Abstract
Injectable hydrogels may potentially be used for augmentation/regeneration of the lamina propria of vocal fold tissue. In this study, hyaluronic acid (HA) and dextran were chemically modified and subsequently crosslinked via formation of hydrazone bonds in phosphate buffer. Swelling ratios, degradation, and compressive moduli of the resulting hydrogels were investigated. It was found that the properties of HA-dextran hydrogels were variable and the trend of variation could be correlated with the hydrogel composition. The biocompatibility of three injectable HA-dextran hydrogels with different crosslinking density was assessed in the vocal fold region using a ferret model. It was found that HA-dextran hydrogels implanted for three weeks stimulated mild foreign-body reactions. Distinct tissue-material interactions were also observed for hydrogels made from different formulations: the hydrogel 7with the lowest crosslinking density was completely degraded in vivo; while material residues were visible for other types of hydrogel injections, with or without cell penetration into the implantation depending on the hydrogel composition. The in vivo results suggest that the HA-dextran hydrogel matrices can be further developed for applications of vocal fold tissue restoration.
Keywords: hydrogel, vocal fold, hyaluronic acid, dextran, tissue restoration
INTRODUCTION
Hydrogels are a category of biocompatible materials that find important applications in biomedical devices for tissue repair. With appropriate modifications of the chemical and physical properties, hydrogels matrices can be fabricated to serve a variety of functions, from space filler, to cellular scaffolding, to sustained delivery of therapeutics.1–6 In this study, a composite hydrogel was designed and assessed for use of reparative implantations in the superficial layer of the vocal fold. The superficial lamina propria (SLP) of the human vocal fold is a soft resilient tissue that plays a critical role in vocal fold vibration and hence phonation.7 Damaged or scarred SLPs often cause voice dysfunction, and the augmentation or repair of the SLP tissue is acknowledged to be a formidable task.8,9
Because hyaluronic acid (HA) is a major constituent of the extracellular matrix (ECM) of the SLP and is thought to be important for the characteristic viscoelasticity of this tissue,10–13 a variety of HA-based hydrogels have been explored for vocal fold tissue repair,13 including Hylan-B,14 Carbylan-SX,15 a PEG-crosslinked HA hydrogel,15 and a HA-gelatin composite material, Carbylan-GSX.16,17 Among these studies, Carbylan-SX was shown to improve healing and the viscoelastic properties of the vocal fold tissue in a rabbit injury model.15 By incorporating a cell-adhesive gelatin component, Carbylan-GSX may further promote an extracellular environment capable of amplifying the normal wound healing responses and facilitating tissue regeneration, as suggested by the gene expression pattern of the vocal fold extracellular matrix components.16,17
Although injectable hydrogels have shown promise, the in vivo vocal fold tissue responses upon biomaterial implantation are often complicated, with the underlying molecular and cellular mechanisms remaining largely unknown. The material design criteria with respects to material degradability, mechanics, and physiochemical properties as required for the SLP functional recovery so far have only been partly explored.14–17 Investigating how to induce predictable and controllable tissue response in vivo using appropriate hydrogel materials and animal models, is therefore of great importance for engineering efficacious devices for vocal fold tissue repair.
In this study, a set of injectable materials were created by crosslinking HA and dextran, which were modified with hydrazide and aldehyde functional groups, respectively. The reaction between hydrazide and aldehyde groups results in hydrazone bonds with water molecules as the only byproduct; the hydrazone linkage is known to be pH sensitive and can hydrolytically dissociate into the original groups under acidic conditions. This reaction has been used for various biomedical applications, such as for designing drug conjugates for pH controlled release18,19 and in situ forming matrices, including HA,20 alginate,21 dextran,22 hydrogels.
Besides the ability to form hydrogels from solutions under mild conditions, as dextran presents different mechanical, protein-adhesion and degradation characteristics compared with HA, it is hypothesized that the blend of these two polymers may offer a broad range of material properties. In this study, a series of HA-dextran composites were implanted in the vocal fold in ferrets, for preliminary assessment of the hydrogel biocompatibility with the vocal fold tissue. It was observed that HA-dextran hydrogels were well-tolerated in vivo and that the degradative and mechanical properties of these gels could be adjusted according to the formulation. It is also the first time that the ferret animal model was used for studying the injectable hydrogel material, and our study demonstrates that HA-dextran composite hydrogel matrices are useful for development of reparative vocal fold implantations.
METHODS
Water was distilled and deionized using a Millipore Milli-RO 10 Plus and Milli-Q UF Plus (Bedford, MA) at 18-MΩ resistance. Dialysis was performed using membrane with MWCO 10,000 (Spectra/Por, Rancho Dominguez, CA). 1-Ethyl-3-[3-(dimethylamino) propyl] carbodiimide (EDC), adipic dihydrazide (ADH), t-butyl carbazate, trinitrobenzenesulfonic acid, ethylene glycol, sodium hydroxide, and sodium periodate were obtained from Aldrich (Milwaukee, WI). Hydroxybenzotriazole (HOBt) was purchased from Alfa Aesar (Ward Hill, MA). Dextran (M.W.140 KDa) and hyaluronidase (Type VIII, from bovine testes) were purchased from Sigma Chemical Co (St. Louis, MO). HA (sodium salt, M.W.490 KDa) was purchased from Genzyme Corp (Cambridge, MA).
Preparation of HA-dextran hydrogels
Modification of HA and dextran polymers
To modify HA with hydrazide groups, the carboxylic acid groups in HA was activated by EDC and HOBt and reacted with excessive ADH. Details of this method were described previously in the literature.23–25 The modification level of HA was determined by the NMR analysis. To functionalize dextran with aldehyde groups, 5 g dextran were dissolved in water at a concentration of 250 mg/mL. An aqueous solution of sodium periodate (0.11–0.33 g dissolved in 5 mL water) was added dropwise, and the reaction was stirred for 2 h at room temperature in the dark. Ethylene glycol was then added to inactivate any unreacted periodate. The solution was purified by exhaustive dialysis against water for 3 days, and the dry product of the oxidized dextran was obtained by freeze drying. The level of aldehyde modification in the dextran product was determined by measuring the number of aldehyde groups in the polymer using t-butyl carbazate and trinitrobenzenesulfonic acid.20,26
Preparation of hydrogel samples
Gel samples for characterization were prepared by mixing reactive HA and dextran polymer solutions at prescribed concentrations. Typically, 30 µL solutions of dextran-aldehyde (0.5–6% w/v in PBS (Invitrogen)) were slowly pipetted into 60 µL HA-ADH (2% w/v) contained in a cylindrical mold made from a truncated 1-mL syringe. The mixture was gently stirred using the pipet tip and then put on a shaker for gelation which typically took about 15 min. The resulting round hydrogel disks were ejected from the cylindrical mold, and immersed and equilibrated with PBS for 1 week for in vitro characterization.
Characterization of HA-dextran hydrogel
Swelling ratios
Swelling ratios of hydrogels made from different formulations were measured gravimetrically. Hydrogel disks formed with prescribed polymer concentrations were prepared as described above and equilibrated with water for 1 week. The gel surfaces were blotting dried with tissue paper and the wet sample weight (Wwet) was recorded. The hydrogel sample was then vacuum dried and the mass of the remaining polymer was weighed (Wdry). The swelling ratio (Q) was calculated according to the equation, Q = Wwet/Wdry.
In vitro degradation assessment
Hydrogel disks made from different formulations were immersed in a hyaluronidase enzyme solution (50 units/mL in PBS), and the degradation was carried out at 37°C. The supernatant solution was replaced and analyzed at predetermined time points. The degraded HA was quantified by a colormetric method27,28 and the cumulative degradation profile over time was plotted and compared.
Mechanical analysis
Compression tests were performed to measure the compressive moduli of fully swollen hydrogels using a mechanical analyzer (DMA Q800, TA Instrument, New Castle, DE). A cylindrical gel disk was placed between two clamps, and compressed at 5%/min to a maximum strain of 25%. The compressive-modulus versus strain was thus measured.
Data analysis
Statistic analysis was performed by one-way nonparametric ANOVA, followed by the Newman-Keuls test to compare selected pairs of assay results obtained for different materials. A p < 0.05 was considered statistically significant. All values are reported as mean ± standard deviation.
In vivo evaluation of biocompatibility of HA-dextran hydrogel in ferret vocal fold tissue
Ferret animal model
Four adult male ferrets weighing 1 kg were housed in separate cages and were cared for in compliance with protocols approved by the Animal Care and Use Committee at the Massachusetts Institute of Technology, and the Principles of Laboratory Animal Care (NIH publication #85-23, revised 1985).
Ferrets have a large, accessible larynx with the gross morphology similar to human. Thus these small mammals are economical and robust for preliminary material screening studies. The ferret vocal fold model has been used in our laboratory for several years and the ECM of the lamina propria in this species has been well characterized.29–31 There is a thick lamina propria containing scattered fibroblasts in a loose ECM, and its major constituents-collagen, elastin, HA, and proteoglycans-show some similarities and differences in their distribution compared to human.29–31 In addition, one characteristic of the ferret lamina propria most similar to human is the looseness of the superficial tissue, which is consistent with the important role of this layer in phonatory vibration across all mammals that have been studied.
Hydrogel preparation
Filter-sterilized HA-ADH and dextran-aldehyde polymers were prepared to perform in vivo studies. After HA and dextran were modified and purified by exhaustive dialysis as afore described, HA-ADH and dextran-aldehyde solutions were adjusted to an approximate concentration of 500 µg/mL. The products were then filtered by passing through 0.2-µm membrane filters and freeze dried under aseptic conditions. Dried polymers were collected and stored at −20 °C. To prepare hydrogels for injection, dextran, and HA solutions were mixed at a volume ratio of 1:2 as the samples prepared for in vitro characterization. HA and dextran were each dissolved to predetermined concentrations (0.3 mL dextran and 0.6 mL HA) under aseptic conditions, and the two solutions were separately loaded into two 3-mL syringes. The content of these syringes were thoroughly mixed by interconnecting the syringes and transferring the materials back and forth several times. The mixture was then maintained in a single syringe for at least 15 min prior to injection.
Hydrogels prepared from 1%, 0.75%, and 0.5% dextran solutions and 2% w/v HA were used for vocal fold injection. These hydrogels were easily passed through a 26-ga. injection needle.
Surgical procedures
Ferrets were anesthetized by IM injection of ketamine (40 mg/kg)/xylazine (4 mg/kg). The animals were positioned in a supine posture with the neck extended. A custom spatula blade laryngoscope was introduced to retract the epiglottis and permit visualization of the vocal folds. An operating microscope (OPMI VISU 160, Zeiss) was used. A topical anesthetic, Cetacaine, was sprayed into the pharynx to reduce reflex movements during positioning of the laryngoscope. The syringe containing an HA-dextran hydrogel sample was connected to an angled 26-gauge needle. Approximately 10–20 µL of HA-dextran hydrogels were injected under the epithelium into the vocal fold SLP using the magnified visual guidance. The injection volume was estimated by weighing the injection syringe and needle before and after injection (assuming the gel density at 1 mg/µL), and subtracting a percentage of the weight based on our estimate of any leakage of the injected material observed during the needle withdrawal. Regardless of partial leakage, each injection resulted in a visible bulging of the mucosal surface which persisted at least one week after injection (as observed under direct micro-laryngoscopic inspection under anesthesia).
Both vocal folds were injected in all four animals, and the bulging of the vocal folds did not cause respiratory stridor or appear to compromise respiration. To minimize the animal-to-animal difference, parallel hydrogel samples were distributed in different animals. HA-dex0.5 and HA-dex0.75 were each injected into three vocal folds of three different ferrets; HA-dex1was injected into two vocal folds of two ferrets. The vocal folds were visually inspected at day 21 prior to euthanasia with intracardiac injection of pentobarbital (100 mg/kg). The larynges were then excised and placed in 10% neutral buffered formalin.
Histology analysis
The larynges were embedded in paraffin and sectioned at 5 µm. Coronal sections were saved at 400-µm intervals along the anterior-posterior extent of the vocal fold. Sections were stained with hematoxylin and eosin and were examined with bright field microscopy (Axiovert 200, Zeiss). The cell types inside gel residues and the tissues surrounding hydrogel implantations were evaluated with the assistance of a certified pathologist.
RESULTS
Synthesis and properties of HA-dextran hydrogels
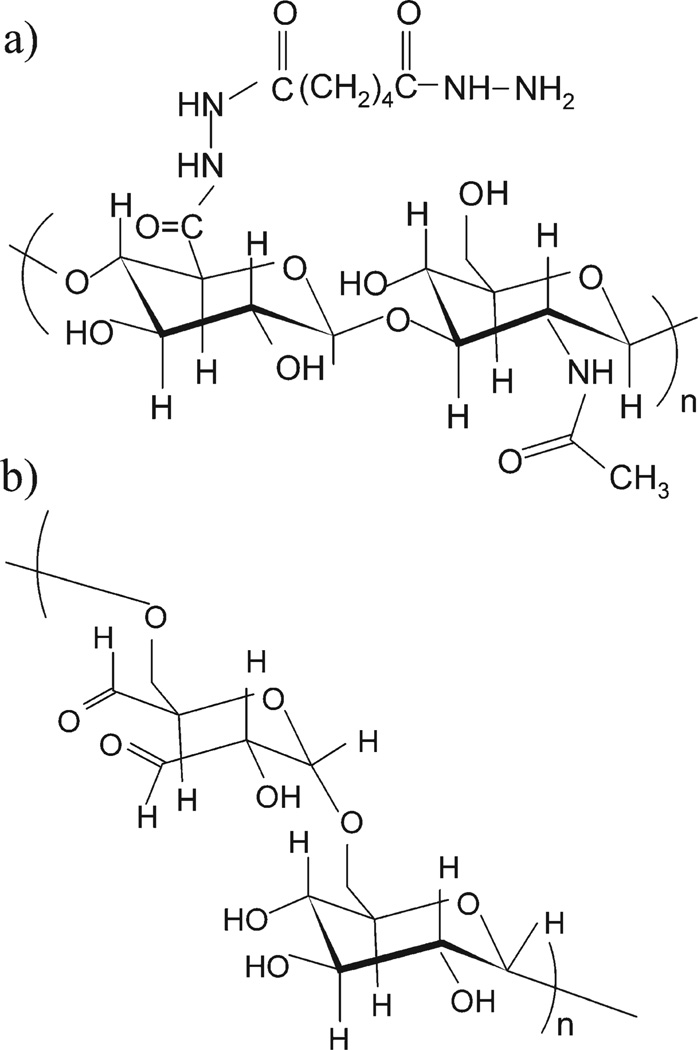
HA carrying hydrazide functionality was synthesized by carbodiimide-mediated coupling of HA with ADH, while aldehyde functionalized dextran was synthesized by periodate oxidation. ADH was linked to the carboxylic acid group of the d-glucuronic acid residue in HA [Figure 1(a)]; periodate oxidized the proximal hydroxyl groups of α-d-1,6-glucose units (dextran has two sets of vicinal hydroxyls, and both sets can be oxidized) and gave rise to aldehyde groups in dextran as illustrated in Figure 1(b). The degree of modification can be controlled by varying the relative amount of the activation reagents (Tables I and II). The modified polymers, HA-ADH and dextran-aldehyde are both soluble and stable in water. The resulting polymer solutions are reactive toward each other and are capable of forming hydrogel matrices under mild conditions.
FIGURE 1.
Illustration of HA and dextran derivatives for synthesis of injectable hydrogels: (a) HA-ADH and (b) dextran-aldehyde derived from periodate oxidization.
TABLE I.
ADH Substitution Levels in Modified HA
| HA-ADH Product | A | B | C | D |
|---|---|---|---|---|
| EDC: HOBt: HA units | 4: 4: 1 | 1: 1: 1 | 0.5: 0.5: 1 | 0.2: 0.2: 1 |
| ADH substitution level (%) | 57 | 50 | 46 | 20 |
TABLE II.
Aldehyde Reactivity in Modified Dextran (Mean ± Standard Deviation, n = 3)
| Dextran-Aldehyde | A | B | C |
|---|---|---|---|
| Dextran: periodate | 45.5 :1 | 15: 1 | 9: 1 |
| Aldehyde reactivity (µmol/mg dextran) | 0.25 ± 0.03 | 0.81 ± 0.07 | 1.19 ± 0.11 |
To study the properties of HA-dextran hydrogels, hydrogel disks with different compositions were prepared (Table III). These hydrogel samples were labeled according to the concentration of dextran-aldehyde solution prior to mixing, as HA-dex0.5, HA-dex0.75, HA-dex1, HA-dex3, and HA-dex6. In all hydrogel formulations, the hydrazide groups of HA were kept in large excess relative to the aldehyde groups of dextran, with the molar ratio greater than 4.1 as shown in Table III. In principle, the amount of hydrazone cross-links in hydrogel matrices increases as the dextran-aldehyde concentration increases from HA-dex0.5 to HA-dex6. These formulations were used mainly because we speculated that the excess ADH groups would facilitate complete reaction of the aldehyde groups of dextran and reduce possible adverse tissue responses in vivo.25
TABLE III.
Formulations of HA-Dextran Hydrogels
| Sample | HA-ADH (%, w/v) |
Dextran-Aldehyde (%, w/v) |
Mixing Volumes (HA/dextran, µL) |
|---|---|---|---|
| HA_Dex6 | 2 | 6 | 60/30 |
| HA_Dex3 | 2 | 3 | 60/30 |
| HA_Dex1 | 2 | 1 | 60/30 |
| HA_Dex0.75 | 2 | 0.75 | 60/30 |
| HA_Dex0.5 | 2 | 0.5 | 60/30 |
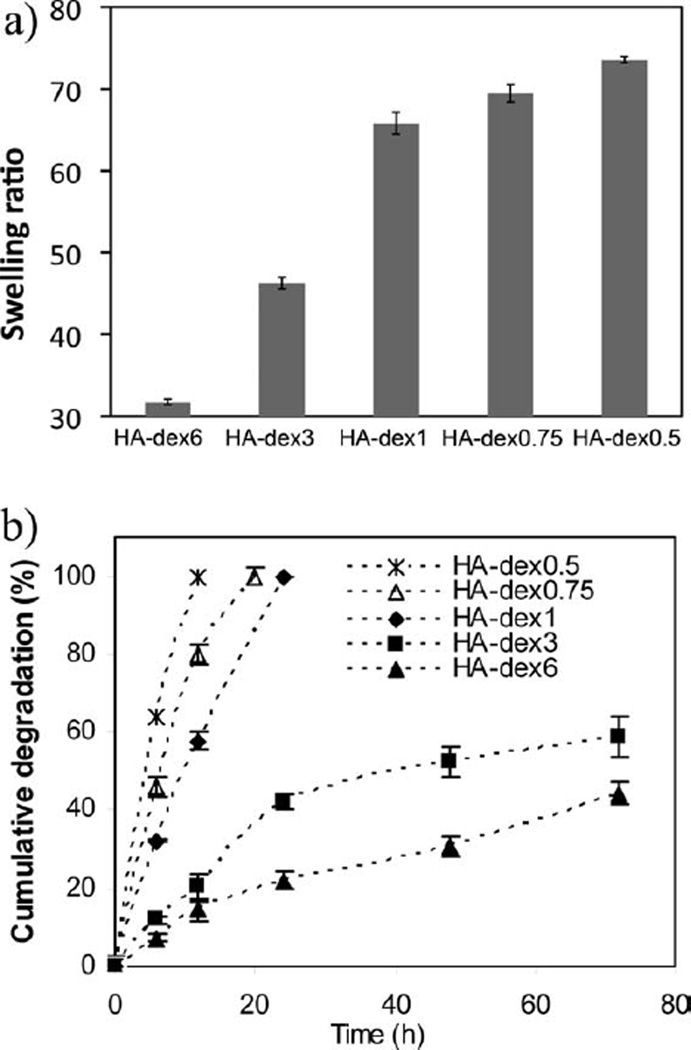
HA-dextran hydrogel samples were evaluated by swelling ratio, degradation, and mechanical tests (Figures 2 and 3). These tests demonstrate variable properties of the HA-dextran composites. In Figure 2(a), swelling ratios of hydrogels made from 0.5 to 6% dextran-aldehyde solutions increased with reducing levels of dextran-aldehyde (p < 0.05), when the hydrogel was supposedly less crosslinked. During the degradation tests, the hydrogel samples steadily decreased in size, thus it is likely that the degradation was mediated through the surface erosion process by hyaluronidase. Figure 2(b) shows that the hydrogels with less dextran contents were more susceptible to enzyme degradation. It is noted that HA-dex0.5, HA-dex0.75, and HA-dex1 completely degraded within 24 h under the assay conditions, exhibiting a much faster degradation rate as compared with HA-dex3 and HA-dex6.
FIGURE 2.
(a) Swelling ratios of HA-dextran hydrogels (mean ± standard deviation, n = 4); (b), in vitro degradation study (in 50 U/mL hyaluronidase solution) using the carbazole assay shows that the degradation rate of HA-dextran hydrogels decreases with increasing dextran content (mean ± standard deviation, n = 3).
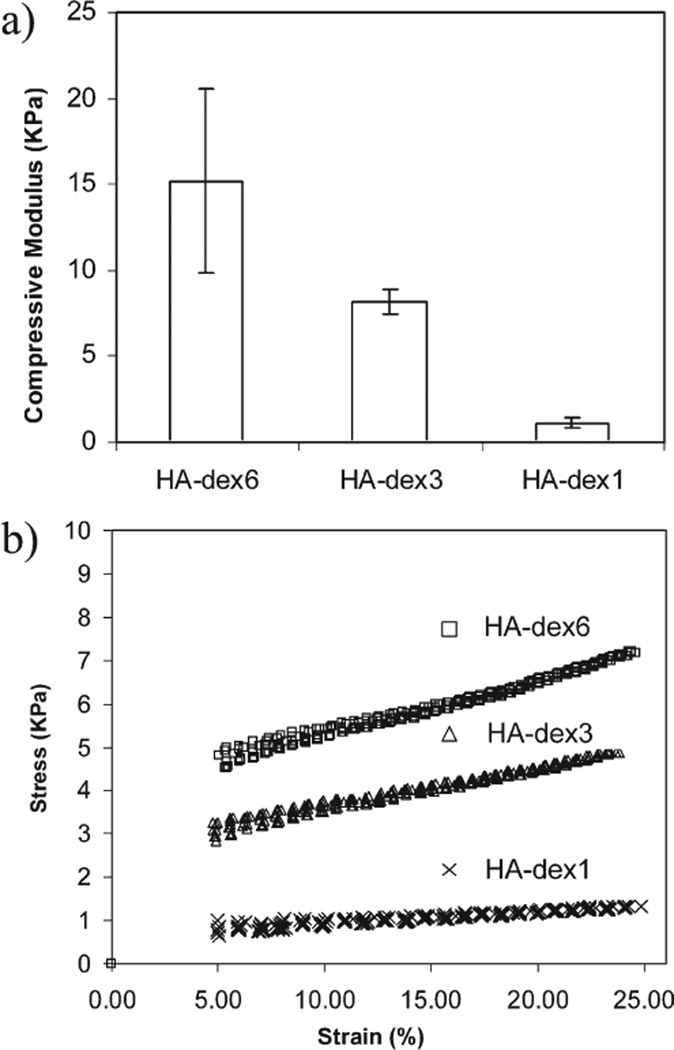
FIGURE 3.
(a) Compressive moduli of fully swollen HA-dextran hydrogels (mean ± standard deviation, n = 3); (b), Representative stress-strain curves of HA-dextran hydrogels. Hydrogel moduli were derived from the linear strain range between 5% and 25%.
The compressive moduli also correlated with the degree of crosslinking in the hydrogel matrices [Figure 3(a)]. Within the effectively measured strain range from 5% to 25%, linear stress-strain curves were observed [Figure 3(b)]. The moduli of HA-dex3 and HA-dex6 hydrogels over this range were around or above 10 kPa, but HA-dex1 was significantly lower (p < 0.05), by almost one order of magnitude. The moduli of HA-dex0.5 and HA-dex0.75 were lower than 1 kPa and beyond the measurable range of the DMA instrument.
In vivo evaluation of HA-dextran hydrogel
The HA-dex1 gel was injected into two vocal folds, and the HA-dex0.75 and HA-dex0.5 gels were each injected into three vocal folds of different animals. The volume of each injection was estimated to be about 10–20 µL (see Methods section), and the vocal fold epithelium was observed to bulge medially after each injection. Some bulging at the injection site was still apparent in each vocal fold after one week in all cases and no acute inflammation was visible.
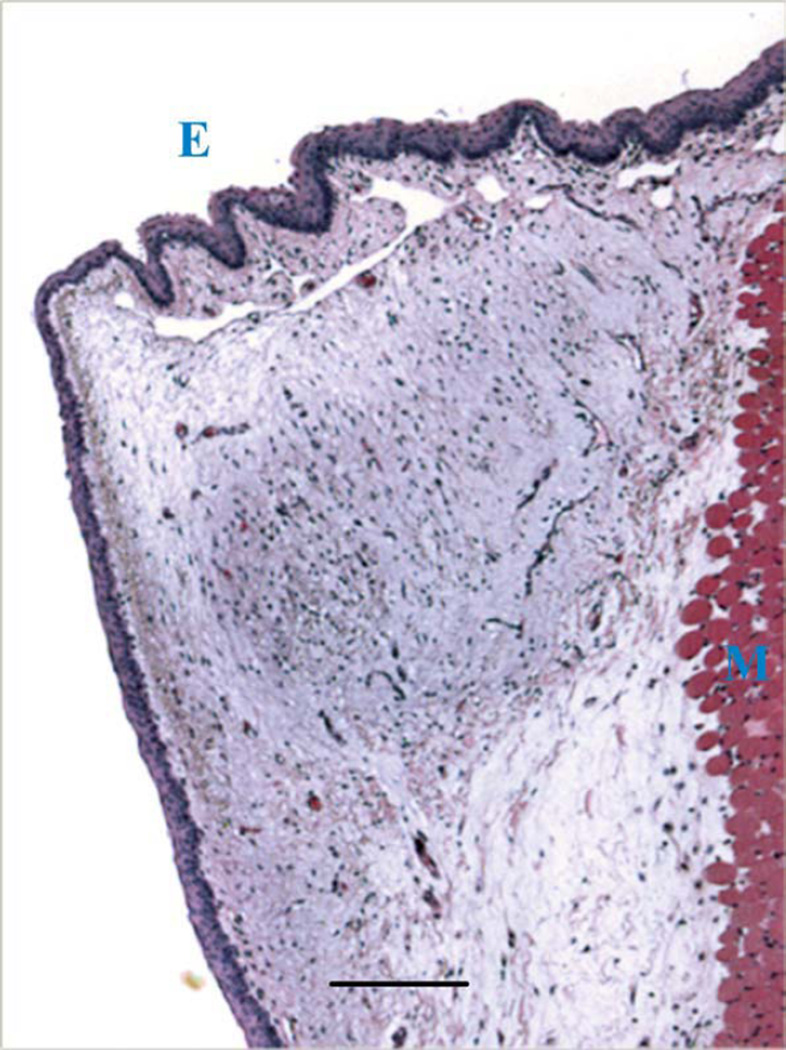
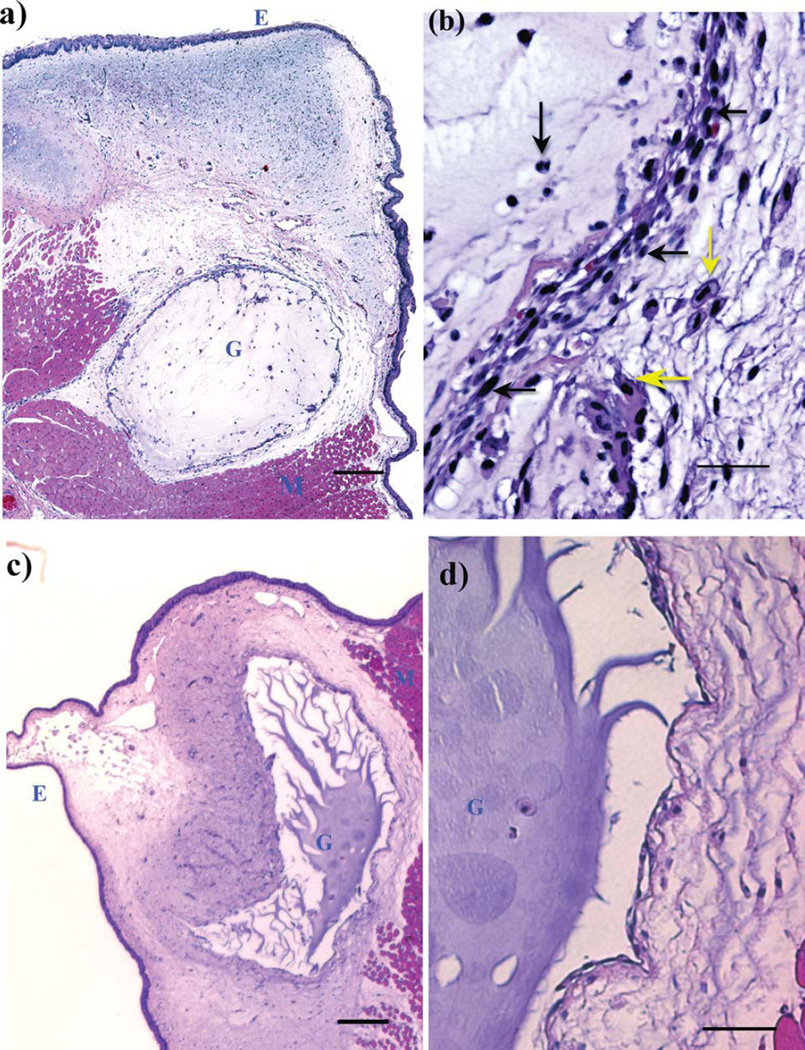
After three weeks, all vocal folds appeared to be normal with little evidence of the bulging observed at earlier time points, suggesting that much of the injected hydrogel had redistributed or resorbed. Animals were euthanized at this time point and vocal fold histology was analyzed. It appeared that the HA-dex0.5 had completely degraded after three weeks, because no gel residues were found in the vocal folds where hydrogels were injected. As shown by an example tissue section in Figure 4, there was no tissue response visible in vocal folds injected with HA-dex0.5, and the histology typically had the normal appearance of ferret vocal fold. In contrast, residual materials were found in the vocal folds injected with HA-dex0.75 [Figure 5(a,b)] or HA-dex1 [Figure 5(c,d)]. In these cases, a thin layer of cells (one to four cells thick) was observed to encapsulate the HA-dextran hydrogels. Most of these cells were visually identifiable as macrophages and fibroblasts, with rare giant cells, indicative of mild inflammatory response of the host to the injected materials [Figure 5(c,d)].
FIGURE 4.
The ferret vocal fold injected with HA-dex 0.5, showing the normal histology of ferret vocal fold tissue with no residue of hydrogel materials. Regions of epithelium and muscle tissue are indicated by letters E and M, respectively (scale bar: 200 µm).
FIGURE 5.
H&E stained tissue slides of HA-Dextran crosslinked hydrogel implanted in the ferret vocal fold at week 3. Regions of epithelium, injected gel and muscle tissue are indicated by letters E, G, and M, respectively. (a) HA-dex0.75 in the host tissue (scale bar: 200 µm); (b), the interface of the host tissue and HA-dex0.75 gel (scale bar: 50 µm); (c), HA-dex1 in the host tissue (scale bar: 200 µm); (d) the interface of the host tissue and HA-dex1 gel (scale bar: 50 µm). Cell types were visually identified in b and pointed by arrows (yellow horizontal arrow: macrophage; yellow vertical arrow: foam cell; black vertical arrow: neutrophil; black horizontal arrows: fibroblasts).
It appeared that more cells had infiltrated HA-dex0.75 compared to HA-dex1 [Figure 5(a)]. Some cells in the vicinity of the HA-dex0.75 gel residue exhibited the morphology of foam cells internalizing foreign materials [Figure 5(b)]. In contrast, few cells invaded the bulk of HA-dex1 [Figure 5(c,d)], which indicates that the more rigid HA-dex1 gel may be more difficult for cells to penetrate. The degradation process appeared to be more-or-less limited to the tissue-material interface as seen in Figure 5(d).
DISCUSSION
In this study, the reaction between hydrazides and aldehydes was employed to synthesize HA-dextran hydrogel materials for implantation applications in the vocal fold tissue. Changing the degradation and mechanical properties of the composite hydrogels was possible by altering the proportions of the two polysaccharides. When HA-dextran hydrogels were injected into the vocal fold region of ferrets, histological analysis was a useful way to observe cell-material interactions in the vocal fold.
The degradation of HA-dextran hydrogels in vivo was observed to be correlated with the degree of crosslinking in the hydrogel matrices. It is noted that the in vivo dextran degradation is typically mediated by different types of α-1-glucosidases in liver, spleen and kidney32; however, it is not known if these enzymes exist in the vocal fold. Therefore, the underlying chemical process may be dominated by the degradation of the HA component under the influence of the hyaluronidase in the tissue. It was observed that cells infiltrated HA-dex0.75 more readily than HA-dex1, which could reflect the differences in the stiffness and physical pore structures in these two types of materials. The morphological, mechanical and physiochemical properties are important factors that affect cell penetration and nutrient permeation in hydrogel matrices, as demonstrated by many previous studies on hydrogel applications as tissue scaffolds.6,33
Mild tissue reactions caused by HA-dextran hydrogels were observed. When formulating the hydrogel samples, the hydrazide groups of HA polymers were intentionally kept in large excess compared to aldehydes; however, unreacted aldehyde groups may still be present due to the reduced mobility and reaction cross-section of polymer chains during gel formation. Aldehydes should readily react with electron-rich functional groups such as amines in biological macromolecules, but its physiological influence on tissue responses has yet to be understood.
In a recent study, Martwiset et al found that oxidizing dextran with aldehydes allow the polymer to resist nonspecific protein adhesion as a result of the interactions between aldehyde and water, and that protein interactions occurred only when dextran had very high levels of oxidization and aldehyde modification (e.g., substitution level >75%).34 This might explain the biocompatibility of HA-dextran hydrogels we observed despite the possible presence of unreacted aldehydes. Also in other recent investigations on polysaccharide hydrogels, aldehyde functionalized polymers crosslinked via hydrazone bonds exhibited good biocompatibility and minimal cytotoxicity.20,21,35–38 In particular, aldehyde-modified dextrans reacted with amine-containing polymers have been studied and used for tissue adhesives.35–37
The compressive mechanical tests performed in this study were useful to compare the stiffness of hydrogels of different formulations. According to the dynamic rheometric characterization performed by Chan et al., the elastic moduli of human vocal fold tissues range from 100 to 1000 Pa.39–41 Therefore, all the injectable hydrogels prepared in this study, that is HA-dex0.5, HA-dex0.75, and HA-dex1, which have a modulus around or below 1 kPa, could potentially match the mechanical properties of vocal fold tissue. On the other hand, optimizing the mechanics of implantations for vocal fold tissue repair has not been fully investigated, and could be complicated and varied with specific tissue environment and different applications. For example, as shown in the study of HA-based hydrogels by Hansen et al., it was found that the material stiffness did not linearly correspond with the stiffness of regenerated vocal fold tissue, implying the interplay of multiple material properties in driving the tissue outcomes.15 The systematic variation of material properties such as made available by HA-dextran composites would potentially be useful to ensure better controlled studies necessary for understanding and engineering injectable materials for clinical use in vocal fold tissue.
CONCLUSION
Hydrogel matrices made from HA and dextran exhibited variable degradation and mechanical properties. Mild tissue reactions and differences in residence time related to hydrogel formulation were observed in vocal folds using a ferret animal model. The results suggest that HA-dextran hydrogels hold promise for use in vocal-fold applications and warrant further study.
ACKNOWLEDGMENTS
The authors thank The Institute of Laryngology and Voice Restoration at MGH. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. The authors thank Dr. William C. Faquin for assistance with histopathological analysis. We thank Rachel Williams for technical assistance.
Contract grant sponsors: The Eugene B. Casey Foundation; the Advisory Board Foundation; Contract grant sponsor: the Institute of Laryngology and Voice Restoration; contract grant number: DE013023
Footnotes
The work presented in this manuscript was conducted at the Massachusetts Institute of Technology, Cambridge of Massachusetts
REFERENCES
- 1.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman AS. Hydrogels for biomedical application. Adv Drug Deliv Rev. 2002;43:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 3.Griffith LG. Polymeric biomaterials. Acta Mater. 2000;48:263–277. [Google Scholar]
- 4.Prestwich GD, Shu XZ, Liu Y, Cai S, Walsh JF, Hughes CW, Ahmad S, Kirker KR, Yu B, Orlandi RR. Injectable synthetic extracellular matrices for tissue engineering and repair. Adv Exp Med Biol. 2006;585:125–133. doi: 10.1007/978-0-387-34133-0_9. [DOI] [PubMed] [Google Scholar]
- 5.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: Material and matrix considerations. J Bone Joint Surg Am. 2008;90(Suppl 1):36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 6.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 7.Zeitels SM, Healy GB. Laryngology and phonosurgery. N Engl J Med. 2003;349:882–892. doi: 10.1056/NEJMra035148. [DOI] [PubMed] [Google Scholar]
- 8.Zeitels SM, Hillman RE, Desloge R, Mauri M, Doyle PB. Phonomicrosurgery in singers and performing artists: Treatment outcomes, management theories, and future directions. Ann Otol Rhinol Laryngol Suppl. 2002;190:21–40. doi: 10.1177/0003489402111s1203. [DOI] [PubMed] [Google Scholar]
- 9.Zeitels SM. Atlas of Phonomicrosurgery and Other Endolaryngeal Procedures for Benign and Malignant Disease. San Diego: Singular; 2001. [Google Scholar]
- 10.Hammond TH, Zhou R, Hammond EH, Pawlak A, Gray SD. The intermediate layer: A morphologic study of the elastin and hyaluronic acid constituents of normal human vocal folds. J Voice. 1997;11:59–66. doi: 10.1016/s0892-1997(97)80024-0. [DOI] [PubMed] [Google Scholar]
- 11.Ward PD, Thibeault SL, Gray SD. Hyaluronic acid: Its role in voice. J Voice. 2002;16(3):303–309. doi: 10.1016/s0892-1997(02)00101-7. [DOI] [PubMed] [Google Scholar]
- 12.Butler JE, Hammond TH, Gray SD. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope. 2001;111:907–911. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143–147. doi: 10.1097/01.moo.0000162261.49739.b7. [DOI] [PubMed] [Google Scholar]
- 14.Hallen L, Johansson C, Laurent C. Cross-linked hyaluronan (Hylan B gel): A new injectable remedy for treatment of vocal fold insufficiency—An animal study. Acta Otolaryngol. 1999;119:107–111. doi: 10.1080/00016489950182043. [DOI] [PubMed] [Google Scholar]
- 15.Hansen JK, Thibeault SL, Walsh JF, Shu XZ, Prestwich GD. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: Early effects on tissue repair and biomechanics in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:662–670. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 16.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 17.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12:3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 18.Kratz F, Beyer U, Collery P, Lechenault F, Cazabat A, Schumacher P, Falken U, Unger C. Preparation, characterization and in vitro efficacy of albumin conjugates of doxorubicin. Biol Pharm Bull. 1998;21:56–61. doi: 10.1248/bpb.21.56. [DOI] [PubMed] [Google Scholar]
- 19.Hruby M, Konak C, Ulbrich K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J Control Release. 2005;103:137–148. doi: 10.1016/j.jconrel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25:4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 2001;56:228–233. doi: 10.1002/1097-4636(200108)56:2<228::aid-jbm1089>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Yeo Y, Highley CB, Bellas E, Kohane DS. Dextran-based in situ cross-linked injectable hydrogels to prevent peritoneal adhesions. Biomaterials. 2007;28:3418–3426. doi: 10.1016/j.biomaterials.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Pouyani T, Prestwich GD. Functionalized derivatives of hyaluronic acid oligosaccharides: Drug carriers and novel biomaterials. Bioconjug Chem. 1994;5:339–347. doi: 10.1021/bc00028a010. [DOI] [PubMed] [Google Scholar]
- 24.Vercruysse KP, Marecak DM, Marecek JF, Prestwich GD. Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid. Bioconjug Chem. 1997;8:686–694. doi: 10.1021/bc9701095. [DOI] [PubMed] [Google Scholar]
- 25.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Bouhadir KH, Housman DS, Mooney DJP. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer. 1999;40:3575–3584. [Google Scholar]
- 27.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 29.Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix. I. Elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- 30.Hahn MS, Kobler JB, Zeitels SM, Langer R. Midmembranous vocal fold lamina propria proteoglycans across selected species. Ann Otol Rhinol Laryngol. 2005;114:451–462. doi: 10.1177/000348940511400607. [DOI] [PubMed] [Google Scholar]
- 31.Hahn MS, Kobler JB, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix II: Collagen. Ann Otol Rhinol Laryngol. 2006;115:225–232. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 32.Larsen C. Dextran prodrugs—Structure and stability in relation to therapeutic activity. Adv Drug Deliv Rev. 1989;3:103–154. [Google Scholar]
- 33.Yu X, Bellamkonda RV. Dorsal root ganglia neurite extension is inhibited by mechanical and chondroitin sulfate-rich interfaces. J Neurosci Res. 2001;66:303–310. doi: 10.1002/jnr.1225. [DOI] [PubMed] [Google Scholar]
- 34.Martwiset S, Koh AE, Chen W. Nonfouling characteristics of dextran-containing surfaces. Langmuir. 2006;22:8192–8196. doi: 10.1021/la061064b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia SK, Arthur SD, Chenault HK, Kodokian GK. Interactions of polysaccharide-based tissue adhesives with clinically relevant fibroblast and macrophage cell lines. Biotechnol Lett. 2007;29:1645–1649. doi: 10.1007/s10529-007-9465-8. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia SK, Arthur SD, Chenault HK, Figuly GD, Kodokian GK. Polysaccharide-based tissue adhesives for sealing corneal incisions. Curr Eye Res. 2007;32:1045–1050. doi: 10.1080/02713680701767876. [DOI] [PubMed] [Google Scholar]
- 37.Araki M, Tao H, Nakajima N, Sugai H, Sato T, Hyon SH, Nagayasu T, Nakamura T. Development of new biodegradable hydrogel glue for preventing alveolar air leakage. J Thorac Cardiovasc Surg. 2007;134:1241–1248. doi: 10.1016/j.jtcvs.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Reyes JM, Herretes S, Pirouzmanesh A, Wang DA, Elisseeff JH, Jun A, McDonnell PJ, Chuck RS, Behrens A. A modified chondroitin sulfate aldehyde adhesive for sealing corneal incisions. Invest Ophthalmol Vis Sci. 2005;46:1247–1250. doi: 10.1167/iovs.04-1192. [DOI] [PubMed] [Google Scholar]
- 39.Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607–614. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 40.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: Theoretical characterization based on constitutive modeling. J Acoust Soc Am. 2000;107:565–580. doi: 10.1121/1.428354. [DOI] [PubMed] [Google Scholar]
- 41.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: Measurement methodology and empirical results. J Acoust Soc Am. 1999;106(Part 1):2008–2021. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 42.Hirano S, Bless DM, Nagai H, Rousseau B, Welham NV, Montequin DW, Ford CN. Growth factor therapy for vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2004;113:777–785. doi: 10.1177/000348940411301002. [DOI] [PubMed] [Google Scholar]
- 43.Hirano S, Nagai H, Tateya I, Tateya T, Ford CN, Bless DM. Regeneration of aged vocal folds with basic fibroblast growth factor in a rat model: A preliminary report. Ann Otol Rhinol Laryngol. 2005;114:304–308. doi: 10.1177/000348940511400409. [DOI] [PubMed] [Google Scholar]
- 44.Luo Y, Kobler JB, Zeitels SM, Langer R. Effects of growth factors on extracellular matrix production by vocal fold fibroblasts in 3-dimensional culture. Tissue Eng. 2006;12:3365–3374. doi: 10.1089/ten.2006.12.3365. [DOI] [PubMed] [Google Scholar]