Abstract
A rat model of bladder reflex contraction (BRC) was used to determine the optimal frequency and intensity of spinal nerve (SN) stimulation to produce neuromodulation of bladder activity and to assess the therapeutic mechanisms of this neuromodulation. In anesthetized female rats (urethane 1.2 g/kg ip), a wire electrode was used to produce bilateral stimulation of the L6 SN. A cannula was placed into the bladder via the urethra, and the urethra was ligated to ensure an isovolumetric bladder. Saline infusion induced BRC. Electrical stimulation of the SN produced a frequency- and intensity-dependent attenuation of the frequency of bladder contractions. Ten-herz stimulation produced maximal inhibition; lower and higher stimulation frequency produced less attenuation of BRC. Attenuation of bladder contraction frequency was directly proportional to the current intensity. At 10 Hz, stimulation using motor threshold pulses (Tmot) produced a delayed inhibition of the frequency of bladder contractions to 34 ± 11% of control. Maximal bladder inhibition appeared at 10 min poststimulation. High current intensity at 0.6 mA (∼6 * Tmot) abolished bladder contraction during stimulation, and the inhibition was sustained for 10 min poststimulation (prolonged inhibition). Furthermore, in rats pretreated with capsaicin (125 mg/kg sc), stimulation produced a stronger inhibition of BRC. The inhibitory effects on bladder contraction may be mediated by both afferent and efferent mechanisms. Lower intensities of stimulation may activate large, fast-conducting fibers and actions through the afferent limb of the micturition reflex arc in SN neuromodulation. Higher intensities may additionally act through the efferent limb.
Keywords: electrical stimulation, parameter, urgency, afferent nerve, delayed inhibition
interstim therapy (Medtronic, Inc.), utilizing electrical stimulation of the sacral spinal nerve (SN; S3), is an established treatment modality for patients with urge incontinence, increased frequency, and urinary retention (15). This therapy is referred to as neuromodulation since the electrical stimulation modulates the pathophysiological control of bladder filling and emptying. Although neuromodulation has been in use for over a decade, its effect on the pathophysiological mechanisms responsible for the bladder overactivity has not been investigated in preclinical models. Furthermore, the optimal stimulation parameters to achieve relief of bladder symptoms have not been evaluated.
Several rat models have been utilized to evaluate the effects of neuromodulation of experimentally induced bladder overactivity via electrical stimulation of the SN (38). For instance, neuromodulation reduced the frequency of micturition in rat models of cystitis induced by intravesical administration of either hydrochloric acid (39) or turpentine oil (23). In spinally transected rats, sacral root neurostimulation abolished bladder hyperreflexia and attenuated the rise in neuropeptide content of the L6 dorsal root ganglion (25). Chronic sacral nerve stimulation significantly eliminated nonvoiding contractions in a rat model of bladder outlet obstruction without changing bladder capacity (11). While these studies all demonstrated the ability of SN stimulation to inhibit elevated bladder activity, the electrical stimulation parameters were not optimized in each experimental study, and the parameters used varied, e.g., 80% motor threshold (Tmot) intensity at 20 Hz used by Wang et al. (39), Wang and Hassouna (40), Zhou et al. (43), and Shaker et al. (25); 3- or 12-fold Tmot intensity at 20 Hz by Riazimand and Mense (23) and 1 V at 16 Hz by Comiter et al. (11). No study has been reported in which stimulation parameters for SN stimulation were optimized in an animal model evaluating bladder function. Our goal was to utilize a “high-throughput” in vivo rat model of bladder reflex contraction (BRC) to optimize the stimulation parameters (current intensity and frequency) for SN stimulation-induced inhibition of bladder contraction.
Spinal nerves are known to contain a mixture of large and small fiber types. It is also known that these fibers have different sensitivities to electrical stimulation. Therefore, it is likely that different stimulation parameters will activate different populations of SN fibers and therefore would have different consequences on BRC. The axons carrying afferent signals from structures of the lower urinary tract are contained primarily in pelvic, hypogastric, and pudendal nerves. The cell bodies of these neurons are found in dorsal root ganglia. Most mechanosensitive afferent fibers, which respond to bladder distension, a natural mechanical stimulus to evoke sensations such as fullness, urgency, and pain, pass through L6 SN in the rat. Half of these are primary C fibers (26, 28). Studies at the cellular level using isolated dorsal root ganglion neurons suggest that ∼50% of visceral sensory neurons are C fiber-type neurons (29, 42). This is based on their sensitivity to capsaicin, a vanillyl amide, which activates, at a low dose, and desensitizes, at a high dose, primary afferent C fibers (10, 13). C-fiber sensory afferents have been suggested to mediate urgency-induced sensation and may represent a target for neuromodulation (25). We measured the inhibitory effect of SN stimulation on the micturition reflex in the BRC model in rats in which the primary afferent C fibers had been desensitized by chronic pretreatment with a high dose of capsaicin. Comparison of the effects in capsaicin-treated and control rats should help to evaluate the role of afferent fibers in the control of bladder activity in this model.
Our study described, for the first time, the effects of different parameters of electrical stimulation of the SN on the micturition reflex in a rat model of isovolumetric bladder contraction. A stimulation frequency range (1–20 Hz) was tested at Tmot intensity. A broader range (0.01–100 Hz) was tested at 0.6 mA (∼6 * Tmot). The response to 10 Hz was measured at Tmot, 2 * Tmot, 3 * Tmot, 4 * Tmot, and 0.6 mA (∼6 * Tmot). When the optimal stimulation parameters had been identified, mechanistic studies were performed using capsaicin to evaluate the role of primary C-fiber afferents (see above) and pancuronium, a neuromuscular blocker, to evaluate a potential role of efferent nerve activation (31, 32).
MATERIALS AND METHODS
Female Sprague-Dawley rats, weighing 200–300 g (n = 164), were anesthetized with urethane (2 ip injections, 4 min apart, total 1.2 g/kg). Anesthetized rats were maintained at 37°C with a heating pad during the studies and were euthanized by CO2 asphyxia upon completion of experimental procedures. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Medtronic and the Non-clinical Research Board of Medtronic (Minneapolis, MN).
To record bladder contractions, a cannula (PE-50) was placed into the bladder via the urethra and secured with a suture tie. The urethral cannula was connected via a T-type connector to a pressure transducer (ADInstrument MLT0380D, Colorado Springs, CO) of the data-acquisition system (ADInstrument, ML880/P), and the signal of intravesical pressure was put through a DC amplifier (ADInstrument, ML228). The other end of the T connector was attached to a syringe pump.
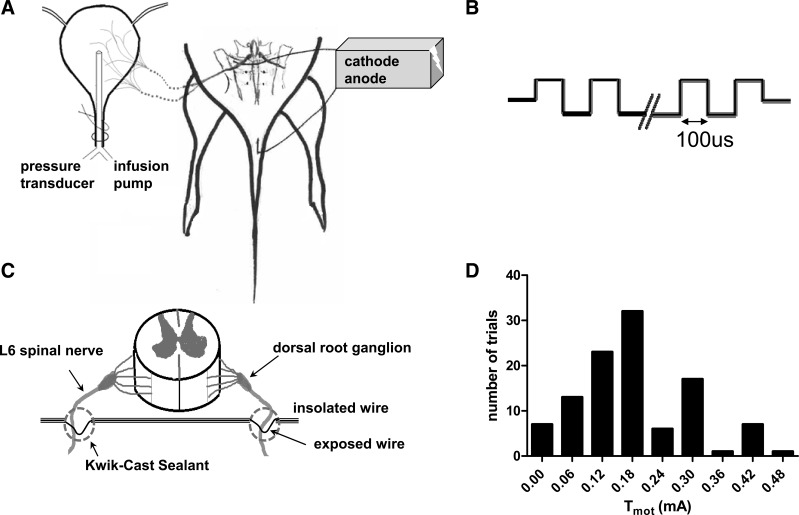
To deliver electrical stimulation, a wire electrode was placed bilaterally under the L6 SN (Fig. 1). The skin around the dorsal sacral and thoracic area was shaved, and a dorsal midline incision was made from approximately SN L3 to S2; the L6/S1 posterior processes were exposed. The S1 processes were removed, and the L6 nerve trunks were localized caudal and medial to the sacroiliac junction. After the wire electrode was placed with two bared portions of teflon-coated, 40-gauge, stainless steel wire (Cooner Wire, Chatsworth, CA) under each nerve, silicone adhesive (Kwik-Cast, World Precision Instruments) was applied to cover the wire around the nerve, and the skin incision was sutured shut. The electrode was connected to a Grass S88 stimulator, through a stimulus isolation unit (SIU-BI, Grass Medical Instruments). A needle electrode under the skin of the tail served as the ground. The stimulator generated pulses to both nerves serially.
Fig. 1.
Diagram representing spinal nerve stimulation system. A: experimental setup. B: schematic drawing of the stimulation waveform. C: bilateral electrode implantation. Two bared portions of a single wire were placed under each of the spinal nerves serially, and bilateral stimulation was achieved by passing current in a parallel circuit. D: histogram of motor threshold (Tmot) to spinal nerve stimulation.
Electrical stimulation of the SN evoked hind-toe twitches and/or pelvic floor muscle contraction. In each rat, the threshold current (Tmot) was defined as the lowest intensity to evoke the first, barely discernable skeletal muscle contraction. Biphasic pulses (pulse width 0.1 ms) of different intensities (Tmot − 6 * Tmot) were used to stimulate the SN at frequencies ranging from 0.01 to 100 Hz.
To induce BRC, saline was infused into the bladder via the syringe pump at a rate of 50 μl/min to induce a micturition reflex (here defined as bladder contraction of a magnitude >10 mmHg). The infusion rate was then lowered to 10 μl/min and continued until three to five consecutive contractions were established. At this time, BRC will continue when saline infusion is terminated. After a 15-min control period, nerve stimulation was applied for 10 min and the BRC was recorded for 20 min poststimulation. Two parameters of BRC were evaluated: frequency/interval and amplitude. Data were calculated in 5-min bins, having three control periods, two periods during stimulation, and four periods after stimulation. In the case of the “shutdown” of BRC induced by high-intensity SN stimulation, the amplitude of the bladder contractions was “0” and data were excluded. All data were compared with the mean response during the last 5 min before stimulation. In an additional 26 rats, SN stimulation to different durations (0, 2, 5, 10, and 20 min) was evaluated. Data during stimulation were the means of each 5-min bin except that 2-min stimulation was calculated in a 2-min bin.
In five rats, an attempt was made to see whether additional saline infusion could overcome SN stimulation-evoked BRC inhibition. After a 5-min period of bladder contractions, 10-Hz SN stimulation at a maximal intensity (>3 * Tmot), which completely abolished bladder contractions, was used to produce a shutdown of BRC. Then saline (60–130 μl) was further infused into the bladder to raise the bladder volume (and therefore, pressure) until periodic BRC was reestablished. Nerve stimulation was maintained during additional saline infusion. All data were calculated in 5-min bins for control, during reestablished BRC, and poststimulation.
In eight rats, one jugular vein and one carotid artery were cannulated with polyethylene tubing for intravenous administration of pancuronium and arterial pressure measurement during neuromuscular blockade, respectively. Pancuronium (1 mg/kg iv) was administered at the start of an experiment, and the effect of stimulation was compared with control rats. The effectiveness of pancuronium was demonstrated by the failure of SN stimulation to produce skeletal muscle twitches even at high stimulation intensity.
Desensitization of primary afferent C fibers.
Thirty-two rats were treated with capsaicin (20 mg/ml, in 10% ethanol, 10% Tween 80, and 80% physiological saline) or vehicle. Capsaicin (125 mg/kg) or vehicle was given subcutaneously (sc) in the hindlimb in divided doses on 2 consecutive days: 25 and 50 mg/kg at a 12-h interval on day 1 and 50 mg/kg on day 2. Injections were performed under isoflurane anesthesia. Four days after the last injection, an eye-wipe test was performed on unanesthetized animals immediately before the bladder experiments. A drop of 100 μg/ml capsaicin solution was instilled into the eye, and the number of defensive wiping movements was counted (9, 13). After the test, the eye was irrigated with physiological saline and then the animals were anesthetized to measure the effect of SN stimulation on BRC as described above.
Compounds.
Urethane (MW: 89.09, dissolved in saline), capsaicin (MW: 305.41, dissolved in 10% ethanol and 10% Tween 80), and pancuronium (MW: 732.67, dissolved in water) were purchased from Sigma-Aldrich (St. Louis, MO).
Data analysis.
All data are expressed as means ± SE. Results were analyzed with Student's t-test or ANOVA with repeated measures by Prism 5 (GraphPad Software, San Diego, CA). A value of P < 0.05 was considered statistically significant.
RESULTS
The threshold current (Tmot) at which first visible motor contraction occurred was 0.18 ± 0.01 mA (n = 107; range: 0.01- 0.45 mA; 95% confidential interval: 0.16–0.20 mA, Fig. 1D). The muscle contraction became stronger, and additional muscle groups were involved as the stimulation current was increased. The experiments employed multiple intensities using multiples (1–4) of Tmot as well as 0.6 mA, which averaged 5.6 ± 2.0 * Tmot.
Effect of high-intensity SN stimulation on bladder contractions.
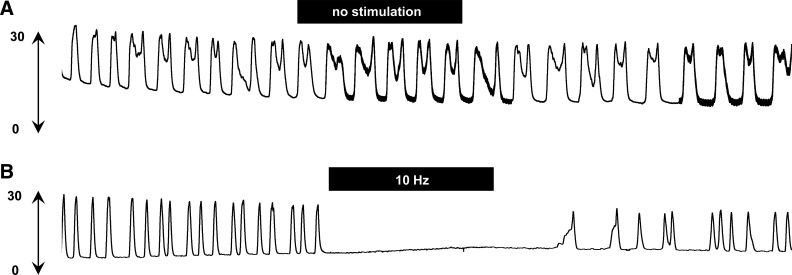
There was no significant change in BRC during a 45-min recording if electrical stimulation was not applied (Fig. 2A). Depending on the stimulation frequency and current intensity, electrical stimulation of the SN attenuated the frequency of bladder contractions, either eliminating bladder contractions (Fig. 2B) or reducing the contraction frequency during electrical stimulation.
Fig. 2.
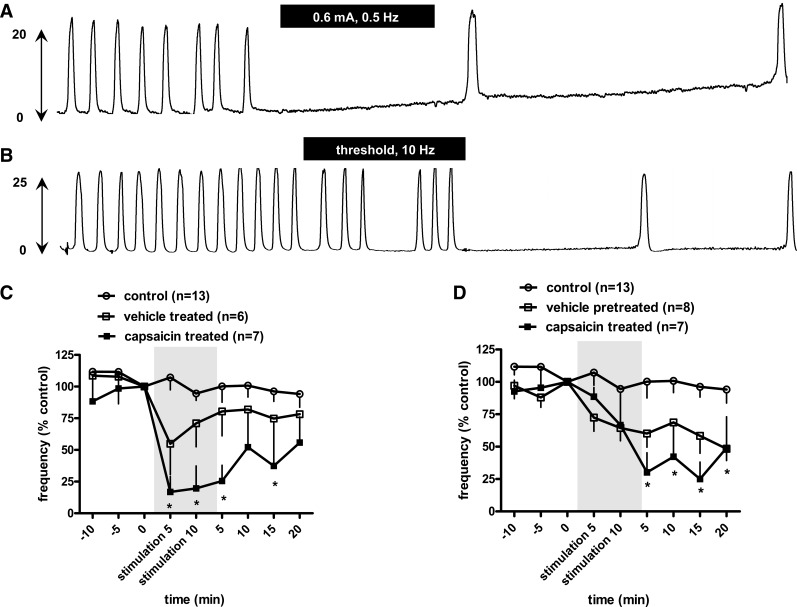
Typical experimental records showing bladder reflex contraction (mmHg) during 45-min recording. A: no significant change in isovolumetric bladder contraction during 45-min recording without electrical stimulation. B: high intensity of spinal nerve stimulation (0.6 mA, 10 Hz, pulse width 0.1 ms) abolished bladder contractions. Black bars indicate 10-min duration of spinal nerve stimulation.
Figure 3 summarizes intensity- and frequency-dependent effects of high-intensity SN stimulation on BRC. Maximal inhibition appeared during stimulation, while after termination of the stimulus, bladder contractions returned to control levels. However, inhibition of BRC at the highest intensities [4 * Tmot or 5.6 * Tmot (0.6 mA) at 10 Hz] of SN stimulation was sustained for 10 min poststimulation (prolonged inhibition). At 10-Hz stimulation, inhibition of the contraction frequency was stronger as the current intensity increased (Fig. 3A). Two * Tmot, 3 * Tmot, 4 * Tmot, and 0.6 mA (5.6 * Tmot) significantly (P < 0.05 vs. control, n = 13) decreased the frequency of contractions during stimulation to 65.56 ± 17 (n = 9), 10.64 ± 8 (n = 8), 6.25 ± 6 (n = 7), and 0% of controls (n = 7), respectively.
Fig. 3.
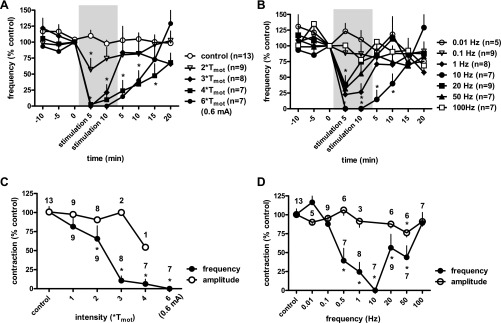
Effects of spinal nerve stimulation at high-intensity (above motor threshold; Tmot) on the frequency of bladder reflex contraction. Responses are represented as a percentage of control (%control), where the baseline response before stimulation is defined as 100%. A: time course response of frequency of bladder reflex contraction to spinal nerve stimulation (10 Hz, pulse width 0.1 ms) at different current intensities. The significance of differences between the tests to 2 * Tmot, 3 * Tmot,4 * Tmot, and 0.6 mA (∼6 * Tmot) stimulation and control values was demonstrated by ANOVA. *P < 0.05 Bonferroni posttest. B: time course response of frequency of bladder reflex contraction to spinal nerve stimulation (0.6 mA, ∼6 * Tmot, pulse width 0.1 ms) at different current frequencies. The significance of differences between the tests to 1-, 10-, 20-, and 50-Hz stimulation and control values was demonstrated by ANOVA. *P < 0.05 Bonferroni posttest. C and D: intensity- and frequency-dependent effects, respectively, of spinal nerve stimulation on the frequency and amplitude of bladder contractions. The response of contraction frequency is the mean value as a percentage of control (%control) during 10-min stimulation (shaded areas in A and B). The significance of differences between the tests and control values was demonstrated by Student's t-test. The numbers of animals are indicated either under or over each symbol. *P < 0.05.
The inhibitory effect of SN stimulation was also frequency dependent, with a U-shaped curve where 10-Hz stimulation produced maximal inhibition of BRC. Figure 3B shows the time course of the mean responses of BRC frequency with different SN stimulation frequencies. Two-way ANOVA analysis demonstrates a significant inhibition of SN stimulation on BRC frequency with stimulation frequencies of 0.5–50 Hz SN stimulation, while 0.01, 0.1, and 100 Hz failed to attenuate bladder contractions. SN stimulation did not reduce the amplitude of bladder contractions (Fig. 3, C and D), except 0.6 mA (5.6 * Tmot), 50 Hz, which produced a mild but significant inhibition of the contraction amplitude to 76.24 ± 6% of control (n = 6, P < 0.05, Student's t-test).
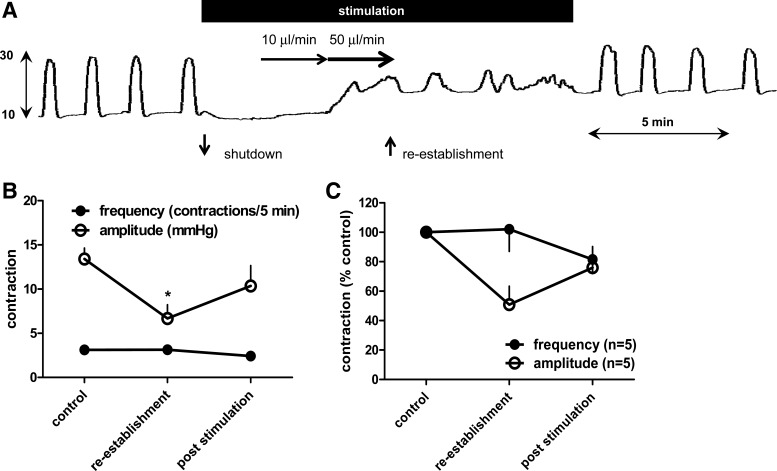
Figure 4A shows that high-intensity SN stimulation abolished the BRC. Further saline infusion increased the bladder threshold pressure (basal pressure). BRC was reestablished with a similar frequency but lower amplitude of bladder contractions, despite the presence of electrical stimulation. Figure 4, B and C, summarizes effects of high-intensity SN stimulation on amplitude and frequency of the reestablished BRC. SN stimulation produced a significant inhibition of the amplitude of bladder contractions but not frequency of bladder contractions (n = 5, P < 0.05, Student's t-test) during this period.
Fig. 4.
Reestablishment of bladder reflex contractions following “shutdown” evoked by high-intensity spinal nerve stimulation. A: raw traces of isovolumetric bladder reflex contraction (mmHg) to high intensity of spinal nerve stimulation (pulse width 0.1 ms) at 10 Hz. Further saline infusion reestablished the bladder contractions. Black bars indicate spinal nerve stimulation period. B and C: effects of high-intensity spinal nerve stimulation on intensity and frequency of bladder contractions.
SN stimulation at current intensities above Tmot triggers a visible skeletal muscle contraction (toes, pelvic floor, tail, or legs) which occurs during the inhibition of BRC. To eliminate the possibility that the inhibition of BRC is an indirect consequence of skeletal muscle contraction, a subset of animals (n = 8) was pretreated with pancuronium (1 mg/kg), which produces paralysis of striated muscle. Stimulation that produced 39 ± 15% inhibition of BRC in untreated rats (5.6 * Tmot at 0.5 Hz) showed an equal degree of bladder inhibition in paralyzed rats (61 ± 14%, P = 0.34), even though skeletal muscle contractions were eliminated.
Effect of SN stimulation at Tmot intensity on bladder contractions.
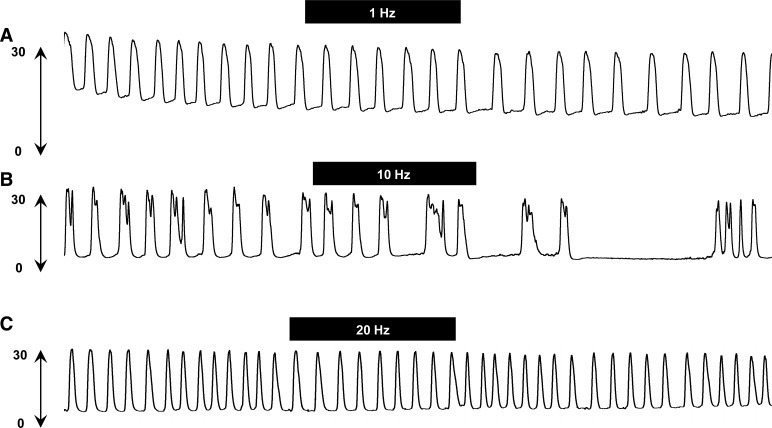
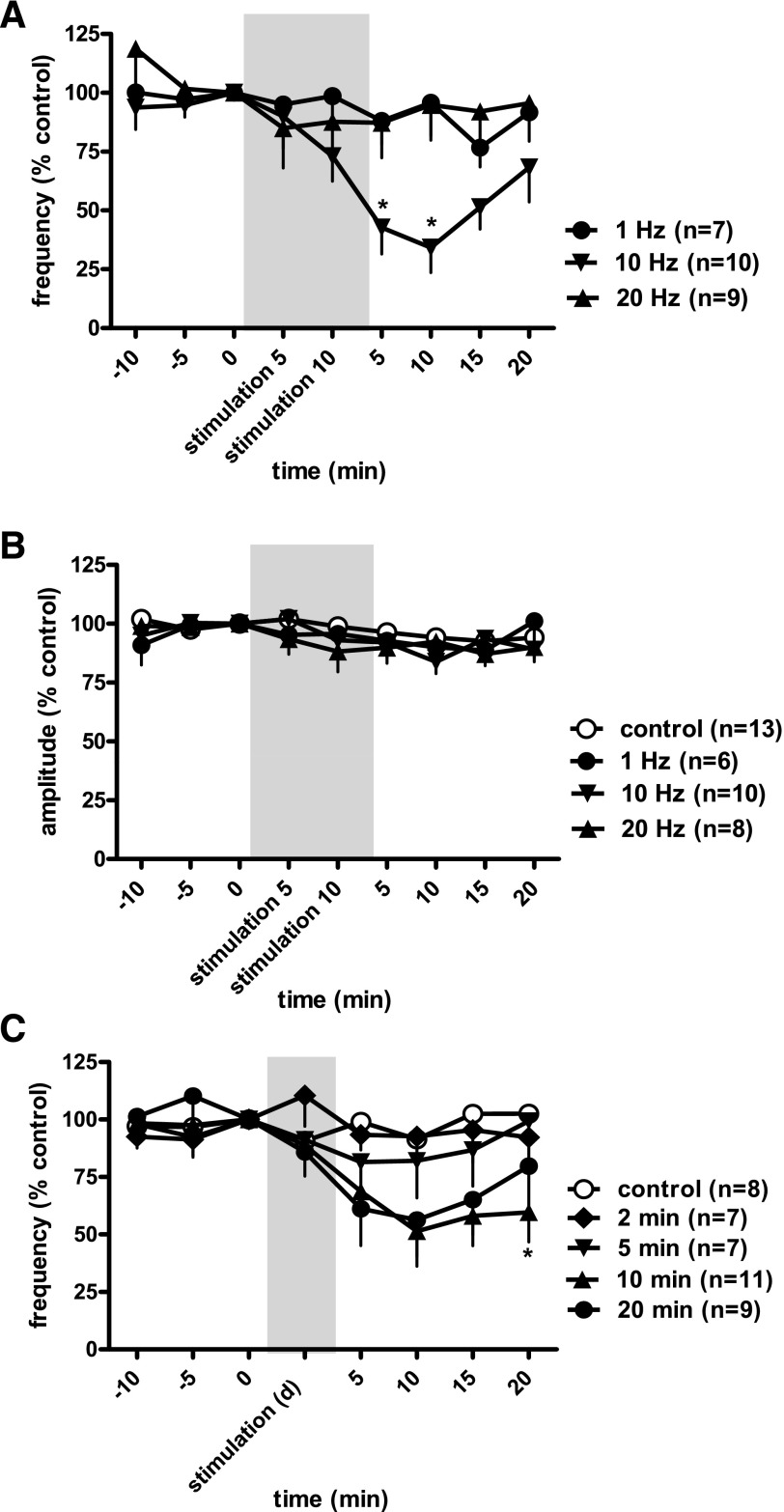
Figures 5 and 6, A and B, show the effect of SN stimulation at Tmot on BRC at different stimulation frequencies. Figure 5, A and C, shows no changes in bladder contractions in response to SN stimulation at 1 and 20 Hz, respectively. Stimulation at 10 Hz also did not produce an obvious inhibition of bladder contractions during stimulation but decreased the frequency of BRC after termination of the electrical stimulation (poststimulation inhibition).
Fig. 5.
Raw traces of isovolumetric bladder reflex contraction (mmHg) following threshold intensity of spinal nerve stimulation (pulse width 0.1 ms) at 1 (A), 10 (B), and 20 Hz (C). Note a strong inhibition of bladder contractions post-spinal nerve stimulation at 10 Hz. The black bars indicate 10-min duration of spinal nerve stimulation.
Fig. 6.
Time course for the effect of spinal nerve stimulation of the threshold intensity (pulse width 0.1 ms) on frequency (A) and intensity (B) of bladder reflex contraction at different current frequencies (10-min stimulation) and different stimulation durations (stimulation d; C). The responses are presented as a percentage of control (%control), where the baseline response before stimulation is defined as 100%. The significance of differences between the test to 10-Hz stimulation for 10 and 20 min at threshold intensity (0.10 ± 0.02 mA) and control values was demonstrated by ANOVA. The shaded areas are responses during electrical stimulation. *P < 0.05 Bonferroni posttest.
SN stimulation at Tmot (0.10 ± 0.02 mA) produced a much narrower range of inhibition with only 10-Hz, but not 1- or 20-Hz, attenuating frequency of bladder contractions (Fig. 6A). Stimulation at 10 Hz decreased BRC to 34 ± 11% of control (n = 10, vs. control, n = 13, P < 0.05), with maximal inhibition appearing at 10 min poststimulation. SN stimulation did not reduce the amplitude of bladder contractions (Fig. 6B).
The poststimulation inhibitory effect on bladder contractions for different durations of SN stimulation was evaluated. Figure 6C shows that 0 (n = 8)-, 2 (n = 7)- and 5-min (n = 7) stimulation periods (10 Hz) failed to attenuate bladder contraction frequency. Ten (n = 11)- and 20-min (n = 9) stimulation significantly decreased the frequency of bladder contractions to 44 ± 14% of control (n = 11) and 56 ± 15% of control (n = 9), 10 min poststimulation, respectively, vs. control, n = 7, P < 0.05, 2-way ANOVA. There was no significant difference in responses to neurostimulation during the 10- or 20-min stimulation periods.
Effect of capsaicin on neuromodulation of bladder contractions.
To examine the involvement of C-fiber sensory afferents in SN stimulation, rats were treated with capsaicin to eliminate capsaicin sensitive C fibers. The effectiveness of this capsaicin treatment was examined using the eye wipe test, where a drop of 100 μg/ml capsaicin solution was applied to the eye and the number of eye wipes in 20 s was counted. Vehicle-treated rats had vigorous wiping in response to the drop of capsaicin (11.38 ± 1.06 wipes, n = 16). In contrast, rats receiving three injections (sc) of capsaicin demonstrated no responsiveness to ocular capsaicin (n = 16), demonstrating a strong loss of capsaicin-sensitive C-fiber afferents. The ability of this treatment to induce desensitization of pain responsiveness was evident, since the rats showed signs of pain behaviors (involuntary twitching, hyperactivity, or immobility) after the first injection of capsaicin. No aversive behaviors were observed with the two subsequent injections.
Tmot values of SN stimulation vary over a wide range, from 0.01 to 0.45 mA in this study, with a mean of 0.18 mA. There is no difference between capcaicin-treated (0.18 ± 0.03 mA) and vehicle-treated rats (0.22 ± 0.05 mA).
Capsaicin- and vehicle-treated rats did not differ in the mean frequency and amplitude of prestimulation BRC (3.19 ± 0.20 contractions/5 min and 19.24 ± 1.89 mmHg, n = 8, vs. 2.81 ± 0.38 contractions/5 min and 20.63 ± 2.73 mmHg, n = 8, respectively).
Figure 7A shows that, in the capsaicin-treated rats, BRC were completely eliminated by SN stimulation at 0.6 mA (∼6 * Tmot), 0.5 Hz. This inhibition persisted for >20 min poststimulation. In another case (Fig. 7B), SN stimulation at Tmot (10 Hz) attenuated bladder contractions, especially after termination of electrical stimulation.
Fig. 7.
Effect of capsaicin on neuromodulation of the micturition reflex. A and B: typical experimental records showing effects of spinal nerve stimulation by high current intensity (0.6 mA, ∼6 * Tmot, 0.5 Hz; A) and threshold current intensity (Tmot, 10 Hz, pulse width 0.1 ms; B) on bladder reflex contraction (mmHg) in rats with capsaicin (125 mg/kg sc) pretreatment 4 days before the bladder reflex contraction study. The black bars indicate 10-min duration of spinal nerve stimulation. C and D: time course for the effect of spinal nerve stimulation at high intensity (0.6 mA, ∼6 * Tmot, 0.5 Hz; C) and threshold intensity (10 Hz; D) on frequency of bladder reflex contraction in rats with vehicle and capsaicin (125 mg/kg sc) pretreatment 4 days before the bladder reflex contraction study. The responses are represented as a percentage of control (%control), where the baseline response before stimulation is defined as 100%. Significant differences between the tests and control values were demonstrated by ANOVA followed by Bonferroni posttest. Shaded areas are responses during electrical stimulation. *P < 0.05 Bonferroni posttest. In capsaicin-pretreated rats, high intensity of current stimulation produced a stronger inhibition of frequency of bladder contractions (P < 0.05 vs. vehicle treated, 2-way ANOVA); Tmot intensity stimulation produced a trend of stronger inhibitory effects but was not statistically significant (P = 0.03 vs. vehicle-treated, 2-way ANOVA).
As seen above, high-intensity stimulation at 0.6 mA (∼6 * Tmot) produced acute inhibition of bladder contraction frequencies. This was true in both vehicle- and capsaicin treated rats (P < 0.05) during current stimulation. However, high-intensity stimulation produced a stronger inhibition of BRC in capsaicin-treated rats (to 18 ± 14% of control, n = 6, vs. 63 ± 17% of control in vehicle-treated rats, n = 7, P < 0.05). Tmot intensity stimulation in both vehicle- and capsaicin-treated rats produced a poststimulation inhibition of bladder contractions, with a trend toward stronger inhibition in capsaicin-treated rats (P = 0.49).
DISCUSSION
In contrast to other rat models which tested the effect of S1 SN stimulation on the micturition reflex (11, 23, 25, 39, 40, 43), we delivered electrical stimulation at the L6 level through which most mechanosensitive afferent fibers innervating the urinary bladder pass in the rat. SN stimulation inhibited the frequency of volume-induced BRC, with the magnitude of the inhibition directly proportional to the applied current (stimulus intensity). Stimulation produced a delayed inhibition at the threshold current and prolonged inhibition at high intensity. Although the reason for this difference in time course is unknown, it is possible that the different stimulation parameters activate different micturition reflex circuits or act via different mechanisms. The inhibitory effect of SN stimulation is likely to be mediated by both afferent and efferent mechanisms. Lower intensities of stimulation may act through the afferent limb of the reflex arc and increase bladder capacity. Higher intensities may additionally act through efferent neural pathways and attenuate amplitude of detrusor contractions as well as by increasing bladder capacity. The inhibitory effects of SN stimulation on bladder contractions were also frequency dependent. For inhibition of bladder contractions by both low- and high-intensity stimulation, 10-Hz stimulation was optimal. Finally, current stimulation produced a greater inhibition of BRC frequency in capsaicin-pretreated vis-à-vis vehicle-treated rats, suggesting that an activation of fast-conducting fibers might be associated with neuromodulation of the bladder micturition reflex.
SN stimulation inhibited BRC frequency and often temporarily eliminated voiding contractions. This effect has been observed in many studies with compounds targeting the bladder afferent pathway, by several different molecular mechanisms (12, 16, 18, 19, 30, 37). Shutdown of BRC is a consequence of an increased pressure threshold for induction of contractions since additional saline can reestablish BRC even in the presence of electrical stimulation. Since the urethra is ligated in this model, voiding cannot occur; however, efficacy in the anesthetized BRC model is predictive of an increased bladder capacity and voided volume, as measured by cystometry in conscious animals (19). The ureters were not ligated in this study, and bladder overactivity could result from enhancement of urine production. The shutdown is not due to a reduction of endogenous urine production. The basal urine production rate for a 250-g female SD rat is very low, only 4 μl/min based on 25 ml·kg−1·24 h−1 (24); it has been well validated that BRC is maintained even when the ureters are ligated to eliminate any urine outflow from the kidney.
Over a wide frequency range (0.1–100 Hz), SN stimulation failed to change the amplitude of BRC, except 0.6 mA, 50 Hz, which produced a mild inhibition of bladder amplitude. This suggests the SN-mediated neuromodulation may not directly depress the contractility of detrusor smooth muscle when BRC was not abolished. However, the SN stimulation-mediated shutdown at high-intensity current may be mediated via both afferent and efferent actions, since SN stimulation suppresses the amplitude of bladder contractions during the reestablishment of BRC.
Our finding that 10-Hz stimulation produced the strongest inhibition of bladder contractions is consistent with clinical applications of SN or pudendal nerve stimulation in patients (36). For sacral nerve stimulation, neuromodulation frequency is typically adjusted to 10–14 Hz (Medtronic InterStim Therapy, Implant Manual). The frequency dependence for bladder inhibition by pudendal nerve and perigenital nerve stimulation in normal and spinal cord-injured cats has also been studied. Maximal inhibition was obtained when the nerve was stimulated at 3–10 Hz (4, 5, 33, 35, 41). Boggs et al. (5) found that, in spinally transacted cats, pudendal nerve stimulation at 10 Hz was more effective in inhibiting bladder activity than either 33 or 100 Hz and that stimulation at 100 Hz had no activity, either inhibitory or excitatory, in at least 50% of the animals studied. Considering the differences in experimental design (species, stimulation site, stimulation duration), the frequency-inhibition relationships are very consistent between the current rat study and the previously reported cat studies.
Over a wide range of stimulation frequencies, we did not observe an excitatory effect which might be clinically relevant for the treatment of urinary retention. This is different from other reports using stimulation of the pudendal or the deep perianal nerves (20–40 Hz) (35, 41). Pudendal nerve stimulation induced bladder contractions in normal cats (33 Hz) (6) and in acute (33 Hz) (5), 20 Hz (27), or chronic spinal cord-injured cats (at 20 Hz) (33, 34). Such bladder excitatory effects were observed when the bladder was filled with saline to 66–80% volume threshold (2, 3, 5, 8). The current study, not using an animal disease model of retention, was not designed to study augmentation of bladder contraction by neuromodulation as an approach to the treatment of urinary retention in humans.
The inhibitory effects of SN stimulation on bladder contractions were intensity dependent as well; attenuation was stronger with increases in the stimulation current. The marked inhibition of BRC frequency by high-intensity SN stimulation (above Tmot) occurs in either normal rats or rats pretreated with pancuronium to block skeletal muscle contractions, showing that the inhibitory effect of SN stimulation is unlikely to be mediated through skeleton muscle contractions. A similar observation has been demonstrated for bladder inhibition in response to vaginal nerve stimulation in the rat (17). Poststimulation inhibitions appeared as a prolonged and delayed inhibition following high-intensity (above Tmot) and low-intensity (Tmot) nerve stimulation, respectively. The poststimulation inhibition is quite reproducible and sustained. Bycroft et al. (7) occasionally observed a delayed “rebound” detrusor contraction following cessation of stimulation of sacral nerve roots in spinal cord-injured patients. We observed no evidence of a rebound in bladder excitation. The poststimulation inhibitory effects are not present when the stimulation durations are short (e.g., seconds long) (35, 41) and were only evoked if stimulation was applied for a sufficient duration, 10 min here in this study and 5 min in that of Jiang and Lindstrom (17). Twenty-minute stimulation produced an equal degree of poststimulation inhibition to 10-min stimulation. The prolonged or delayed inhibitory effects have been observed in InterStim Therapy in humans, where neuromodulation leads to lasting continence, e.g., the patients remain continent even when stimulation is off (clinical observation).
It seems that mechanisms of the inhibitory effects during electrical stimulation with high-intensity currents differ from poststimulation inhibition. The former is an “on-and-off” switch and needs only a few seconds to a few minutes to have a full effect while poststimulation inhibition by SN stimulation appears to be another mechanism of neuromodulation, which needs a longer time to develop. Prolonged inhibition may resemble the long-term depression of the central micturition reflex (17). Delayed inhibition by low-stimulation currents suggests that the effect requires some time to develop or that, during SN stimulation, both excitation and inhibition occur and that the excitation, but not inhibition is lost immediately after termination of nerve stimulation.
The inhibitory effects have been reported not only in spinal cord-intact animals [e.g., in cats to pudendal nerve stimulation (5), in rats to SN stimulation (23)] but also in spinal cord-injured animals where the spinobulbospinal micturition reflex is not reserved (5, 33, 39, 43). Thus, although it is difficult to attribute the inhibitory effect on neuromodulation solely to a spinal reflex, at least a spinobulbospinal pathway is not the only mechanism.
A centrally mediated long-term depression in the micturition reflex pathway may be more important than a stimulation-induced modification of peripheral afferent nerve activity, since neither tonic after-discharge nor mechanical sensitivity of afferent nerves is altered by electrical stimulation (17). Even though the relative contribution of peripheral and central actions are not yet known, acute peripheral nerve activation evoked by SN stimulation is the trigger pointing toward neuromodulation of the bladder micturition reflex.
Administration of capsaicin 4 days before the experiments in a dose that is known to desensitize bladder C-fiber afferents blocked the eye-wipe response but did not significantly alter BRC, showing that bladder sensory C fibers are not essential for micturition in the rat (9, 22). In these rats, the inhibitory effects of SN stimulation on bladder contractions remain, supporting the primary involvement of fast-conducting fibers.
Pretreatment of rats with capsaicin to desensitize the primary afferent C fibers appears to potentiate the inhibitory effect of SN stimulation on BRC frequency. This potentiation is clear in the case of high-current SN stimulation (Fig. 7C). In rats stimulated at the threshold current, no difference is observed during stimulation, but the magnitude of the delayed inhibitory effect tended to be greater following C-fiber desensitization (Fig. 7D).
It is possible that activation of Aδ fibers in the sacral nerve root by low-current stimulation excites central inhibitory pathways. The SN is composed of a wide range of fiber types, including myelinated Aβ and Aδ fibers, as well as unmyelinated C fibers (28, 30, 42). The large myelinated Aδ or Aβ fibers should be stimulated by low-current intensity while the small unmyelinated C fibers are activated by high current intensity (20). Tmot values of the SN stimulation did not differ between capcaicin- or vehicle-treated rats. Accordingly, the delayed bladder inhibition by Tmot current stimulation may result from an action only at the large myelinated fibers.
Activation of C-fiber afferents is not necessary for SN stimulation action; however, high-current stimulation may activate the unmyelinated C fibers (unwanted) in addition to myelinated Aδ or Aβ fibers (desired) in vehicle-treated rats. Many bladder afferent C fibers in the anesthetized rat are mechanosensitive afferent fibers responding to bladder distension (28). Activation of these C fibers would stimulate BRC, and consequently weaken the inhibitory effect of bladder contractions associated with SN stimulation. This could explain the potentiation of high-current SN-mediated inhibition by capsaicin-induced C-fiber desensitization.
In summary, we have optimized the parameters of SN stimulation in a preclinical model and demonstrated time course responses of bladder inhibition to different intensities of SN stimulation. Current explanations of peripheral or central neuromodulation are based on the longer stimulation periods regularly used in patients or on repeated stimulation over several days in experimental animals (see Ref. 14 for a review). The present study, using only 10-min stimulation, cannot directly address the sustained bladder inhibition seen in overactive bladder patients after InterStim therapy. In addition, isovolumetric bladder contraction is a nonpathological micturition reflex and the study was performed in urethane-anesthetized rats although urethane has minimal influence on cardiovascular function in the rat compared with other general anesthetics (1, 21). Further experiments using conscious cystometry in chronic models of bladder overactivity will target the mechanisms by which neuromodulation acts to relieve the symptoms of overactive bladder.
DISCLOSURES
The authors are Medtronic employees.
AUTHOR CONTRIBUTIONS
Author contributions: X.S. and D.E.N. provided conception and design of research; X.S. and A.N. performed experiments; X.S. and A.N. analyzed data; X.S., A.N., and D.E.N. interpreted results of experiments; X.S. and D.E.N. prepared figures; X.S. drafted manuscript; X.S. and D.E.N. edited and revised manuscript; X.S., A.N., and D.E.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Drs. Greg Molnar and Thaddeus Brink for helpful comments and Kent Wika for study coordination. The manuscript was edited by J. Paul Hieble Scientific Writing.
REFERENCES
- 1. Angel A. Central neuronal pathways and the process of anaesthesia. Br J Anaesth 71: 148–163, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Barrington FJF. The component reflexes of micturition in the cat. Parts I and II. Brain 54: 177–188, 1931 [Google Scholar]
- 3. Barrington FJF. The component reflexes of micturition in the cat. Part III. Brain 64: 239–243, 1941 [Google Scholar]
- 4. Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol 93: 2688–2697, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol 577: 115–126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boggs JW, Wenzel GJ, Gustafson KJ, et al. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neuroal Eng 3: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bycroft JA, Craggs MD, Sheriff M, Knight S, Shah PJR. Does magnetic stimulation of sacral nerve roots cause contraction or suppression of the bladder? Neurourol Urodyn 23: 241–245, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Chang HU, Cheng CL, Chen JJ, Peng CW, DeGroat WC. Reflexes evoked by electrical stimulation of afferent axons in the pudendal nerve under empty and distended bladder conditions in urethane-anesthetized rats. J Neurosci Meth 150: 80–89, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng CL, Ma CP, De Groat WC. Effects of capsaicin on micturition and associated reflexes in rats. Am J Physiol Regul Integr Comp Physiol 265: R132–R138, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Cheng CL, Ma CP, De Groat WC. Effects of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res 678: 40–48, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Comiter CV, Mazar C, Phull H, Salkini M. Chronic sacral nerve stimulation prevents detrusor structural and functional changes associated with bladder outlet obstruction—a rat model. Neurourol Urodyn 29: 783–788, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Dray A, Metsch R. Inhibition of urinary bladder contractions by a spinal action of morphine and other opioids. J Exp Pharmacol Ther 231: 254–361, 1984 [PubMed] [Google Scholar]
- 13. Gamse R, Leeman SE, Holzer P, Lembeck F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn Schmiedebergs Arch Pharmacol 317: 140–148, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Griebling TL. Neuromodulation: mechanisms of action. In: The Overactive Bladder: Evaluation and Management, edited by Kreder K, Dmochowski R. London, UK: Informa Healthcare, 2007, p. 293–302 [Google Scholar]
- 15. Hussain Z, Harrison SVW. Neuromodulation for lower urinary tract dysfunction—an update. Sci World J 7: 1036–1045, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hisamitsu T, De Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res 298: 51–65, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Jiang CH, Lindstrom S. Prolonged increase in micturition threshold volume by anogenital afferent stimulation in the rat. Br J Urol 82: 398–403, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Leon LA, Hoffman BE, Gardner SD, Laping NJ, Evans C, Lashinger ESR, Su X. Effects of the β3-adrenergic receptor agonist CL-316243 on bladder micturition reflex in spontaneously hypertensive rats. J Exp Phar Thera 326: 178–185, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Leonardi A, Guarneri L, Poggesi E, Angelico P, Velasco C, Cilia A, Testa R. N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-nitrophenyl) cyclohexanecarboxamide: a novel pre- and postsynaptic 5-hydroxytryptamine 1A receptor antagonist active on the lower urinary tract. J Exp Pharmacol Ther 299: 1027–1037, 2001 [PubMed] [Google Scholar]
- 20. Li CL, Bak A. Excitability characteristics of the A- and C-fibers in a peripheral nerve. Exp Neurol 50: 67–79, 1976 [DOI] [PubMed] [Google Scholar]
- 21. Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: general considerations. Cell Mol Life Sci 42: 109–114, 1986 [DOI] [PubMed] [Google Scholar]
- 22. Mallory B, Steers WD, De Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol Regul Integr Comp Physiol 257: R410–R421, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Riazimand SH, Mense S. Interaction between neurotransmitter antagonists and effects of sacral neuromodulation in rats with chronically hyperactive bladder. BJU Int 96: 900–908, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt F, Yoshimura Y, Ni RX, Kneesel S, Constantinou CE. Influence of gender on the diurnal variation of urine production and micturition characteristics of the rat. Neurourol Urodyn 20: 287–295, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Shaker H, Wang Y, Loung D, Balbaa L, Fehlings MG, Hassouna MM. Role of C-afferent fibers in the mechanism of action of sacral nerve root neuromodulation in chronic spinal cord injury. BJU Int 85: 905–910, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the rat's urinary bladder. J Neurophysiol 84: 1924–1933, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Shefchyk SJ, Buss RR. Urethral pudenda afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett 244: 137–140, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Su X, Sengupta JN, Gebhart GF. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 77: 1566–1580, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Su X, Wachtel RE, Gebhart GF. Capsaicin sensitivity and voltage-gated sodium currents in colon sensory neurons from rat S1 dorsal root ganglia. Am J Physiol Gastrointest Liver Physiol 277: G1180–G1188, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Su X, Lashinger ES, Leon LA, Hoffman BE, Hieble JP, Gardner SD, Fries HE, Edwards RM, Li J, Laping NJ. An excitatory role for peripheral EP3 receptors in bladder afferent function. Am J Physiol Renal Physiol 295: F585–F594, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Su X, Nickles A, Nelson DE. Interstim therapy in a rat model of rhythmic bladder contraction. International Continence Society/International Urogynecological Association Joint Meeting, http://www.icscoffice.org/Abstracts/Publish/105/000521.pdf, 521. Toronto, Canada, 2010a
- 32. Su X, Nickles A, Nelson DE. Inhibition of bladder contractions by electrical stimulation of the spinal nerve: a study of the stimulation duration. Society For Neuroscience annual meeting, 904.12. JJJ46. 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2010b [Google Scholar]
- 33. Tai C, Smerin SE, De Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol 197: 225–234, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Tai C, Wang J, Wang X, Roppolo JR, De Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn 26: 879–886, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tai C, Shen B, Wang J, Chancellor MC, Roppolo JR, De Groat WC. Inhibitory and excitatory perigenital-to-bladder spinal reflexes in the cat. Am J Physiol Renal Physiol 294: F591–F602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanagho EA. Electrical stimulation. J Am Geriatr Soc 38: 352–355, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Testa R, Guarneri L, Angelico P, Velasco C, Poggesi E, Cilia A, Leonardi A. Effect of different 5-hydroxytryptamine receptor subtypeantagonists on the micturition reflex in rats. BJU Int 87: 256–264, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Vignes JR, Deloire M, Petry K. Animal models of sacral neuromodulation for detrusor overactivity. Neurourol Urodyn 28: 8–12, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Zhou Y, Mourad MS, Hassouna MM. Neuromodulation reduces urinary frequency in rats with hydrochloric acid-induced cystitis. BJU Int 86: 726–730, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Hassouna MM. Neuromodulation reduces c-fos gene expression in spinalized rats: a double-blind randomized study. J Urol 163: 1966–1970, 2000 [PubMed] [Google Scholar]
- 41. Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol 294: R1880–R1889, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshimura N, Seki S, Erickson KA, Erickson VL, Chancellor MB, De Groat WC. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neruosci 23: 4355–4361, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou Y, Wang Y, Abdelhady M, Mourad MS, Hassouna MM. Change of vanilloid receptor 1 following neuromodulation in rats with spinal cord injury. J Surg Res 107: 140–144, 2002 [DOI] [PubMed] [Google Scholar]