Abstract
This study aimed to examine the hepatoprotective effects of the superfine particles of Radix Tetrastigma (SPRT) against CCl4-induced acute liver damage in rats. Animals were treated with SPRT (0.3, 0.6, and 1.2 g/kg) and showed remarkable hepatoprotection against CCl4-induced hepatotoxicity. CCl4 altered various biochemical parameters in rat liver, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), superoxide dismutase (SOD), histopathological changes, and Bax and caspase-3 expressions. SPRT significantly prevented increases in ALT and AST levels, reduced MDA level, decreased Bax and caspase-3 protein expression, enhanced SOD activity, and provided significant amelioration in the histopathological lesions. These findings suggested that SPRT has significant protective effect against acute hepatotoxicity induced by CCl4 in rats.
1. Introduction
Herbal medicine has been applied for disease prevention and treatment for thousands of years [1, 2]. Herbal medicine can boost immunity and protect the liver, regulate liver function, improve blood circulation in the liver, effectively repair liver cell damage, and relieve symptoms of liver diseases [3–5].
Radix Tetrastigma, also called San ye-qing, one of the oldest tonic herbal medicines, has attracted increasing attention as one of the most popular and valuable herbal medicines in clinical application. Radix Tetrastigma can be used as medicine, healthy food, and cosmetics because of its biological and pharmacological activities, including anti-inflammation, antivirus, antioxidation, heat-removing, detoxification, antihepatotoxicity, and analgesia [6, 7]. The root of Radix Tetrastigma contains various flavonoids, such as rutin, quercetin, and kaempferol [8, 9].
Radix Tetrastigma is a rare plant and could not be cultured. Unfortunately, its natural supply is now significantly decreasing. Traditional processing procedures of Radix Tetrastigma include section baking and drying, sulfur fumigation, and storage in a woven bag. The disadvantages of these procedures include high water content, mold growth, infestation, and unsuitability for long-term storage. More importantly, the essences can be lost during the baking process and the residual sulfur dioxide produced during sulfur fumigation can be harmful to humans. Traditional decoction of Radix Tetrastigma can achieve efficacy of only 3% to 5%, resulting in the loss of 95% of the bioactive components. Even when 3% to 5% of bioactive components are obtained from water, the decoction tends to be volatilized. Evidently, the traditional processing technique is not efficient. Thus, improving the processing technique is urgently needed to avoid unnecessary loss of precious Radix Tetrastigma. This study was conducted to develop a superfine processing technique using superfine particles to process Radix Tetrastigma. The proposed technique allows active components to be absorbed without relying on their active transportation through the cell membrane. Superfine particles present large surface areas to interact with target molecules. When these particles are conjugated with medicine, the conjugates offer higher surface charges and surface adhesion, resulting in higher efficacies and better medicine absorption. The efficacy and medicine absorption could be improved by more than 90%, and the medicinal dosage can be saved by more than 90%. This proposed technique can efficiently preserve the bioactive constituents in the original medicine, accelerate the release of bioactive constituents, and improve their bioavailability [10, 11].
Previous studies have reported that polysaccharides in Radix Tetrastigma have strong antioxidant and antihepatotoxic activities [12, 13]. However, information on the hepatoprotective effect of the superfine herbal particles of Radix Tetrastigma in vivo is limited. This study was undertaken to evaluate the protective effect of the superfine particles of Radix Tetrastigma (SPRT) against CCl4-induced hepatotoxicity.
2. Materials and Methods
2.1. Preparation of SPRT
Radix Tetrastigma was obtained from Henan suppliers and authenticated by an expert in the field. SPRT was prepared by using the mechanical ball milling technique in the Research Center of TCM Processing Technology, Zhejiang Chinese Medical University. The power particle size was less than 13 μm, and the recovery rate was more than 98%.
2.2. Reagents
Carbon tetrachloride (CCl4) was purchased from Guangdong Dajinhua Chemical Co., Ltd. (Guangdong, China). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) assay kits were purchased from Ningbo Meikang Biotech Co., Ltd. (Zhejiang, China). Superoxide dismutase (SOD) and malondialdehyde (MDA) assay kits were purchased from Jiancheng Biotech Co., Ltd. (Nanjing, China). Bax and caspase-3 antibodies were purchased from Wuhan Boshide Biotechnology Co., Ltd. (Wuhan, China). All other chemicals were purchased from Nanjing Ronghua Reagent Co. (Nanjing, China).
2.3. Animals
Adult male imprinting control region rats (20 ± 2 g body weight) were purchased from the Laboratory Animal Center of Zhejiang Academy of Medical Sciences (Zhejiang, China) and maintained at the Experimental Animal Center of Zhejiang Chinese Medical University. Animals were acclimatized for at least five days with alternating 12 h dark/light cycles in a climate-controlled room where the temperature was maintained at 25 ± 2°C and relative humidity of 60% ± 10%. Water and standard laboratory food were made available ad libitum. All experiments were performed according to the guidelines for the care and use of animals as established by Zhejiang University.
2.4. Experimental Design
Hepatotoxicity was orally induced with 0.1% of CCl4 (20 mL/kg body weight in peanut oil). SPRT (0.3, 0.6, and 1.2 g/kg) and the standard hepatoprotective drug, bifendate, were prepared in 1% sodium carboxymethyl-cellulose (CMC). Rats were randomly divided into six groups (10 rats/group). The rats in group A served as normal controls and were only treated per orem (p.o.) with the vehicle (20 mL/kg of 1% CMC-Na) daily for seven days. The rats in group B served as the disease models and received intraperitoneal injection with 0.1% of CCl4 (0.1 : 100 of CCl4 in peanut oil, 20 mL peanut oil/kg body weight) on the seventh days and using a vehicle for the rest of the days. The rats in groups C, D, and E were given SPRT p.o. at 0.3, 0.6, and 1.2 g/kg body weight for seven days, respectively, and 20 mL/kg o.p. of 0.1% CCl4 on the seventh day.
All rats were sacrificed 16 h after the final treatment. Blood was collected and serum was separated to evaluate the biochemical markers of hepatic injury. Liver was harvested for biochemical and histopathological studies as described previously.
2.5. Histopathology
A portion of the right lobe of the liver was preserved in 10% formalin solution and cut into 5 μm sections and stained with hematoxylin and eosin (HE) for microscopic observation.
2.6. Immunohistochemistry
Immunoperoxidase staining was performed on formalin-fixed and paraffin-embedded 5 μm tissue sections. Sections were fixed on microscope slides. The microscope slides were treated with poly-L-lysine to prevent capping. Microwave antigen retrieval was performed. The expressions of Bax and caspase-3 were quantitatively evaluated using a digital microscope equipped with a computer-aided image analysis system. The intensity index was determined using average integral optical density (AIOD).
2.7. Statistical Analysis
Data were presented as mean ± SD of ten animals each group. Statistical significance was determined using one-way analysis of variance. If the variances were unequal, LSD's multiple range tests and rank-sum tests were used. P value of <0.05 was considered statistically significant.
3. Results and Discussion
3.1. Effect of SPRT on Serum Marker Enzymes
The effects of SPRT on serum marker enzymes are presented in Figure 1. CCl4 increased the enzymatic activity of AST and ALT compared with the control group, indicating liver damage. Administration of SPRT at the doses of 0.3, 0.6, and 1.2 g/kg remarkably prevented hepatotoxicity induced by CCl4 in a dose-dependent manner.
Figure 1.
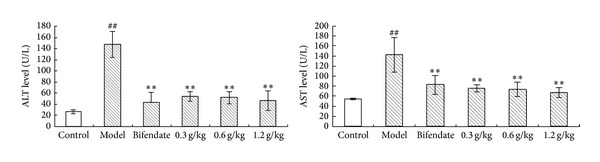
Effects of SPRT on biochemical parameters in CCl4-induced hepatotoxicity in rats. Data are expressed as mean ± SD. ## P < 0.01, versus normal controls. **P < 0.01, versus disease model group.
3.2. Effect of SPRT on Liver MDA and SOD Activity
CCl4 treatment significantly increased the level of MDA in liver tissue compared with the control group. Meanwhile, CCl4 decreased the activity of hepatic antioxidant enzyme SOD compared with the respective control group. Treatment with SPRT at doses of 0.3, 0.6, and 1.2 g/kg significantly reduced the MDA level compared with the CCl4-treated rats. The liver of the animal treated with 0.6 and 1.2 g/kg of SPRT showed significant increase in SOD levels compared with the CCl4-treated rats. The results are shown in Figure 2.
Figure 2.
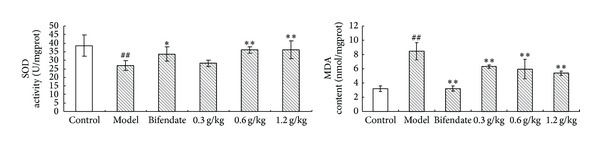
Effects of SPRT on antioxidant profile of CCl4-induced hepatotoxicity in rats. Data are expressed as mean ± SD. ## P < 0.01, versus normal controls. **P < 0.01, versus disease model group. *P < 0.05, versus disease model group.
3.3. Histological Analysis
Figure 3 showed the well-formed hepatocytes in an intact hepatic lobule of normal rat liver. The liver sections of CCl4-treated rats showed massive vacuolization, fatty changes, ballooning degeneration, and inflammatory cell infiltration, especially in the periportal hepatocytes. The histopathological hepatic lesions were significantly improved by the preadministration of SPRT or bifendate compared with the disease model group. These results indicate the hepatoprotective potential of SPRT.
Figure 3.
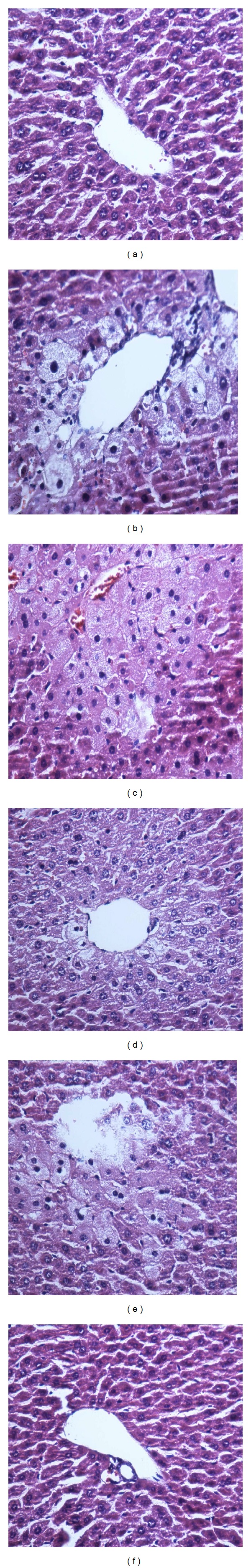
Effects of SPRT on the histopathological changes in liver of rats treated with CCl4. (a) Normal controls; (b) disease model group; (c) bifendate + 0.1% of CCl4; (d) SPRT (0.3 g/kg) and 0.1% of CCl4 group; (e) SPRT (0.6 g/kg) and 0.1% of CCl4 group; (f) SPRT (1.2 g/kg) and 0.1% of CCl4 group. HE staining; original magnification ×400.
3.4. Bax and Caspase-3 Expression in Liver
CCl4 treatment caused a significant increase in Bax and caspase-3 in the liver tissue. SPRT or bifendate treatment significantly inhibited both Bax and caspase-3 expressions (Figure 4). Table 1 shows that Bax and caspase-3 in the disease model group were higher than those in normal control group (P < 0.01). Compared with the disease model group, the Bax and caspase-3 ICH indexes were significantly lower in the two doses of SPRT-treated group (0.6 and 1.2 g/kg; P < 0.05). No significant difference was observed between the disease model group and SPRT treatment group at 0.3 g/kg.
Figure 4.
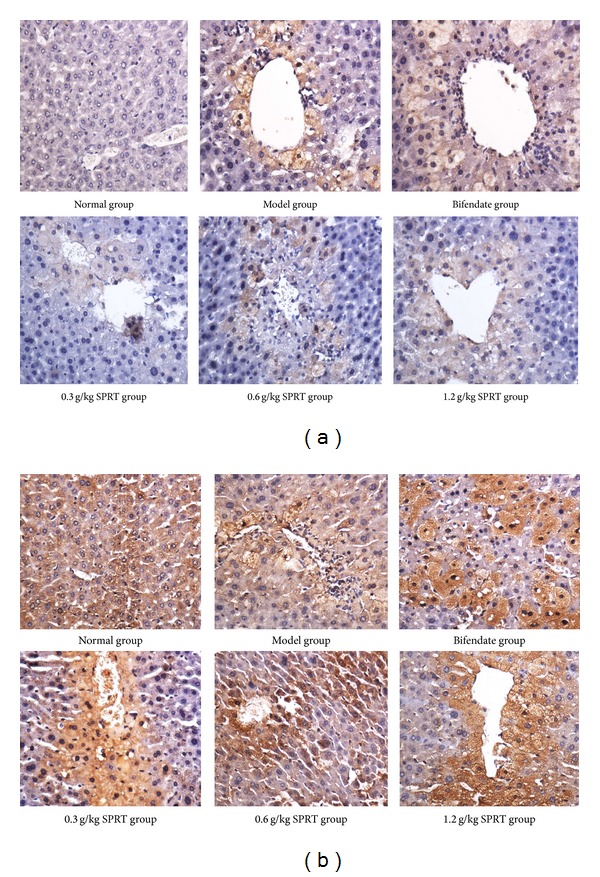
Effects of SPRT on the Bax (a) and caspase-3 (b) expression in rat liver treated with CCl4.
Table 1.
Effects of SPRT on bax and caspase-3 expression in rat liver treated with CCl4.
| Group | Concentration | Mean density | Mean density |
|---|---|---|---|
| Bax | Caspase-3 | ||
| Control | — | 0 | 0 |
| Model | — | 0.0683 ± 0.0161## | 0.0536 ± 0.0188## |
| Bifendate | 200 mg/kg | 0.0539 ± 0.0192 | 0.0394 ± 0.0154 |
| SPRT-1 | 0.3 g/kg | 0.0624 ± 0.0170 | 0.0388 ± 0.0203 |
| SPRT-2 | 0.6 g/kg | 0.0450 ± 0.0179* | 0.0358 ± 0.0148* |
| SPRT-3 | 1.2 g/kg | 0.0359 ± 0.0245** | 0.0311 ± 0.0064* |
Data are expressed as mean ± SD (n = 10). ## P < 0.01 compared to normal controls; **P < 0.01, *P < 0.05 compared to disease model group.
Hepatotoxicity induced by CCl4 is a classic disease model used for evaluation of hepatoprotective activity of herbal medicines. This study showed a significant increase in the activities of AST and ALT in serum after CCl4 treatment. These changes by preadministration of SPRT are reversible, implying that SPRT prevents liver damage.
SOD is a cellular antioxidant with a strong capability to remove superoxide anion and inhibit lipid peroxidation in which MDA is the main product. CCl4 treatment increased the SOD levels in the liver, leading to a low MDA level. The reduced activity of SOD and increased MDA level indicated hepatic damage in rats, which was reversed by the preadministration of SPRT at 0.3, 0.6, and 1.2 g/kg, suggesting the antioxidation effect of SPRT.
Hepatocyte apoptosis is a complex process regulated by multiple genes. Positive immunohistochemical staining of Bax and caspase-3 confirmed liver cell apoptosis induced by CCl4. The positive expression of Bax and caspase-3 decreased with increasing doses of SPRT treatment, suggesting that hepatocyte apoptosis was reversed by SPRT. This finding agreed with those of other studies [14].
Based on our experimental findings, SPRT has a protective effect against hepatotoxic CCl4. The hepatoprotective effects of SPRT are likely related to the free radical scavenging effect, which increases antioxidant activity, against membrane lipid peroxidation, and protects membrane integrity and function of liver cells. Further studies are needed to investigate the molecular mechanism of the hepatoprotective effect of SPRT.
4. Conclusion
A novel superfine particle processing technique was developed for Radix Tetrastigma. SPRT can prevent liver cell apoptosis and create long-term clinical application for sustainable Radix Tetrastigma. Superfine grinding technology can be used for the processing of other rare herbal medicines.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 81202918); the Open Project of National First-Class Key Discipline for Science of Chinese Materia Medica, Nanjing University of Chinese Medicine (no. 2011ZYX2-006); the Project of Science and Technology for Chinese Medicine of Zhejiang Province, China (no. 2013KYB183); the Chinese Medicine Research Program of Zhejiang Province, China (nos. 2008ZA002 and 2014ZQ008); the Science and Technology Project of Hangzhou, China (nos. 20130533B68 and 20131813A23); and the Science Foundation of Zhejiang Chinese Medical University (nos. 2011ZY25 and 2013ZZ12).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Gang Cao, Qinglin Li, and Hao Cai contributed equally to this work.
References
- 1.Cao G, Zhang Y, Cong XD, Cai H, Cai BC. Advances in research on polysaccharides from Fructus Corni. Asian Journal of Traditional Medicines. 2009;4:205–209. [Google Scholar]
- 2.Cao G, Zhang Y, Cong XD, Cai H, Cai BC. Research progress on the chemical constituents and pharmacological activities of Fructus corni. Journal of Chinese Pharmaceutical Sciences. 2009;18:208–213. [Google Scholar]
- 3.Lu A-F, Qi M-J, Li Z-L, Lv H-F. Callus cultivation and determination of flavonoids from Tetrastigma hemsleyanum. Journal of Chinese Medicinal Materials. 2010;33(7):1042–1045. [PubMed] [Google Scholar]
- 4.Wang XL, Zeng J, Zhou H. The effect of extract of radix tetrastigma hemsleyani on lung cancer cell line A549. Anti-Tumor Pharmacy. 2012;5:347–350. [Google Scholar]
- 5.Ma DD, Li WP, Ma ZL, He LY, Jiang FS, Ding ZS. Anti-liver damage activity analysis of polysaccharide in radix tetrastigmatis hemsleyani. Journal of Medical Research. 2012;41:33–36. [Google Scholar]
- 6.Shao Q, Deng Y, shen H, Fang H, Zhao X. Optimization of polysaccharides extraction from Tetrastigma hemsleyanum Diels et Gilg using response surface methodology. International Journal of Biological Macromolecules. 2011;49(5):958–962. doi: 10.1016/j.ijbiomac.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Sharma N, Shukla S. Hepatoprotective potential of aqueous extract of Butea monosperma against CCl4 induced damage in rats. Experimental and Toxicologic Pathology. 2011;63(7-8):671–676. doi: 10.1016/j.etp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Yadav NP, Pal A, Shanker K, et al. Synergistic effect of silymarin and standardized extract of Phyllanthus amarus against CCl4-induced hepatotoxicity in Rattus norvegicus. Phytomedicine. 2008;15(12):1053–1061. doi: 10.1016/j.phymed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Hung M-Y, Fu TY-C, Shih P-H, Lee C-P, Yen G-C. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibits CCl4-induced hepatic damage in rats. Food and Chemical Toxicology. 2006;44(8):1424–1431. doi: 10.1016/j.fct.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Ren ZM, Dai GH, Tong YL, Chen Y, Yang F. The anti-inflammatory effect of radix tetrastigmae lyophilized powder. China Modern Doctor. 2013;51:13–14, 17. [Google Scholar]
- 11.Pu JB, Liang WQ, Zheng JX, Hu TJ, Wei KM. Simultaneous determination of quercetin and kaempferol in Tetrastigmatis hemsleyani by HPLC. Chinese Journal of Traditional Medical Science and Technology. 2011;18:134–135. [Google Scholar]
- 12.Fu TT, Lv XY, Li YH. Study on the process for extracting total flavonoids from radix tetrastigmae. Natural Product Research and Development. 2009;21:502–505. [Google Scholar]
- 13.Kluk MJ, Ashworth T, Wang H, et al. Gauging NOTCH1 activation in cancer using immunohistochemistry. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0067306.e67306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bafna PA, Balaraman R. Protective effect of DHC-1, a polyherbal formulation, against CCL4 induced liver damage. Hygeia: Journal for Drugs and Medicines. 2013;5(1):10–18. [Google Scholar]


