Abstract
Vancomycin-resistant Enterococcus faecium represents a growing threat in hospital-acquired infections. Two outbreaks of this pathogen from neighboring Warsaw hospitals have been analyzed in this study. Pulsed-field gel electrophoresis (PFGE) of SmaI-digested DNA, multilocus VNTR analysis (MLVA), and multilocus sequence typing (MLST) revealed a clonal variability of isolates which belonged to three main lineages (17, 18, and 78) of nosocomial E. faecium. All isolates were multidrug resistant and carried several resistance, virulence, and plasmid-specific genes. Almost all isolates shared the same variant of Tn1546 transposon, characterized by the presence of insertion sequence ISEf1 and a point mutation in the vanA gene. In the majority of cases, this transposon was located on 50 kb or 100 kb pRUM-related plasmids, which lacked, however, the axe-txe toxin-antitoxin genes. 100 kb plasmid was easily transferred by conjugation and was found in various clonal backgrounds in both institutions, while 50 kb plasmid was not transferable and occurred solely in MT159/ST78 strains that disseminated clonally in one institution. Although molecular data indicated the spread of VRE between two institutions or a potential common source of this alert pathogen, epidemiological investigations did not reveal the possible route by which outbreak strains disseminated.
1. Introduction
Since the first isolation of vancomycin-resistant enterococci (VRE) in 1986 [1, 2], this phenotype has spread rapidly and now is present in hospitals worldwide [3]. In Poland, the first VanA outbreak took place in the adult hematology ward of Gdansk Medical University in December 1996, followed by outbreaks in other centers [4]. The predominant species among VRE is Enterococcus faecium (VREfm). The majority of worldwide VREfm belongs to the meroclone CC17 (ciprofloxacin- and ampicillin-resistant and enriched in putative virulence traits), recently split into three distinct lineages, 17, 18, and 78, that evolved in hospital environment through horizontal gene transfer (HGT) and recombination processes [5]. These hospital-adapted lineages play a crucial role in the emergence and spread of VREfm.
The vanA gene cluster is a widely studied vancomycin/teicoplanin resistance determinant, described as part of Tn1546-type transposons, generally carried on plasmids and thus effectively disseminated by HGT [6]. An acquisition of vanA plasmid by a strain of E. faecium representing hospital-adapted lineage may result in a spread of VREfm, first colonizing patients and then causing symptomatic infections. Therefore, both characterization of the Tn1546 structure and its linkage to particular plasmid groups is crucial for understanding of VRE dissemination in hospital environments. Several studies have shown the presence of various Tn1546 types on Inc18, pRUM-like, pMG1-like, and pLG1 plasmids [7–13]; however, our knowledge of vanA plasmids and their epidemiology is still far from being satisfactory and the common presence of plasmids with Tn1546, belonging to unknown replicon types, has been shown [10, 14].
The aim of this study was to characterize E. faecium VanA isolates from the outbreaks that concomitantly took place in hospital wards of two neighboring medical centers, The Institute of Oncology (IO) and The Institute of Hematology and Transfusion Medicine in Warsaw (IH). The investigation focused on the clonal relationships among isolates as well as analysis of the Tn1546 transposon structure and colocalization of vanA with other plasmid genes in order to elucidate the role of particular MGE during a VREfm outbreak in hospital settings.
2. Materials and Methods
2.1. Outbreak Description, Bacterial Isolates, and Susceptibility Testing
Forty-four vancomycin-resistant E. faecium outbreak isolates were collected between February and June 2009 in two neighboring hospitals in Warsaw, The Institute of Oncology (IO) and The Institute of Hematology and Transfusion Medicine (IH), 776- and 198-bed hospitals, respectively. First VREfm was isolated from stool of 46-year-old patient on 4th February at the Gastroenterology Clinic of IO. Until the end of February, eight more cases were reported, in majority from the Clinic of Lymphatic System Cancers of IO. From the 31st March till the 18th of April, 18 VREfm were isolated, mainly from patients of this clinic (16 cases) and from two patients of the Gastroenterology Clinic. Simultaneously, VREfm cases were reported in IH wards, with the first two isolations on the 5th February from rectum and stool of the Hematology Ward patient and a patient from the ICU, respectively. One more isolate was obtained 10 days later in the Surgery Ward and till the end of June, 14 other VREfm cases were reported in the Hematology Ward of IH. Altogether, the outbreaks affected 42 patients, including 27 patients of IO (27 stool isolates) and 15 patients of IH (13 stool, 1 urine, 3 blood isolates). Antimicrobial susceptibility of collected isolates was determined using the Etest method (bioMérieux, Marcy l'Etoile, France) for glycopeptide susceptibility testing and broth microdilution method for other antimicrobials. The results were interpreted following the breakpoints of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [15]; for chloramphenicol, erythromycin, ciprofloxacin, and tetracycline the Clinical and Laboratory Standards Institute (CLSI) [16] breakpoints were applied, and in the case of kanamycin and clindamycin, the breakpoints proposed by the Société Française de Microbiologie (SFM) [17] were used. The Enterococcus faecalis strain ATCC29212 was used for quality control purposes during testing. E. faecium BM4147 was used as a control VanA strain in this study.
2.2. DNA Isolation and Genotyping of Isolates
Total DNA of isolates was extracted using Genomic DNA Prep Plus kit (A&A Biotechnology, Gdansk, Poland), following the manufacturer's instructions. Additionally, as the above method may result in a low yield of small plasmids, plasmid DNA was isolated using the alkaline lysis method [18]. Pulsed-field gel electrophoresis (PFGE) was performed according to de Lancastre et al. [19] for agarose plugs preparation, followed by the procedure of Clark et al. [20] for total genomic DNA purification. Purified DNA in plugs was digested with the SmaI restriction enzyme (Fermentas, Vilnius, Lithuania). Electrophoresis was performed at 14°C for 22 h with a pulse time of 1–30 s at 6 V/cm² in 0.5x TBE buffer and the results were interpreted according to criteria proposed by Tenover et al. [21]. The Bionumeric software (Applied Maths, Kortrijk, Belgium) was used to analyze the similarity of PFGE-banding patterns, with an unweighted pair group method with arithmetic average (UPGMA) algorithm and Dice coefficient. Multilocus variable-number tandem repeat (VNTR) analysis (MLVA) was performed as described by Top et al. [22] with modifications given on the website (http://www.mlva.umcutrecht.nl). Multilocus sequence typing (MLST) was performed as described previously [23]. Allele numbers and sequence types (STs) were assigned using E. faecium MLST database (http://efaecium.mlst.net/; 16th December 2013, date last accessed). PCR detection of IS16 was performed as described by Werner et al. [24]. The Simpson index and Wallace index were calculated using the online tool available at http://darwin.phyloviz.net/ComparingPartitions/ (14th January 2014, date last accessed).
2.3. Detection of Virulence Genes, Antimicrobial Resistance Determinants, and Plasmid Functional Modules by PCR
Enterococcal virulence genes hyl Efm, esp Efm, gel, asa, and cyl were screened as described by Vankerckhoven et al. [25]. Genes representing four E. faecium pilus gene clusters (PGC) were detected by the amplification of representative components of particular PGC, namely, fms21/pilA (PGC-1), fms17 (PGC-2), fms5 (PGC-3), and fms19 (PGC-4) [26]. Antimicrobial resistance determinants were investigated using primers and conditions described by others: vanA [20], cat [27], erm(B) [28], tet(M) [29], tet(O) [30], aad6 [31], aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(2′′)-Ic, aph(2′′)-Id, aph(3′)-IIIa, and ant(4′)-Ia [32]. The presence of the vanA gene for 13 isolates from the IO was established in our previous study [33]. Detection of 19 rep families and the unique rep pMG1 gene was performed according to Jensen at al. [34]. PCR for the rep pLG1 [9] was performed with primers designed previously [35]. The presence of plasmid addiction systems, relaxase genes, and the intA integrase gene of integrative and conjugative element ICEEfm1 was also verified by PCR [36–38].
2.4. Plasmid Profiling, Hybridization Analyses, and Tn1546 Typing
DNA in agarose plugs obtained as described above was treated with 14U of S1 nuclease (Takara Bio, Japan) for 15 minutes at 37°C and separated by PFGE at 14°C for 22 h with pulse time 5–35 s at 6 V/cm² in 0.5x TBE buffer [39]. This method allows visualization and determination of the number and size of plasmids larger than approximately 30 kb. After electrophoresis, DNA was blotted onto the Hybond-N+ membrane (GE Healthcare, Buckinghamshire, UK) by capillary transfer. Probe labeling and signal detection for PFGE-S1 membranes were carried out using the Amersham ECL Random-Prime Labeling and Detection System (GE Healthcare), according to the manufacturer's protocol.
The 4.4 kb fragment of the vanRSHAX operon was amplified using Expand Long Template System (Roche Diagnostics GmbH, Mannheim, Germany) according to Palepou et al. [40] with the following amplification conditions: 94°C for 2 min; 10 cycles of 94°C for 10 s, 56°C for 30 s, and 68°C for 4 min; 20 cycles of 94°C for 10 s, 56°C for 30 s, and 68°C for 4 min (with the elongation time increased by 20 s/cycle); and 68°C for 7 min. L-PCR amplicons were analyzed by restriction fragment length polymorphism (RFLP) with DdeI (New England Biolabs, UK). The whole Tn1546 transposon was investigated by PCR mapping and sequencing (Table 1 and references therein).
Table 1.
Primers used in the analysis of Tn1546 transposon.
| Primer pair | Primer names | Sequence (5′-3′) | Position in Tn1546 | Application in this study | Reference |
|---|---|---|---|---|---|
| 1 | vanRSHAX-1 | AGACAAGTCTGAGATTGACCTTGCC | 4141–4165 | PCR | [40] |
| vanRSHAX-2 | ATATGCTTCAAACCCACTGTTTTCC | 8565–8589 | PCR | [40] | |
| 2 | Tn1546 | GGAAAATGCGGATTTACAACGCTAAG | 13–38 | PCR | [40] |
| ORF1-5 | CACGTCCTGCCGACTATGATTATTT | 1900–1876 | PCR | [41] | |
| 3 | ORF2-F | TCATTCCATTTCTGTATTTTCAATTT | 3050–3086 | PCR | [42] |
| ORF2-R | GCCCATTAGCGGAATACAGA | 3770–3751 | PCR | [42] | |
| 4 | ORF2-F2 | ACTAATGTATCTAGGGCTTCA | 3710–3731 | PCR | [42] |
| vanR-R | GCAATTTCATGTTCATCATCCA | 4000–3979 | PCR | [42] | |
| 5 | vanS | AACGCTATTCCAAACTAGAA | 4690–4710 | PCR, sequencing | [41] |
| vanS-R | GTCGGAAGCTCTACCCTAAA | 5760–5741 | PCR, sequencing | [41] | |
| 6 | vanS1 | ATTGTTCAGCATGGAGGGC | 5700–5719 | PCR, sequencing | [34] |
| vanH2 | GAGCATGGAATGCATCTGCC | 6060–6041 | PCR | [34] | |
| 7 | vanA1 | CATGAATAGAATAAAAGTTGCAATA | 6978–7002 | PCR | [20] |
| vanX2 | TTATTTAACGGGGAAATC | 8600–8583 | PCR, sequencing | [43] | |
| 8 | vanX-F | ATGGGTATTTTCAGAAGTCCC | 8580–8601 | PCR, sequencing | [42] |
| vanZ2 | AATGGGTACGGTAAACGAGC | 10555–10536 | PCR | [34] | |
| ORF1-4 | GCATGTAGTGATGAAACACCTAGCTGC | 960–987 | sequencing | [41] | |
| vanA2 | CCCCTTTAACGCTAATACCATCAA | 8007–7894 | sequencing | [20] | |
| vanY1 | AGAGACGAACCATACCCCAA | 9200–9181 | sequencing | [42] | |
| vanY2-R | AGTATGTGTTGATCCGGGAAAC | 9900–9922 | sequencing | this study | |
2.5. Conjugation Experiments
Conjugation transfer of vancomycin resistance was examined by cross-streak mating procedure with E. faecium strain 64/3 resistant to rifampin and fusidic acid as recipient. Fresh colonies of donors were cross-streaked with recipient on BHI-Agar plates and incubated overnight at 37°C. Bacterial cells from the streak crossing area were then incubated overnight in 37°C on selective media. Transconjugants were then confirmed by MLVA. For isolates negative for conjugation in this assay, a technique specific for bacteria with low frequency of transfer was used [44].
3. Results
3.1. Antibiotic Resistance Phenotypes, Antimicrobial Resistance Determinants, and Virulence Genes
All analyzed isolates were resistant to vancomycin and teicoplanin and exhibited the presence of vanA determinant (Table 2). Additionally, all of them were penicillin-, ampicillin-, ciprofloxacin-, clindamycin-, and erythromycin-resistant. The vast majority of isolates from both IO and IH showed resistance to rifampin. High-level resistance to gentamicin (HLGR), kanamycin (HLKR), and streptomycin (HLSR) was more prevalent among IH isolates, which were particularly enriched in aminoglycoside resistance genes aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, and aad6 (Figure 1). The aph(2′′)-Ib gene occurred in nine isolates and three other tested genes, coding for aminoglycoside resistance; that is, aph(2′′)-Ic, aph(2′′)-Id, and ant(4′)-Ia were not detected. Isolates from both groups commonly carried erm(B) and tet(M) genes. Resistance and intermediate susceptibility to tetracycline was typical for 61% and 18% of isolates, respectively. Intermediate susceptibility to chloramphenicol and quinupristin-dalfopristin was shown for 51% and 29% of isolates, respectively. All isolates were susceptible to linezolid and tigecycline.
Table 2.
Clonal relatedness, antimicrobial resistance profiles, and distribution of resistance and virulence determinants among IO and IH outbreak isolates.
| Isolate | MT/PT/ST | MIC VAN (mg/L) | MIC TEI (mg/L) | Resistance phenotypesa,b | Resistance determinantsb,c | Cotransferred resistance | Virulence genes | Pili genesd |
|---|---|---|---|---|---|---|---|---|
| Institute of oncology | ||||||||
| 988 | 1/2-A/17 | >256 | 32 | RIF | ermB | ERY | — | fms17, fms5, fms19 |
| 989* | 159/3/78 | >256 | 256 | CHL, GEN, KAN, RIF | aac(6′)-Ie-aph(2′′)-Ia, cat, tetM | — | esp, hyl | fms5, fms19 |
| 990 | 1/4-A/18 | >256 | 32 | RIF | ermB, tetM | ERY | hyl | fms5, fms19 |
| 991 | 3/1-A/18 | >256 | 32 | RIF | ermB, tetM | ERY | hyl | fms5, fms19 |
| 992 | 3/1-A/nd | >256 | 32 | RIF | ermB, tetM | ERY | hyl | fms5, fms19 |
| 993 | 3/1-A/nd | >256 | 32 | RIF | ermB, tetM | ERY | hyl | fms5, fms19 |
| 994 | 3/1-A/nd | >256 | 32 | RIF | ermB, tetM | ERY | hyl | fms5, fms19 |
| 995 | 3/1-A/nd | >256 | 24 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 996 | 3/1-A/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3612 | 3/1-C/nd | >256 | 48 | GEN, RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3613 | 3/1-C/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3614 | 3/1-C/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3615 | 3/1-C/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3616 | 3/1-C/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3617 | 3/1-C/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3618 | 3/1-C/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3620 | 10/5/262 | >256 | 48 | GEN, KAN, RIF, STR, TET | aac(6′)-Ie-aph(2′′)-Ia, aad6, ermB, tetM | ERY | hyl | fms17, fms5, fms19 |
| 3621 | 3/1-C/nd | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3622 | 3/1-B/18 | >256 | 32 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3623 | 3/1-C/nd | >256 | 48 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3624 | 10/5/262 | >256 | 48 | GEN, KAN, RIF, STR, TET | aac(6′)-Ie-aph(2′′)-Ia, aad6, ermB, tetM | no | — | fms17, fms5, fms19 |
| 3625 | 10/5/nd | >256 | 48 | GEN, KAN, RIF, STR, TET | aac(6′)-Ie-aph(2′′)-Ia, aad6, ermB, tetM | no | — | fms17, fms5, fms19 |
| 3626 | 10/5/nd | >256 | 48 | GEN, KAN, RIF, STR, TET | aac(6′)-Ie-aph(2′′)-Ia, aad6, ermB, tetM | no | — | fms17, fms5, fms19 |
| 3627 | 10/5/nd | >256 | 48 | GEN, KAN, RIF, STR, TET | aac(6′)-Ie-aph(2′′)-Ia, aad6, ermB, tetM | ERY | — | fms17, fms5, fms19 |
| 3628 | 3/1-C/nd | >256 | 64 | RIF, TET | ermB, tetM | ERY | hyl | fms5, fms19 |
| 3629 | 7/2-B/18 | >256 | 48 | GEN, KAN, TET | aac(6′)-Ie-aph(2′′)-Ia, ermB, tetM | ERY | hyl | fms17, fms5, fms19 |
| 3630 | 10/5/nd | >256 | 64 | GEN, KAN, RIF, STR, TET | aac(6′)-Ie-aph(2′′)-Ia, aad6, ermB, tetM | ERY, TET | — | fms17, fms5, fms19 |
| IO (N = 27) | CHL (1), GEN (9), KAN (8), STR (6), TET (20), RIF (26) | cat (1), aac(6′)-Ie-aph(2′′)-Ia (8), aad6, (6), ermB (26), tetM (26) | ERY (23), TET (1) | hyl (21), esp (1) | fms17 (8), fms5 (27), fms19 (27) | |||
|
| ||||||||
| Institute of hematology | ||||||||
| 3549 | 11/7-A/202 | >256 | 32 | GEN, KAN, RIF, TET | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, ermB, tetM | ERY, GEN | esp, hyl | fms17, fms5, fms19 |
| 3550x | 7/8/18 | >256 | 48 | GEN, KAN, RIF, STR | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(3′)-IIIa, aad6, ermB | ERY | esp, hyl | fms17, fms5, fms19 |
| 3551 | 159/9/78 | >256 | 32 | GEN, KAN, RIF, STR | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(3′)IIIa, aad6, ermB | ERY | esp, hyl | fms17, fms19 |
| 3552* | 7/10/18 | >256 | >256 | KAN, RIF, STR | aph(3′)-IIIa, aad6, ermB, tetM | ERY | esp, hyl | fms17, fms5, fms19 |
| 3553 | 11/7-A/nd | >256 | 32 | GEN, KAN, RIF, TET | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)Ib, aph(3′)IIIa, ermB, tetM | ERY | esp, hyl | fms17, fms5, fms19 |
| 3554 | 159/6-A/192 | >256 | 48 | GEN, KAN, RIF, STR | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(3′)-IIIa, aad6, ermB | no | esp, hyl | fms17, fms5, fms19 |
| 3555 | 159/6-B/nd | >256 | 48 | GEN, KAN, RIF, STR | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(3′)-IIIa, aad6, ermB | no | esp, hyl | fms17, fms5, fms19 |
| 3556 | 159/6-C/nd | >256 | 32 | GEN, KAN, STR | aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, aad6, ermB | no | esp, hyl | fms17, fms5, fms19 |
| 3557x# | 296/11/17 | >256 | 48 | KAN, RIF | aph(3′)-IIIa, aad6, cat, ermB, tetM | ERY | esp, hyl | fms17, fms19 |
| 3558 | 159/6-A/nd | >256 | 48 | GEN, KAN, PEN | aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, aad6, ermB | no | esp, hyl | fms17, fms5, fms19 |
| 3559 | 159/6-D/nd | >256 | 48 | GEN, KAN, PEN | aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, aad6, ermB | no | esp, hyl | fms17, fms5, |
| 3560 | 1/7-B/18 | >256 | 48 | GEN, KAN, RIF, TET | aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ermB, tetM | ERY | hyl | fms5, fms19 |
| 3561 | 11/4-C/202 | >256 | 32 | GEN, KAN, RIF, TET | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(3′)-IIIa, ermB, tetM | ERY | esp, hyl | fms17, fms5, |
| 3562# | 1/4-B/18 | >256 | 32 | GEN, KAN, RIF, TET | aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ermB, tetM | no | hyl | fms5, fms19 |
| 3563 | 159/6-E/192 | >256 | 48 | GEN, KAN, RIF | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(3′)-IIIa, aad6, ermB | no | esp, hyl | fms17, fms5, fms19 |
| 3564y | 7/12/18 | >256 | 48 | GEN, KAN, RIF, TET | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, aph(3′)-IIIa, ermB, tetM | ERY | esp, hyl | fms17, fms5, fms19 |
| 3567y# | 144/13/18 | >256 | 48 | GEN, KAN, RIF, TET | aac(6′)-Ie-aph(2′′)-Ia, aph(2′′)-Ib, ermB | ERY | hyl | fms17, fms5, fms19 |
| IH (N = 17) | GEN (15), KAN (17), STR (6), TET (7), RIF (15) | cat (1), aac(6′)-Ie-aph(2′′)-Ia (15), aph(2′′)-Ib (10), aph(3′)-IIIa (15), aad6 (10), ermB (17), tetM (8) | ERY (11), GEN (1) | hyl (17), esp (14) | fms17 (15), fms5 (15), fms19 (15) | |||
|
| ||||||||
| IO and IH (N = 44) | CHL (1), GEN (24), KAN (25), STR (12), TET (27), RIF (41) | cat (2), aac(6′)-Ie-aph(2′′)-Ia (23), aph(2′′)-Ib (10), aph(3′)-IIIa (15), aad6 (16), ermB (43), tetM (34) | ERY (34), TET (1), GEN (1) | hyl (38), esp (15) | fms17 (23), fms5 (42), fms19 (42) | |||
nd: not determined; no: no transconjugants obtained; two isolates with the prototype Tn1546 marked with an asterisk; #isolates from blood; aall isolates resistant to vancomycin, teicoplanin, penicillin, ampicillin, ciprofloxacin, erythromycin, and clindamycin; bfive isolates with tet(M) showed intermediate susceptibility to tetracycline and one isolate with tet(M) was susceptible to this compound, one tetracycline-resistant isolate (3567) was negative for the determinants tested; for four isolates with aad6 the MIC values for streptomycin increased (512 mg/L); one isolate with cat showed an intermediate resistance to chloramphenicol; call isolates were positive for the vanA gene; dall isolates positive for the pilA gene; x,yisolates from the same patients “X” and “Y”; RIF: rifampin; CHL: choramphenicol; GEN: gentamicin (HLGR); STR: streptomycin (HLSR); KAN: kanamycin (HLKR); TET: tetracycline. For the summarized results, the number of isolates is given in brackets.
Figure 1.
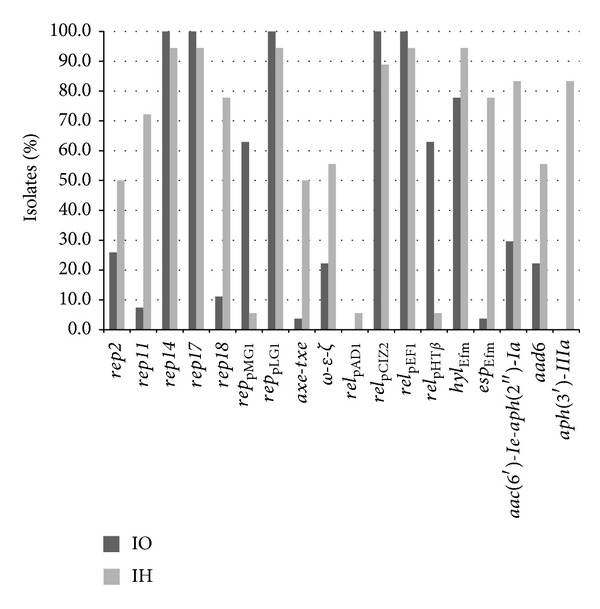
Distribution (% of isolates) of plasmid-specific genes, selected virulence genes, and resistance determinants among isolates from outbreak at the Institute of Oncology and Institute of Hematology, Warsaw.
Among virulence determinants studied, the hyl Efm gene was prevalent in both outbreaks, while the esp Efm gene was present mainly in IH (Table 2 and Figure 1). All esp Efm-positive isolates harbored the intA integrase gene. PGC genes fms21 (PGC-1), fms5 (PGC-3), and fms19 (PGC-4) commonly occurred in the whole studied collection, while the fms17 (PGC-2) was more prevalent among IH than IO isolates. Genes gel, asa, and cyl were not detected.
3.2. Clonal Relationships among Isolates
The clonal structure of outbreak isolates was evaluated with the use of three typing methods (Table 2 and Figure 2). PFGE analysis and MLVA were performed for the whole set of isolates, yielding altogether 13 PFGE types (PTs) and eight MLVA types (MTs). Two PTs, PT1 and PT6, were further diversified into three and five subtypes, respectively. A single novel MT296 was detected for the isolate recovered from blood in the IH. Generally, the IH isolates showed higher diversity of PTs and MTs, compared to the isolates obtained from the IO. For both hospitals, the predominance of specific PTs and MTs was observed, namely, PT1/MT3 and PT5/MT10 among the IO isolates and PT6/MT159 in the IH. Isolates with MT1/PT4 were observed in the two institutions (a single isolate in both IO and IH). In the case of two patients, two VREfm isolates with different PFGE and MLVA types were obtained from different body sites (rectum, stool, and blood). The comparison of MLVA and PFGE typing results showed a good correlation of both methods (Wallace indices: PT/MT 0.994, MT/PT 0.887) and a higher discriminatory power of PFGE over MLVA (Simpson's indices 81.7 and 79.5, resp.). Further MLST analysis for 20 representatives of different PTs and MTs yielded six sequence types (STs), all belonging to lineages: 17 (STs 17, 202), 18 (STs 18, 262), and 78 (STs 78, 192) of meroclone CC17. The most common ST18 occurred in both hospitals, however, in association with various PTs/MTs; this ST was also characteristic for two MT1/PT4 isolates mentioned above.
Figure 2.
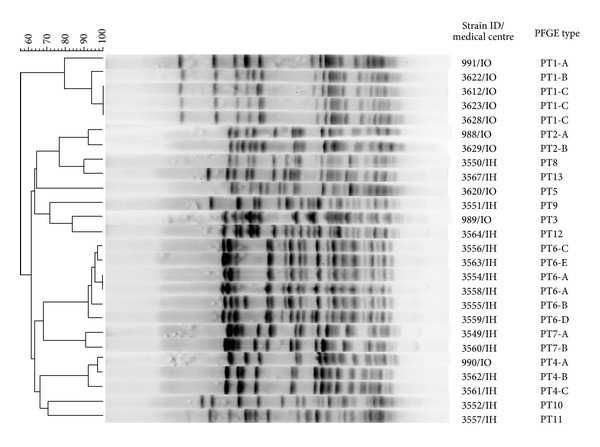
PFGE-based dendrogram of selected isolates from outbreaks in IO and IH, representing all PTs. Normalization performed by the use of reference Lambda Ladder PFG Marker (New England BioLabs, UK). The phylogenetic tree was constructed by the use of Dice coefficient (optimization, 0.5%; band tolerance, 1.3%) and UPGMA clustering.
3.3. Tn1546 Structures and Transferability of Vancomycin Resistance
All isolates exhibited the presence of 4.4 kb L-PCR product containing the vanRSHAX operon and showed that the DdeI restriction pattern is identical to the E. faecium BM4147 control VanA strain. Further PCR mapping and sequencing showed the presence of ISEf1, inserted at the position 9147 nt of Tn1546 (numbering according to the GenBank sequence M97297), that is, within the vanX-vanY intergenic region. The 5′GACTGAAA duplication was observed at the insertion site. ISEf1 was present in all but two isolates with the prototype Tn1546. One of the isolates was derived from IO and the other from IH, and each of them showed a unique PFGE type, PT3 and PT10, respectively (Table 2). Similarly, all isolates, except for the two mentioned above, exhibited the G7747C point mutation of Tn1546 located within the vanA gene, resulting in the amino acid substitution V257F. Both isolates with the prototype Tn1546 showed higher teicoplanin MIC values compared to the isolates with Tn1546: ISEf1.
Conjugation experiments were performed for all 44 isolates and 34 of them were able to transfer vancomycin resistance to the E. faecium 64/3 recipient. All donors produced transconjugants with cross-streak mating except for a single isolate, which required use of the method designed for strains with low-level conjugation frequencies [44]. Susceptibility testing of transconjugants (a single transconjugant for each donor) showed a concomitant transfer of erythromycin resistance in 32 cases. One of these transconjugants showed also HLGR phenotype and one was additionally resistant to tetracycline.
3.4. Diversity of Plasmid-Associated Gene Content
PCR screening revealed the presence of plasmid replication genes of the rep14pRI and rep17pRUM families as well as rep pLG1 in all isolates. Four other rep groups, rep2pRE25 (n = 16), rep11pEF1071 (n = 15), rep18pEF418 (n = 19), and rep pMG1 (n = 18) were also detected. First three of them were characteristic mainly for the IH outbreak, while rep pMG1 occurred mainly in isolates from IO (Figure 1). The number of rep genes per isolate varied from three to seven, and the average number of plasmid rep genes per isolate was 4.50; however, this value was lower for IO (4.07) compared to IH (5.18). Analysis of distribution of relaxase genes revealed the common presence of two relaxases, rel pCIZ2 and rel pEF1, while rel pHTβ was predominantly detected in IO and the distribution of this gene was completely concordant with the presence of rep pMG1. Additionally, one IH isolate had the rel pAD1 gene. Screening for plasmid toxin-antitoxin systems (TA) resulted in the detection of axe-txe and ω-ε-ζ, while other TA genes, including ccd, higBA, mazEF, par, parDE, phd-doc, relBE, and vagCD were absent in the studied group. All but one ω -ε-ζ-positive isolates were also rep2pRE25-positive and only one rep2pRE25-positive lacked the ω -ε-ζ gene.
3.5. Colocalization of vanA Determinant and Other Plasmid Genes
Twenty-seven selected isolates (11 from IO and 16 from IH) of various clonal types, as defined by MLVA, MLST, and PFGE, were subjected to PFGE-S1 analysis, followed by Southern blot hybridization (Figure 3 and Table 3) with probes specific for genes detected earlier by PCR, such as vanA, seven rep genes (rep2pRE25, rep11pEF1071, rep14pRI1, rep17pRUM, rep18pEF418, rep pLG1, and rep pMG1), genes of two plasmid TA systems (ω -ε-ζ and axe-txe), and three other plasmid-associated genes (pilA, hyl Efm, and aac(6′)-Ie-aph(2′′)-Ia). Altogether, 122 plasmid bands were visualized in PFGE-S1, with 56 megaplasmids bands greater than 100 kb. The average number of plasmid bands in PFGE-S1 analysis was 4.35, with very similar values for both IO and IH outbreaks. Thirty plasmid bands hybridized with the vanA probe; that is, three of the analyzed isolates carried two vanA plasmids. The majority of vanA plasmids were <30–100 kb in size; additionally, four megaplasmids (170, 200, 240, and 315 kb) were associated with the vanA determinant. Among vanA plasmids, 24 were rep17pRUM replicons, mostly of 50 kb and 100 kb. The 100 kb plasmid was present in 15 isolates of various clonal backgrounds in both IH and IO and most of these isolates easily produced transconjugants. Moreover, the first observed VREfm isolates in both IH and IO carried such plasmids but in different clonal backgrounds. The 50 kb plasmid was associated exclusively with MT159 isolates, which differed, however, in their PFGE patterns. Six such isolates occurred exclusively in IH and all of them were deficient in conjugation. Five of vanA-rep17pRUM plasmids of various sizes hybridized also with other rep genes, such as rep pLG1 (two 100 kb plasmids and one 315 kb plasmid), rep18pEF418 (one 100 kb plasmid), and both rep2pRE25 and rep18pEF418 (a 40 kb plasmid). Two isolates of MT1/PT4/ST18 from IO and IH both had 100 kb rep17pRUM plasmids but they differed in the content of other plasmids (Table 3, isolates labeled C and X); moreover, 100 kb plasmids from IO isolate additionally carried rep18pEF418. Among the remaining plasmids, other than rep17pRUM replicons, vanA plasmids, a single 70 kb plasmid had rep2pRE25 and a 240 kb megaplasmid hybridized with rep pLG1 and rep18pEF418. Considering other tested genes, the pilA gene was associated with 18 vanA plasmids, including all the 100 kb plasmids with rep17pRUM; genes of the ω -ε-ζ TA system were present on a single 40 kb plasmid with rep2pRE25, rep17pRUM, and rep18pEF418 and on a 70 kb plasmid carrying rep2pRE25. The 240 kb vanA megaplasmid carried also the aac(6′)-Ie-aph(2′′)-Ia resistance gene. Each of the two isolates with the prototype Tn1546 carried two vanA plasmids (<30 kb in both isolates and megaplasmids of 170 and 200 kb) that did not hybridize with any of probes used in this study. In summary, almost all IO isolates showed the presence of 100 kb vanA plasmids with rep17pRUM and pilA genes, while in IH the diversity of vanA plasmids was higher, with pRUM-like replicons of both 50 kb and 100 kb Information concerning other than vanA plasmids of E. faecium that was obtained during the study is summarized in Table 3.
Figure 3.
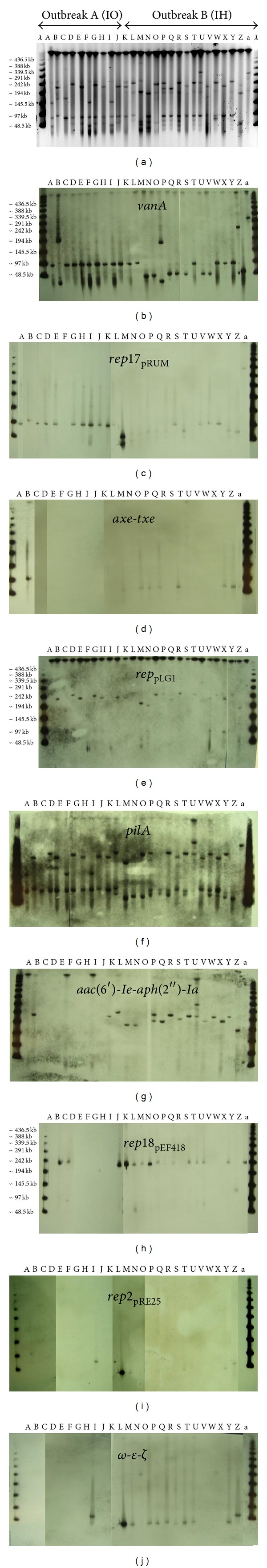
PFGE of S1-digested total DNA of selected 27 VREfm isolates, visualized by ethidium bromide staining (a) and subjected to Southern hybridization with the following probes: vanA (b), rep17pRUM (c), axe-txe (d), rep pLG1 (e), pilA (f), aac(6′)-Ie-aph(2′′)-Ia (g), rep18pEF418 (h), rep2pRE25 (i), and ω -ε-ζ (j). Lanes A–a, isolates designation as described in Table 3.
Table 3.
Plasmid profiles and colocalization of particular genes on vanA and other plasmids among selected 27 VREfm isolates.
| Letter code | Isolate | MT/PT/ST | vanA plasmids in kb (hybridizing probes) | Other plasmids in kb (hybridizing probes) |
|---|---|---|---|---|
| IO (N = 11) | ||||
| A | 988# | 1/2-A/17 | 100 (rep17, pilA) | <30, 150 (pilA), 180, 270 |
| B | 989∗# | 159/3/78 | <30, 200 | 110 (rep2, rep17, pilA, axe-txe), 175, 230 (rep18, rep pLG1 , hyl, aac (6′)-Ie-aph (2′′)-Ia, pilA), 310, >400 |
| C | 990# | 1/4-A/18 | 100 (rep17, rep18, pilA) | 175, 230 (rep18), 280, 340 |
| D | 991# | 3/1-A/18 | 100 (rep17, pilA) | 65 (rep pMG1), 240 (rep pLG1 , hyl, pilA), 295 |
| E | 3612# | 3/1-C/nd | 100 (rep17, pilA) | 70 (rep pMG1), 235 (rep pLG1 , hyl, pilA), 290 |
| F | 3620# | 10/5/262 | 100 (rep17, pilA) | 65, 150 (pilA), 290 |
| G | 3622# | 3/1-B/18 | 100 (rep17, pilA) | 70 (rep pMG1), 240 (rep pLG1 , hyl, pilA), 290 |
| H | 3623# | 3/1-C/nd | 100 (rep17, pilA) | 70 (rep pMG1), 235 (rep pLG1 , hyl, pilA), 290 |
| I | 3624 | 10/5/262 | 100 (rep17, pilA) | 40, 65 (rep2, ω-ε-ζ), 150 (pilA), 290 |
| J | 3628# | 3/1-C/nd | 100 (rep17, pilA) | 70 (rep pMG1), 235 (rep pLG1 , hyl, pilA), 290 |
| K | 3629# | 7/2-B/18 | 100 (rep17, pilA) | 235 (rep18, rep pLG1 , hyl, aac (6′)-Ie-aph (2′′)-Ia, pilA), 270 |
|
| ||||
| IH (N = 16) | ||||
| L | 3549# | 11/7-A/202 | 100 (rep17, pilA) | 70 (rep pMG1 , pilA), 240 (rep18, rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA), 310 |
| M | 3550x# | 7/8/18 | 45 (rep2, rep17, rep18, ω-ε-ζ) | 60 (rep17, pilA), 70, 195 (rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA), 230 (rep18), 315 |
| N | 3551# | 159/9/78 | 45 (rep17) | 40 (rep2, ω-ε-ζ), 70 (rep17, pilA, axe-txe), 100, 190 (rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia), 240 (rep18), 290 |
| O | 3552∗# | 7/10/18 | <30, 170 | 65 (rep17, pilA), 240 (rep18, rep pLG1 , pilA, ω-ε-ζ) |
| P | 3554 | 159/6-A/192 | 50 (rep17) | 40 (rep2, ω-ε-ζ), 75 (rep17, pilA, axe-txe), 100 (pilA), 220 (aac (6′)-Ie-aph (2′′)-Ia), 240 (rep18, rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| Q | 3555 | 159/6-B/nd | 50 (rep17) | 40 (ω -ε-ζ), 75 (rep17, pilA, axe-txe), 100 (pilA), 220 (rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia), 240 (rep18) |
| R | 3556 | 159/6-C/nd | 50 (rep17) | 40 (ω -ε-ζ), 75 (rep17, pilA, axe-txe), 100 (pilA), 240 (rep18, rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| S | 3557x# | 296/11/17 | 100 (rep17, pilA) | 40 (ω -ε-ζ), 235 (pilA) |
| T | 3558 | 159/6-A/nd | 50 (rep17) | 40 (rep2, ω-ε-ζ), 75 (rep17, pilA, axe-txe), 100 (pilA), 240 (rep18, rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| U | 3559 | 159/6-D/nd | 50 (rep17) | 40 (ω -ε-ζ), 75 (rep17), 100 (rep17), 240 (rep18, aac (6′)-Ie-aph (2′′)-Ia, pilA), 330 (rep pLG1 , axe-txe, aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| V | 3560# | 1/7-B/18 | 100 (rep17, rep pLG1 , pilA) | 85 (pilA), 235 (rep18, rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| W | 3561# | 11/4-C/202 | 100 (rep17, rep pLG1 , pilA) | 240 (rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| X | 3562 | 1/4-B/18 | 100 (rep17, pilA) | 240 (rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| Y | 3563 | 159/6-E/192 | 50 (rep17), 240 (rep18, rep pLG1 , pilA, aac(6′)-Ie-aph(2′′)-Ia) | 40 (rep2, ω-ε-ζ), 75 (rep17, pilA, axe-txe), 100 (pilA), 240 (rep18, rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia, pilA) |
| Z | 3564y# | 7/12/18 | 70 (rep2, pilA,ω-ε-ζ) | 60 (rep pLG1), 75 (rep17, axe-txe), 130 (rep18), 200 (rep pLG1 , aac (6′)-Ie-aph (2′′)-Ia), 250 |
| a | 3567y# | 144/13/18 | 315 (rep17, rep pLG1 , pilA) | 75 (axe-txe, aac (6′)-Ie-aph (2′′)-Ia), 240 (rep18) |
Letter code of each isolate corresponds to the designation used in Figure 2; nd: not determined; two isolates with the prototype Tn1546 marked with an asterisk; x,yisolates from the same patients “X” and “Y”; #isolates positive in conjugation.
4. Discussion
This study provides the molecular characteristics of VREfm outbreak isolates with the special focus on the role of MGE, such as Tn1546-type transposons and vanA plasmids, acting as mediators of vancomycin resistance transfer. The investigated group of isolates originated from two hospitals, The Institute of Oncology and The Institute of Hematology and Transfusion Medicine in Warsaw, where two VREfm outbreaks occurred concomitantly. Immunocompromised patients of oncological and hematological wards are known to be of special risk for VRE colonization and infection [45]. Such susceptibility was especially evident during the outbreak in the IH, where three bloodstream infections caused by VRE were reported. The proximity of the two hospitals in the city and the simultaneous emergence of both outbreaks, which lasted for a few months, suggested the possibility of VRE transmission between the two institutions, although the investigation of available medical documentation, done independently in IO and IH, revealed no obvious routes, such as patient transfer between the two hospitals or from the same third hospital or utilization of common diagnostic equipment just before or during the outbreak period. The involvement of hospital personnel in VREfm transfer was also excluded.
Molecular typing methods, such as PFGE analysis and MLVA, have shown a divergent clonal structure of isolates. Such a situation is typical for VanA hospital outbreaks [46–49]. MLST performed for representative isolates included all of them into the hospital-associated lineages 17, 18, and 78, formerly described as CC17 complex [3]. Isolates belonging to this meroclone display common features such as ampicillin and ciprofloxacin resistance, the prevalence of IS16, esp Efm, and hyl Efm, and enrichment in microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), including pili genes [26, 50]. However, isolates from each institution showed some specific features, such as predominance of certain PTs/MTs and differences in distribution of virulence, resistance, and plasmid-specific genes. This observation would suggest the existence of two separate endemic subpopulations without much exchange of strains prior to the introduction of vanA-carrying MGE.
VanA phenotype in both outbreaks was associated, in the vast majority of cases, with an acquisition of the same specific variant of Tn1546 transposon with ISEf1 and a mutation in the vanA gene. Tn1546-type transposons show significant variability due to point mutations, deletions, and presence of various ISs [41, 42, 47, 51], and thus analysis of transposon structure provides valuable epidemiological information for investigation of VRE outbreaks. The ISEf1 insertion in the vanX-vanY intergenic region was described previously for hospital VREfm in Portugal and Germany [47, 51], and the mutation in the vanA gene was not reported before. The presence of the same type of Tn1546 in both outbreaks provides a strong indication of either a common source or transmission of VREfm between the two hospitals.
Hybridization studies on representative isolates revealed the presence of a 100 kb plasmid carrying the vanA determinant as well as rep17, typical for pRUM plasmid and the pilA gene in several isolates from both institutions. Most of these isolates readily produced transconjugants, suggesting that this plasmid might play the principal role in the outbreak. The observed concomitant transfer of erythromycin resistance is in agreement with the colocalization of the ermB gene on pRUM [52]. Other, frequently encountered vanA plasmid was 50 kb in size and also represented the rep17pRUM replicon; however, it lacked pilA and all isolates with this plasmid were negative in conjugation. The 50 kb plasmid was exclusively associated with isolates of MT159/ST78 and observed solely in IH. The recently emerged lineage 78 of the hospital-adapted E. faecium shows increased epidemic properties and plays an important role in HAIs [53]. Thus, strains of this lineage, harboring the 50 kb nonconjugative vanA plasmid, were likely to be spreading by efficient clonal dissemination during the outbreak in IH. Association of van determinants with pRUM-type plasmids was described also by others [7, 10, 54]. Both 100 kb and 50 kb plasmids lacked the axe-txe TA system genes, typical for pRUM [10, 52], suggesting the possible common origin of these two plasmids. Further studies, based on whole plasmid sequencing, are indispensable to elucidate the possible evolution of these plasmids during the outbreak. Although 100 kb plasmids were found in two isolates of the same clonal characteristics from IO and IH (Table 3, isolates C and X), differences in the plasmid content do not allow us to indicate these isolates as a direct epidemiological link between two hospitals. In a few cases, the vanA gene was associated with other replicons, typical for pLG1, pRE25, and pEF418. Such vanA plasmids were observed also in other studies [7, 10]. The prevalent distribution of rep pLG1 as well as its predominant presence on plasmids over 200 kb in size is also in agreement with earlier studies which showed that all VREfm megaplasmids with the defined replicon type were always pLG1-like [7, 8]. The association of VanA determinants with various plasmids during one outbreak may be caused by the Tn1546 transposition among plasmids and/or plasmid recombination. The latter process may yield plasmids with more than a single rep gene, which was also observed in the current study, both for vanA- and other plasmids. The role of plasmid mosaics in the dissemination of Tn1546 among VRE was emphasized recently by Freitas et al. [7]. Finally, for some vanA plasmids and other plasmids the replicon types could not be established (13% and 34% of observed plasmids, resp.), indicating that the pool of E. faecium plasmids remains only partly explored [10] and that there is the need for further studies of these epidemiologically important elements.
5. Conclusions
Molecular analysis of VanA VREfm outbreaks revealed that Tn1546::ISEf1 elements associated with pRUM-like plasmids were the key mediators of vancomycin-resistant E. faecium dissemination among the investigated group of oncological/hematological patients. Horizontal gene transfer of the whole vanA plasmids and/or Tn1546 transposons in endemic populations of nosocomial E. faecium is suggested as the potential way of VanA phenotype spread in the analyzed outbreaks. The enrichment in different plasmid-associated genes, antimicrobial resistance, and potential virulence determinants in the investigated population emphasizes the impact of mobile genetic elements on the epidemiology and evolution of VREfm.
Acknowledgments
The authors thank J. Top for assigning new MTs. This publication made use of the Enterococcus faecium MLST website (http://efaecium.mlst.net/) hosted at the Imperial College of the University of Oxford and funded by the Wellcome Trust. This work was supported by Grant no. N401588540 from the National Science Centre, Poland, and by the National Progamme for the Protection of Antibiotics (Narodowy Program Ochrony Antybiotyków—NPOA) funding from the Ministry of Health, Poland.
Conflict of Interests
The authors declare that they have no conflict of interests regarding to the publication of this paper.
References
- 1.Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium . The New England Journal of Medicine. 1988;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 2.Uttley AHC, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. The Lancet. 1988;1(8575-8576):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 3.Willems RJL, Top J, van Santen M, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerging Infectious Diseases. 2005;11(6):821–828. doi: 10.3201/eid1106.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner G, Coque TM, Hammerum AM, et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Eurosurveillance. 2008;13(47):p. 19046. [PubMed] [Google Scholar]
- 5.Willems RJ, Top J, van Schaik W, et al. Restricted gene flow among hospital subpopulations of Enterococcus faecium . MBio. 2012;3(4):e00151–e00112. doi: 10.1128/mBio.00151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arthur M, Molinas C, Depardieu F, Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. Journal of Bacteriology. 1993;175(1):117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freitas AR, Novais C, Tedim AP, et al. Microevolutionary events involving narrow host plasmids influences local fixation of vancomycin-resistance in Enterococcus populations. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0060589.e60589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freitas AR, Coque TM, Novais C, et al. Human and swine hosts share vancomycin-resistant Enterococcus faecium CC17 and CC5 and Enterococcus faecalis CC2 clonal clusters harboring Tn1546 on indistinguishable plasmids. Journal of Clinical Microbiology. 2011;49(3):925–931. doi: 10.1128/JCM.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laverde Gomez JA, van Schaik W, Freitas AR, et al. A multiresistance megaplasmid pLG1 bearing a hylEfm genomic island in hospital Enterococcus faecium isolates. International Journal of Medical Microbiology. 2011;301(2):165–175. doi: 10.1016/j.ijmm.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Rosvoll TCS, Pedersen T, Sletvold H, et al. PCR-based plasmid typing in Enterococcus faecium strains reveals widely distributed pRE25-, pRUM-, pIP501- and pHTβ-related replicons associated with glycopeptide resistance and stabilizing toxin-antitoxin systems. FEMS Immunology and Medical Microbiology. 2010;58(2):254–268. doi: 10.1111/j.1574-695X.2009.00633.x. [DOI] [PubMed] [Google Scholar]
- 11.Sletvold H, Johnsen PJ, Wikmark O-G, Simonsen GS, Sundsfjord A, Nielsen KM. Tn1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. Journal of Antimicrobial Chemotherapy. 2010;65(9):1894–1906. doi: 10.1093/jac/dkq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita H, Pierson C, Lim SK, Clewell DB, Ike Y. Possible connection between a widely disseminated conjugative gentamicin resistance (pMG1-like) plasmid and the emergence of vancomycin resistance in Enterococcus faecium . Journal of Clinical Microbiology. 2002;40(9):3326–3333. doi: 10.1128/JCM.40.9.3326-3333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu W, Murray PR, Huskins WC, et al. Dissemination of an Enterococcus Inc18-like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy. 2010;54(10):4314–4320. doi: 10.1128/AAC.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu W, Clark NC, McDougal LK, Hageman J, McDonald LC, Patel JB. Vancomycin-resistant Staphylococcus aureus isolates associated with Inc18-like vanA plasmids in Michigan. Antimicrobial Agents and Chemotherapy. 2008;52(2):452–457. doi: 10.1128/AAC.00908-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing (EUCAST) Breakpoint tables for interpretation of MICs and zone diameters. Version 3.0. 2013, http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_Breakpoint_table_v_3.0.pdf.
- 16.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. 20th informational supplement, CLSI document M100-S20. CLSI, Wayne, Pa, USA, 2010.
- 17. Recommandations 2011 du Comité de l'antibiogramme de la SFM. Société Française de Microbiologie (SFM). Paris, France.
- 18.Handwerger S, Skoble J, Discotto LF, Pucci MJ. Heterogeneity of the vanA gene cluster in clinical isolates of enterococci from the northeastern United States. Antimicrobial Agents and Chemotherapy. 1995;39(2):362–368. doi: 10.1128/aac.39.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lencastre H, Severina EP, Roberts RB, et al. Testing the efficacy of a molecular surveillance network: methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) genotypes in six hospitals in the metropolitan New York City area. Microbial Drug Resistance. 1996;2(3):343–351. doi: 10.1089/mdr.1996.2.343. [DOI] [PubMed] [Google Scholar]
- 20.Clark NC, Cooksey RC, Hill BC, Swenson JM, Tenover FC. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrobial Agents and Chemotherapy. 1993;37(11):2311–2317. doi: 10.1128/aac.37.11.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed- field gel electrophoresis: criteria for bacterial strain typing. Journal of Clinical Microbiology. 1995;33(9):2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Top J, Schouls LM, Bonten MJM, Willems RJL. Multiple-locus variable-number tandem repeat analysis, a novel typing scheme to study the genetic relatedness and epidemiology of Enterococcus faecium isolates. Journal of Clinical Microbiology. 2004;42(10):4503–4511. doi: 10.1128/JCM.42.10.4503-4511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz-Garbajosa P, Bonten MJM, Robinson DA, et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. Journal of Clinical Microbiology. 2006;44(6):2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner G, Fleige C, Geringer U, van Schaik W, Klare I, Witte W. IS element IS16 as a molecular screening tool to identify hospital-associated strains of Enterococcus faecium . BMC Infectious Diseases. 2011;11, article 80 doi: 10.1186/1471-2334-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vankerckhoven V, van Autgaerden T, Vael C, et al. Development of a multiplex PCR for the detection of asaI, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among european hospital isolates of Enterococcus faecium . Journal of Clinical Microbiology. 2004;42(10):4473–4479. doi: 10.1128/JCM.42.10.4473-4479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galloway-Peña JR, Nallapareddy SR, Arias CA, Eliopoulos GM, Murray BE. Analysis of clonality and antibiotic resistance among early clinical isolates of Enterococcus faecium in the United States. Journal of Infectious Diseases. 2009;200(10):1566–1573. doi: 10.1086/644790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widdowson CA, Adrian PV, Klugman KP. Acquisition of chloramphenicol resistance by the linearization and integration of the entire staphylococcal plasmid pC194 into the chromosome of Streptococcus pneumoniae . Antimicrobial Agents and Chemotherapy. 2000;44(2):393–395. doi: 10.1128/aac.44.2.393-395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrobial Agents and Chemotherapy. 1996;40(11):2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty N, Trzcinski K, Pickerill P, Zawadzki P, Dowson CG. Genetic diversity of the tet(M) gene in tetracycline-resistant clonal lineages of Streptococcus pneumoniae . Antimicrobial Agents and Chemotherapy. 2000;44(11):2979–2984. doi: 10.1128/aac.44.11.2979-2984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trzcinski K, Cooper BS, Hryniewicz W, Dowson CG. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus . Journal of Antimicrobial Chemotherapy. 2000;45(6):763–770. doi: 10.1093/jac/45.6.763. [DOI] [PubMed] [Google Scholar]
- 31.Poyart C, Jardy L, Quesne G, Berche P, Trieu-Cuot P. Genetic basis of antibiotic resistance in Streptococcus agalactiae strains isolated in a French hospital. Antimicrobial Agents and Chemotherapy. 2003;47(2):794–797. doi: 10.1128/AAC.47.2.794-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vakulenko SB, Donabedian SM, Voskresenskiy AM, Zervos MJ, Lerner SA, Chow JW. Multiplex PCR for detection of aminoglycoside resistance genes in enterococci. Antimicrobial Agents and Chemotherapy. 2003;47(4):1423–1426. doi: 10.1128/AAC.47.4.1423-1426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabicka D, Strzelecki J, Wozniak A, et al. Efficiency of the Cepheid Xpert vanA/vanB assay for screening of colonization with vancomycin-resistant enterococci during hospital outbreak. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology. 2012;101(3):671–675. doi: 10.1007/s10482-011-9681-z. [DOI] [PubMed] [Google Scholar]
- 34.Jensen LB, Garcia-Migura L, Valenzuela AJS, Løhr M, Hasman H, Aarestrup FM. A classification system for plasmids from enterococci and other Gram-positive bacteria. Journal of Microbiological Methods. 2010;80(1):25–43. doi: 10.1016/j.mimet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Wardal E, Gawryszewska I, Hryniewicz W, Sadowy E. Abundance and diversity of plasmid-associated genes among clinical isolates of Enterococcus faecalis . Plasmid. 2013;70(3):329–342. doi: 10.1016/j.plasmid.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Moritz EM, Hergenrother PJ. Toxin-antitoxin systems are ubiquitous and plasmid-encoded in vancomycin-resistant enterococci. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(1):311–316. doi: 10.1073/pnas.0601168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goicoechea P, Romo M, Coque TM, et al. Identification of enterococcal plasmids by multiplex-PCR-based relaxase typing. Clinical Microbiology and Infection. 2008;14(supplement 7):p. S484. [Google Scholar]
- 38.Sadowy E, Sieńko A, Gawryszewska I, Bojarska A, Malinowska K, Hryniewicz W. High abundance and diversity of antimicrobial resistance determinants among early vancomycin-resistant Enterococcus faecium in Poland. European Journal of Clinical Microbiology and Infectious Diseases. 2013;32(9):1193–1203. doi: 10.1007/s10096-013-1868-y. [DOI] [PubMed] [Google Scholar]
- 39.Barton BM, Harding GP, Zuccarelli AJ. A general method for detecting and sizing large plasmids. Analytical Biochemistry. 1995;226(2):235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 40.Palepou M-FI, Adebiyi A-MA, Tremlett CH, Jensen LB, Woodford N. Molecular analysis of diverse elements mediating VanA glycopeptide resistance in enterococci. Journal of Antimicrobial Chemotherapy. 1998;42(5):605–612. doi: 10.1093/jac/42.5.605. [DOI] [PubMed] [Google Scholar]
- 41.Huh JY, Lee WG, Lee K, Shin WS, Yoo JH. Distribution of insertion sequences associated with Tn1546-like elements among Enterococcus faecium isolates from patients in Korea. Journal of Clinical Microbiology. 2004;42(5):1897–1902. doi: 10.1128/JCM.42.5.1897-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talebi M, Pourshafie MR, Oskouii M, Eshraghi SS. Molecular analysis of vanHAX element in vancomycin resistant enterococci isolated from hospitalized patients in Tehran. Iranian Biomedical Journal. 2008;12(4):223–228. [PubMed] [Google Scholar]
- 43.Yu H-S, Seol S-Y, Cho D-T. Diversity of Tn1546-like elements in vancomycin-resistant enterococci isolated from humans and poultry in Korea. Journal of Clinical Microbiology. 2003;41(6):2641–2643. doi: 10.1128/JCM.41.6.2641-2643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manson JM, Hancock LE, Gilmore MS. Mechanism of chromosomal transfer of Enterococcus faecalis pathogenicity island, capsule, antimicrobial resistance, and other traits. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(27):12269–12274. doi: 10.1073/pnas.1000139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel R. Clinical impact of vancomycin-resistant enterococci. Journal of Antimicrobial Chemotherapy. 2003;51(3):iii13–iii21. doi: 10.1093/jac/dkg272. [DOI] [PubMed] [Google Scholar]
- 46.Valdezate S, Miranda C, Navarro A, et al. Clonal outbreak of ST17 multidrug-resistant Enterococcus faecium harbouring an Inc18-like::Tn1546 plasmid in a haemo-oncology ward of a Spanish hospital. Journal of Antimicrobial Chemotherapy. 2012;67(4):832–836. doi: 10.1093/jac/dkr545. [DOI] [PubMed] [Google Scholar]
- 47.Werner G, Klare I, Fleige C, Witte W. Increasing rates of vancomycin resistance among Enterococcus faecium isolated from German hospitals between 2004 and 2006 are due to wide clonal dissemination of vancomycin-resistant enterococci and horizontal spread of vanA clusters. International Journal of Medical Microbiology. 2008;298(5-6):515–527. doi: 10.1016/j.ijmm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Kawalec M, Kȩdzierska J, Gajda A, et al. Hospital outbreak of vancomycin-resistant enterococci caused by a single clone of Enterococcus raffinosus and several clones of Enterococcus faecium . Clinical Microbiology and Infection. 2007;13(9):893–901. doi: 10.1111/j.1469-0691.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 49.Kawalec M, Gniadkowski M, Hryniewicz W. Outbreak of vancomycin-resistant enterococci in a hospital in Gdansk, Poland, due to horizontal transfer of different Tn1546-like transposon variants and clonal spread of several strains. Journal of Clinical Microbiology. 2000;38(9):3317–3322. doi: 10.1128/jcm.38.9.3317-3322.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sillanpää J, Prakash VP, Nallapareddy SR, Murray BE. Distribution of genes encoding MSCRAMMs and pili in clinical and natural populations of Enterococcus faecium . Journal of Clinical Microbiology. 2009;47(4):896–901. doi: 10.1128/JCM.02283-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novais C, Freitas AR, Sousa JC, Baquero F, Coque TM, Peixe LV. Diversity of Tn1546 and its role in the dissemination of vancomycin-resistant enterococci in Portugal. Antimicrobial Agents and Chemotherapy. 2008;52(3):1001–1008. doi: 10.1128/AAC.00999-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grady R, Hayes F. Axe-Txe, a broad-spectrum proteic toxin-antitoxin system specified by a multidrug-resistant, clinical isolate of Enterococcus faecium . Molecular Microbiology. 2003;47(5):1419–1432. doi: 10.1046/j.1365-2958.2003.03387.x. [DOI] [PubMed] [Google Scholar]
- 53.Lam MM, Seemann T, Tobias NJ, et al. Comparative analysis of the complete genome of an epidemic hospital sequence type 203 clone of vancomycin-resistant Enterococcus faecium . BMC Genomics. 2013;1(14):p. 595. doi: 10.1186/1471-2164-14-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjørkeng E, Rasmussen G, Sundsfjord A, Sjöberg L, Hegstad K, Söderquist B. Clustering of polyclonal VanB-type vancomycin-resistant Enterococcus faecium in a low-endemic area was associated with CC17-genogroup strains harbouring transferable vanB2-Tn5382 and pRUM-like repA containing plasmids with axe-txe plasmid addiction systems. APMIS. 2011;119(4-5):247–258. doi: 10.1111/j.1600-0463.2011.02724.x. [DOI] [PubMed] [Google Scholar]


