Abstract
Glial cell-derived neurotrophic factor (GDNF) has shown beneficial effects in models of Parkinson's disease. The mild results observed in the double-blind clinical trial by intraputamenal infusion of recombinant GDNF proteins warrant a search for alternative delivery methods. In this study, we investigated the function of autologous mesenchymal stem cells (MSCs) expressing GDNF (GDNF-MSCs) for protection against MPTP-induced injury in cynomolgus monkeys. MSCs were obtained from the bone marrow of individual monkeys and gene-modified to express GDNF. Following unilateral engraftment of GDNF-MSCs into the striatum and substantia nigra, the animals were challenged with MPTP to induce a stable systemic Parkinsonian state. The motor functions were spared in the contralateral limbs of monkeys receiving GDNF-MSCs, but not in those receiving MSCs alone. In the striatum of the grafted hemisphere, dopamine levels were higher and dopamine uptake was enhanced. The results suggest that autologous MSCs may be a safe vehicle to deliver GDNF for enhancing nigro-striatum functions.
Parkinson's disease (PD) is a devastating neurodegenerative disease that afflicts more than 1% of the population over 65 years old1. Although treatments have been developed to target certain symptoms of PD, still no clinically approved therapy is available to stop or reverse the disease progression.
Cell therapy holds great promise for treating PD. Two strategies have been contemplated by researchers, which are transplantation of dopaminergic neurons into striatum to replenish dopamine supply, and introducing neurotrophic factors to nourish the remaining neurons to slow down or prevent further degeneration of dopaminergic neurons. Among the tested neurotrophic factors, GDNF has shown the most robust beneficial effect on dopaminergic neurons in rodent and primate PD models2,3,4,5. Several clinical trials have been carried out to test the effect of recombinant GDNF. In two open-label trials, GDNF infused into the putamen through pumps led to significant improvement in the clinical symptoms of patients6,7,8. However, the double-blind trial only revealed mild effect by intraputamenal injection of GDNF9. A careful review of the clinical trials suggest that, in addition to patient selection and the strong placebo effect involved in open-surgery procedures, GDNF delivery might be a possible reason to hamper its beneficial effect10.
Recombinant GDNF cannot cross blood brain barrier. Even after injection into the cerebral ventricle, which is anatomically close to caudate nucleus and putamen, GDNF shows difficulty to reach the target regions11. This property of GDNF indicated that only cells in the immediate vicinity of the needle tip might have been affected in the intraputamenal GDNF trials. To overcome the delivery problems, researchers have attempted alternative means to introduce GDNF. Using lenti- or adeno-viral vectors for intracerebral delivery of GDNF, significant benefits have been observed in primate PD models12,13. However, safety concerns may prevent the direct injection of viral vectors from being widely accepted in clinic. Instead, ex vivo modification of cells as a vehicle to express GDNF may represent a safer method. Svendson and colleagues employed this strategy and tested the effects of human cortical neural progenitor cells (NPCs) expressing GDNF in both rodent and primate PD models14,15. GDNF-hNPCs can increase tyrosine hydroxylase (TH) and VMAT2 expression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated cynomolgus monkeys14. NPCs undoubtedly possess advantages, such as being able to differentiate into neurons which may further integrate into host tissues. Nevertheless, the efficiency for NPCs to differentiate into dopaminergic neurons is generally low and the establishment of synaptic connections with inappropriate neuronal subtypes may not necessarily improve outcome, and sometimes can even lead to undesirable effects16. In addition, although brain is an immune-privileged organ, allografts such as NPCs still elicit immune recognition and inflammatory responses17,18; Inflammation and the resulted oxidative stress normally accompanies the course of PD and are often viewed as a negative factor for outcome19,20.
Mesenchymal stem cells (MSCs) are a cell population residing in various tissues, such as peripheral blood, bone marrow and fat tissue. MSCs can be easily obtained as autologous donor cells and therefore circumvent the immune-recognition problems associated with allografts. MSCs also show immunomodulatory properties and could suppress the inflammatory responses that already exist in many chronic diseases21,22. In addition, MSCs are able to scavenge reactive oxygen species (ROS) and secret various trophic factors23,24,25,26. In light of the above properties, MSCs have been recently introduced into the subventricular zone of PD patients in a phase I clinical trial and no serious adverse effects were observed27. In the present study, we attempted to combine the potentially beneficial functions of MSCs and GDNF and examine whether they can protect against MPTP-induced damage in a cynomolgus PD model.
Results
Characterization of MSCs
Bone marrow mononuclear cells were isolated by gradient centrifugation (Fig. 1A) from the 6 cynomolgus monkeys in the transplantation groups, and plated at a density of 2 × 105 cells/cm2. Proliferating MSC colonies emerged in 2 days (Fig. S2A) and grew as adherent cells with a spindle-like shape (Fig. S2B–D). MSCs were passaged upon 80% confluence (Fig. 1B). As the population doubling level increased, more cells entered a quiescence state, indicated by β-galactosidase activity (Fig. S2E–H). In this study, we only used MSCs below passage number 7 for gene modification and transplantation (Table 2). All the cell lines showed a normal karyotype (Fig. 1C).
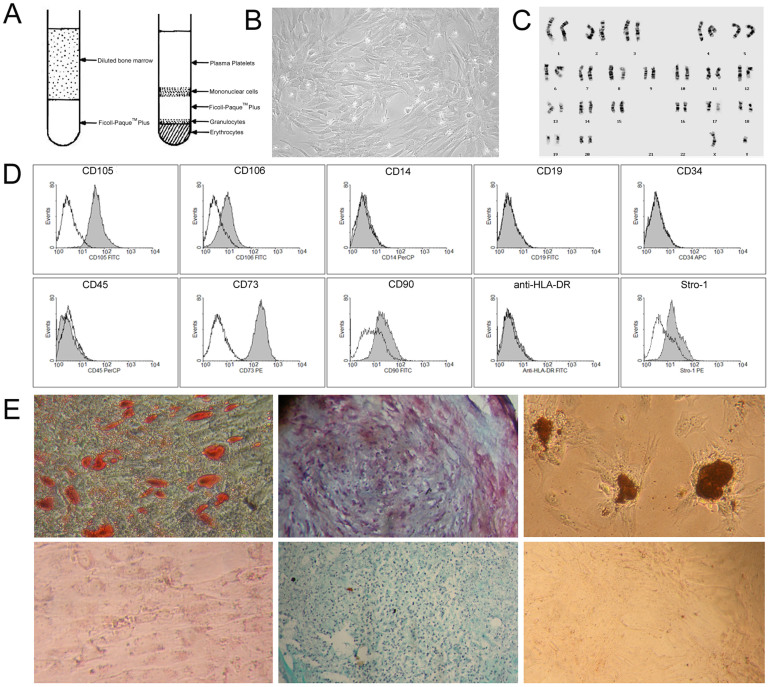
Figure 1. Culture and characterization of bone marrow mesenchymal stem cells.
(A) Schematic diagram of MSC isolation from bone marrow by using Ficoll- Paque™ Plus. (B) Primary culture of MSCs at day 8 (200×). (C) Cynomolgus monkey MSCs showed a normal karyotype of 42, XY. (D) Surface marker expression on MSCs at passage 3 analyzed by flow cytometry. Unfilled lines represent isotype controls. (E) In vitro differentiation of MSCs into adipocytes (Oil O Red staining, upper left), chondrocytes (Safranin O staining, upper middle) and osteocytes (von Kossa Staining, upper right). Staining of undifferentiated MSCs served as controls (lower panels).
Table 1. Animal information.
| Group | Monkey ID | Sex | Date of birth | Age (Year) | Weight (kg) |
|---|---|---|---|---|---|
| 8–10 years old (GDNF+) | M0011201 | male | 2000–11 | 8.1 | 6.71 |
| M0009203 | male | 2000–09 | 8.5 | 7.25 | |
| 3–4 years old (GDNF+) | M0509999 | male | 2005–09 | 3.7 | 2.54 |
| M0510559 | male | 2005–10 | 3.8 | 3.84 | |
| 8–10 years old (GDNF-) | M0009205 | male | 2000–09 | 8.5 | 7.46 |
| M0009209 | male | 2000–09 | 8.5 | 9.85 | |
| 8–10 years old Naive control | M0010204 | male | 2000–10 | 8.5 | 7.89 |
| M0010495 | male | 2000–10 | 8.5 | 8.45 |
Note: GDNF (+) group received GDNF-expressing MSCs followed by MPTP treatment; GDNF (-) group received MSCs alone followed by MPTP treatment; naïve controls received no transplantation or MPTP administration.
Table 2. Detailed information of transplanted cells.
| Monkey ID | Passage No. | Quantity | GDNF (pg/ml.106cells) |
|---|---|---|---|
| M0009205 | Passage 4 | 5.14 × 106 | 464.16 ± 97.79 |
| M0009209 | Passage 4 | 5.05 × 106 | 418.48 ± 93.49 |
| M0510559 | Passage 5 | 6.23 × 106 | 3089.29 ± 250.56 |
| M0509999 | Passage 6 | 5.92 × 106 | 3232.31 ± 437.69 |
| M0009203 | Passage 6 | 4.63 × 106 | 3178.54 ± 383.29 |
| M0011201 | Passage 5 | 4.24 × 106 | 3340.65 ± 305.38 |
Note: Passage No., cell passage number when autologous transplantation was performed; Quantity, the total number of transplanted cells for that monkey; GDNF (pg/ml.106cells), the amount of GDNF released into medium per 1 × 106 cells in 24 h in vitro.
In agreement with previous reports28, MSCs expressed high levels of CD73 and CD105, moderate levels of CD90, CD106 and Stro-1, and did not express the following cell surface markers: CD14, CD19, CD34, CD45 or HLA-DR (Fig. 1D). In addition, MSCs possessed the capacity to differentiate into adipocytes, chondrocytes and osteocytes (Fig. 1E).
Generation of GDNF-secreting mesenchymal stem cells
MSCs were infected with GDNF-encoding lentivirus in the absence of Tenofovir. Previous study shows that up to 100% of captive and free-ranging Asian nonhuman primate over 3 years old are infected with simian foamy viruses (SFV)29 (Table S1). Therefore, the antiviral drug Tenofovir30 was added to the medium to maintain proper culture of MSCs. However, Tenofovir interfered with lentivirus infection and had to be temporally removed from culture before the infection process started; the short period of Tenofovir removal did not cause adverse effect or cell fusion (Fig. S3). Following infection, the MSCs were selected by hygromycine treatment for 1 week (Fig. 2A). Transcription of GDNF in the infected MSCs was confirmed by conventional RT-PCR (Fig. 2B) and quantified by real-time PCR at various MOIs (Fig. 2C). GDNF production at protein levels was examined by using ELISA. More GDNF protein was released into the medium with higher MOI, and reached a plateau at MOI 10 (Fig. 2D). GDNF production was also consistent across different lines. MSCs isolated from the 4 monkeys showed similar levels of GDNF expression at MOI 10 (Fig. 2E). To test the stability of expression, GDNF concentrations in the medium were measured over a period of 40 days. The infected cells could maintain a continuous and stable production of GDNF at least for the period of time tested (Fig. 2F). We also examined whether the infection process affected the cells' ability to differentiate and/or proliferate. After infection, the differentiation capacity did not change, but the proliferation was slightly decreased (Fig. S4A–C).
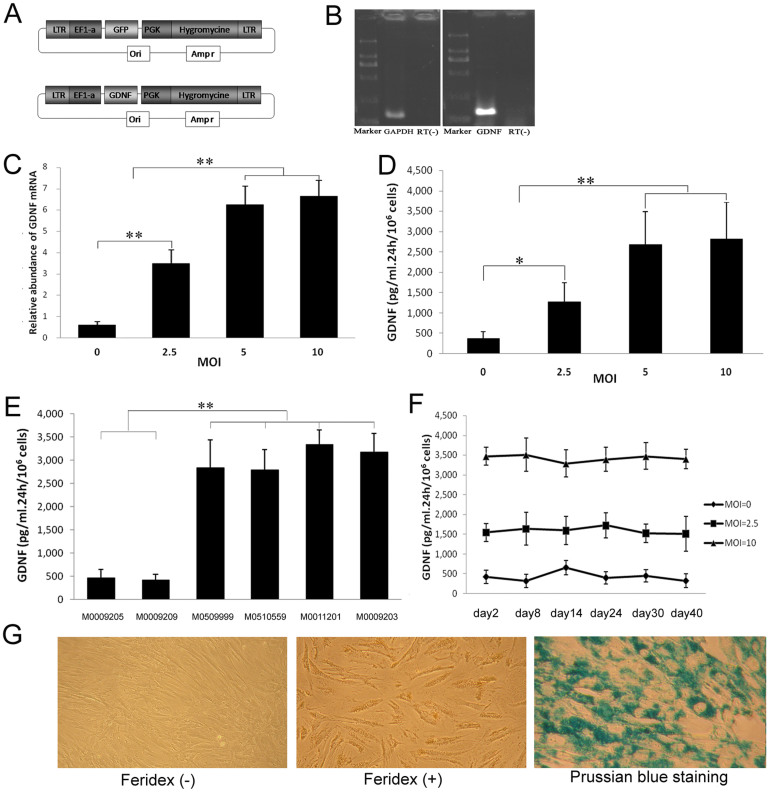
Figure 2. Lentivirus infection and Feridex-labeling of MSCs.
(A) Schematic diagram of vector construction. (B) Expression of GDNF mRNA in MSCs after lentivirus infection. (C) Relative abundance of GDNF mRNA analyzed by real-time PCR in GDNF-MSCs at various MOIs. (D) GDNF secretion analyzed by ELISA in GDNF-MSCs at various MOIs. (E) Compared to the two non-infected MSC lines, GDNF secretion was significantly higher in MSCs infected with lenti-GDNF at MOI 10. (F) Stable expression of GDNF for 40 days. (G) Feridex-labeled MSCs appeared yellowish under bring field microscopy and could be visualized by Prussian blue staining. (C–E) *P < 0.05, **P < 0.01).
For tracking purposes, MSCs were labeled with Feridex, which is a superparamagnetic iron oxide contrast agent approved by FDA for MRI use. After labeling with Feridex, MSCs appeared yellowish under light microscope and exhibited blue in cytoplasm following Prussian blue staining (Fig. 2G). The labeled MSCs reduced GDNF production by around 25% (Fig. S4D) and still showed a normal karyotype (Fig. S4E).
Cell transplantation
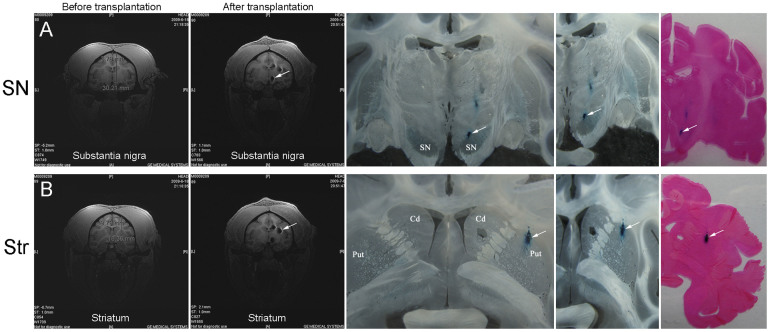
The transplant sites in SN and striatum were determined by MRI (Fig. 3A and B, left panel). Autologous MSCs were injected into 3 sites in caudate nucleus, 3 sites in putamen and 1 site in SN (Table S3). The detailed procedure was described in Methods and the information about the MSCs in Table 2. Two weeks after transplantation, MRI was performed again to confirm the position of transplanted cells in SN and striatum (Fig. 3). On T2-weighted MRI images, the superparamagnetic iron oxide, incorporated by MSCs prior to engraftment, exhibited a dark signal, which was not seen in control brains injected with Feridex (-) cells (data not shown). The imaging results showed that the Feridex-labeled cells had been correctly deposited in the SN (Fig. 3A) and striatum (Fig. 3B). At the end of the experiments (11 weeks after transplantation), the animals were euthanized and brains taken out and sliced at 40 μm thickness. Prussian blue staining further confirmed the correct targeting in SN (Fig. 3A) and striatum (Fig. 3B). The transplanted cells displayed a limited degree of migration and mostly remained in the target sites (Fig. S6A and B). Some migrating cells could be detected spreading out of the edge of the bolus (Fig. S6C). Possibly through the vasculature transport system, a few grafted cells were observed at further distance in the ipsilateral (Fig. S6D) and contralateral side (Fig. S6E and F). We also examined the level of gliosis by staining for GFAP and Iba1, markers for astrocytes and microglia, respectively. GFAP staining showed no significant difference in the left versus right hemisphere. Even in the right hemisphere receiving engraftment, GFAP staining was similar in areas in close proximity to and distant from the injection sites (Fig. S7). Similar phenomenon was observed with regard to Iba1 staining (Fig. S8). The failure to detect activation of astrocytes or microglia may be due to the autologous nature of transplanted cells, the immunosuppressing property of MSCs, and/or the late examination time point (11 weeks after transplantation, when the glia may have come back to the resting state).
Figure 3. Tracking of transplanted cells.
(A) Transplantation into substantia nigra. MRI images, Prussian blue staining, and eosin staining all confirmed that the cells had been correctly targeted at the substantia nigra. (B) Transplantation into striatum. MRI images, Prussian blue staining, and eosin staining confirmed that the cells had been correctly deposited at the striatum. SN, substantia nigra; Str, striatum.
Transplantation of GDNF-expressing MSCs partially spares the motor function of the contralateral limb
Three weeks after transplantation, the animals were dosed with MPTP to elicit parkinsonian symptoms until reaching a PPRS score over 10. The older monkeys 8–9 years of age received 21 injections and 16 ± 2.7 mg MPTP in total (n = 4) to reach a score of 10 – 12. In contrast, the younger ones (3–4 years old) only needed 16–19 injections and 5.5 ± 0.9 mg MPTP (n = 2) to reach a score of 13–14, suggesting that juvenile monkeys are more sensitive to MPTP treatment (Tables S5).
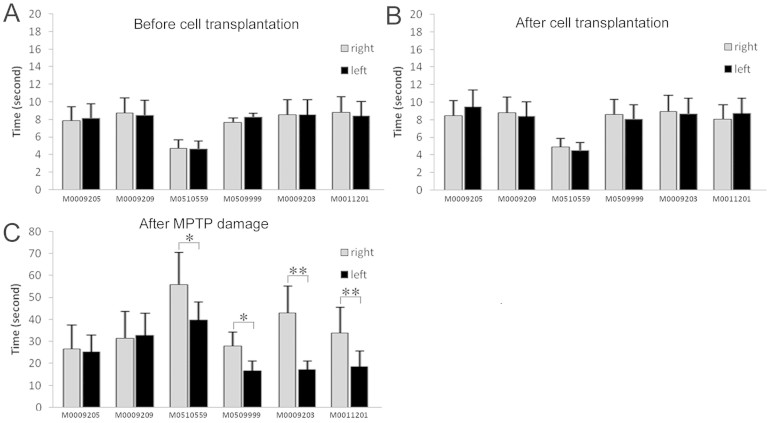
The upper limb motor function was assessed by using an automated movement assessment panel (mMAP) to record the time the monkey spent to take the food through left or right limb. The monkeys normally spent less than 10 seconds to get the food and showed no difference between left and right limb either before (Fig. 4A) or after (Fig. 4B) cell transplantation. After MPTP treatment, there was a 2–6 fold increase in time that monkeys needed to take the food, reminiscent of the slowness feature of PD patients (Fig. 4C). Interestingly, the 4 monkeys that had received GDNF-producing MSCs exhibited differential speeds between left and right limbs, with the left limbs performing faster than the right limbs, suggesting that the GDNF-MSCs engrafted in the right brain hemisphere may have exerted a protective role against MPTP-induced damage.
Figure 4. Motor functions are protected by GDNF-MSCs.
The left and right upper limb motor performance was recorded and the time spent to take the food treat was analyzed. (A) Before cell transplantation; (B) After transplantation but before MPTP treatment; (C) After transplantation and MPTP treatment. No difference was noticed in motor functions after transplantation. However, following MPTP treatment, all the 6 monkeys spent more time to get the food. All the 4 monkeys that had received GDNF-MSCs in the right hemisphere showed faster movement in the left upper limb (C). *P < 0.05, **P < 0.01.
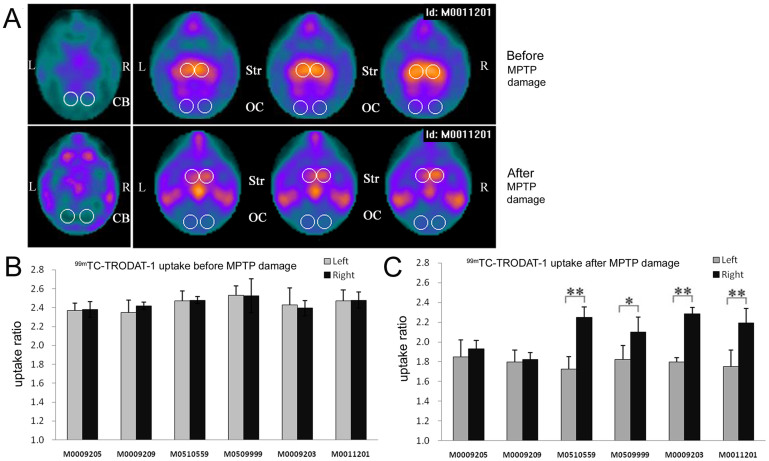
Higher 99mTc-TRODAT-1 uptake in the hemisphere receiving GDNF-MSCs
Dopamine transporter (DAT) proteins are located on presynaptic dopaminergic neuron terminals and are involved in the re-uptake of dopamine released at the synapses31. Degeneration of the projection from SN to striatum results in loss of DATs. We used 99mTc-TRODAT-1, a technetium labeled dopamine transporter ligand, to image the dopaminergic system. SPECT images were captured prior to and following MPTP treatment and analyzed in a semi-quantitative manner (Fig. 5). Before MPTP administration, a high intensity of symmetrical signals was detected in both sides of basal ganglia (Fig. 5A, upper panel). However, after MPTP treatment, 99mTc-TRODAT-1 uptake in striatum was markedly reduced. The ratio of signal intensities at the striatum relative to those at cerebellum and occipital cortex, where no DAT normally exists, was measured and presented in Fig. 5B and C. Animals without MPTP treatment showed similar ratios in left versus right striatum (Fig. 5B). In contrast, MPTP-treated animals presented with reduced uptake at both sides. In the 4 monkeys receiving GDNF-MSCs, the ratio values at the right striatum were significantly greater than those at the left striatum (Fig. 5C).
Figure 5. 99mTc-TRODAT-1 uptake analyzed by SPECT imaging.
(A) Representative SPECT images for one monkey before (upper panels) and after (lower panels) MPTP treatment. The signal intensities in striatum were reduced after MPTP treatment. However, in the MPTP-treated animals, the right striatum showed higher signal intensities compared with the left striatum. The circled areas indicate the regions of interest. CB, cerebellum; OC, occipital cortex; Str, striatum. (B and C) The ratio of signal intensities in striatum relative to those in cerebellum and occipital cortex was measured (Str/[(CB + OC)/2]) before (B) and after (C) MPTP treatment. *P < 0.05, **P < 0.01.
Survival of dopaminergic neurons in substantia nigra is not protected by graft
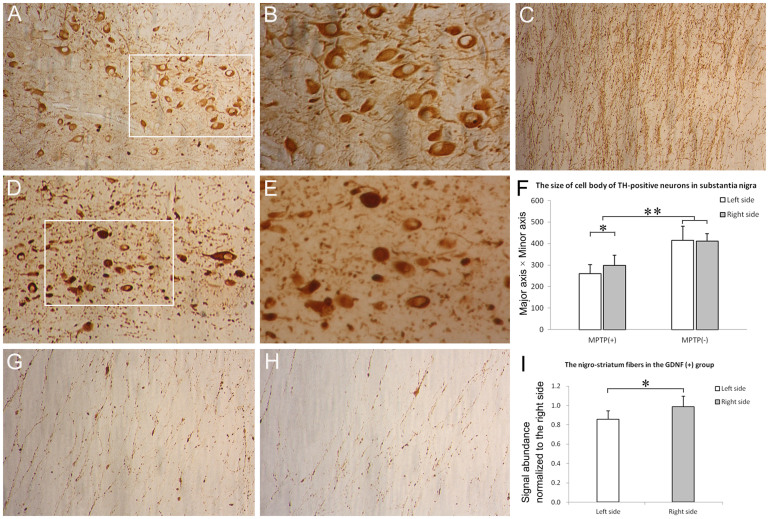
Coronal sections throughout the substantia nigra pars compacta were stained for TH by immunohistochemistry. Compared to naive controls (no engraftment or MPTP, Fig. 6A and B), the number of TH-positive neurons at SN was dramatically reduced in all MPTP-treated monkeys (41.45 ± 8.47%, relative to naïve controls, n = 6, Fig. 6D and E). The number of TH-positive neurons at SN did not differ significantly between GDNF (+) group (44.27 ± 9.36%, n = 4, relative to naïve controls) and GDNF (-) group (43.55 ± 6.34%, n = 2, relative to naïve controls). Moreover, no significant differences was found between the left (43.78 ± 2.64%, n = 4, relative to naïve controls) and right (44.57 ± 7.58%, n = 4, relative to naïve controls) side of SN within GDNF (+) group.
Figure 6. Immunohistochemical analysis of dopaminergic system.
Compared with naive controls (A and B), the number of TH-positive neurons in the substantia nigra pars compacta was dramatically reduced in all MPTP-treated monkeys (D and E). (B) and (E) represent the magnified views of the insets in (A) and (D), respectively. However, no significant difference existed in the number of TH+ neurons in the left versus right half of substantia nigra either in the GDNF (+) or GDNF (-) group after MPTP treatment. The signal abundance of TH immunoreactivity on the nigro-striatum fibers was also reduced after MPTP treatment (C versus G and H). (F) The size of the remaining TH-positive neurons (minor axis equal or more than 10 μm) in substantia nigra was quantified. The neurons were smaller after MPTP treatment and displayed slight but significant difference in size in the right versus left half of substantia nigra. (I) The signal abundance of the nigro-striatum fibers was quantified within the GDNF(+) group. (A), (D) and (C), 200 ×; (B), (E), (G) and (H), 400 ×. *P < 0.05, **P < 0.01.
The result that GDNF-MSCs did not affect the dopaminergic neuron survival at SN was contrary to our expectation. So we next examined whether the sizes of the remaining TH+ neurons were changed by the treatments. We measured the major axis (longest distance across a cell) and minor axis (shortest distance across a cell) and used the value (major axis multiplies minor axis) to reflect the cell size. Following MPTP treatment, many dopaminergic neurons died and disappeared, and some were still undergoing apoptosis by the time of analysis (Fig. 6D and E). Due to the heterogeneity of the remaining cells, we selected those with minor axis equal or more than 10 μm for analysis (Fig. 6F). The average cell size was smaller after MPTP treatment. Interestingly, the dopaminergic neurons were slightly but significantly larger in the right half side of SN receiving GDNF-MSCs than those in the contralateral half side.
We also measured the TH signal abundance on the fibers projecting from SN to striatum. Compared to naive controls (Fig. 6C), the signal abundance was markedly reduced in all MPTP-treated monkeys (Fig. 6G and H). However, within the GDNF (+) group, the TH signal abundance on the fibers in the left hemisphere (Fig. 6H), was modestly but significantly smaller than that in the right hemisphere (Fig. 6G and I), where GDNF-MSCs were received.
We further measured the GDNF amount at the transplantation sites. Tissue samples were dissected out from the SN and striatum areas in the frozen brain slabs and subjected to ELISA analysis. In the monkeys receiving GDNF-MSCs, GDNF expression was significantly higher on the right side in the striatum but not in the SN (Fig. S5).
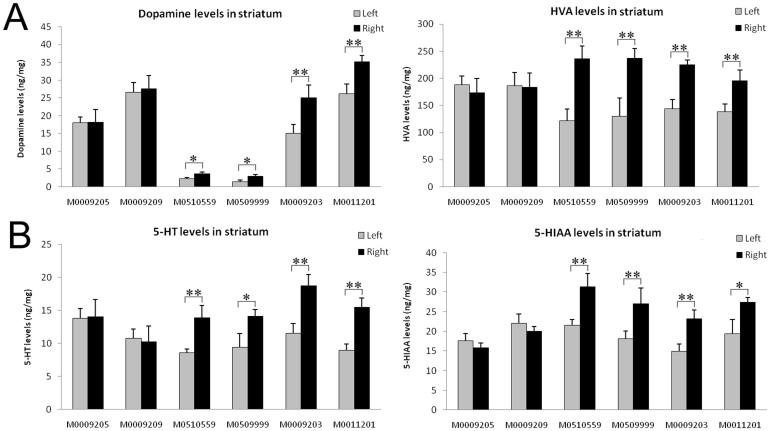
Dopamine levels are higher in the striatum with GDNF-MSC engraftment
Monoamine neurotransmitters and their metabolites were measured by HPLC at the end of the study. Dopamine and its metabolites HVA, as well as 5-HT and its metabolite 5-HIAA were all remarkably reduced in the striatum in all MPTP-treated monkeys compared to those in naive controls (data not shown). In keeping with the enhanced level of GDNF which is known to stimulate dopamine and serotonin synthesis32, the levels of dopamine, HVA, 5-HT and 5-HIAA in the right striatum were significantly higher than those in the left striatum in the 4 monkeys receiving GDNF-MSCs (Fig. 7). We also stained the brain sections for choline acetyltransferase (ChAT), and dopamine receptors D1R and D2R. The numbers of ChAT+, D1R+, or D2R+ neurons in striatum did not differ in the left versus right striatum (data not shown). However, the intensities of ChAT and D1R, but not D2R staining were significantly higher on the positive neurons in the right striatum, as well as on the fibers at the internal capsule on the transplantation side (Fig. S9 and S10), suggesting that GDNF may also exert influences on cholinergic neurons and D1R+ medium spiny neurons in striatum.
Figure 7. Monoamine neurotransmitter levels in striatum.
The levels of monoamine neurotransmitters in striatum were determined by HPLC. (A) The levels of dopamine and its metabolite HVA; (B) 5-HT and its metabolite 5-HIAA. *P < 0.05, **P < 0.01.
Discussion
PD symptoms are presented only after 50–60% of dopaminergic neurons have been lost in the SN33, emphasizing the importance of early diagnosis and early intervention. In the current study, we tested the idea of autologous intracerebral engraftment of MSCs expressing GDNF as an early intervention strategy to prevent or slow down the disease progression. A series of MPTP injections were administered in cynomolgus monkeys to induce a progressive loss of dopaminergic neurons. Engraftment of GDNF-MSCs prior to MPTP treatment significantly improved the motor performance of the contralateral limb, enhanced the dopamine uptake through DATs, and increased the dopamine level present in the striatum.
The results suggest that ex vivo modification of culture-expanded MSCs may be a safe and viable means for delivering trophic factors into brain. Eleven weeks after transplantation, no overgrowth of the grafts was observed. The transplants basically remained where they were deposited and only showed a limited degree of migration. The behavioral performance, body weight, blood and CSF parameters of the monkeys were all similar to those prior to transplantation (Figs. 4 and S11 and Tables S6 and S7). Although spontaneous transformation of cynomolgus MSCs can be detected in vitro after extended cell passaging34,35, the cells seemed relatively stable under in vivo conditions. The fact that the endogenous MSCs could live well with simian foamy virus29,36 (most cynomolgus monkeys are positive for SFV but all remain asymptomatic), but required antiviral treatment to prevent cell fusion when cultured in dish, may also reflect this great difference of in vitro versus in vivo environments (Fig. S3A). It is possible that the immune surveillance may decrease the transformation rate of the MSCs after being introduced into the live animals. However, a longer experimental paradigm is required to assess the long-term safety of MSCs for transplantation therapies.
MSCs possess properties such as scavenging ROS, secreting trophic factors, and suppressing inflammatory responses in host, all of which may be beneficial to the remaining dopaminergic neurons in PD patients22,23,24,26. An open-label clinical trial has been carried out in India to examine the safety of MSC transplantation for treating PD27. Seven patients entered the trial and around 22% and 38% improvement from baseline were observed in the “off” and “on” scores of Unified Parkinson's Disease Rating Scale (UPDRS), respectively. However, given the strong placebo effect reported in previous fetal mesencephalon tissue transplantation trials37,38,39, it would be difficult to determine the efficacy of such open-label trials. One advantage of using primate models is that the brain organization and functions are similar to human brain, yet monkeys do not anticipate an improvement from open surgery as human patients normally do. In contrast to the four cynomolgus monkeys which received GDNF-MSCs and displayed alleviated motor deficit in the contralateral limbs, the two control monkeys receiving MSCs alone did not present with obvious improvement in outcome (Fig. 4C). Although our results imply that transplanting MSCs alone may not be an efficacious preventive strategy for PD, using a larger number of animals and various experimental paradigms will be informative in confirming the efficacy of MSCs.
One possible reason that accounts for the mild effect observed in the double-blind GDNF clinical trial is that using pumps to inject recombinant GDNF proteins may not be an optimal delivery method, resulting in limited and uneven diffusion, and possibly inappropriate dosing of the protein9,10. Gene modified MSCs may serve as a more stable and constant supply source for GDNF. In vitro, 1 million MSCs infected at MOI 10 produced about 3 ng/ml GDNF per 24 h, and this production was consistently stable over at least a period of 40 days (Fig. 2F). Given the limited migration capacity, we injected the MSCs at 3 sites in the caudate nucleus, 3 sites in the putamen, and 1 site in the SN (Table S3), in hope of achieving a wider distribution of the cells. Eleven weeks after transplantation, the GDNF concentration in the right striatum was 15–30% higher than that in the contralateral side, but did not show such a difference in the SN (Fig. S5). One interpretation for the discrepancy in striatum versus SN is that multiple injection sites and/or a larger number of cells are needed to provide significant amount of GDNF. That no change in GDNF amount was observed in SN with only one injection site was reminiscent of the situation where only one needle was placed into the putamen in the double-blind trial9. The other interpretation is that, although GDNF-MSCs showed stable production of GDNF in vitro, after being introduced into the live animals, GDNF expression might have been dynamically changing, possibly due to the dying of transplants over time and/or the regulation of the GDNF element by local signals/factors present in the niche. The endpoint measure of GDNF may not reflect what had taken place during the 11-week course following engraftment. Future studies applying an inducible system that can regulate GDNF production in vivo will be helpful in addressing this issue. If the GDNF levels were indeed higher at previous time points, it may be one possible reason accounting for the larger size of TH+ neurons observed in the right half of SN receiving GDNF-MSCs. Or, more likely, the larger size may have resulted from a retrograde transport of GDNF originated from striatum.
In contrast to SN, GDNF levels in the half of striatum receiving GDNF-MSCs were higher than those in the contralateral control side. Accordingly, in the grafted hemisphere, dopamine and its metabolite HVA levels were also higher; dopamine uptake as indicated by SPECT was greater; and the TH-positive fibers connecting SN and striatum were more abundant (Fig. 6). Not surprisingly, the above neurochemical changes were presented with an improved behavioral readout – a better motor performance of the contralateral limb. The results in the present study suggest that GDNF produced in striatum can enhance the functions of nigro-striatum circuit, although the survival of dopaminergic neurons in SN was not spared in the current experimental setting. The finding that preceding transplantation of autologous GDNF-MSCs did not cause obvious motor behavioral alterations by itself (Fig. 4A and B), yet could protect against MPTP-induced damage suggests a viable strategy for early intervention of PD. However, in clinic, many patients diagnosed as PD are already at a late stage, and therefore future study is needed to examine the curative potential of such strategies by implementing engraftment after MPTP challenges. With increasingly improved technologies to achieve targeted gene therapy for sustained and specific transgene expression, this study provides proof-of-principle for a combined stem cell/gene therapy for degenerative diseases such as PD.
Methods
Ethics statement
The animal experiments were performed at Wincon TheraCells Biotechnologies Co. Ltd (Guangxi, China), which is an AAALAC-accredited non human primate research facility. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC). All efforts were made to minimize the number of animals used and to ameliorate any distress.
Subjects
Male cynomolgus monkeys were housed individually with a 12-h light/dark cycle, and received food and water ad libitum.
The animals were divided into three groups, GDNF (+), GDNF (-), and naïve control group. GDNF (+) group included four cynomolgus monkeys (two 8–10 years old and two 3–4 years old), which received autologous MSCs modified to express GDNF, followed by MPTP treatment. GDNF (-) group comprised two monkeys 8–9 years of age that received autologous MSCs only, followed by MPTP treatment. Two 8-year-old cynomolgus monkeys without treatment (engraftment or MPTP) were used as naive controls. The detailed information of the cynomolgus monkeys was shown in Table 1. All monkeys had gone through a 1-month quarantine period before the experiments started and the quarantine report was shown in Table S1. The experimental paradigm was illustrated in Fig. S1.
Mesenchymal stem cell culture and characterization
Bone marrow aspirate (5 ml) of individual cynomolgus monkeys were collected from iliac crest following anesthetization with ketamine (10 mg/kg), as previously described40. The mononuclear cells (MNCs) were isolated by centrifugation using Ficoll-Paque TM Plus (StemCell), and cultured in Alpha-MEM (Life technologies, Grand Island, NY) medium containing 10% MSC Qualified Fetal Bovine Serum (Life technologies), 1% GlutaMAX™-I (Life technologies), 10 μM Tenofovir (LGM Pharmaceuticals), and 1% Penicillin-Streptomycin liquid (Life technologies). The medium was replaced every other day until the cells reached 80% confluence. Adherent MSCs were harvested using 0.25% Trypsin-EDTA (Life technologies), and replated at a concentration of 1 × 104 cells/cm2.
MSCs surface marker expression was examined by flow cytometry. The cells were collected and washed twice with cold PBS, then re-suspended at 4°C in PBS containing 1% bovine serum albumin (BSA, Life technologies), followed by staining with fluorescent antibodies at room temperature for 30 min. Negative cells were gated by the forward and side scatter coordinates with cell debris excluded. Five thousand events were acquired using a FACS Calibur Flow Cytometer (Becton) and analyzed with CellQuest software. The detailed information of fluorescent antibodies used was shown in Table S2.
Cynomolgus monkey MSCs were characterized for their capacity to differentiate into osteogenic, adipogenic, and chondrogenic lineages using the differentiation kit (Lonza Walkersville) as previously described40. For adipogenic differentiation, the cells were incubated in three cycles of Induction/Maintenance medium (3 days each), and then cultured for 7 days in supplemented Adipogenic Maintenance medium, before being fixed and stained with Oil Red O. For chondrogenic differentiation, 2.5 × 105 cells were pelleted at 150 × g followed by incubation in complete chondrogenic induction medium containing 10 ng/ml transforming growth factor-β3 (TGF-β3) for 28 days. At the end of the differentiation, the cells were fixed and stained with Safranin O. For osteogenic differentiation, MSCs were plated at 3.1 × 103 cells/cm2, and incubated in proliferation medium for 24 h. The medium was changed to osteogenic induction medium and cultured for 21 days, before being fixed and stained with Von Kossa stain to assess mineralization.
For karyotype analysis, the adherent MSCs were treated with 20 μg/ml Colcemid (Sigma) at 37°C for 30 min, detached by 0.125% trypsin (Life technologies) and treated with 0.075 M KCl (Sigma). The cells were stained with freshly prepared 4% Giemsa stain in Gurr buffer (both from Life technologies) and examined under light microscope. The karyotype was printed by assigning a number to each sister chromatid pair.
Infection of MSCs with lentivirus encoding GDNF
The lentiviral vectors were obtained as a gift from Dr. Cheng's lab41. Lenti-GDNF was constructed from a base vector DUET101 (lenti-GFP) by replacing GFP with human GDNF gene (NM_199231.2) as shown in Fig. 2A. Lenti-GDNF and lenti-GFP vectors both contain a cassette of hygromycine resistant gene. The lentivirus was packaged in 293T cells transfected with lenti-GDNF and two helper vectors, PMD.G encoding VSV-G and CMV8.91 encoding gag and pol41.
Measurement of GDNF expression
MSCs were infected with GDNF-encoding lentivirus at multiplicity of infection (MOIs) 0, 2.5, 5 and 10, with temporal removal of Tenofovir. To measure GDNF secretion, the medium was collected after 24 h, and the concentration of GDNF analyzed using an ELISA kit (Promega, Madison, WI) according to the manufacturer's instructions.
To measure the GDNF expression by MSCs at RNA levels, total RNA was extracted and reverse transcribed to cDNA using Superscript II reverse transcriptase (Life technology). Conventional PCR was performed in a PTC-100 thermal cycler (Bio-Rad Laboratories) using rTaq polymerase (Takara). Real-time PCR was performed using SYBR green with the DNA Engine Opticon2 real time PCR system (MJ Research). GDNF primers used were: Forward 5′-ACTGACTTGGGTCTGGGCTATG-3′; Reverse 5′-TTTGTCACTCACCAGCCTTCTATTT-3′. GAPDH primers used were: forward 5′-ATGACATCAAGAAGGTGGTG-3′; Reverse 5′-CATACCAGGAAATGAGCTTG-3′. The reaction conditions were as follows. Step I: 10 seconds at 95°C, 1 cycle; step II: 5 seconds at 95°C, 30 seconds at 60°C, 1 second at 79°C, 34 cycles; 60 seconds at 30°C. The expression was normalized to GAPDH mRNA levels.
Feridex labeling of cells
Prior to cell transplantation, MSCs were labeled with contrast agent, Feridex IV (100 μg/ml, Advanced Magnetics Inc.), including 1 μg/ml Ploy-L-Lysine (PLL, Sigma-Aldrich) as facilitator agent. The solutions containing Feridex and PLL (FE-PLL) were added to MSCs medium and incubated for 12 h. The cells that have incorporated Feridex can be visualized by Prussian blue staining in culture. Briefly, MSCs were fixed with 4% paraformaldehyde, washed and incubated with Perls' reagent (20% potassium ferrocyanide and 20% hydrochloric acid) for 20 min at room temperature. After another wash with deionized water, the cells can be observed under light microscopy.
Magnetic resonance imaging and cell transplantation
Magnetic resonance imaging was performed 1 week before and 2 weeks after cell transplantation. Cynomolgus monkeys were pre-medicated with atropine (0.025 mg/kg subcutaneously) and anesthetized by using isoflurane. Animals were then installed in a stereotaxic frame and placed in the Magnetom Vision 1.5 T MR scanner (Siemens, Germany). T2 weighted sequences, previously employed for the detection of Feridex-labeled cells42, were applied to assess MRI sensitivity to Feridex-labeled MSCs.
Before cell transplantation, the cells were collected and re-suspended at a concentration of 100, 000 cells/μl in sterile PBS. The cells remained on ice for the duration of the transplantation procedure. The monkeys received MRI-guided stereotaxic intracerebral injection of Feridex-labeled MSCs, as previously described14. Transplantation was restricted to the caudate nucleus, putamen and substantia nigra (SN) in the right side of brain. The coordinates of transplant sites were shown in Table S3. At each site, 5 μl or 10 μl cell suspension was deposited, and the average number of injected cells was 5.2 × 106 per monkey. The detailed information about transplanted cells was shown in Table 2. The rate of infusion was 1 μl/min and the syringe remained in place for an additional 3 min after injection. At the end of the experiment, the monkeys were sacrificed and the survival of transplanted cells was estimated by counting the number of Prussian blue positive cells (Table S4).
MPTP treatment and behavioral assessment
The method of MPTP treatment has been described previously43,44. Two weeks after autologous cell transplantation, animals were treated with MPTP hydrochloride (Sigma, USA; 0.1 mg/kg) by intravenous injection through the lower limb to elicit parkinsonian symptoms until they reached a score over 10 on Primate Parkinsonian Rating Scale (PPRS). The total dose for individual monkeys ranged from 1.6 to 2.1 mg/kg. The total amount of MPTP administered per monkey was 12.7 ± 5.99 mg (mean ± S.E.). For 8 ~ 10 age group, MPTP dose was 16.31 ± 2.72 mg/monkey; for 3 ~ 4 age group, the dose was 5.48 ± 0.92 mg/monkey. Fluid diet, massage and additional heating were provided as needed. The detailed information of MPTP dosing was shown in Table S5.
At the end of MPTP dosing, animals showed stable parkinsonian symptoms, and exhibited a narrow range of PPRS from 10 to 14 points. The motor functions were evaluated four times per week for two weeks using video recordings analyzed by a third examiner blind to monkey identities. Assessment of inter-rater reliability was 0.95, calculated using the Spearman correlation coefficient. Test-retest variability for PPRS scores (intrasubject day-to-day variability) was 0.80, calculated using the Spearman-Brown reliability coefficient43. We assessed the following symptoms as previously described45: facial expression (0–3), static tremor (0–3), intentional tremor (0–3), body posture (flexion of spine; 0–2), gait (0–3), body bradykinesia (0–4), balance/synergy (0–3), the general level of activity 0–3 for upper limb, the general level of activity 0–3 for lower limb, and defensive reaction (0–2). The minimal score was 0 and the maximum disability score was 29. A score of 10 corresponds to moderate parkinsonian state. The PPRS scores of individual monkeys were shown in Table S5.
The upper limb motor functions were recorded by using an automated monkey movement assessment panel (mMAP) for nonhuman primates46,47. The animals were allowed to retrieve small food objects from a food receptacle and the time spent was recorded as previously described48. Prior to the test, the monkeys were trained twice per week for two consecutive weeks. Before and after cell transplantation, the motor time of upper limb (right and left) was recorded four times per week for one week. Two weeks after MPTP administration, the motor time of upper limb was assessed again, and analyzed by using the software developed by University of Kentucky Medical Center47.
SPECT imaging and data analysis
SPECT imaging was carried out as previously described49. Each of the 6 animals received SPECT scans both before and 6 weeks after MPTP administration. In brief, after overnight fasting, the monkeys were injected with atropine 20 min before anesthetization with ketamine (10 mg/kg, I.M.). The monkeys were placed in a supine position and fixed with a head-holder. Intravenous instillment (0.9% NaCl, 5 ml/kg/hr) into the cephalic vein was used for hydration as well as for ligand administration. Monkeys were gavaged with potassium perchlorate (10 mg/kg) 30 min before a single bolus (I.V.) injection of 110 mCi of 99mTc-TRODAT-1 (40 mCi/1 ml). 3–3.5 hr later, SPECT scan was performed.
The maging was performed using a GE Millennium VG/Discovery VH SPECT, equipped with double-head gamma cameras and ultrahigh resolution fan-beam collimators. The SPECT images were reconstructed using filtered back-projection (Butterworth filter) with a cut-off frequency of 0.8 per cm and an order of 12, and corrected for attenuation using Chang's method. No attempt was made to correct for partial volume effects. The slice thickness and in-plane pixel size was 2.21 mm. If motion was detected, the acquisition was repeated without new injection of TRODAT-1.
Three reconstructed transaxial slices were summed together, with the slice containing the highest signal in the basal ganglia used as the central one. The summed image was analyzed to determine the 99mTc-TRODAT-1 activity in the striatum. The cerebellum and occipital areas were used as non-specific sites devoid of DAT. Four regions of interests were positioned on the summed slice: left and right striatum, cerebellum (CB) and the occipital cortex (OC). The evaluation of TRODAT-1 SPECT images was done in a semi-quantitative manner as previously described50. The specific to non-specific 99mTc-TRODAT-1 binding ratios were calculated by analyzing the pixels in the striatum/cerebellum + occipital areas (Str/[(CB + OC)/2]).
Tissue preparation and histology
Eight weeks after the first injection of MPTP, the monkeys were euthanized using pentobarbital (25 mg/kg, I.V.) and perfused transcardially with precooled saline that contains heparin. The brain was removed and placed in precooled saline in a dish, and slabbed in the coronal plane with a calibrated Lucite brain slice apparatus. For HPLC and ELISA analysis, slabs were stored at −80 degrees before processing. For the histochemical staining, slabs were immersed in 4% paraformaldehyde for 48 h and then cryoprotected by immersion in a graded (10–40%) sucrose/0.1 M PBS (PH 7.2) solution. Two naive animals were used as controls.
The coronal sections through the SN and striatum was cut (40 μm) using a sliding cryotome. Prussian blue staining was used to detect iron within the transplanted cells. Part of tissues were embedded in paraffin and cut (5 μm) using a microtome, and processed for immunohistochemical staining as previously described14. Antibodies used were mouse anti-monkey tyrosine hydroxylase (TH) (1:500, Chemicon), mouse anti-human glial fibrillary acidic protein (GFAP) polyclonal antibody (1:1000, Chemicon), goat anti-human Iba1 polyclonal antibody (1:100, abcam), mouse anti-monkey choline acetyltransferase monoclonal antibody (ChAT) (1:500, abcam), rat anti-human dopamine receptor D1 monoclonal antibody (D1R) (1:200, Sigma-aldrich), rabbit anti-human dopamine receptor D2 (L/S) polyclonal antibody (D2R) (1:1000, Chemicon). Secondary antibodies used were rabbit anti-mouse IgG (1:1000, Jackson), fluorescein (FITC) conjugated donkey anti-mouse IgG (1:1000, Jackson), donkey anti-goat IgG (1:200, santa cruz), goat anti-mouse IgG (1:200, Zymed), goat anti- rabbit Ig G (1:200, Zymed), and goat anti- rat Ig G (1:200, Zymed) conjugated with biotin.
For quantitative analyses of TH-positive neurons, one out of every five serial sections (5 μm) throughout the SN was picked up and counted bilaterally. The number of TH-positive cells was determined by counting at 100 × magnification. The values were expressed as a percentage of TH-positive cell counts relative to those at SN from the naive controls. To estimate transplant survival, the number of Prussian blue signals was counted in every fifth section. For analysis of GFAP, Iba1, ChAT, D1R, D2R staining, the number of positive cells were counted in every fifth brain section. The signal intensities were measured by randomly choosing 10 fields in areas of interest under 400 × magnification on every fifth section, and analyzing the staining intensity of each cell within that field by using Image J. To evaluate cell size, the major and minor axis of TH-positive cells on every fifth section across SN were measured by using Image J. The cells with minor axis equal or more than 10 μm were selected for comparison of size, as indicated by the value of major axis multiplies minor axis. For measurement of signal abundance for TH, ChAT, D1R, and D2R on the fibers projecting from SN to striatum and fibers between caudate nucleus and putman, every fifth section that include the fibers was picked and the area with positive signals analyzed by using Image J.
HPLC analysis
The bilateral striatum and SN were dissected out from a 2-mm thick coronal brain section and weighed. Perchloric acid (0.2 N, 500 μl) (containing 0.062 mM EDTA and 0.01% L-cysteine, pH 3, 4°C) was added to the tube, and the tissue was homogenized and sonicated (ultrasonic for 3 seconds, interval 3 seconds, 20 cycles). After centrifugation (15 min, 14,000 rpm, 4°C), the supernatant was stored at −80°C until analyzed. The concentrations of dopamine and its metabolites HVA, and 5-HT and its metabolite (5-hydroxyindoleacetic acid, 5HIAA) were measured by high-performance liquid chromatography-electrochemical detection (HPLC-ECD) as described51. Briefly, 20 μl of each sample was injected using a HP1050 manual injector, and separation was achieved on Hpersil ODS (C18) reverse-phase column (125 mm × 4.6 mm, 5 μm) (Cole-Parmer, USA). Mobile phase containing 90% of 32 mM citric acid, 0.23 mM 1-octanesulfonic acid sodium salt, 0.13 mM EDTA, 90 mM anhydrous sodium acetate, and 10% of methanol HPLC (adjusted to pH 4.63 with KOH 10 M), was pumped at 0.1 ml/min through the C-18 column connected to the electrochemical detectors (HP1049A, Agilent). Concentrations of the neurotransmitters and its metabolites were measured relative to standard solutions, and data capture was performed using Waters Empower software for HPLC. Each sample was run three times and the mean content was calculated and normalized to the wet weight of tissue, giving a final value of ng/mg of wet tissue. The sample of cerebrospinal fluid (CSF) was examined by the same method.
Statistical analysis
The results were expressed as means ± S.E.M. for all groups. Multiple comparisons among groups were analysed by ANOVA (analysis of variance). Pairwise comparisons were made by Student's t test. A value of P < 0.05 was considered significant.
Author Contributions
Z.C. and Y.A.Z. designed the study, analyzed the data and wrote the paper. Z.R., J.W., S.W., C.Z., X.L. and Y.G. conducted the experiments. All authors reviewed and approved the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CBA01307 and 2011CB965103), National High Technology Research and Development Program of China (2011AA020106), National Natural Science Foundation of China (31070946, 81141014), High Level Talent Fund of the Beijing Healthcare System (2009-2-14), and Research Assistance Fund of Anhui Medical University (Grant No. XJ201008). We thank Dr. Feng Yue (Wincon TheraCells Biotechnologies Co., Ltd.) for technical assistance.
References
- de Rijk M. C. et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54, S21–23 (2000). [PubMed] [Google Scholar]
- Choi-Lundberg D. L. et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 275, 838–841 (1997). [DOI] [PubMed] [Google Scholar]
- Gash D. M. et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380, 252–255 (1996). [DOI] [PubMed] [Google Scholar]
- Tomac A. et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373, 335–339 (1995). [DOI] [PubMed] [Google Scholar]
- Tomac A. et al. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A 92, 8274–8278 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S. et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9, 589–595 (2003). [DOI] [PubMed] [Google Scholar]
- Slevin J. T. et al. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 102, 216–222 (2005). [DOI] [PubMed] [Google Scholar]
- Patel N. K. et al. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 57, 298–302 (2005). [DOI] [PubMed] [Google Scholar]
- Lang A. E. et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59, 459–466 (2006). [DOI] [PubMed] [Google Scholar]
- Sherer T. B., Fiske B. K., Svendsen C. N., Lang A. E. & Langston J. W. Crossroads in GDNF therapy for Parkinson's disease. Mov Disord 21, 136–141 (2006). [DOI] [PubMed] [Google Scholar]
- Nutt J. G. et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60, 69–73 (2003). [DOI] [PubMed] [Google Scholar]
- Kordower J. H. et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290, 767–773 (2000). [DOI] [PubMed] [Google Scholar]
- Eslamboli A. et al. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease. J Neurosci 25, 769–777 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg M. E. et al. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant 17, 383–395 (2008). [PubMed] [Google Scholar]
- Behrstock S. et al. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther 13, 379–388 (2006). [DOI] [PubMed] [Google Scholar]
- Grealish S. et al. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson's disease. Brain 133, 482–495 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. et al. MHC mismatch inhibits neurogenesis and neuron maturation in stem cell allografts. PLoS One 6, e14787, http://dx.doi.org/10.1371/journal.pone.0014787 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. & Palmer T. D. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet 17, R84–92 (2008). [DOI] [PubMed] [Google Scholar]
- Dauer W. & Przedborski S. Parkinson's disease: mechanisms and models. Neuron 39, 889–909 (2003). [DOI] [PubMed] [Google Scholar]
- Foley P. & Riederer P. Influence of neurotoxins and oxidative stress on the onset and progression of Parkinson's disease. J Neurol 247 Suppl 2, II82–94 (2000). [DOI] [PubMed] [Google Scholar]
- Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 5, 485–489 (2003). [DOI] [PubMed] [Google Scholar]
- Keyser K. A., Beagles K. E. & Kiem H. P. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transplant 16, 555–562 (2007). [DOI] [PubMed] [Google Scholar]
- Chen M. F. et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys 66, 244–253 (2006). [DOI] [PubMed] [Google Scholar]
- Valle-Prieto A. & Conget P. A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19, 1885–1893 (2010). [DOI] [PubMed] [Google Scholar]
- Kurozumi K. et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther 11, 96–104 (2005). [DOI] [PubMed] [Google Scholar]
- Cova L. et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res 1311, 12–27 (2010). [DOI] [PubMed] [Google Scholar]
- Venkataramana N. K. et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res 155, 62–70 (2010). [DOI] [PubMed] [Google Scholar]
- Ke H. et al. Derivation, characterization and gene modification of cynomolgus monkey mesenchymal stem cells. Differentiation 77, 256–262 (2009). [DOI] [PubMed] [Google Scholar]
- Jones-Engel L. et al. Sensitive assays for simian foamy viruses reveal a high prevalence of infection in commensal, free-ranging Asian monkeys. J Virol 81, 7330–7337 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Ye F. & Tarantal A. F. Comparison of growth and differentiation of fetal and adult rhesus monkey mesenchymal stem cells. Stem Cells Dev 15, 209–220 (2006). [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol 41, 571–591 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K. D. et al. GDNF induces a dystonia-like state in neonatal rats and stimulates dopamine and serotonin synthesis. Neuron 16, 665–673 (1996). [DOI] [PubMed] [Google Scholar]
- German D. C., Manaye K., Smith W. K., Woodward D. J. & Saper C. B. Midbrain dopaminergic cell loss in Parkinson's disease: computer visualization. Ann Neurol 26, 507–514 (1989). [DOI] [PubMed] [Google Scholar]
- Ren Z. et al. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp Cell Res 317, 2950–2957 (2011). [DOI] [PubMed] [Google Scholar]
- Ren Z., Zhang Y. A. & Chen Z. Spontaneous transformation of cynomolgus mesenchymal stem cells in vitro: further confirmation by short tandem repeat analysis. Exp Cell Res 318, 435–440 (2012). [DOI] [PubMed] [Google Scholar]
- Switzer W. M. et al. Ancient co-speciation of simian foamy viruses and primates. Nature 434, 376–380 (2005). [DOI] [PubMed] [Google Scholar]
- Lindvall O. et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science 247, 574–577 (1990). [DOI] [PubMed] [Google Scholar]
- Olanow C. W. et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 54, 403–414 (2003). [DOI] [PubMed] [Google Scholar]
- Freed C. R. et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 344, 710–719 (2001). [DOI] [PubMed] [Google Scholar]
- Kim B. S. et al. Growth, differentiation, and biochemical signatures of rhesus monkey mesenchymal stem cells. Stem Cells Dev 17, 185–198 (2008). [DOI] [PubMed] [Google Scholar]
- Ye Z., Yu X. & Cheng L. Lentiviral gene transduction of mouse and human stem cells. Methods Mol Biol 430, 243–253 (2008). [DOI] [PubMed] [Google Scholar]
- Kim S. H., Lee W. J., Lim H. K. & Park C. K. SPIO-enhanced MRI findings of well-differentiated hepatocellular carcinomas: correlation with MDCT findings. Korean J Radiol 10, 112–120 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E., Imbert C., Deloire X., Bioulac B. & Gross C. E. A chronic MPTP model reproducing the slow evolution of Parkinson's disease: evolution of motor symptoms in the monkey. Brain Res 766, 107–112 (1997). [DOI] [PubMed] [Google Scholar]
- Stephenson D. T. et al. The effects of a selective dopamine D2 receptor agonist on behavioral and pathological outcome in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated squirrel monkeys. J Pharmacol Exp Ther 314, 1257–1266 (2005). [DOI] [PubMed] [Google Scholar]
- Kurlan R., Kim M. H. & Gash D. M. Oral levodopa dose-response study in MPTP-induced hemiparkinsonian monkeys: assessment with a new rating scale for monkey parkinsonism. Mov Disord 6, 111–118 (1991). [DOI] [PubMed] [Google Scholar]
- Gash D. M. et al. An automated movement assessment panel for upper limb motor functions in rhesus monkeys and humans. J Neurosci Methods 89, 111–117 (1999). [DOI] [PubMed] [Google Scholar]
- Maswood N. et al. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged Rhesus monkeys. Neurobiol Aging 23, 881–889 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang Z. et al. Motor slowing and parkinsonian signs in aging rhesus monkeys mirror human aging. J Gerontol A Biol Sci Med Sci 55, B473–480 (2000). [DOI] [PubMed] [Google Scholar]
- Huang W. S. et al. 99mTc-TRODAT-1 SPECT in healthy and 6-OHDA lesioned parkinsonian monkeys: comparison with 18F-FDOPA PET. Nucl Med Commun 24, 77–83 (2003). [DOI] [PubMed] [Google Scholar]
- Geng Y. et al. Investigating the role of 99mTc-TRODAT-1 SPECT imaging in idiopathic Parkinson's disease. J Zhejiang Univ Sci B 6, 22–27 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C., Coomber B., Gibson C. L. & Young A. M. Effect of pre-ischaemic conditioning on hypoxic depolarization of dopamine efflux in the rat caudate brain slice measured in real-time with fast cyclic voltammetry. Neurochem Int 59, 714–721 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information