Abstract
When asked to choose between immediate versus future gains, individuals tend to fast-track benefits and, congruently, they tend to delay costs. But, despite the ever-increasing importance of the topic, few studies have investigated this behavior among senior citizens. The handful of studies that have been conducted have led to conflicting results and focused on gains as opposed to losses. These conflicting results may in part be due to demographic confounds and the inherent variability that comes with aging. Here, demographic confounds and variability due to aging were minimized by studying three groups: middle-aged, unimpaired-older, and impaired-older adults. Participants were asked to choose between sooner-smaller and later-larger monetary rewards and losses. Results indicated that impaired-older adults discounted the future more than unimpaired-older adults. Interestingly, middle-aged adults discounted future gains at a similar rate to impaired-older adults, but discounted future losses less than impaired-older adults (and similarly to unimpaired-older adults). This may suggest that unimpaired-older adults have developed a compensatory mechanism that leads to more cautious, patient choices. We discuss these results in the context of the neurobiology and neuropsychology of aging and decision making.
Keywords: Temporal discounting, aging, prefrontal cortex, reward, loss
Senior citizens are faced with numerous difficult decisions in the face of deteriorating cognitive ability. This includes choices about retirement savings, long-term care, dietary habits, and medical treatment. Importantly, many of these decisions involve weighing future outcomes versus proximate desires. In general, people tend to ascribe a lesser subjective value to gains as the delay to receive them increases (Ainslie, 1975) – a preference behavior termed time discounting (Frederick, Loewenstein & O’Donoghue, 2002) or temporal discounting (Kable & Glimcher, 2007). Temporal discounting also occurs in the domain of losses: individuals tend to assign a larger subjective cost to sooner, smaller losses than later, but objectively larger losses (Frederick, Loewenstein & O’Donoghue, 2002). In other words, just as individuals have difficulty in delaying gratification, they would often rather delay negative consequences. Much progress has been made on this research topic in the past decades in younger populations. However, the adult lifespan, and especially older adulthood, has largely been overlooked. In the following section, we will provide a brief overview of temporal discounting in the domains of economics, psychology, and neuroscience. Then we will proceed to discuss the relationship between aging and the prefrontal cortex, which we propose is a critical region involved in temporal discounting. We will then discuss our goals and hypotheses as they pertain to earlier research in older adults and temporal discounting.
Temporal Discounting in Economics, Psychology, and Neuroscience
In younger adults, it has been shown on numerous occasions that humans tend to discount the future (Ainslie & Haslam, 1992; Mischel & Staub, 1965; Thaler, 1981). In fact, greater rates of temporal discounting have been associated with less favorable real world outcomes such as poorer academic outcomes (Mischel, Shoda, & Peake, 1988), gambling, and drug addiction (see Reynolds, 2009, for a review). Importantly, the tendency to discount is prevalent in both the domains of gains and losses (for reviews, see Frederick, Loewenstein, & O’Donoghue, 2002; Loewenstein & Prelec, 1992). However, there is substantial asymmetry in how individuals discount future gains versus future losses. For example, in a seminal study, Thaler (1981) found that individuals’ discount rates for gains are 3 to 10 times higher than for losses. Although later research has shown less dramatic effects, the tendency to discount gains more steeply than losses has remained a robust finding (Loewenstein, 1988; Xu, Liang, Wang, Li, & Jiang, 2009).
More recent research has begun to address the neural processes that underlie temporal discounting using lesion studies and neuroimaging methods, and a number of brain regions have been linked repeatedly to discounting. For example, the striatum has been implicated in reward prediction and time delay, the posterior cingulate in processing larger reward outcomes, and the insula in delaying gratification (Wittmann, Lovero, Lane, & Paulus, 2010). It has also been posited that because the ventral striatum is active during all types of intertemporal choices, that it integrates a value signal (Mohr, Li, & Heekeren, 2010). Subregions of the prefrontal cortex have commonly been linked to temporal discounting as well; however, there has been substantial variability in the interpretation of activation patterns observed.
To elaborate, early functional imaging work characterized the neural processing of intertemporal choices in terms of a hot/cold or dual processing system (McClure, Laibson, Loewenstein, & Cohen, 2004). This line of research has shown that the lateral prefrontal cortex (LPFC) and parietal cortex are active during all intertemporal choices and are hypothesized to relate to cognitive control processes, or the “cool” system. By contrast, the medial orbitofrontal cortex (mOFC), medial prefrontal cortex (MPFC), posterior cingulate cortex, and the striatum seem to be preferentially active when an immediate reward is available and are thought to be associated with greater impulsivity, or the “hot” system (McClure et al., 2004).
It has also been proposed that the LPFC acts as a self-control mechanism. In fact, disruption of neural activity in the LPFC during transcranial magnetic stimulation (TMS) has been shown to decrease “patient” choices in an intertemporal choice task (Figner, Knoch, Johnson, Krosch, Lisanby, Fehr, & Weber, 2010).
In an alternative model, it has been proposed that the ventral striatum, posterior cingulate, and MPFC track the subjective value of rewards during all intertemporal choices, rather than functioning as an impulsive signal (Kable & Glimcher, 2007). This model proposes a single valuation system, rather than a two competing systems (Kable & Glimcher, 2010). In line with with this, Sellitto, Ciaramelli, and di Pellegrino (2010) found that middle-aged patients with stable lesions to the mOFC discounted the future at a greater rate than patients with brain damage in nonfrontal regions or normal controls. If the mOFC was associated with greater impulsivity, as proposed in the dual system models, they should have observed a reduction, rather than increase in discounting. Overall, the finding that damage to the mOFC leads to greater discounting is consistent with the view that the mOFC/ventromedial prefrontal cortex (VMPC) represents and integrates reward and loss signals (Basten, Biele, Heekeren, & Fiebach, 2010).
The modular functional findings are likely secondary to changes in the underlying systems that subserve these interrelated regions. For example, the involvement of dopaminergic, and, to some extent, serotoninergic, frontostriatal loops seems to play a role in discounting. Indeed, increased dopamine via the administration of L-DOPA leads to steeper discounting (Pine, Shiner, Seymour, & Dolan, 2010; but see Kayser, Allen, Navarro-Cebrian, Mitchell, & Fields, 2012 for a different view). Relatedly, dopamine agonists prescribed in Parkinson’s disease have been associated with, on occasion, impulse control behaviors (Voon et al., 2009). There has been evidence that serotonin, too, influences intertemporal choice, such that lower levels of serotonin lead to increased discounting of delayed rewards (Schweighofer et al., 2008). Though there is likely an interactive relationship between these two systems, little is understood how this interaction relates to behavioral performance. It has been hypothesized, though, that serotonin function may mirror dopamine function such that serotonin plays a role in avoiding aversive outcomes, whereas dopamine plays a role in appetitive and activating behaviors through a reward error prediction mechanism (Cools, Nakamura, & Daw, 2011).
Although less thoroughly examined, discounting of future losses seems to engage similar brain regions as discounting of future gains (Bickel, Pitcock, Yi, & Angtuaco, 2009; Xu et al., 2009). Losses engage regions such as the MPFC, striatum, and LPFC in a manner similar to gains. However, some regions, such as the VMPC, striatum, and insula, seem to show even greater activation when making intertemporal choices between losses as compared to gains (Xu et al., 2009). It was suggested that this augmented neural activity is because of increased sensitivity to losses versus gains. This sensitivity may be because of an enhanced emotional bias to avoid losses that places a greater cognitive demand on the brain when assessing losses compared to gains (Xu et al., 2009). The authors suggest this could be one reason, on a neural level, why there is asymmetric discounting of losses versus gains.
The relationship of the prefrontal cortex to intertemporal choice would explain on an evolutionary level why animals that have less developed frontal cortices, such as rodents (see Roesch, Bryden, Cerri, Haney, & Schoenbaum, 2012) and non-human primates (see Woolverton, Myerson, & Green, 2007), discount the future at a substantially greater rate than humans (Mazur, 2001). The prerequisite of developed and functioning prefrontal cortices in order to make more patient intertemporal choices is also consistent with the observations that children discount future rewards at a greater rate than adults (Green, Fry, & Myerson, 1994). Importantly, the prefrontal cortex is one of the last regions of the brain to fully develop (Casey, Giedd, & Thomas, 2000) and one of the first to deteriorate in older age (Jernigan, Archibald, Fennema-Notestine, Garnst, Stout, Bonner, & Hesselink, 2001; Raz et al., 1997; Salat et al., 2004).
Indeed, research has repeatedly shown that the prefrontal cortex (Backman & Farde, 2001; Cabeza, Raz, & Park, 2005; Raz et al., 1997; West, 1996) and its associated neuromodulatory systems (e.g., dopaminergic and serotonergic; see Eppinger, Hämmerer, & Li, 2011, for a review) selectively deteriorate or decline in the elderly. Damage to the prefrontal cortex (Sellitto, Ciaramelli, & di Pellegrino, 2010), increased dopamine (Pine et al., 2010), and decreased serotonin (Schweighofer et al., 2008) have all been implicated in altering intertemporal choice preferences; similarly, activation in the prefrontal cortex has been associated with intertemporal choice (Figner et al., 2010; Kable & Glimcher, 2007; McClure et al., 2004; Wittmann et al., 2010). Despite this, it is not well-understood how temporal discounting changes in old age. The few studies that have been conducted have led to conflicting results. Green and colleagues (1994) found evidence that younger adults discounted the future at a steeper rate than older adults; however, Green, Myerson, Lichtman, Rosen, and Fry (1996) later reported that this effect did not hold and that older adults discounted the future at a similar rate to younger adults when accounting for socioeconomic status. By contrast, Read and Read (2004) found that older adults discounted the future more than younger adults. In part, these discrepant results may be due to lack of demographic and socioeconomic controls. For example, college-aged samples tend to have socioeconomic differences from older adults that can affect results (Green et al., 1996). Moreover, there is considerable variability in how individuals age cognitively and neurologically – while some remain healthy and mentally sharp, others are affected by considerable neuropsychological decline. This variability could easily contribute to discrepant findings in the literature. Taking into account the heterogeneity of the aging process seems considerably important as it can aid in a more comprehensive understanding of age-related effects.
Controlling age-related cognitive and neurological differences can be costly and difficult. Cognitive factors like working memory and IQ can be evaluated using standardized tests and questionnaires, but neuroimaging exams like magnetic resonance imaging (MRI) and positron emission tomography (PET) are cost prohibitive for many purposes. It is therefore of great interest to identify non-imaging means to classify neuropsychological decline. We suggest that the Iowa Gambling Task (IGT; Bechara, 2007; Denburg, Tranel, & Bechara, 2005), which incorporates aspects of learning, ambiguity, uncertainty, reward, and punishment, may be an effective alternative to neuroimaging. The IGT seems like a promising candidate for several reasons. First, prior research has suggested that IGT performance is affected by aging and that some healthy (i.e., those with normal neuropsychological functioning) older adults perform advantageously while others perform disadvantageously on the task (Denburg, Bechara, & Tranel, 2005). Second, impaired performance on the IGT by older adults has been linked to hypometabolism in the medial prefrontal cortex (MPFC) (Denburg & Harshman, 2010), an area of the brain that has been implicated in many decision making tasks including temporal discounting. Third, a study on individuals with cocaine dependence found that higher rates of discounting were negatively associated with performance on the Iowa Gambling Task, indicating functioning on these measures may be related (Monterosso, Ehrman, Napier, O’Brien, & Childress, 2001). Combined, these studies suggest processing deficits in the MPFC and the ability to value future outcomes may be correlated with performance on the IGT.
In the current study, we focus on the fact that there is substantial variability in the healthy aging process and present a study investigating differences between identified IGT impaired- and unimpaired-older adults and middle-aged adults. We do this in the context of neurocognitive theories of aging, assuming a triadic relationship between 1) economic decision making, 2) neurofunctional systems, and 3) aging, consistent with the one proposed by Mohr, Li, and Heekeren (2010). This framework is useful as it emphasizes the idea that the regions of the brain that are most susceptible to the aging process are often the same regions that are linked to decision making. This will help to clarify which aspects of the decision making process are the most susceptible to the effects of aging and further, how to identify those senior citizens with divergent decision biases.
It is our hypothesis that impaired-older adults have undergone a faster rate of neurocognitive decline in the MPFC and related neuromodulatory systems than the unimpaired-older adults. Based on the evidence that individuals with damage to the mOFC, a region of the MPFC on the orbital aspect, show greater rates of discounting and the implication of prefrontal regions, along with related systems, in intertemporal choice, we predict that the impaired-older adults will discount the future more than middle-aged and unimpaired-older adults. In other words, we predict that impaired-older adults will choose sooner-smaller rewards more frequently than middle-aged and unimpaired-older adults. We hypothesize that the unimpaired-older adults have a slowed rate of neurocognitive decline and thus their neurocognitive functioning will largely be intact. Thus, we predict that unimpaired-older adults will not differ from middle-aged adults in their discounting behavior. Lastly, we predict that the results for gains will be congruent with the results for losses. For example, we predict that impaired-older adults will discount future gains (e.g., choose more sooner options) and future losses (e.g., choose more later options) more than either the middle-aged or unimpaired-older adults.
Methods
Participants
Three groups of participants were included in the study: middle-aged adults (n = 13; mean age = 39.2, SD = 8.95 years), impaired-older adults (n = 15; mean age = 80.5, SD = 7.04 years), and unimpaired-older adults (n = 20; mean age = 77.6, SD = 8.10 years). All participants were recruited from a registry compiled at the Department of Neurology, University of Iowa College of Medicine1. Exclusion criteria for the registry included head injury with extended loss of consciousness, stroke, Type 1 diabetes, neurosurgery, seizure disorder, demyelinating disorder, substance abuse, uncontrolled medical condition, vision/hearing loss, psychiatric illness necessitating inpatient treatment, and self-reported depression and/or anxiety exceeding mild severity. Extensive neuropsychological batteries were administered to the three participant groups at an earlier testing session and relevant scores are summarized in Table 1. All participants’ neuropsychological performances were within the normal range for their age and level of educational attainment (Lezak, Howieson, Bigler, & Tranel, 2012). As is typically observed (Bechara, Tranel, & Damasio, 2000), all of the middle-aged adults performed advantageously (i.e., “unimpaired”) on the IGT. All participants were consented in accordance with the University of Iowa’s Institutional Review Board policy, and compensated at a set rate of $15 for completion of this task as well as some additional questionnaires. Participants took an average of one hour to complete the task and questionnaires.
Table 1.
Descriptive statistics for middle-aged, unimpaired-older, and impaired-older adults. A one-way ANOVA indicated that there were no group differences in sex, education, or IQ. There was a group difference on IGT (post-hoc analysis showed a difference between impaired-older adults compared to both unimpaired-older adults and middle-aged adults). There was a group difference in Age such that middle-aged adults were significantly younger than either group of older adults.
| Group | Age (SD) | % Female | Education (SD) |
IQ (SD) | IGT Score (SD) |
|---|---|---|---|---|---|
|
Middle-
Aged |
39.2 (8.95) |
62% | 16.1 (1.89) |
115.5 (111.08) |
50.6 (21.38) |
|
Unimpaired-
Older |
77.6 (8.10) |
45% | 16.5 (3.17) |
121.6 (6.74) |
43.95 (18.47) |
|
Impaired-
Older |
80.5 (7.04) |
60% | 15.4 (2.95) |
115.0 (10.32) |
−36.4 (14.19) |
Procedure
The procedure for this study closely followed the behavioral portion of the intertemporal choice task in Figner et al. (2010). Each participant was tested individually on a computerized version of the task using E-Prime (version 2.0). Participants were instructed to make a series of hypothetical choices between a smaller gain that came sooner versus a larger gain that came later. For instance, participants were asked to choose between $25 Today versus $30 in 2 weeks. They were told that there were no correct answers, and they should choose the option that they preferred most. Participants completed two practice choices in order to familiarize them with the procedure. Participants selected the option they preferred by pushing a button on the keyboard labeled “S” (for sooner) or “L” (for later). After making their selection, an X appeared below the option that the individual chose for 2 seconds followed by 3-second inter-trial interval. The task was then repeated for a second time, with loss trials, in which participants chose between a sooner, smaller loss and a later, larger loss. Order of loss and gain blocks was randomized across subjects.
The possible values of the sooner amount varied between $13 and $48 whereas the possible values of the later amount was 0.5%, 1%, 5%, 10%, 15%, 20%, 25%, 30%, 50%, or 75% greater than the sooner amount (up to 50% for loss trials because of asymmetry in loss aversion compared to gain-seeking behavior). The sooner gain was offered either Today or in 2 weeks while the later gain was offered 2 weeks or 4 week after the sooner reward. There were 36 randomly ordered binary choices for each block (gain and loss) for a total of 72 trials.
Analysis
We conducted several preliminary analyses to investigate predicted trends and patterns, which were followed by a more detailed analysis using generalized estimating equations (GEE; Liang and Zeger, 1986). For our preliminary analyses, we separately fit each participant’s gain and loss data to a hyperbolic curve and calculated a single discounting parameter (k) using and (following Kable & Glimcher, 2007), where SV is the subjective value, A is the objective value, D is the delay, and k is the discount parameter. Thus, a separate discounting parameter was produced for both loss and gain data. For these measures, higher scores indicate steeper discounting. We further calculated a simple tally of each individual’s “sooner” choices, generating an overall proportion for both loss and gain data. For gains, higher values indicate less “patient” choices overall. For losses, higher values indicate more patient choices. Means and standard errors from these analyses are summarized in Table 2. We assessed the correlational relationship between temporal discounting and both age and IGT score within each group separately for k values generated from the hyperbolic model, as well as proportion of sooner choices.
Table 2.
Means and standard errors for discount rate and the estimated proportion of sooner choices for gains and losses. For gains, the same general trend was seen across measures: Unimpaired-older adults had lower discount rates than impaired-older adults or middle-aged adults (smaller numbers indicate less discounting). For losses, impaired-older adults chose the sooner option less often than middle-aged and unimpaired-older adults (note that for the estimated proportion of sooner choices among losses, smaller numbers indicate more discounting).
| Group | Discount Rate – Gains |
Est. Average Proportion of Soon Choices – Gains |
Discount Rate – Losses |
Est. Average Proportion of Soon Choices – Losses |
|---|---|---|---|---|
| Middle-Aged | 0.0078 (0.00132) |
0.47 (0.023) |
0.0021 (0.00061) |
0.82 (0.018) |
|
Unimpaired-
Older |
0.0065 (0.00213) |
0.35 (0.018) |
0.00342 (0.00087) |
0.79 (0.016) |
|
Impaired-
Older |
0.0097 (0.00342) |
0.44 (0.022) |
0.0031 (0.00080) |
0.71 (0.020) |
Our primary data analysis was the GEE technique conducted in SPSS 19.0.0.1 (IBM SPSS, 2010). GEE is an alternative generalized regression method to the generalized linear mixed model (Liang & Zeger, 1986). The GEE technique takes into account repeated measurements, dichotomous choices, and correlations between choices across the task, while concurrently modeling within- and between-subject factors. Thus, GEE is uniquely suited for this dataset. Choice was modeled using a binary logistic distribution. Trials were handled as repeated measurements with group (middle-aged, impaired-older, unimpaired-older adults) as the between subject factor and delay to the sooner (today or two weeks) reward and the delay between rewards (two or four weeks) reward as the within-subject factors.
Results
Gains
There was a wide range of discounting behavior, as is typically reported in the literature. Descriptively, as reported in Table 2, unimpaired-older adults had the lowest discount rates (mean = 0.0065, SE = 0.00213) and the lowest estimated proportion of overall sooner choices. Impaired-older adults had the highest discount rate (mean = 0.0097, SE = 0.00342). Middle-aged adults discount rate fell between the other two groups (median = 0.0077, mean = 0.0078, SE = 0.00132), falling within a range characteristic of young and middle-aged adults in similar studies (range = 0.006-0.010; see Kable & Glimcher, 2007; Kable & Glimcher, 2010; Peters, Miedl, & Buechal, 2012 for comparisons).
Among older adults (collapsed across unimpaired- and impaired-older adults), there was a negative correlation between IGT score and discount rate, k, such that higher scores on the IGT were associated with lower discount rates (ρ = −0.41, p = 0.007). This result held for proportion of sooner choices (ρ = −0.38, p = 0.01). There was not a significant relationship between age and discount rate among older adult participants (ps > 0.07), though the relationship between proportion of sooner choices and age trended in the expected direction (ρ = 0.26, p = 0.07), such that increasing age was correlated with an increasing proportion of sooner choices.
Among middle-aged adults, there was not a significant correlation between IGT and discount rate or any other measures of discounting (ps > 0.46). However, there was a negative correlation between age and discount rate (ρ = −0.50, p = 0.04). Thus, increasing age was associated with lower discount rates. This result held for proportion of sooner choices (ρ = −0.50, p = 0.04).
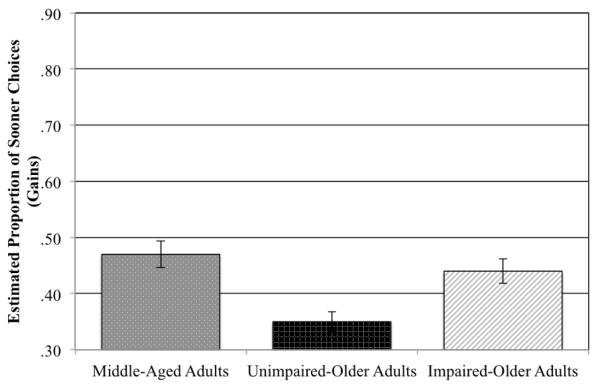
Using the GEE technique, we found a significant effect of the delay to the sooner option (Wald = 4.7, p = 0.03) and delay between options (Wald = 11.2, p = 0.001). As can be seen in Figure 1, middle-aged, impaired-older, and unimpaired-older adults differed in how often they chose the sooner versus later option (Wald = 19.5, p = 0.0006). Consistent with our preliminary analyses, unimpaired-older adults were less likely than either middle-aged (mean difference = 0.12, 95% C.I. [0.06-0.18], p = 0.00005, pairwise comparison) or impaired-older adults (mean difference = 0.09, 95% C.I. [0.04-0.15], p = 0.001, pairwise comparison) to choose the sooner, smaller option. There was no statistically significant difference between impaired-older adults and middle-aged adults, (mean difference = 0.03, 95% C.I. [−0.04-0.09], p = 0.41, pairwise comparison). There were no interactions between delay to the sooner option, delay between options, and group (ps > 0.13).
Figure 1.
Estimated proportion of sooner choices in the gain condition for middle-aged, unimpaired-older, and impaired-older adults. Unimpaired-older adults chose the sooner option significantly less frequently than either middle-aged or impaired-older adults. Middle-aged and impaired-older adults did not differ in the proportion of sooner choices. Error bars indicate standard error.
Losses
It should be noted that choosing the sooner option between a sooner, smaller loss and a later, larger loss is analogous to paying costs up front rather than putting them off – thus the observed relationship utilizing proportion of sooner choices should be in the opposite direction to those of the gains. The discount parameter, however, is still higher for steeper discounting. Descriptively, middle-aged adults chose the sooner option (mean = 0.82, SE = 0.018) more frequently than unimpaired-older (mean = 0.79, SE = 0.016) and impaired-older adults (mean = 0.71, SE = 0.020; Table 2). Middle-aged adults also had the lowest mean discount rate, whereas unimpaired-older adults had the highest mean discount rate.
Among older adults (collapsed across impaired-older and unimpaired-older adults), the proportion of sooner choices was correlated with IGT score such that individual’s with higher scores on the IGT chose a greater proportion of sooner losses (ρ = 0.41, p = 0.007). This relationship trended in the expected direction when utilizing discount rate as the measure of discounting (ρ = −0.25, p = 0.07). There was no relationship between age and the discounting of losses (ps > 0.40).
Among middle-aged adults, there was no relationship between IGT score and proportion of sooner choices (ps > 0.41). By contrast to the gain results, we did not observe a significant relationship among middle-aged adults age and proportion of sooner choices (ps >0.14).
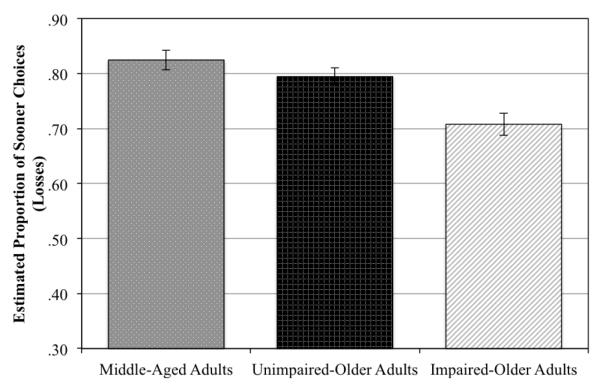
The GEE analysis revealed a significant effect of the delay to the sooner option (Wald = 4.4, p = 0.04). There was not a significant effect of delay between the sooner and later loss (p = 0.90). As can be seen in Figure 2, middle-aged, impaired-, and unimpaired-older adults differed in how often they chose the sooner versus later option, (Wald = 20.5, p = 0.0003; Figure 2). By contrast to the discounting of future gains, impaired-older adults differed from both unimpaired-older (mean difference = 0.09, 95% C.I. [0.04-0.14], p = 0.0002, pairwise comparison) and middle-aged adults (mean difference = 0.11, 95% C.I. [0.06-0.17], p = 0.00004, pairwise comparison). Both unimpaired-older adults and middle-aged adults were more likely to choose the sooner option than impaired-older adults. Unimpaired-older adults and middle-aged adults did not differ (mean difference = 0.02, 95% C.I. [−0.03-0.07], p = 0.21). There were no significant interactions (ps > 0.13).
Figure 2.
Estimated proportion of sooner choices in the loss condition for middle-aged, unimpaired-older, and impaired-older adults. Impaired-older adults chose the sooner option significantly less frequently than either middle-aged or unimpaired-older adults. Middle-aged and unimpaired-older adults did not differ in the proportion of sooner choices. Error bars indicate standard error.
Relationship between the discounting of gains versus losses
We next sought out to investigate the relationship between temporal discounting in gains and losses. Older adults who had greater rates of discounting in the gains condition were also considerably more likely to have greater rates of discounting in the loss condition (i.e., delaying costs; ρ = 0.44, p = 0.004). This result held for proportion of sooner choices (ρ = −0.41, p = 0.008). Middle-aged adults showed the same tendency (ρ = 0.55, p = 0.03); this held utilizing proportion of sooner choices (ρ = −0.48, p = 0.05). Note that proportion of sooner choices was always used as the measure for both gain and loss choices.
Discussion
Optimal decision making involves at least occasionally delaying gratification and bearing smaller misfortunes that come sooner rather than allowing for larger, later losses. The types of choices individual’s make on a daily basis often involve this trade-off. This includes decisions about finances, health, and personal affairs, all of which become increasingly important in older age. Here, we demonstrate that previously characterized aging trajectories (i.e., strong versus weak decisional capacity on the IGT) are associated with distinct changes in decision biases in intertemporal choice compared to middle-aged individuals.
In the domain of gains, our results indicated that increasing age is associated with reduced discounting of future rewards among middle-aged adults and that increasing scores on the IGT among older adults are associated with reduced discounting of future rewards. More fine-grained analysis indicated that middle-aged adults, impaired-older adults, and unimpaired-older adults differed in how they discounted future rewards. Middle-aged adults and impaired-older adults tended to choose the sooner, smaller option more frequently than unimpaired-older adults. In other words, the unimpaired-older adults seemed to be the most patient. Based on these findings, it appears that increasing age affects temporal discounting behavior to a certain point. After that point, in older adulthood, discounting appears to be related to strong versus weak decision making abilities.
To elaborate, one might expect the negative association between age and discounting among middle-aged adults to continue into older age. Indeed, it seems that some older adults, the unimpaired-older adults, tend to discount less than middle-aged adults. But the impaired-older adults do not differ from middle-aged adults, suggesting the trend of reduced discounting with advanced age does not continue in this particular group. This provides further evidence that healthy aging leads to heterogeneous changes in preferences and that temporal discounting may be a weakened component process in more complex decision processing leading to divergent decision biases in some older adults relative to their peers.
Additionally, we demonstrated that middle-aged, unimpaired-older, and impaired-older adults differed in how they discounted future losses. Impaired-older adults were more likely to choose a larger future loss than unimpaired-older adults, which, like the gain results, suggests impaired-older adults discount the future more than unimpaired-older adults. However, by contrast to our gain findings, middle-aged adults were less likely to discount future losses and more frequently chose the sooner, smaller loss over the future, large loss, akin to the unimpaired-older adults. This seems to be congruent with the finding that age was not associated with the discounting of future losses, as it was with gains.
The finding that unimpaired-older adults discount future gains less than either middle-aged adults or impaired-older adults is not entirely consistent with our initial predictions. While we predicted that unimpaired-older adults would discount the future less than impaired-older adults, we did not predict that they would also be more patient than middle-aged adults. By contrast, our original predictions were more accurate in the loss domain: impaired-older adults discounted future losses more so than either middle-aged or unimpaired-older adults. This pattern of behaviors may be secondary to asymmetric declines in dopaminergic and serotonergic systems in the unimpaired-older adults relative to the impaired-older adults. To elaborate, it has been proposed that dopamine and serotonin play mirroring roles in brain function, where dopamine promotes appetitive/activation and serotonin promotes avoidance/aversion (Cools, Nakamura, & Daws, 2011). If the unimpaired-older adults have a relatively greater decrease in dopaminergic function compared to serotonergic function, they may discount future gains less than either middle-aged or impaired-older adults, though discounting future losses less than only impaired-older adults. If, by contrast, the impaired-older adults have a relatively greater decrease in serotonergic function relative to dopaminergic function they may discount gains and losses at a higher rate. Though overly simplified with regard to possible interactions between the two systems, the idea that differential changes in either of these two systems leads to divergent behavioral changes is both consistent with our findings and promising for future research in identifying interventions specific to individual deficits. It is also consistent with the findings that lower levels of serotonin are associated with higher discount rates (Schweighofer et al., 2008) and higher levels of dopamine are associated with steeper discounting (Pine et al., 2010).
Moving beyond neurobiological explanations, the finding that unimpaired-older adults discount gains less than either middle-aged or impaired-older adults, while impaired-older adults discount losses more than either middle-aged or unimpaired-older adults is intriguing in relation to aging and positivity biases (Carstensen, Isaacowitz, & Charles, 1999). The hypothesis that advanced age is associated with a positivity bias is part of a larger framework that describes aging in the context of socioemotional selectivity (Carstensen et al., 1999). More specifically, Carstensen and colleagues (1999) posit that the perception of time on a large scale influences how individuals prioritize their goals. Relatedly, it has been suggested that the subjective perception of time is associated with choice preferences in intertemporal choices (Kim & Zauberman, 2009; Wittmann & Paulus, 2009).
In respect to aging, Carstensen and colleagues (1999) further explicate that as individuals advance in age, time horizons become shorter (i.e., there is less time remaining in their lives), and thus these aged adults prioritize social and emotional goals over knowledge-seeking goals. This, in turn, can shape decision biases. For example, it has been suggested that older adults show reduced neural processing of loss information relative to gain information as compared to their younger counterparts (Samanez-Larkin, Gibbs, Khanna, Nielsen, Carstensen, & Knutson, 2007). Moreover, older adults are more likely to show impairments on versions of the IGT that emphasize choosing lower immediate reward for lower delayed punishment, as opposed to versions that require choosing higher immediate punishment for higher delayed reward (Bauer, Timpe, Edmonds, Bechara, Tranel, & Denburg, 2012). The finding that age-related changes in how individuals respond to differentially valenced rewards (e.g., gains versus losses) is also consistent with the recent finding that increased age leads to a reduction in risky choices that involve gains, but increased age does not lead to a change in risky choices that involve losses (Weller, Levin, & Denburg, 2011). Importantly, it seems likely that emotional aspects of these choices (involving the valence) play a role in how aging affects biases toward sooner versus later rewards and losses.
Likewise, Loeckenhoff, O’Donoghue, and Dunning (2011) found that older age was associated with reduced discounting of future rewards, but not associated with reduced discounting of losses. They interpret in terms of positivity bias, proposing that older age is associated with better emotion regulation leading to more patient choices (Loeckenhoff et al., 2011). In the case of the current study, it may be that impaired-older adults are experiencing changing time horizons relative to unimpaired-older adults. These changes in time horizons and emotional goals may be secondary to changes in structural or functional changes in the brain. For example, unimpaired-older adults may be utilizing increases in emotion regulation, and impaired-older adults may be showing increases in the processing of emotionally salient positive information. This possibility would be interesting to test more directly in future research, in conjunction with structural and functional imaging to elucidate mediating brain changes.
Our results may resolve some of the conflicting findings on temporal discounting in older adults. While Green et al. (1994) found that older adults discounted less, Read et al. (2004) found the opposite. Here, we propose that advancing age indeed leads to changes in discounting, but these changes are heterogeneous and may be best explained by functional differences in decisional capacity. These differences in decision making ability are likely associated with structural and functional changes in the brain. For example, healthy aging is associated with changes in the prefrontal cortex (Backman & Farde, 2001; Cabeza, Raz, & Park, 2005; Raz et al., 1997; West, 1996), a critical region for the valuation of rewards that has been repeatedly linked to temporal discounting. Perhaps we are observing behavioral changes in the impaired-older adults that are a result of speeded dysfunction in the prefrontal cortex relative to their peers. This is partly supported by a recent finding the ventral striatum is active when choosing both sooner and later options in older adults, but not in young adults (Samanez-Larkin, Mata, Radu, Ballard, Carstensen, & McClure, 2011). By contrast, Eppinger, Nystrom, and Cohen (2012) report reduced activity in striatal regions among older relative to younger adults. Although neither group observed differences in the prefrontal cortex, the ventral striatum is intricately connected to the prefrontal cortex, and dysfunctional activity in the ventral striatum may lead to dysfunction in the prefrontal cortex. Future research should explore further the neurofunctional and neurostructural correlates of temporal discounting across the lifespan.
Studying age-related changes in cognitive functions is often a challenging task. Researchers often use college-aged samples to serve as comparisons to the elderly because they are convenient. However, we would argue that college-aged samples are often not the best comparison to the elderly when investigating how cognitive processes change across the lifespan. College-aged samples tend to have socioeconomic differences from older adults that can confound results (Green et al., 1996). Additionally, older adults are often treated as a homogenous group. But even among the healthiest elderly, there are substantial differences in how individuals age, particularly at the neurocognitive level. In fact, taking into account the heterogeneity of the aging process seems considerably important as it can aid in the identification of declining individuals early in cognitive regression. Thus, in the current study we were able to avoid many of the commonly seen limitations.
Of course, the current study is not without limitations. It should be noted that we used hypothetical rewards rather than real payments. Although some research suggests hypothetical rewards produce similar results to incentive compatible, real payments (Bickel et al., 2009), the results of this study should be replicated using real monetary outcomes. Second, this study utilized a cross-sectional design, and thus we cannot make definitive claims about the causal effects of age versus cohort effects on temporal discounting. Future research should follow cohorts longitudinally to better understand the effects of age versus cohort. However, we would argue that regardless of whether the effect is due to age or this particular cohort, it is imperative to our understanding of this group’s economic preferences. Lastly, future research should directly address the functional and structural correlates of temporal discounting across the lifespan. Though we based our hypotheses largely on neural underpinnings of temporal discounting and how they interact with age-related neural decline, we did not directly test these claims using functional or structural neuroimaging.
Footnotes
Participants included in this study had no statistically significant differences in demographics (age, sex, or education) or in cognitive status (IQ, IGT) from the participants in the larger registry (all ps > 0.23).
References
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Ainslie G, Haslam N. Hyperbolic discounting. In: Loewenstein George, Elster Jon., editors. Choice over Time. Russell Sage; NY: 1992. pp. 57–92. [Google Scholar]
- Backman L, Farde L. The role of dopamine systems in cognitive aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford University Press; New York: 2005. pp. 58–84. [Google Scholar]
- Basten U, Guido B, Heekeren HR, Fiebach CJ. How the brain integrates costs and benefits during decision making. Proceedings of the National Academy of Sciences. 2010;14:21767–21772. doi: 10.1073/pnas.0908104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AS, Timpe JC, Edmonds EC, Bechara A, Tranel D, Denburg NL. Myopia for the future or hypersensitivity to reward? Age-related changes in decision making on the Iowa Gambling Task. Emotion. 2012;13:19–24. doi: 10.1037/a0029970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Iowa Gambling Task professional manual. Psychological Assessment Resources; Lutz, FL: 2007. [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain: A Journal of Neurology. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJC. Congruence of BOLD response across intertemporal choice conditions: Fictive and real money gains and losses. The Journal of Neuroscience. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, Park DC. Cognitive neuroscience of aging: Emergence of a new discipline. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford University Press; New York: 2005. pp. 3–15. [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, Huettel SA. Functional neuroimaging of intertemporal choice models: A review. Journal of Neuroscience, Psychology, and Economics. 2010;3:27–45. [Google Scholar]
- Casey BJ, Giedd J, Thomas K. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: Unifying affective, activational, and decisional functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Harshman L. Cerebrum 2010: Emerging Ideas in Brain Science. Dana Press; New York: 2010. Why so many seniors get swindled: Brain anomalies and poor decision-making in older adults; pp. 123–131. [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Hammerer D, Li S-C. Neuromodulation of reward-based learning and decision making in human aging. Annals of the New York Academy of Sciences. 2011;1235:1–17. doi: 10.1111/j.1749-6632.2011.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS ONE. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nature Neuroscience. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, O’Donoghue T. Time discounting and time preference: A critical review. Journal of Economic Literature. 2002;40:351–401. [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: The role of age and income. Psychology and Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Garnst, Stout AC, Bonner J, Hesselink JR. Effects of age on tissues and regions of cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The as soon as possible effect in human intertemporal decision making: Behavioral evidence and neural mechanisms. Journal of Neurophysiology. 2010;103:2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. The Journal of Neuroscience. 2012;32:9402–9409. doi: 10.1523/JNEUROSCI.1180-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Zauberman G. Perception of anticipatory time in temporal discounting. Journal of Neuroscience, Psychology, and Economics. 2009;2:91–101. [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th ed. Oxford University Press; New York, New York: 2012. [Google Scholar]
- Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Loeckenhoff CE, O’Donoghue T, Dunning D. Age differences in temporal discounting: The role of dispositional affect and anticipated emotions. Psychology and Aging. 2011;26:274–284. doi: 10.1037/a0023280. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF. Frames of mind in intertemporal choice. Management Science. 1988;34:200–214. [Google Scholar]
- Loewentstein G, Prelec D. Anomalies in intertemporal choice: Evidence and interpretation. The Quarterly Journal of Economics. 1992;107:573–597. [Google Scholar]
- Mazur JE. Hyperbolic value addition and general models of animal choice. Psychological Review. 2001;108:96–112. doi: 10.1037/0033-295x.108.1.96. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. Journal of Personality and Social Psychology. 1988;54:687–699. doi: 10.1037//0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- Mischel W, Staub E. Effects of expectancy on working and waiting for larger rewards. Journal of Personality and Social Psychology. 1965;2:625–633. doi: 10.1037/h0022677. [DOI] [PubMed] [Google Scholar]
- Mohr PNC, Li S-C, Heekeren HR. Neuroeconomics and aging: Neuromodulation of economic decision making in old age. Neuroscience and Biobehavioral Reviews. 2010;34:678–688. doi: 10.1016/j.neubiorev.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: Do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Peters J, Miedle SF, Buechel C. Formal comparison of dual-parameter temporal discounting models in controls and pathological gamblers. PLoS ONE. 2012;7:e47225. doi: 10.1371/journal.pone.0047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. Journal of Neuroscience. 2010;30:8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in Vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:1047–3211. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Read D, Read NL. Time discounting over the lifespan. Organizational Behavior and Human Decision Processes. 2004;94:22–32. [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behavioral Pharmacology. 2009;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Bryden DW, Cerri DH, Haney ZR, Schoenbaum G. Willingness to wait and altered encoding of time-discounted reward in the orbitofrontal cortex with normal aging. The Journal of Neuroscience. 2012;32:5525–5533. doi: 10.1523/JNEUROSCI.0586-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielson L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Mata R, Radu PT, Ballard IC, Carstensen LL, McClure SM. Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in Neuroscience. 2011;5:126. doi: 10.3389/fnins.2011.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto M, Ciaramelli E, di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. The Journal of Neuroscience. 2010;30:16429–16436. doi: 10.1523/JNEUROSCI.2516-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, Doya K. Low-serotonin levels increase delayed reward discounting in humans. Journal of Neuroscience. 2008;28:4528–4532. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler RH. Some empirical evidence on dynamic inconsistency. Economic Letters. 1981;8:201–207. [Google Scholar]
- Voon V, Reynolds B, Bresing C, Gallea C, Skaljic M, Ekanayake V, Fernandez H, Potenza MN, Dolan RJ, Hallett M. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology. 2009;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Denburg NL. Trajectory of risky decision making for potential gains and losses from ages 5 to 85. Journal of Behavioral Decision Making. 2011;24:331–344. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Lovero KL, Lane SD, Paulus MP. Now or later? Striatum and insula activation to immediate versus delayed rewards. Journal of Neuroscience, Psychology, and Economics. 2010;3:15–26. doi: 10.1037/a0017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Temporal horizons in decision making. Journal of Neuroscience, Psychology, and Economics. 2009;2:1–11. [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by Rhesus monkeys. Experimental and Clinical Psychopharmacology. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liang Z, Wang K, Li S, Jiang T. Neural mechanism of intertemporal choice: From discounting future gains to future losses. Brain Research. 2009;1261:65–74. doi: 10.1016/j.brainres.2008.12.061. [DOI] [PubMed] [Google Scholar]