds. Int J Dent. 2012;2012:190561. doi: 10.1155/2012/190561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishimatsu H, Kitamura C, Morotomi T, Tabata Y, Nishihara T, Chen KK, Terashita M. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatin hydrogels. J Endod. 2009;35:858–865. doi: 10.1016/j.joen.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 81.Kikuchi N, Kitamura C, Morotomi T, Inuyama Y, Ishimatsu H, Tabata Y, Nishihara T, Terashita M. Formation of dentin-like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. J Endod. 2007;33:1198–1202. doi: 10.1016/j.joen.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 82.Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–114. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- 83.Bouletreau PJ, Warren SM, Spector JA, Steinbrech DS, Mehrara BJ, Longaker MT. Factors in the fracture microenvironment induce primary osteoblast angiogenic cytokine production. Plast Reconstr Surg. 2002;110:139–148. doi: 10.1097/00006534-200207000-00025. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, Chotkowski G, Eisig SB, Wong A, Mao JJ. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res. 2011;90:1013–1018. doi: 10.1177/0022034511408426. [DOI] [PubMed] [Google Scholar]
- 85.He H, Yu J, Liu Y, Lu S, Liu H, Shi J, Jin Y. Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int. 2008;32:827–834. doi: 10.1016/j.cellbi.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 86.Rutherford RB, TrailSmith MD, Ryan ME, Charette MF. Synergistic effects of dexamethasone on platelet-derived growth factor mitogenesis in vitro. Arch Oral Biol. 1992;37:139–145. doi: 10.1016/0003-9969(92)90009-w. [DOI] [PubMed] [Google Scholar]
- 87.Denholm IA, Moule AJ, Bartold PM. The behaviour and proliferation of human dental pulp cell strains in vitro, and their response to the application of platelet-derived growth factor-BB and insulin-like growth factor-1. Int Endod J. 1998;31:251–258. doi: 10.1046/j.1365-2591.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 88.Nakashima M. The effects of growth factors on DNA synthesis, proteoglycan synthesis and alkaline phosphatase activity in bovine dental pulp cells. Arch Oral Biol. 1992;37:231–236. doi: 10.1016/0003-9969(92)90093-n. [DOI] [PubMed] [Google Scholar]
- 89.Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006;81:1241–1257. doi: 10.4065/81.9.1241. [DOI] [PubMed] [Google Scholar]
- 90.Yokose S, Kadokura H, Tajima N, Hasegawa A, Sakagami H, Fujieda K, Katayama T. Platelet-derived growth factor exerts disparate effects on odontoblast differentiation depending on the dimers in rat dental pulp cells. Cell Tissue Res. 2004;315:375–84. doi: 10.1007/s00441-003-0839-5. [DOI] [PubMed] [Google Scholar]
- 91.Davidson B, Reich R, Lazarovici P, Flørenes VA, Risberg B, Nielsen S, Sert B, Bedrossian C. Expression of the nerve growth factor receptors TrkA and p75 in malignant mesothelioma. Lung Cancer. 2004;44:159–165. doi: 10.1016/j.lungcan.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 92.Woodnutt DA, Wager-Miller J, O’Neill PC, Bothwell M, Byers MR. Neurotrophin receptors and nerve growth factor are differentially expressed in adjacent nonneuronal cells of normal and injured tooth pulp. Cell Tissue Res. 2000;299:225–236. doi: 10.1007/s004419900129. [DOI] [PubMed] [Google Scholar]
- 93.Byers MR, Wheeler EF, Bothwell M. Altered expression of NGF and P75 NGF-receptor by fibroblasts of injured teeth precedes sensory nerve sprouting. Growth Factors. 1992;6:41–52. doi: 10.3109/08977199209008870. [DOI] [PubMed] [Google Scholar]
- 94.Luukko K, Moshnyakov M, Sainio K, Saarma M, Sariola H, Thesleff I. Expression of neurotrophin receptors during rat tooth development is developmentally regulated, independent of innervation, and suggests functions in the regulation of morphogenesis and innervation. Dev Dyn. 1996;206:87–99. doi: 10.1002/(SICI)1097-0177(199605)206:1<87::AID-AJA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 95.Arany S, Koyota S, Sugiyama T. Nerve growth factor promotes differentiation of odontoblast-like cells. J Cell Biochem. 2009;106:539–545. doi: 10.1002/jcb.22006. [DOI] [PubMed] [Google Scholar]
- 96.Six N, Lasfargues JJ, Goldberg M. Differential repair responses in the coronal and radicular areas of the exposed rat molar pulp induced by recombinant human bone morphogenetic protein 7 (osteogenic protein 1) Arch Oral Biol. 2002;47:177–187. doi: 10.1016/s0003-9969(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 97.Rutherford RB, Gu K. Treatment of inflamed ferret dental pulps with recombinant bone morphogenetic protein-7. Eur J Oral Sci. 2000;108:202–206. doi: 10.1034/j.1600-0722.2000.108003202.x. [DOI] [PubMed] [Google Scholar]
- 98.Jepsen S, Albers HK, Fleiner B, Tucker M, Rueger D. Recombinant human osteogenic protein-1 induces dentin formation: an experimental study in miniature swine. J Endod. 1997;23:378–382. doi: 10.1016/S0099-2399(97)80187-2. [DOI] [PubMed] [Google Scholar]
- 99.Saito T, Ogawa M, Hata Y, Bessho K. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod. 2004;30:205–208. doi: 10.1097/00004770-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 100.Chen S, Gluhak-Heinrich J, Martinez M, Li T, Wu Y, Chuang HH, Chen L, Dong J, Gay I, MacDougall M. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J Biol Chem. 2008;283:19359–19370. doi: 10.1074/jbc.M709492200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 102.Nakashima M. Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and -4. J Dent Res. 1994;73:1515–1522. doi: 10.1177/00220345940730090601. [DOI] [PubMed] [Google Scholar]
- 103.Nakashima M, Nagasawa H, Yamada Y, Reddi AH. Regulatory role of transforming growth factor-beta, bone morphogenetic protein-2, and protein-4 on gene expression of extracellular matrix proteins and differentiation of dental pulp cells. Dev Biol. 1994;162:18–28. doi: 10.1006/dbio.1994.1063. [DOI] [PubMed] [Google Scholar]
- 104.Rutherford RB, Spångberg L, Tucker M, Rueger D, Charette M. The time-course of the induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol. 1994;39:833–838. doi: 10.1016/0003-9969(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 105.Rutherford RB, Wahle J, Tucker M, Rueger D, Charette M. Induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol. 1993;38:571–576. doi: 10.1016/0003-9969(93)90121-2. [DOI] [PubMed] [Google Scholar]
- 106.Nakashima M, Iohara K, Ishikawa M, Ito M, Tomokiyo A, Tanaka T, Akamine A. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11) Hum Gene Ther. 2004;15:1045–1053. doi: 10.1089/hum.2004.15.1045. [DOI] [PubMed] [Google Scholar]
- 107.Nakashima M, Tachibana K, Iohara K, Ito M, Ishikawa M, Akamine A. Induction of reparative dentin formation by ultrasound-mediated gene delivery of growth/differentiation factor 11. Hum Gene Ther. 2003;14:591–597. doi: 10.1089/104303403764539369. [DOI] [PubMed] [Google Scholar]
- 108.American Association of Endodontists AAE Foundation Releases RFP for Regenerative Endodontics Research. 2011 Nov 18; http://www.aae.org/AAE_News_Room/Press_Releases.aspx.