Abstract
Migration and differentiation of cranial neural crest cells are largely controlled by environmental cues, whereas pathfinding at the trunk level is dictated by cell-autonomous molecular changes owing to early specification of the premigratory crest. Here, we investigated the migration and patterning of vagal neural crest cells. We show that: 1) vagal neural crest cells exhibit some developmental bias and 2) they take separate pathways to the heart and to the gut. Together these observations suggest that prior specification dictates initial pathway choice. However, when we challenged the vagal neural crest cells with different migratory environments, we observed that the behavior of the anterior vagal neural crest cells (somite-level 1-3) exhibit considerable migratory plasticity whereas the posterior vagal neural crest cells (somite-level 5-7) are more restricted in their behavior. We conclude that the vagal neural crest is a transitional population that has evolved between the head and the trunk.
Keywords: neural crest, circumpharyngeal crest, specification, enteric crest, cardiac crest, migratory pathways, single-somite level electroporation, chick-quail transplantations, morphogenesis
Introduction
Neural crest cells originate from the dorsal neural tube and migrate throughout the embryo where they differentiate into neurons, glial cells, melanocytes and craniofacial connective tissue (Le Douarin and Kalcheim, 1999). At all axial levels, they migrate to their final destination following two stereotypical migratory pathways, termed the dorsolateral and ventral pathways. The overarching question we wish to answer is how different neural crest derivatives are positioned in the embryo and how they access the appropriate pathway: do they migrate by following extrinsic cues and then differentiate according to the environment in which they find themselves (i.e., they are ‘plastic’ in terms of migratory potential and fate), or are they prespecified to become a particular cell type and to follow a specific pathway (i.e., their fate is ‘restricted’ and consequently they display cell-autonomous pathfinding)?
At the trunk level in birds, neural crest cells initially invade the ventral pathway between somites and through the anterior-half of the somites and give rise to neurons and glial cells of the peripheral nervous system (Rickmann et al., 1985; Bronner-Fraser, 1986; Loring and Erickson, 1987; Teillet et al., 1987). Twenty-four hours later, trunk neural crest cells migrate dorsolaterally between the ectoderm and dermamyotome, where they differentiate into pigment cells (Serbedzija et al., 1989; Erickson et al., 1992). Analysis of single cells and cell populations has shown that avian melanoblasts are fate-restricted when they leave the neural tube (Henion and Weston, 1997; Reedy et al., 1998a). Similar conclusions have been reached about zebrafish neural crest cells (Raible et al., 1992; Raible and Eisen, 1994). Furthermore, melanoblasts are the only neural crest cells that have the ability to take the dorsolateral pathway (Erickson and Goins, 1995; Reedy et al., 1998a). Therefore, at the trunk level, melanoblasts are specified at the time they emigrate and, as a result of the molecular changes that accompany specification (Santiago and Erickson, 2002; Harris et al., 2008; Thomas and Erickson, 2009), their choice of migratory pathways is regulated (reviewed by Harris and Erickson, 2007).
At the cranial level, mesencephalic neural crest cells disperse predominantly in the dorsolateral pathway, with only a modest number of cells invading the underlying paraxial mesoderm (Noden, 1975; Noden, 1988). Both early- and late-emigrating neural crest cells can give rise to melanocytes, neurons, glia, cartilage and bone (Le Lievre, 1974; Noden, 1978; Le Douarin, 1982; Couly et al., 1993), but the early-migrating neural crest cells contribute to both dorsal structures (i.e. melanocytes) and ventral structures (i.e. neurons, glial cells and skeletal derivatives of the jaw), whereas the late-migrating neural crest cells give rise to more dorsal structures (i.e. melanocytes and parasympathetic neurons in the ciliary ganglia) and much less cartilage and bone (Baker et al., 1997). Analysis of heterochronic transplantations of early neural crest cells into late embryos, and vice-versa, reveals that the grafted cranial neural crest cells behave like the endogenous cells. Therefore the mesencephalic neural crest cells are plastic in their migratory behavior and differentiation (Baker et al., 1997), which contrasts with the more restricted nature of the trunk neural crest cells.
The vagal neural crest, which arises from the axial level of somites 1-7, has been described as a hybrid between the head and the trunk populations (Kuratani et al., 1991; Kuratani and Kirby, 1992; Kuratani, 1997; Ferguson and Graham, 2004). The neural crest cells from somite-levels 1-3 includes a subset of the cardiac crest (defined as the neural crest cells that originate from the mid-otic placode to somite-level 3 (Kirby et al., 1983; Kirby et al., 1985)), which plays a role in the septation of the outflow tract of the heart, and contributes to the connective tissue and smooth muscle of the great vessels (Kirby et al., 1983; Kirby and Waldo, 1995). Experimental analysis has also shown that no other neural crest cells can substitute for the cardiac crest that arise from somite-levels 1-3 (Kirby, 1989), which suggests that cardiac neural crest cells may be specified prior to migration. The vagal neural crest also produces the neurons and glial cells of the enteric nervous system (Yntema and Hammond, 1954; Le Douarin and Teillet, 1973; Epstein et al., 1994; Burns et al., 2000; Burns and Le Douarin, 2001; Burns et al., 2002). Finally, like neural crest cells from other axial levels, the vagal neural crest contributes to the neurons and glial cells of the peripheral nervous system and the melanocytes that populate the skin (Reedy et al., 1998a; Reedy et al., 1998b).
Previous mapping studies using HNK-1 or E/C8 antibody and DiI labeling have provided some information regarding the early migratory behavior and distribution of vagal neural crest cells [HNK-1(Tucker et al., 1988; Kuratani and Kirby, 1992; Reedy et al., 1998a), an antibody against the neurofilament-associated protein, E/C8 (Tucker et al., 1986), and DiI labeling (Kuratani and Kirby, 1991; Kuratani and Kirby, 1992; Shigetani et al., 1995; Suzuki and Kirby, 1997; Ferguson and Graham, 2004)]. However, these studies, unlike those focused on the head and trunk levels (Serbedzija et al., 1989; Kuratani and Kirby, 1991; Kuratani and Kirby, 1992; Kontges and Lumsden, 1996), do not trace from beginning to end the pathways that the vagal neural crest cells take to reach their final destinations. We were particularly interested to know if there are unique pathways for specific derivatives. For instance, do the neural crest-derived components of the heart strictly take the dorsolateral pathway and do the neurons and glial cells of the enteric nervous system take the ventral pathway? Additionally many of the previous studies labeled large segments of the neural tube so that the precise axial origins of different neural crest derivatives are not known. We therefore wanted to determine the pathways taken by the neural crest cells from each somite level to reach the heart and the gut. Also, the destination of all the vagal neural crest cells that initially collect in the circumpharyngeal ridge (Kuratani and Kirby, 1991; Kuratani and Kirby, 1992) (Supplementary Figure 1) has not been ascertained. In particular, since the circumpharyngeal ridge forms a nexus between the dorsolateral and ventral pathways (diagrammed in Figure 3), we wondered if neural crest cells use this intersection to switch pathways. Finally, several reports are in disagreement regarding the axial level and the embryonic stage at which the vagal neural crest cells migrate segmentally through the anterior half of the somite (Lim et al., 1987; Kuratani et al., 1991; Ferguson and Graham, 2004) and we wished to resolve this controversy.
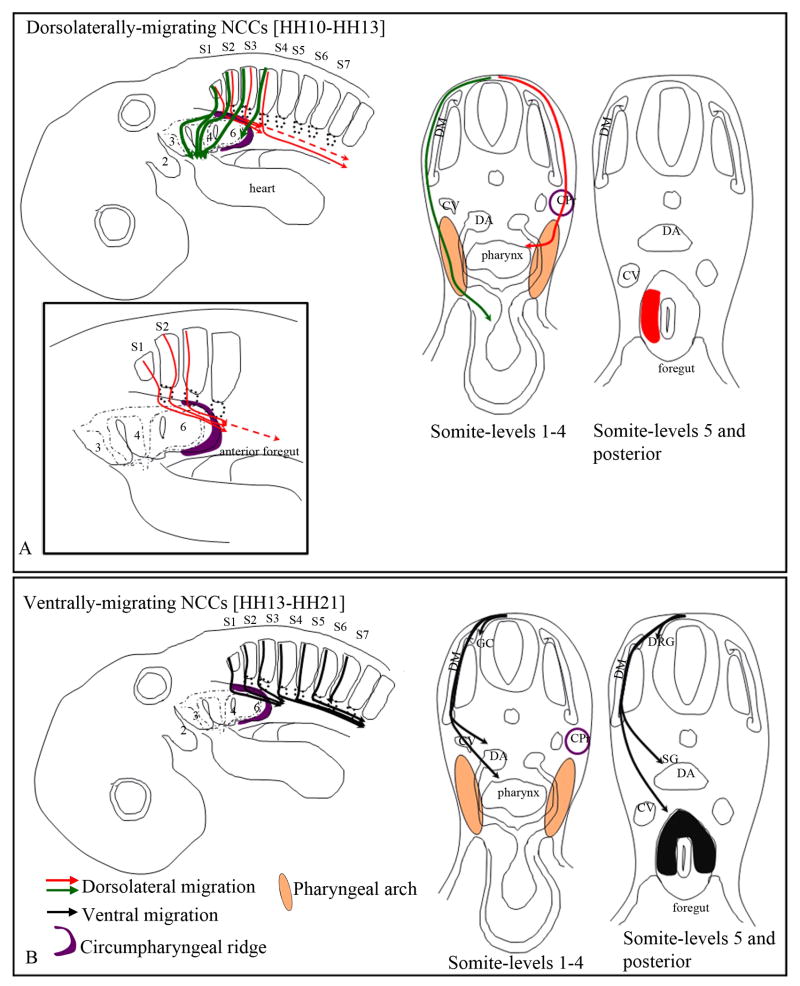
Figure 3. Final destination of the vagal neural crest cells.
Schematic representations of neural crest pathways shown in whole mount and in cross sections. The destination of neural crest cells taking the dorsolateral pathway are shown in A and the ventral pathway is illustrated in B. Although the migratory pathways are only depicted on one side, these events occur bi-laterally.
(A) The dorsolaterally-migrating vagal neural crest cells from somite-level 1-3 populate the pharyngeal arches 3-6 and the outflow tract of the heart, whereas the neural crest cells from somite-level 4 only populate the posterior region of pharyngeal arch 6 (green arrows). Additionally, the dorsolaterally-migrating neural crest cells invade the gut by migrating along the circumpharyngeal ridge (red arrows in inset in A) and populate the lateral region of the foregut (red patch in A) by subsequently moving medially from the pharyngeal arch (red arrow in cross section). Neural crest cells from somite-level 1-2 reach the anterior foregut (esophagus/lung bud region), while the cells from somite-level 3 and 4 migrate more posteriorly in the stomach, by stage 22-23.
(B) At stage 13 the vagal neural crest cells from somite-level 1-4 begin migrating ventrally. By stage 23, at somite-level 1-3 they coalesce to form the ganglionic crest (GC), and reach the anterior foregut (black arrows). These cells populate the dorsal and lateral regions of the foregut (black patch in cross section). The ventrally-migrating neural crest cells from somite-levels 4-6 form the ganglionic crest, and reach the stomach. The neural crest cells from somite-level 7 only populate the dorsal root and sympathetic ganglia.
Cardinal vein (CV), Dorsal aorta (DA), Dermomyotome (DM) Circumpharyngeal ridge (CPr; purple), Ganglionic crest (GC), Dorsal root ganglia (DRG).
The unique ability of the vagal neural crest cells to contribute to the heart suggests that there may be a causal relationship between prespecification and pathfinding at the vagal level, as there is for trunk neural crest cells. To test this hypothesis, we used a combination of experimental approaches, including cell culture, cell lineage labeling and neural tube heterotopic transplantations. First, we demonstrate using cell culture studies that the vagal neural crest cells exhibit some developmental bias: the early-migrating neural crest cells differentiate predominantly into smooth muscle cells, whereas the later-migrating neural crest cells differentiate principally into pigment cells. Next, we define the timing of migration of the vagal neural crest cells into the dorsolateral and the ventral pathways and establish a detailed map of the contribution of vagal neural crest cells from each somite level to the heart and the gut. We find that the neural crest cells that populate the pharyngeal arches, great vessels and heart take the dorsolateral pathway, whereas those that give rise to the neurons and glial cells of the peripheral nervous system, including the enteric nervous system, primarily take the ventral pathway. Together, these data suggest that there is a correlation between early specification and pathfinding. However, when we challenged vagal neural crest cells with alternative migratory environments using heterotopic and heterochronic neural tube transplants, we observed that the migratory behavior of the vagal neural crest cells from the level of somites 1-3 exhibit considerable migratory plasticity, whereas the neural crest cells from the level of somites 5-7 display cell autonomous migratory behavior. The data presented here further extend the evidence that the vagal neural crest represents a transition between the head and the trunk populations (Kuratani and Kirby, 1991; Ferguson and Graham, 2004).
Results
Vagal neural crest cells are developmentally biased
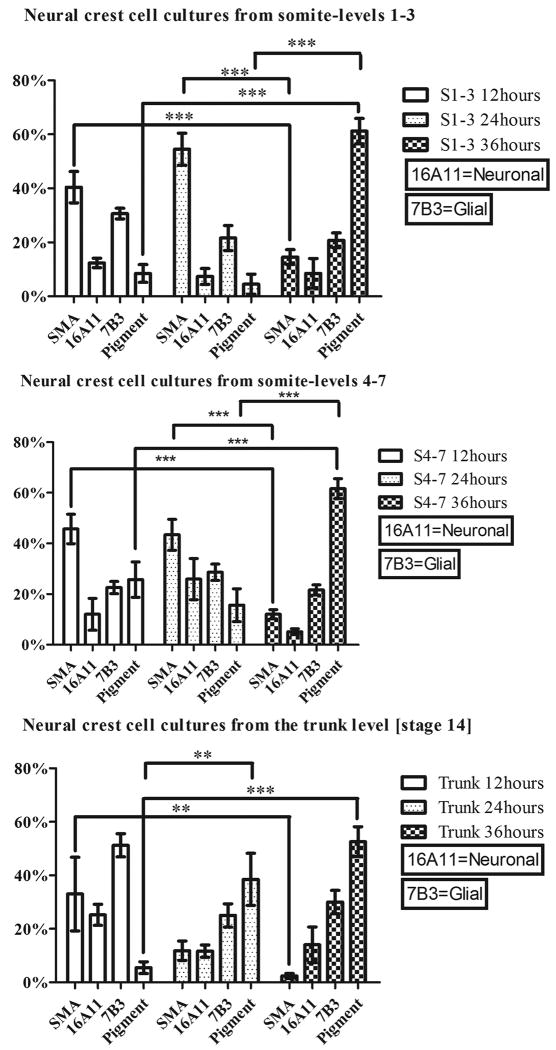
If early specification of the vagal neural crest controls pathway choice, then neural crest cells should be specified at the time they emigrate from the neural tube. We therefore first investigated the developmental potential of the vagal neural crest by performing neural tube serial-replating experiments. Briefly, we isolated quail neural tubes from Hamburger and Hamilton stage-10 embryos (Hamburger and Hamilton, 1951) at the level of somites 1-3 and 4-7. The neural tubes were severed between somite-level 3 and 4 because the cardiac crest is believed to come from somite-levels 1-3 at the vagal level. The neural tube segments were replated every twelve hours to produce three cultures representing sequential waves of neural crest emigration (0-12 hours, 12-24 hours and 24-36 hours). The cultures were subsequently incubated for ninety-six hours in order for the cells to differentiate (as described in Henion and Weston, 1997; Reedy et al. 1998a; Reedy et al., 1998b). Neural tube segments from the trunk level were used for comparison. The developmental potential of the neural crest cells was analyzed using antibodies against α-smooth-muscle-actin (Kirby et al., 1983; Kirby and Waldo, 1990) to label the smooth- muscle cell lineage (SMA in Figure1), which differentiates into the vascular smooth muscle that form the tunica media of the arteries and the mesenchyme responsible for outflow tract septation (Beall and Rosenquist, 1990). Antibodies against 16A11 and 7B3 were used as markers for neuronal and glial differentiation, respectively (Weston, 1991; Yao et al., 1993; Marusich et al., 1994). Melanocytes were identified by the presence of melanin. This experiment was repeated three times and one representative dataset is shown in Figure 1.
Figure 1. Vagal neural crest cells exhibit some developmental bias.
Stage-10 quail neural tubes from the level of somites 1-3 and 4-7 and stage-14 trunk neural tubes were serially replated to generate successive waves of migrating neural crest cells (labeled 12 hours, 24 hours and 36 hours), and immunolabeled with lineage markers for smooth muscle (α-smooth-muscle-actin), neural (16A11) and glial fates (7B3). Melanocytes were identified by the presence of melanin. Smooth muscle cells (SMA lineage) are predominant in the first (twelve hours) and second (twenty-four hours) cultures, whereas the pigment cells are most abundant in the last culture (labeled 36 hours). The percentage of neurons and glial cells does not vary significantly with the time of migration. Fewer myofibroblasts differentiate in the trunk cultures. **p<0.01 and ***p<0.001 by two-way ANOVA with Bonferroni correction.
Neural crest cells that emigrated during the first and second twelve-hour time periods (cultures labeled 12 hours and 24 hours, respectively, in Figure 1), from both somite-level segments 1-3 and 4-7, differentiate primarily into smooth muscle as revealed by the α-smooth-muscle-actin-positive immunoreactivity. In the third twelve-hour period (labeled 36 hours in Figure 1), the pigment cell population increases significantly (p<0.001) and represents the predominant cell type in both cultures, whereas the α-smooth-muscle-actin-positive cell population declines significantly (p<0.001). The percentage of glial and neuronal cells is similar for all three time points. These results indicate that the vagal neural crest cells exhibit some developmental bias: the early neural crest cells favor the smooth muscle cell lineage, whereas the late-migrating neural crest cells are biased towards a melanogenic fate. Although there are some α-smooth-muscle-actin-positive cells in the early migratory trunk neural crest cells, neurons and glial cells predominate.
Migratory pathways taken by the vagal neural crest cell subpopulations
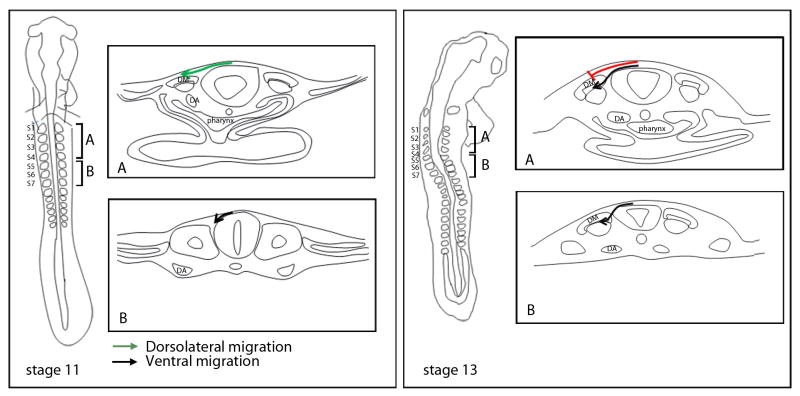
In order to test our hypothesis that specification dictates pathway choice, we needed to know what pathways are taken by the vagal neural crest cells to the heart and the gut from the time they emigrate from the neural tube until they arrive at their final destination. We first determined the initial pathways (dorsolateral or ventral) invaded by neural crest cells at the vagal level by electroporating plasmids expressing GFP or TdTomato into the premigratory neural crest, starting at stage 10, and then analyzing their distribution at stages 12, 13 and 14. By stage 12 (9 hours after electroporation), the neural crest cells from the level of somites 1-4 have initiated migration in the dorsolateral pathway but not the ventral pathway (Supplementary Figure 2). They begin to enter the ventral pathway by stage 13 (12 hours after electroporation), as the migration in the dorsolateral pathway continues (Supplementary Figure 3). By stage 14 (18-20 hours after electroporation), migration into the dorsolateral pathway has ceased and neural crest migration is entirely in the ventral pathway (Supplementary Figure 4). Emigrating neural crest cells re-establish migration into the dorsolateral pathway at stage 21, which prior studies from our lab show to be melanoblasts (Reedy et al., 1998b). We also noted that the neural crest cells migrate in the anterior half of the somite beginning at the level of somite 2 (Supplemental Figure 5).
In contrast, the neural crest cells originating from the level of somites 5-7 initiate migration in the ventral pathway at stage 12 (Supplementary Figure 2), and persist in their ventral migration (Supplementary Figure 3 and 4) until stage 21, at which time newly-migrating cells enter the dorsolateral pathway (Reedy et al., 1998b). These data are summarized in Figure 2.
Figure 2. Summary of the early migratory pathways taken by the vagal neural crest cells.
Whole-mount representations are on the left and cross-sections taken from somite-levels 1-4 (A) and 5-7 (B) are to the right. The neural crest cells from the level of somites 1-4 initiate their migration in the dorsolateral pathway (between the ectoderm and the dermomyotome) at stage 10 (box A; stage-11 schematic; green arrow), whereas the neural crest cells from the level of somites 5-7 are only in the early stages of their emigration from the neural tube (box B; stage-11 schematic; black arrow). By stage 13, the neural crest cells from the level of somites 1-4 stop migrating dorsolaterally (box A; stage -13 schematic; red line) and begin to migrate in the ventral pathway (box A; stage-13 schematic; black line) up until stage 21, when they subsequently migrate into the dorsolateral pathway (see Reedy et al., 1998a). At the level of somites 5-7 migration persists in the ventral pathway (box B; stage-13 schematic; black line). Dermomyotome (DM), Dorsal aorta (DA), Somites 1-7 (S1-S7).
Since only neural crest cells from somite levels 1-4 take the dorsolateral pathway and previous studies have shown that the cardiac crest are uniquely derived from this same axial level, our data suggest that the cardiac neural crest (smooth-muscle-cell lineage in our culture study) take the dorsolateral pathway to the arches and the heart, whereas the enteric neural crest are more likely to follow the ventral pathway.
Neural crest cells that arrive in the arches and the heart take the dorsolateral pathway whereas neural and glial precursors in the peripheral nervous system mostly take the ventral pathway
We next investigated the entire length of the pathway that neural crest cells take to the heart and to the gut. First, to determine what subpopulation of vagal neural crest cells along the anterior/posterior axis populates the heart vs. the enteric nervous system of the gut, GFP- or TdTomato-expressing plasmids were focally electroporated in the neural tube at individual somite levels and the migration of the labeled neural crest cells was followed in progressively older embryos (Supplementary Table 1). By stage 20, neural crest cells from the level of somites 1, 2 and 3 migrate to the pharyngeal arches 3-6 and the outflow tract of the heart. We also observe aggregation of cells next to the neural tube, which have been referred to as the ganglionic crest of the cranial nerve IX and X (Kuratani et al., 1988). The neural crest cells from somite-level 1-2 reach the anterior foregut (extending posteriorly to the site where the laryngotracheal groove has split into the esophagus and the lung buds), whereas the neural crest cells from somite-level 3 reach the stomach (at the forelimb level).
Neural crest cells from the level of somite 4, by stage 20, aggregate into the ganglionic crest (as described above), populate the posterior edge of pharyngeal arch 6, and posterior regions of the foregut (as far posterior as the forelimb level, into the stomach).
The neural crest cells from the level of somites 5-6 colonize the Froriep's (sensory) ganglia (Froriep, 1889; Froriep and Beck, 1895) and as far posterior in the foregut as the stomach, similar to the neural crest cells from the level of somite 4 (Supplementary Figure 6). Finally, the neural crest cells from the level of somite 7 coalesce to form the first permanent dorsal root and sympathetic ganglia, but do not migrate into the foregut. Mapping results are summarized in Figure 3.
We next wanted to determine the final destination of the neural crest cells depending on whether they initiated migration in the dorsolateral or ventral pathway and to discover whether neural crest cells could cross over from one pathway to the other at the circumpharyngeal ridge. To ascertain the destination of the neural crest cells that take the dorsolateral pathway, we labeled the neural tube at a single-somite level in stage-10 embryos by electroporation of a fluorescent construct, and then ablated the fluorescently-labeled neural tube twelve-hours later (stage 13) before ventral migration from the level of somites 1-4 begins.
Forty-eight hours following the ablation (stage 23), the labeled dorsolaterally-migrating neural crest cells from the level of somites 1-3 populate the pharyngeal arches 3-6, the outflow tract of the heart and the foregut. The neural crest from somite-level 1-2 reach the point where the laryngotracheal groove has split into the esophagus and the lung buds (Supplementary Figure 7), whereas the neural crest cells from the level of somite 3 migrate more posteriorly into the stomach, confirming earlier experiments (see Figure 3). Interestingly, the labeled neural crest cells selectively occupy the lateral portion of the foregut, which is contiguous with the pharyngeal arch mesenchyme. This same neural crest subpopulation has been shown previously to be E/C8-immunoreactive by Kuratani and Kirby (1992). We noted that the neural crest cells from the level of somites 1-3 reach the foregut by migrating along the circumpharyngeal ridge, whose mesenchyme fuses with that of the foregut, and not by migrating medially to the ventral pathway. At stage 23, the neural crest cells from the level of somite 4 populate the posterior edge of the pharyngeal arch 6 and the foregut as far as the stomach (forelimb level), but are absent from the heart. Therefore, our data show that the vagal neural crest cells from somite-levels 1-4 initiate migration dorsolaterally and cells in this pathway predominantly populate the pharyngeal arches and the outflow tract of the heart (see Figure 3 for summary). The cells that reach the foregut migrate from the circumpharyngeal ridge directly into the foregut mesenchyme.
We further predicted that the ventrally-migrating neural crest cells from the vagal level would populate the gut and contribute to the development of the enteric nervous system (but not the pharyngeal arches or the heart). We selectively labeled the ventrally-migrating neural crest cells by electroporating a GFP construct in the neural tube at each somite level in stage-13 embryos (time of initiation of ventral migration) and then incubated the embryos for forty-eight hours (until stage 23) (Supplementary Table 1). Neural crest cells from the level of somites 1-2 form the ganglionic crest, and colonize the foregut, up to the level where the laryngotracheal groove splits into the esophagus and the lung buds (Supplementary Figure 8), whereas the neural crest cells from somite-level 3 reach the stomach. In contrast to the neural crest cells that migrate to the gut through the circumpharyngeal ridge, these neural crest cells populate the dorsal and lateral portions of the anterior foregut. A previous study from Kuratani and Kirby (1992) showed that neural crest cells that occupy the anterior dorsal foregut are HNK-1 positive and are E/C8-negative. Here, we provide evidence that the E/C8-negative cells are those that exclusively take the ventral pathway. The neural crest cells from the level of somites 4-6 form the ganglionic crest (at the level of somite 4) and Froriep's ganglia (somite level 5 and 6), and colonize the foregut, as far posteriorly as the stomach (Supplementary Figure 9). The neural crest cells from the level of somite 7 only contribute to the dorsal root ganglia and the sympathetic ganglia that form at somite-levels 7 and 8. Mapping results are summarized in Figure 3.
In conclusion, neural crest cells that migrate to the pharyngeal arches and the heart take the dorsolateral pathway, whereas neural crest cells that form the peripheral nervous system and the enteric nervous system follow the ventral pathway. There is minimal cross over between these pathways at the circumpharyngeal ridge. Taken together with the observation that vagal neural crest cells have developmental biases that correlate with the time they leave the neural tube, we hypothesized that early specification dictates whether neural crest cells will follow the dorsolateral or ventral pathway.
Do the vagal neural crest cells have cell-autonomous pathfinding ability?
To test the cell-autonomous pathfinding abilities of the vagal neural crest cells, we transplanted quail donor neural tubes (stage 10) from the level of somites 1-3 to the same axial level of older chick embryo hosts (stage 13) or to the level of somites 5-7 in stage-10 chick embryo hosts (Figure 4 and Table 1). In both situations, the grafted neural crest cells would normally be migrating in the dorsolateral pathway whereas the endogenous neural crest cells should be migrating in the ventral pathway. After twelve hours of incubation, the transplanted quail neural crest cells invade the ventral pathway only. These results suggest that any cell-autonomous migratory properties possessed by the early migrating neural crest cells from the level of somites 1-3 are overridden by local environmental directional signals.
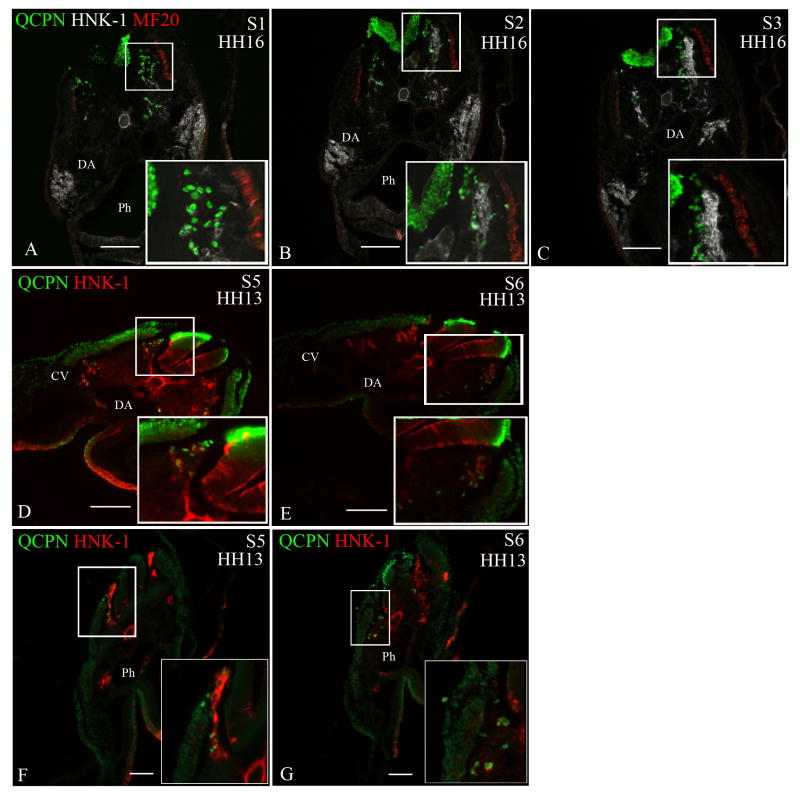
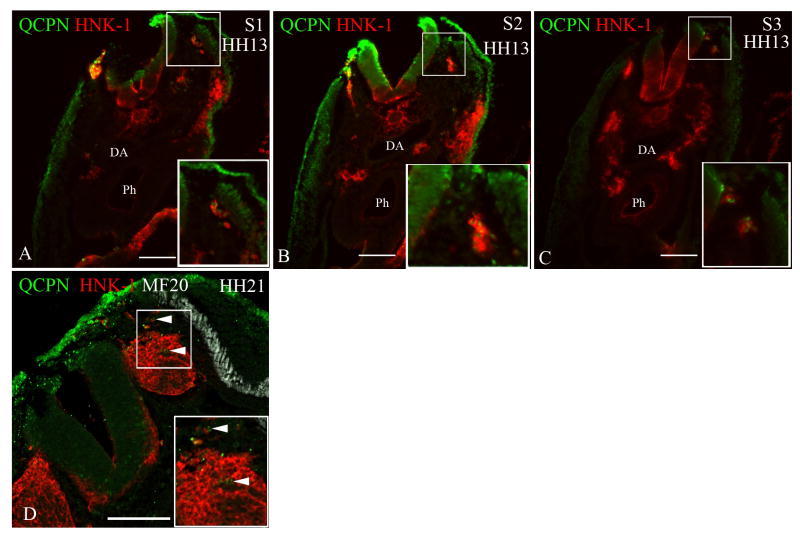
Figure 4. Neural crest cells from the level of somites 1-3 [stage-10 embryos] exhibit migratory plasticity.
Quail neural tubes (labeled with QCPN antibody; green) from somite-level 1-3 were heterotopically or heterochronically transplanted into a stage-10 chicken embryo to test for cell autonomous behavior. Neural crest cells are labeled with HNK-1 antibody (red) and the myotome labeled with MF20 (white A-C only) to indicate unambiguously the boundary between the dorsolateral and ventral pathways. The boxed areas are shown at higher magnification in the insets. Representative sections are shown from somite-levels 1,2,3, (A-C) or somite levels 5 and 6 (D,E; F-G).
When a quail neural tube from the level of somites 1-3 [stage 10] is isotopically transplanted into a stage-13 chick embryo host (A-C) or heterotopically transplanted to the level of somites 5-7 in a stage-10 chick host (D-E), the transplanted neural crest cells (QCPN-positive) switch their migration to the ventral pathway, 12 hours later [stage 16 and 13, respectively], thus mimicking the migratory behavior of the endogenous neural crest. (F-G) The ventrally-migrating neural crest cells from the level of somites 1-3 [stage 13] migrate in the ventral pathway when transplanted to the level of somites 5-7 in stage-10 embryo hosts, 12 hours later [stage 13] like the endogenous cells.
Somite 1 (S1), Somite 2 (S2), Somite 3 (S3), Somite 5 (S5), Somite 6 (S6), Dermomyotome (DM), Dorsal aorta (DA), Neural tube (NT), Pharynx (Ph). Scale bar = 100μm.
Table 1. Summary of transplantation experiments.
| [Donor]: host migratory behavior | Expected migratory behavior of the transplanted cells | Actual migratory behavior of the transplanted cells | n | |
|---|---|---|---|---|
| somite-level 1-3 (stage 10) transplanted to somite-level 5-7 (stage 10) | [DL]:VL | DL | VL migration after 12 hours | 4 |
| somite-level 1-3 (stage 10) transplanted to somite-level 1-3 (stage 13) | [DL]:VL | DL | VL migration after 12 hours | 4 |
| somite-level 1-3 (stage 10) transplanted to somite-level 5-7 (stage 13) | [DL]:VL | DL | VL migration after 12 hours | 2 |
| somite-level 5-7 (stage 10) transplanted to somite-level 1-3 (stage 10) | [VL]:DL | VL | VL migration after 12 hours | 4 |
| somite-level 1-3 (stage 13) transplanted to somite-level 1-3 (stage 10) | [VL]:DL | VL | No migration after 12 hours | 21 |
| VL after 20 hours | 4 | |||
| somite-level 5-7 (stage 13) transplanted to somite-level 1-3 (stage 10) | [VL]:DL | VL | No migration after 12 hours | 11 |
| VL after 20 hours | 6 |
To determine whether the neural crest cells from the level of somites 5-7 would maintain their initial migration in the ventral pathway in an environment where the endogenous neural crest cells migrate dorsolaterally, we performed two transplantation experiments: 1) we transplanted a stage-10 quail neural tube from somite-levels 5-7 into a stage-matched chicken embryo at the level of somites 1-3; and 2) we transplanted a stage-10 quail neural tube into stage-21 chicken embryos at the trunk level (Table 1). In both cases, the transplanted neural crest cells are found in the ventral pathway twelve hours later (Figure 5). The former result is especially surprising since the trunk neural crest cells transplanted to the level of somites 1-3 do use the dorsolateral space (Harris et al., 2008).
Figure 5. Neural crest cells from the level of somites 5-7 [stage-10 embryos] always migrate in the ventral pathway.
Quail neural tubes (labeled with QCPN antibody; green) from somite-level 5-7 were transplanted heterotopically and heterochronically to test for cell autonomous behavior. Neural crest cells are labeled with HNK-1 (red) and the myotome labeled with MF20 (white in D only) to indicate unambiguously the boundary between the dorsolateral and ventral pathways. The boxed areas are shown at higher magnification in the insets. Representative sections are shown from somite-levels 1,2,3 (A-C) or the trunk level (D).
The transplanted neural crest cells from the level of somites 5-7 from stage-10 embryos maintain their migration into the ventral pathway when they are transplanted to the level of somites 1-3 in stage-10 embryo hosts,12 hours later [stage 13] (A-C) or to the trunk level in stage-21 embryo hosts (arrowheads D).
Somite 1 (S1), Somite 2 (S2), Somite 3 (S3), Dermomyotome (DM), Dorsal aorta (DA), Neural tube (NT), Pharynx (Ph).
Scale bar = 100μm.
This suggests that neural crest cells in the first migratory wave from the level of somites 5-7 are predisposed to migrate ventrally or at a minimum have endogenous migratory properties that differ from ventrally-migrating neural crest cells at other axial levels.
The “young” environment (stage-10 embryo) at somite-levels 1-3 does not support the migration of “older” (stage-13 embryo) vagal neural crest cells populations
To test whether the late-migrating neural crest cells from the level of somite 1-3 (stage 13) also exhibit similar plasticity in their migratory behavior as the early-migrating neural crest cells from stage-10 embryos, we transplanted quail donor neural tubes to the same axial level, but in younger chicken embryo hosts (stage 10) when the endogenous neural crest cells migrate in the dorsolateral pathway (Table 1). After twelve hours of incubation, the quail neural crest cells do not initiate migration from the transplanted neural tube (Supplementary Figure 10). However, after twenty hours of incubation, which corresponds to the time at which the endogenous neural crest cells migrate in the ventral pathway, the transplanted neural crest cells now migrate ventrally (Supplementary Figure 10). Because these stage-13 (older) neural crest cells immediately migrate in the ventral pathway when transplanted heterotopically at the level of somites 5-7 in stage-10 embryos (Figure 4), this suggests that the “young” environment at the level of somites 1-3 is not compatible with the migration of these older neural crest cells.
Similarly we transplanted the neural tube from somite level 5-7 from a stage-13 embryo into a stage-10 embryo at the level of somites 1-3 (Table 1). After twelve hours of incubation, the transplanted quail neural crest cells did not migrate (Supplementary Figure 10), whereas, twenty hours after the transplantation, the quail neural crest cells were found in the ventral pathway (Supplementary Figure 10). When the same neural crest cells were transplanted into a young host, but isotopically, the neural crest cells initiated migration immediately and ventrally. Again it appears that the “early” environment at the level of somites 1-3 is not permissive for “older” neural crest cell migration.
All vagal neural crest cells can migrate into the pharyngeal arches 3-6, the outflow tract of the heart and the stomach
Fate mapping shows that at stage 20, the contribution of neural crest cells from the level of somites 1-2 to the enteric nervous system is restricted to the anterior foregut up to the laryngotracheal groove split (posterior to the pharyngeal arch 6). In contrast, the neural crest cells from the level of somites 3-6 migrate more posteriorly into the foregut, as far as the stomach (forelimb level) (also see Barlow et al., 2008). In addition, only the vagal neural crest cells from the level of somites 1-3 reach the outflow tract of the heart. We asked whether these distinctive distributions reflect a difference in migratory ability or result only from different start sites.
To determine whether the neural crest cells from the level of somites 1-2 are capable of invading the posterior regions of the foregut (by stage 20), we performed heterotopic transplantations using quail neural tubes and chick hosts. When the neural crest cells from the level of somites 1-2 (stage-10 embryo) are transplanted posteriorly to somite-levels 5-7 (stage-10-embryo host), they do not migrate anteriorly into the pharyngeal arches 3-6 or the outflow tract of the heart, but rather migrate posteriorly into the stomach, reaching regions normally populated by neural crest cells from the level of somites 5-7 (Figure 6). Note that the transplanted cells occupy the dorsal and lateral portion of the foregut, similar to the endogenous neural crest cells (Figure 3).
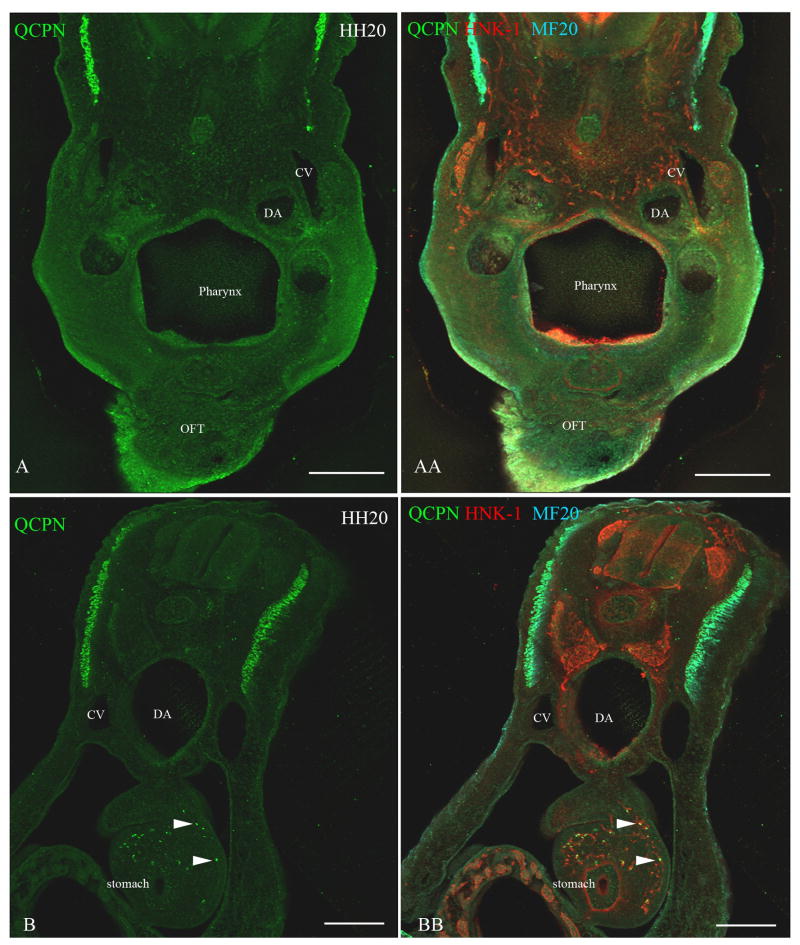
Figure 6. Neural crest cells from the level of somites 1-2 [stage-10 embryos] transplanted to the level of somites 5-7 [stage-10 embryos] migrate into the stomach and not into the heart.
To test whether neural crest cells from somite-level 1-2, which normally reach the lung buds, can migrate more posteriorly to the stomach, a quail neural tube (labeled with QCPN, green) from somite level 1-2 was grafted to the axial level of somites 5-7 in a stage-10 embryo. Neural crest cells were labeled with HNK-1 (red) and the myotome labeled with MF20 (cyan). Forty-eight hours later [stage 20], the transplanted neural crest cells (QCPN-positive) migrated posteriorly into the stomach (arrowheads B, BB), which is normally populated by neural crest cells from the level of somites 5-7. However, the transplanted neural crest cells did not migrate anteriorly into the pharyngeal arches 4-6 and the outflow tract, which they normally populate (A, AA). Dorsal aorta (DA), Cardinal vein (CV). Scale bar = 100μm.
Conversely we asked whether neural crest cells from the level of somites 4-6 can migrate to the pharyngeal arches 3-6 and the outflow tract of the heart. When neural crest cells from these somite-levels (stage-10 embryo) are transplanted to the level of somites 1-3 (in a stage-10 host), they only migrate in the ventral pathway. At later time points (forty-eight hours after the surgery, stage 20), the transplanted cells populate the pharyngeal arches 3-6 and the outflow tract of the heart, but also continue their posterior migration into the stomach along the ventral pathway (Figure 7). The transplanted cells also occupy the dorsal and lateral portions of the foregut. These observations suggest that neural crest cells from the level of somites 4-6, when moved anteriorly, can find their way into anterior structures normally filled by the neural crest cells that originate from the level of somites 1-3. This means that the transplanted neural crest cells crossed over from the ventral to the dorsolateral pathway in order to invade the pharyngeal arches. In addition, the results strongly suggest that the cardiac developmental potential as reflected by the bias toward the smooth muscle cell lineage observed in cell culture (Figure 1), might allow the neural crest cells from the level of somites 4-6 to invade or be attracted to the pharyngeal arches and the heart.
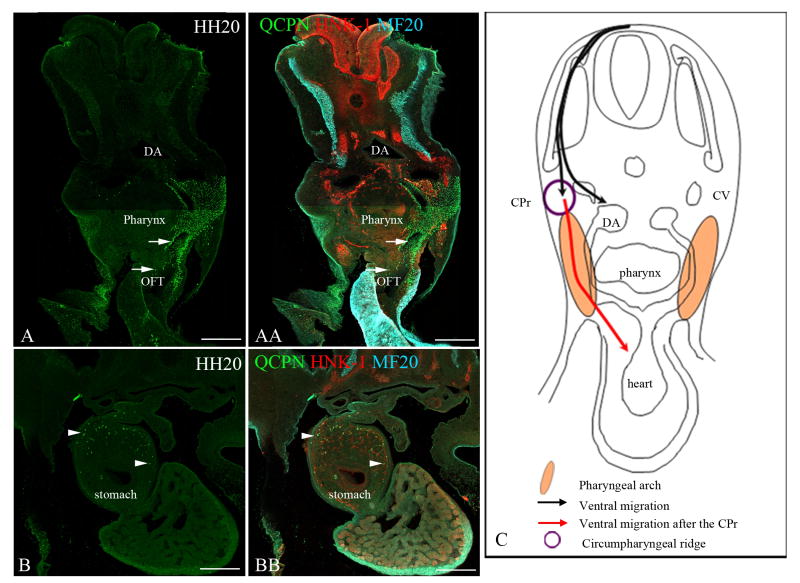
Figure 7. Neural crest cells from the level of somites 5-7 [stage-10 embryos] transplanted to the level of somites 1-3 [stage-10 embryos] migrate into the pharyngeal arches 4-6, the heart and the stomach.
To test whether neural crest cells from somite-levels 5-7 are capable of migrating into the pharyngeal arches and the outflow tract, a quail neural tube (labeled with QCPN, green) from somite-levels 5-7 [stage 10] was transplanted to the level of somites 1-3 in a stage-10 chick embryo host. All neural crest cells are labeled with HNK-1 (red). The myotome is indicated by MF-20 label (cyan). Forty-eight hours later [stage 20], the transplanted neural crest cells (QCPN, green) migrated into the pharyngeal arches 4-6 (top arrow in A and AA) and the outflow tract (lower arrow in A and AA), normally occupied by the neural crest cells from the level of somites 1-3. Additionally, they migrate posteriorly into the stomach region, which they normally reach (arrowheads B and BB). This shows that the neural crest cells from the level of somites 5-7 can migrate into the pharyngeal arches 4-6 and the outflow tract of the heart even when they initially migrate ventrally. (C) Schematic depicting the ventral migration of the transplanted neural crest cells, and their entry in the pharyngeal arch and the heart [red arrow] once they reach the circumpharyngeal ridge. Note: The migration is only depicted on one side in panel C, but the migration is bilateral.
Dorsal aorta (DA), Cardinal vein (CV), Sympathetic ganglion (SG), Outflow tract (OFT), Circumpharyngeal ridge (CPr). Scale bar = 100μm.
The neural crest cells from somite-levels 3-6 prevent the migration of the neural crest cells from somite-levels 1-2 into the stomach
We hypothesized that the failure of neural crest cells from the level of somites 4-7 to migrate to the heart during normal development could be the result of contact inhibition or population pressure generated by the neural crest cells that have already migrated from the level of somites 1-3. To determine if there is a role for endogenous neural crest cells from the level of somites 1-3 in restricting the migration of the more posterior neural crest cells into the heart, we ablated the neural tube from the level of somites 1-3 in stage-10 embryos and concurrently labeled the remaining neural tube from the level of somites 4-7 by electroporating GFP. By stage 20, in the absence of neural crest cells from the level of somites 1-3, the neural crest cells from the level of somites 4-7 are still missing from pharyngeal arches 3-6 and the heart (Figure 8). This suggests that the neural crest cells from the level of somites 1-3 do not block the anterior migration of posterior vagal crest into the pharyngeal arches and the heart.
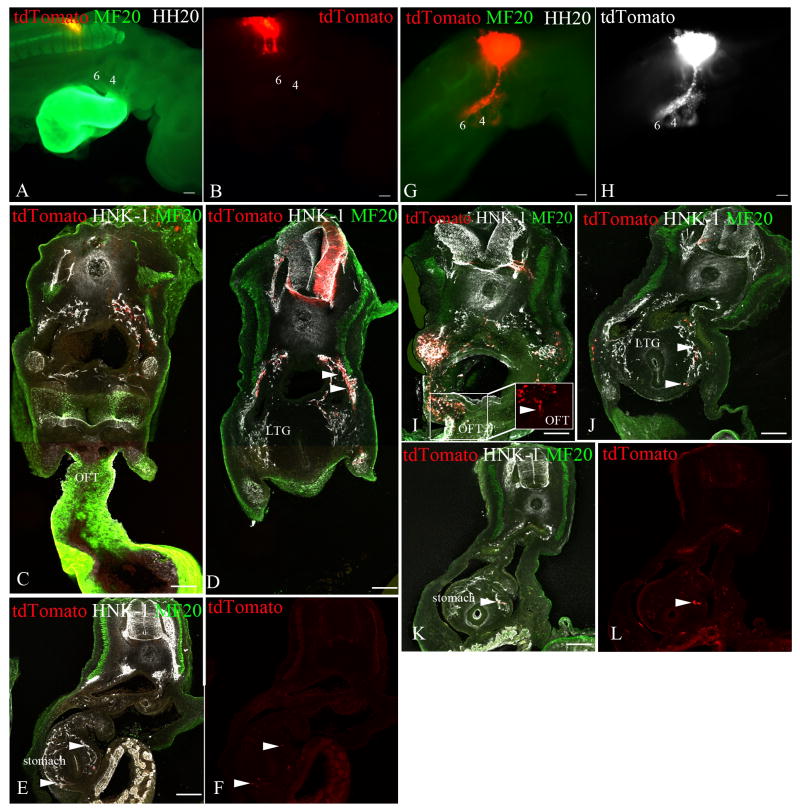
Figure 8. Effects of neural crest interactions on migratory behavior.
To address the effect of the neural crest cells from somite-levels 1-3 on the migration of the neural crest cells from somite-levels 5-7, and vice-versa, we performed partial neural tube ablation with concurrent labeling of the remaining neural tube with tdTomato by electroporation. The migrating the neural crest (tdTomato, red A-B, G and white H), also labeled with HNK-1 (white), was analyzed forty-eight hours later [stage 20]. The myotome is labeled with MF20 (green) to identify the location of the somites. Whole-mount representations of stage-20 embryos are in the top panels, and representative cross-sections at the levels of the outflow tract (C and I), the laryngotracheal groove (D and J), and the stomach (E-F and K-L) are in the lower panels.
(A-F) The neural tube from somite-levels 1-3 from a stage-10 embryo was removed, whereas the neural tube at somite-level 5-7 was fluorescently labeled. Forty-eight hours later [stage 20], the labeled neural crest cells still do not migrate anteriorly into the pharyngeal arches 4-6 (labeled 4 and 6 in A and B) or the outflow tract (C), but they still populate the foregut (arrowheads D) as far posteriorly as the stomach (arrowheads E,F). (G-L) The neural tube from somite-levels 3-7 from a stage-10 embryo was removed and the neural tube from the level of somites 1-2 was fluorescently labeled. Forty-eight hours following the procedure, the labeled neural crest cells colonize the pharyngeal arches 4-6 (labeled 4 and 6 in G and H), the outflow tract (arrowhead I), and the anterior foregut (arrowheads J), as they normally do, but they also migrated posteriorly into the stomach (arrowhead K,L), which they do not normally colonize.
Pharyngeal arch 6 (PA6), Laryngotracheal groove (LTG), Esophagus (E), Outflow tract of the heart (OFT). Scale bar = 100μm.
Conversely, to determine if the endogenous crest from somites 3-6 prevent neural crest cells from somite level 1-2 from migrating into the stomach, we ablated the neural tube from the level of somites 3-6 and simultaneously labeled the remaining neural tube from the level of somites 1-2 in stage-10 embryos. Under these conditions, the neural crest cells from the level of somites 1-2 colonize the pharyngeal arches and the outflow tract, and also migrate posteriorly into the stomach (Figure 8). We conclude that the neural crest cells from the level of somites 3-6 prevent the migration of the neural crest cells from the level of somites 1-2 as far as posterior as the stomach.
Discussion
How neural crest cells find their way to their proper location is a matter of considerable importance to the embryo and still debated in the scientific community. At the trunk level, only fate-specified melanoblasts can take the dorsolateral pathway, whereas neural crest cells that are restricted to the neural or glial lineage are prevented from entering the dorsolateral pathway. There is also evidence suggesting that fate-restricted neural and glial precursors are directed to the appropriate sites in the ventral pathway (Harris and Erickson, 2007). For example, George et al. (2007) have discovered that different subsets of sensory neurons are specified prior to migration and they take either an ipsilateral or contralateral direction to arrive at the developing dorsal root ganglion. Similarly, a subset of ventrally migrating neural crest cells express the receptor CXCR4, are attracted to a source of the ligandSDF-1, near the dorsal aorta, and are destined to become the core of the sympathetic ganglia (Kasemeier-Kulesa et al., 2010). At the cranial level, neural crest cells largely take the dorsolateral pathway beneath the ectoderm (Noden, 1988) and although there may be some cranial neural crest cells that are specified at the time they initiate migration, heterotopic and heterochronic transplantation experiments (Baker et al., 1997) reveal that the mesencephalic crest are plastic in their behavior and that development is normal when early- and late-migrating neural crest cells are exchanged.
We explored the possibility that neural crest cells from the vagal level might be directed to the appropriate target by molecular changes that occur during specification. We made the following discoveries: 1) Tissue culture studies suggest that there is some developmental bias of neural crest cells depending upon the order in which they leave the neural tube. Most early migrators develop into myofibroblasts, late migrators into pigment cells, and an equal number of neural and glial cells emigrate at all times. This pattern of developmental bias correlates with the timing of the neural crest cells dispersing into the dorsolateral vs. ventral pathways, at least at somite levels 1-4. 2) Neural crest cells that will populate the pharyngeal arches and heart initiate migration in the dorsolateral pathway and do not cross over into the ventral pathway at the circumpharyngeal ridge. Conversely, neural crest cells that give rise to the neurons and glial cells of the peripheral nervous system and the enteric nervous system initiate their migration in the ventral pathway and do not normally cross over into the arches at the circumpharyngeal ridge. 3) Despite the correlation between developmental bias and pathway choice, transplantation experiments do not suggest a rigid molecular control of pathway determination depending on order of migration. For example, neural crest cells from the axial level of somites 5-7, which normally migrate ventrally and do not migrate to the heart, still migrate ventrally when grafted to somite level 1-3 and not dorsolaterally, as the host cells do, and yet will arrive in the heart by taking an atypical pathway. Our results suggest that there are some migratory differences in neural crest cells that arise from various regions of the vagal level, but that the pathways are not rigidly assigned, as at the trunk level.
What are the pathways at the vagal level?
Most early studies of the vagal neural crest using chick/quail chimeras (Le Douarin and Teillet, 1973; Le Lievre and Le Douarin, 1975) determined the final destination of the neural crest from this region but not the pathways they took or differences in pathways depending on the somite level from which they originated. Later detailed studies of the cardiac neural crest using HNK-1 and DiI (Kuratani and Kirby, 1991) and E/C8 (Kuratani and Kirby, 1992) labeling described the pathways taken by the circumpharyngeal crest, but did not address whether the pathways to the heart and the gut overlapped or were completely separate. In order to define the pathways more completely we introduced several experimental strategies, including: labeling of the premigratory neural crest at individual somite levels; early vs. late labeling of premigratory neural crest cells to follow separately the cell populations that migrate dorsolaterally and ventrally; and simultaneous labeling of the somites and the neural crest cells in order to identify unequivocally the position of the somites, since somite 1 eventually disintegrates (Huang et al., 1997). We resolved the timing of migration into the dorsolateral and ventral pathway as well as the destinations (i.e. heart or gut) associated with these migratory pathways. We conclude that: 1) the ectomesenchymal contribution to the pharyngeal arches 3-6 originates from the level of somites 1-4 and these neural crest cells migrate in the dorsolateral path; 2) the cardiac neural crest cells originate from the level of somites 1-3 (and also the post-otic crest, which we did not study) and migrate through the pharyngeal arches; 3) the enteric neural crest cells are derived primarily from the neural crest cells from the level of somites 3-6 that take the ventral path.
Importantly, we observe a minimal cross-over between the dorsolateral and the ventral pathways at the circumpharyngeal ridge, indicating that the dorsolateral pathway is largely reserved for ectomesenchyme and the ventral pathway for nervous system. An exception to this generality is a subpopulation of enteric neural crest cells that are derived from the dorsolaterally-migrating neural crest cells from the level of somites 1-4, and which are seeded medially from the pharyngeal arches. Even in this latter case, the neural crest cells are still not in the ventral pathway.
Mouse and chick neural crest cells behave differently at the trunk level, and this seems to be the case at the vagal level as well. Chan and colleagues (2004) labeled mouse neural crest cells with DiI or gold-conjugated WGA and observed that at the vagal level, cell migration occurred simultaneously in both the ventral and dorsolateral pathway (as opposed to migrating initially in the dorsolateral pathway, as we have reported here). Interestingly in the splotch mutant mouse, which shows severe defects in heart development, neural crest cells were not found in the dorsolateral pathway. This suggests that even in the mouse, those crest cells that migrate dorsolaterally are critical for normal heart development.
Segmental migration of the vagal neural crest cells begins at the level of somite 2
Previous reports have addressed the segmental migration of the vagal neural crest cells through the anterior half of the somite, but the results do not agree about where this segmental behavior begins. Lim et al. (1987) show, by HNK-1 immunolabeling, that segmental migration of the neural crest cells begins at the level of somite 2 in stage-15 embryos. Kuratani and Kirby (1991 and 1992) reported that the segmental migration in the ventral pathway begins at the level of somite 3 in stage-13 embryos, using similar antibody labeling. More recently, using a lipophilic dye to label the neural tube, Ferguson and Graham (2004) claimed that segmental migration is initiated in somite 4 in stage-14/15 embryo. In our study, we followed the migration of the vagal neural crest cells by labeling them with GFP and co-labeled the somites with a muscle marker. The sections were subsequently analyzed by confocal microscopy. We found that the neural crest cells migrate in a segmental fashion through the anterior half of the somite starting with somite 2, in accord with Lim et al. (1987). Our conclusions may differ from that of Kuratani and Kirby (1991 and 1992) and Ferguson and Graham (2004) because we labeled neural crest cells with a longer-lasting fluorophore, and we visualized the embryos with confocal microscopy, which allowed us to observe the lesser number of neural crest cells in the anterior somites and to distinguish whether they were in the somite or the intersomitic space. Additionally we were able to keep track of somite number even after somite 1 is no longer recognizable morphologically, using a skeletal-muscle-specific marker.
Are the vagal neural crest cells specified prior to emigration from the neural tube?
Recent evidence suggests that most neural crest cells are not pluripotent when they initiate migration, but rather display some degree of fate-restriction. Henion and Weston (1997) showed in the chick that virtually all trunk neural crest cells are developmentally restricted at the time they initiate migration. By labeling individual cultured neural crest cells, they discovered that the first cells to migrate are either neurogenic or gliagenic, whereas the last to leave are melanogenic. Similar tissue culture studies have not been done using cranial neural crest cells, but when early- and late-migrating neural crest cells are reciprocally exchanged in vivo (Baker et al., 1997), development is normal, suggesting that as a population either these cells are not developmentally restricted or that they are extremely plastic.
In this study we show, by serially replating neural tubes, that there is developmental bias, at a population level, in the vagal neural crest. The first neural crest cells to migrate from neural tubes taken from either somite level 1-3 or from 4-7 differentiate largely into alpha-smooth-muscle-actin-positive cells (which populate the aorticopulmonary septum of the outflow tract of the heart and the great vessels (Beall and Rosenquist, 1990; Rosenquist and Beall, 1990). This was not surprising since Kirby et al. (1983) discovered, by ablation experiments, that neural crest cells from the level of somites 1-3 are the only neural crest cells that can give rise to elements of the heart, therefore also suggesting early fate restriction. Additionally, previous limit-dilution clonal analysis of the vagal crest demonstrated that there are two lineage-committed cells: smooth muscle and pigment cells (Ito and Sieber-Blum, 1991; Youn et al., 2003; Sieber-Blum, 2004). What is surprising is that the initial wave of neural crest cells from somite level 5-7, which do not normally migrate to the heart or the pharyngeal arches, are also biased towards a smooth muscle fate and have the capability of invading the pharyngeal arches and the heart when grafted to a more anterior axial level (our data). Under normal in vivo circumstances, we do not know how the first wave of migrating neural crest cells at somite level 5-7 differentiate since they do not contribute to the pharyngeal derivatives or the heart. Similarly, the last cells to migrate differentiate into pigment cells, which we had shown previously (Reedy et al., 1998b). Curiously, we saw a small percentage of pigment cells develop from the first wave of migration in culture, which is consistent with recent reports that pigment cells in the heart are associated with repression of atrial arrhythmias (Levin et al., 2009).
We observe a differential colonization of the foregut by neural crest cells that migrate along the dorsolateral or the ventral pathway: the former populate the lateral region of the foregut, whereas the latter occupy the dorsal and lateral regions. Kuratani and Kirby (1992) have also reported two subpopulations of enteric neural crest based on their HNK-1 and E/C8 immunoreactivity. Specifically, they show that the circumpharyngeal crest that fills the pharyngeal arches are E/C8-positive and, from fixed tissue sections, that they occupy the lateral and ventral region of the gut whereas the ventrally-migrating cells are E/C8-negative and populate the dorsolateral region of the gut. Together, these data suggest that: 1) there are vagal neural crest cells prespecified as enteric neural crest cells, and 2) that these cells take specific pathways to reach different regions of the gut.
Does early specification dictate migratory pathway?
Although there is clear evidence of developmental bias from our tissue culture studies, we wished to address directly whether this bias might confer behavioral differences. Our heterotopic transplant studies show that there are some migratory differences between early vs. late-migrating neural crest and between neural crest cells arising from somite level 1-3 and those from 5-7.
When early-migrating neural crest cells from somite level 5-7 are grafted in place of the early migrating neural crest cells at somite level 1-3, the grafted cells do not enter the dorsolateral path and instead migrate ventrally. Interestingly, even though the transplanted cells are developmentally biased toward the smooth muscle lineage, based on our tissue culture studies, they do not enter the usual pathway at somite level 1-3. This is in contrast to the early-migrating neural crest cells from somite levels 1-3, which also exhibit a bias toward the smooth muscle lineage, but whose migratory behavior changes to that of the endogenous neural crest cells when heterotypically grafted to the level of somites 5-7. The migratory plasticity of the neural crest cells from somite-levels 1-3 that we describe is consistent with a report from Boot et al., (2003) where they report two temporally-separated cardiac crest cell populations (early and late from somite-levels 1-3) whose apparent developmental bias does not impart differences in cell behavior. Nevertheless, after migrating ventrally, these cells cross over to the dorsolateral path at the circumpharyngeal ridge, invade the pharyngeal arches, and eventually reach the heart. Therefore, even though the grafted cells do not initially use the dorsolateral path, they still colonize the heart by taking an atypical pathway. Thus, at least some aspects of pathfinding are cell autonomous in this developmentally biased subpopulation of neural crest cells. We do not know what attracts the cardiac neural crest to the outflow tract of the heart, but the presence of Plexin-A2, Plexin-D1 and neuropilin-1 receptors on the cardiac neural crest and its associate ligand, Semaphorin 3C, in the outflow tract suggest that these neural crest cells may find their way to the heart by chemotaxis (Toyofuku et al., 2008). FGF8, expressed in the pharyngeal endoderm and ectoderm (Wendling et al., 2000; Farrell et al., 2001), has recently been shown to be a chemotactic signal to the cardiac neural crest cells as they migrate into the caudal pharyngeal arches (Sato et al., 2011). In the aforementioned report, the authors show that the cardiac neural crest cells migrate towards an ectopic source of FGF8 in vivo, a response is mediated by FGFR1 and 3, and that this signaling activates the Ras (Mek/Erk, p38). This leaves unanswered why neural crest cells from the axial level of somites 5-7 do not normally find their way to the outflow tract. One possibility is that migration anteriorly is blocked by neural crest cells that have migrated from somite level 1-3. However, when we ablate the more anterior neural tube, neural crest cells from somite level 5-7 are not diverted anteriorly to the heart. These results suggest that either there is some morphological block to anterior migration, or that the chemotactic cues do not diffuse far enough to attract the more posterior neural crest cells.
A second difference in migratory behavior is the enhanced ability of neural crest cells from somite-levels 3-6 to migrate along the developing gut. During normal development, we show that the more anterior neural crest cells (from the level of somites 1-2) only invade the anterior foregut, up to the esophagus and the lung buds regions, whereas the neural crest cells from somite-levels 3-6 migrate into the foregut, as far posteriorly as the stomach by stage 20. Our data are consistent with a previous report from the Burns laboratory, which showed that neural crest cells from the level of somites 3-5 are the major contributor to the enteric nervous system (Burns et al., 2000). A later study extended these data by demonstrating that the neural crest cells from somite-level 3 are able to populate the entire gut (Barlow et al., 2008). However, heterotypic transplantations were not performed in either of these studies. Here, we show that when these two subpopulations are heterotopically exchanged, the neural crest from somite-levels 1-2 can now migrate into the stomach. However, neural crest from somite-levels 4-6, even when transplanted to the more anterior level, still migrate along the length of the foregut into the stomach. In addition, we show that the neural crest cells from the level of somites 3-6 prevent the neural crest cells from somite-levels 1-2 from entering the stomach. The most parsimonious explanation is that, in addition to population pressure, there is an environmental block to the more anterior neural crest, which can be overcome by the posterior neural crest. Since RET has been shown to enhance invasive behavior of the vagal neural crest over the sacral crest (Delalande et al., 2008), potentially there is a difference in RET expression in the anterior vs. posterior vagal neural crest that accounts for this difference in migratory behavior.
The vagal neural crest as a transition between the head and the trunk
The border between rhombomere 8 and the spinal cord is considered to be at the intersomitic space between somite 4 and 5 (Kalcheim and Le Douarin, 1986; Lumsden, 1991; Muller and O'Rahilly, 1994). This is also the position of the occipitocervical junction (but see Christ and Wilting, 1992; Ferguson and Graham, 2004, who suggest that the boundary is between somites 5-6). The vagal neural crest from somite level 1-7 appears to be a molecular and behavioral interface between the cranial and trunk neural crest, and reflects an evolutionary transition from the brain to the spinal cord. The surrounding embryonic structures, namely the somites and the pharyngeal arches, are similarly superimposed in this region to suggest an overlapping transitional zone (the posterior arch 6 is at the same axial level as the somite 4/5 boundary (Kuratani, 1997).
Neural crest behavior can also help to define this transition zone. We propose the following to extend the description of the vagal neural crest as a head-trunk hybrid cell population. First, the neural crest cells from the level of somites 1-4 begin their migration in the dorsolateral pathway, which reflects a pattern typical of the cranial neural crest. Conversely, the neural crest cells from the level of somite 5 and posterior begin their migration in the ventral pathway, like the trunk neural crest cells, which favors the location of the head-trunk neural crest interface at the boundary between somite 4/5, as proposed by Ferguson and Graham (2004). This is contradictory to the view of Kuratani (1997), who proposed the dorsal interface between the head and spinal chord is at the somite 1/2 boundary based on his view that the neural crest cells that migrate into the arches do so by migrating around the somites rather than over them. We clearly show that neural crest cells do migrate dorsolaterally over somites 1-4 to reach the branchial arches and the heart. Secondly, we propose that the hybrid nature of the vagal neural crest cells can be based on the contribution to the pharyngeal mesenchyme (a characteristic found at the cranial level), which we show originates from the neural crest cells at the level of somites 1-4, and not somite 1-5 as reported by Shigetani et al. (1995). Third, our heterotopic transplantations suggest that cell-autonomous guidance signals direct the neural crest cells from the level of somites 5-7 into the ventral pathway. This is in contrast to the neural crest cells from a more anterior level, which are guided into pathways depending on the surrounding environmental cues. The latter reflects a cranial behavior (Baker et al., 1997). This differs from the trunk neural crest cells, which are lineage-restricted at the time they migrate, and whose behavior is dependent on their lineage. Together these observations show that the vagal crest cells represent a transitional zone where cranial neural crest behavior morphs into trunk neural crest behavior. Understanding the molecular, cellular and behavioral differences between these three populations of neural crest cells will be of assistance when trying to understand the evolution of the head-neck junction.
Experimental Procedures
Embryo Preparation
Fertilized White Leghorn chicken (Gallus gallus domesticus) eggs (Animal Sciences, UC Davis) were incubated at 37°C in a humidified incubator until Hamburger and Hamilton stage 10 or stage 13 (Hamburger and Hamilton, 1951). The eggs were windowed after removing 4ml of albumin using a 26-gauge needle. A solution of 10% India ink (Rapidograph, Ultradraw 3785-F) in phosphate buffer saline (PBS) (Fisher Sciences) was injected below the embryo for visualization.
Quail neural crest cultures
Quail neural tubes from the vagal level (stage-10 embryos; somite-level 1-7) were dissected as described previously and divided into two segments between somites 3 and 4 (Reedy et al., 1998a). For comparison, we also cultured trunk segments taken from the axial level of the six last-formed somites from stage-14 embryos. The segments were cultured in 35-mm culture dishes for twelve hours, during which time the neural crest cells migrated onto the dishes. Neural tubes were then peeled away with tungsten needles and replated in a fresh dish for another twelve hours. This replating was performed a total of three times, which resulted in cultures of progressive waves of neural crest cell migration. The resulting neural crest cells were cultured for ninety-six hours (as described in Reedy et al. (1998a)) before fixation and immunohistochemical analysis. There were at least seven neural tubes for each time point.
Lineage analysis of cell cultures
To determine the percentage of myofibroblasts (α-smooth-muscle-actin-immunoreactivity), glial (7B3-immunoreactivity), neuronal (16A11-immunoreactivity) and pigment (presence of melanin) cells, cell counts were performed as follows. Each field of analysis extended from the location of the neural tube, prior to its removal, to the lateral edge of the cell outgrowth. The entire region was captured using a Leica DM5000B microscope and a 20× objective (2048 × 2048 resolution). Depending on the extent of cell dispersal, each field encompassed 4-6 images. We recorded at least two fields per outgrowth. The statistical analysis was performed using GraphPad Prism version 5.
Neural crest cell labeling in vivo
Neural tubes at the vagal level (somite-level 1 through 7) were labeled using a pcaβ-GFP construct (gift from Dr. Jonathan Gilthorpe, King's College, London), or a pcaβ-TdTomato construct (the TdTomato sequence, a gift from Dr. Robert Tsien, was inserted into the pcaβ vector). After windowing the eggshell, the vitelline membrane was torn open above the neural tube at the desired axial level using a sharpened tungsten needle. The construct (2mg/ml) was injected into the lumen of the neural tube at the desired axial level in stage-10 embryos using a pulled borosilicate glass needle (Sutter; BF100-50-10, Novato, CA). Thin (26-gauge or finer) platinum wire was held on either side of the neural tube with electrode holders (Genetronics, Inc., San Diego) and a current was applied (12Volts, 3× 50ms pulses at 1ms intervals) using an electroporator (Electro Square Porator ECM 830; BTX). The electrodes were buffed or sanded so that the tips were beveled or pointed and could be precisely positioned alongside the neural tube. The eggs were sealed with adhesive tape and re-incubated at 37°C in a humidified incubator for forty-eight hours or seventy-two hours.
Neural crest cell labeling at a single-somite level
The neural tube at an individual somite level (somites 1 through 7) was labeled using the same constructs described above by electroporation using custom-made electrodes. The electrodes consisted of a microinjection needle (Sutter Instrument) through which a thin platinum wire (thinner than the wire described above), approximately the width of a somite, was inserted. The end of the needle was sealed with nail polish after leaving a portion of the platinum wire extending from the needle. The needle was held using a needle holder (Fine Science Tools). An electrical wire connected to the electroporator was then inserted in the needle holder to contact the platinum wire. After windowing the eggshell, the vitelline membrane was opened. The GFP construct (2mg/ml) was injected into the lumen of the neural tube as described above. Two platinum wires were placed on top of the neural tube adjacent to the somite level of interest and a current was applied (12Volts, 2× 45ms pulses at 1ms intervals). The eggs were sealed with adhesive tape and re-incubated.
Neural tube transplantation
In all transplantation experiments, the quail embryos (Coturnix coturnix japonica) were incubated at 37°C in a humidified incubator until the equivalent of Hamburger and Hamilton stage 10 or stage 13. The embryos were then collected and tissue sections spanning the entire vagal region were removed and placed in Pancreatin (Sigma) to loosen the neural tubes from the adjacent somites and overlying ectoderm. Using sharpened tungsten needles, the neural tubes were peeled away from somites and ectoderm, and transferred to Ham's F12 medium (Invitrogen) supplemented with horse serum to inhibit Pancreatin activity. The host chicken embryos were windowed and the neural tubes spanning the regions of somites 1-2 or somites 5-7 were removed using sharpened needles. Briefly, incisions were made between the somites and neural tube, followed by cuts perpendicular to the anterior-posterior axis. The neural tube was then removed by teasing it away from the notochord. Once the host neural tube was removed, the donor quail neural tube was maneuvered into place. The eggs were then sealed with adhesive tape and re-incubated at 37°C.
Immunohistochemistry
Fertile chicken eggs were incubated at 37°C, and the embryos were collected at the appropriate developmental stages. Embryos were fixed 2-3 hours in 4% paraformaldehyde at room temperature and then rinsed several times in phosphate buffer saline (PBS) (Fisher Scientific). For antibody labeling, the embryos or tissue sections were incubated with primary antibody [1:10 MF20 (Developmental Studies Hybridoma Bank); 1:10 HNK-1 (supernatant made from cells purchased from ATCC); 1:10 QCPN (Developmental Studies Hybridoma Bank)] in PBS+ 3% BSA (Sigma) +0.1% TritonX (Sigma) and 0.1% Tween20 (Sigma) at 4°C overnight. The primary antibody was then washed off with PBS and the embryos were incubated with secondary antibody [1:1000 Goat anti-mouse Alexa 488 (Invitrogen); 1:1000 Goat anti-mouse Alexa 555 (Invitrogen); 1:1000 Goat anti-mouse Cy5 (Jackson Immunoresearch)] in the same solution used previously overnight at 4°C. The embryos were then washed extensively with PBS at room temperature.
Tissue Sectioning
For paraffin sections, the embryos were dehydrated in an alcohol gradient (50% to 100%), cleared with Histoclear, embedded in paraffin, and then sectioned at 7μm. The sections were rehydrated in an alcohol gradient (100% to 15%), rinsed in PBS, mounted with Fluoromount-G mounting medium (Southern Biotech), and imaged using a Leica DM5000 microscope. For thick tissue sections, the embryos were embedded in 4% PBS/low melting agar (Invitrogen) and sectioned in cold PBS using a vibratome 1000plus (Vibratome) at 300-μm intervals. The sections were then mounted with Fluoromount-G mounting medium (Southern Biotech) and imaged using an Olympus Fluoview FV1000 confocal laser scanning microscope (Olympus).
Supplementary Material
Acknowledgments
We thank Drs. Richard Tucker, Lesilee Rose, Melissa Harris and Aaron Thomas for critical comments on the manuscript. We also thank Dr. Jian Zuo from St. Jude Children's Research Hospital for providing additional experimental support. Research was funded by grants from the American Heart Association (0455041Y; 0655034Y) and a grant from the NIH (R01 GM53258) to CAE. BK was supported, in part, on a training grant from the NIH (T32 GM7377).
References
- Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development. 1997;124:3077–3087. doi: 10.1242/dev.124.16.3077. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Beall AC, Rosenquist TH. Smooth muscle cells of neural crest origin form the aorticopulmonary septum in the avian embryo. Anat Rec. 1990;226:360–366. doi: 10.1002/ar.1092260313. [DOI] [PubMed] [Google Scholar]
- Boot MJ, Gittenberger-De Groot AC, Van Iperen L, Hierck BP, Poelmann RE. Spatiotemporally separated cardiac neural crest subpopulations that target the outflow tract septum and pharyngeal arch arteries. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1009–1018. doi: 10.1002/ar.a.10099. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Champeval D, Le Douarin NM. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000;219:30–43. doi: 10.1006/dbio.1999.9592. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Delalande JM, Le Douarin NM. In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development. 2002;129:2785–2796. doi: 10.1242/dev.129.12.2785. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec. 2001;1:16–28. doi: 10.1002/1097-0185(20010101)262:1<16::AID-AR1007>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Chan WY, Cheung CS, Yung KM, Copp AJ. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development. 2004;131:3367–3379. doi: 10.1242/dev.01197. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Delalande JM, Barlow AJ, Thomas AJ, Wallace AS, Thapar N, Erickson CA, Burns AJ. The receptor tyrosine kinase RET regulates hindgut colonization by sacral neural crest cells. Dev Biol. 2008;313:279–292. doi: 10.1016/j.ydbio.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Epstein ML, Mikawa T, Brown AM, McFarlin DR. Mapping the origin of the avian enteric nervous system with a retroviral marker. Dev Dyn. 1994;201:236–244. doi: 10.1002/aja.1002010307. [DOI] [PubMed] [Google Scholar]
- Erickson C, Duong T, Tosney K. Descriptive and experimental analysis of the dispersion of neural crest cells along the dorsolateral path and their entry into ectoderm in the chick embryo. Dev Biol. 1992;151:251. doi: 10.1016/0012-1606(92)90231-5. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Goins TL. Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development. 1995;121:915–924. doi: 10.1242/dev.121.3.915. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Burch JL, Wallis K, Rowley L, Kumiski D, Stadt H, Godt RE, Creazzo TL, Kirby ML. FGF-8 in the ventral pharynx alters development of myocardial calcium transients after neural crest ablation. The Journal of clinical investigation. 2001;107:1509–1517. doi: 10.1172/JCI9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CA, Graham A. Redefining the head-trunk interface for the neural crest. Dev Biol. 2004;269:70–80. doi: 10.1016/j.ydbio.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Froriep A. Ueber ein Ganglion des Hypoglossus und Wirbelanlagen in der Occipitalregion. Beitrag zur Entwickelungsgeschichte des Säugethierkopfes. chiv Anat Physiol Anat. 1889;15 [Google Scholar]
- Froriep A, Beck W. Ueber des Vorkommen dorsaler Hypoglossus-wurzeln mit Ganglion in der Reihe der Säugethiere. Anat Anz. 1895;10:688–696. [Google Scholar]
- George L, Chaverra M, Todd V, Lansford R, Lefcort F. Nociceptive sensory neurons derive from contralaterally migrating, fate-restricted neural crest cells. Nat Neurosci. 2007;10:1287–1293. doi: 10.1038/nn1962. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. Dev Biol. 1951;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Harris ML, Erickson CA. Lineage specification in neural crest cell pathfinding. Dev Biol. 2007;236:1–19. doi: 10.1002/dvdy.20919. [DOI] [PubMed] [Google Scholar]
- Harris ML, Hall R, Erickson CA. Directing pathfinding along the dorsolateral path - the role of EDNRB2 and EphB2 in overcoming inhibition. Development. 2008;135:4113–4122. doi: 10.1242/dev.023119. [DOI] [PubMed] [Google Scholar]
- Henion PD, Weston JA. Timing and pattern of cell fate restrictions in the neural crest lineage. Development. 1997;124:4351–4359. doi: 10.1242/dev.124.21.4351. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Ordahl CP, Christ B. The fate of the first avian somite. Anat Embryol (Berl) 1997;195:435–449. doi: 10.1007/s004290050063. [DOI] [PubMed] [Google Scholar]
- Ito K, Sieber-Blum M. In vitro clonal analysis of quail cardiac neural crest development. Dev Biol. 1991;148:95–106. doi: 10.1016/0012-1606(91)90320-3. [DOI] [PubMed] [Google Scholar]
- Kalcheim C, Le Douarin NM. Requirement of a neural tube signal for the differentiation of neural crest cells into dorsal root ganglia. Dev Biol. 1986;116:451–466. doi: 10.1016/0012-1606(86)90146-6. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, McLennan R, Romine MH, Kulesa PM, Lefcort F. CXCR4 controls ventral migration of sympathetic precursor cells. J Neurosci. 2010;30:13078–13088. doi: 10.1523/JNEUROSCI.0892-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML. Plasticity and predetermination of mesencephalic and trunk neural crest transplanted into the region of the cardiac neural crest. Dev Biol. 1989;134:402–412. doi: 10.1016/0012-1606(89)90112-7. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Stewart DE. Neural crest origin of cardiac ganglion cells in the chick embryo: identification and extirpation. Dev Biol. 1983;97:433–443. doi: 10.1016/0012-1606(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Turnage KL, 3rd, Hays BM. Characterization of conotruncal malformations following ablation of “cardiac” neural crest. Anat Rec. 1985;213:87–93. doi: 10.1002/ar.1092130112. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL. Role of neural crest in congenital heart disease. Circulation. 1990;82:332–340. doi: 10.1161/01.cir.82.2.332. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL. Neural crest and cardiovascular patterning. Circ Res. 1995;77:211–215. doi: 10.1161/01.res.77.2.211. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Spatial distribution of postotic crest cells defines the head/trunk interface of the vertebrate body: embryological interpretation of peripheral nerve morphology and evolution of the vertebrate head. Anatomy Embryology. 1997;195:1–13. doi: 10.1007/s004290050020. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Tanaka S, Ishikawa Y, Zukeran C. Early development of the hypoglossal nerve in the chick embryo as observed by the whole-mount nerve staining method. Am J Anat. 1988;182:155–168. doi: 10.1002/aja.1001820206. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Kirby ML. Initial migration and distribution of the cardiac neural crest in the avian embryo: an introduction to the concept of the circumpharyngeal crest. Am J Anat. 1991;191:215–227. doi: 10.1002/aja.1001910302. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Kirby ML. Migration and distribution of circumpharyngeal crest cells in the chick embryo. Formation of the circumpharyngeal ridge and E/C8+ crest cells in the vertebrate head region. Anat Rec. 1992;234:263–280. doi: 10.1002/ar.1092340213. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Miyagawa-Tomita S, Kirby ML. Development of cranial nerves in the chick embryo with special reference to the alterations of cardiac branches after ablation of the cardiac neural crest. Anat Embryol (Berl) 1991;183:501–514. doi: 10.1007/BF00186439. [DOI] [PubMed] [Google Scholar]
- Le Douarin L, Kalcheim C. The neural crest. 2nd. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- Le Douarin N. The neural crest. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Le Lievre C. Rôle des cellules mésectodermiques issues des crêtes neurales céphaliques dans la formation des arcs branchiaux et du squelette viscéral. J Embryol Exp Morphol. 1974;31:453–477. [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Levin MD, Lu MM, Petrenko NB, Hawkins BJ, Gupta TH, Lang D, Buckley PT, Jochems J, Liu F, Spurney CF, Yuan LJ, Jacobson JT, Brown CB, Huang L, Beermann F, Margulies KB, Madesh M, Eberwine JH, Epstein JA, Patel VV. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J Clin Invest. 2009;119:3420–3436. doi: 10.1172/JCI39109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TM, Lunn ER, Keynes RJ, Stern CD. The differing effects of occipital and trunk somites on neural development in the chick embryo. Development. 1987;100:525–533. doi: 10.1242/dev.100.3.525. [DOI] [PubMed] [Google Scholar]
- Loring JF, Erickson CA. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev Biol. 1987;121:220–236. doi: 10.1016/0012-1606(87)90154-0. [DOI] [PubMed] [Google Scholar]
- Lumsden A. Cell lineage restrictions in the chick embryo hindbrain. Philos Trans R Soc Lond B Biol Sci. 1991;331:281–286. doi: 10.1098/rstb.1991.0017. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Muller F, O'Rahilly R. Occipitocervical segmentation in staged human embryos. J Anat. 1994;185(Pt 2):251–258. [PMC free article] [PubMed] [Google Scholar]
- Noden DM. An analysis of migratory behavior of avian cephalic neural crest cells. Dev Biol. 1975;42:106–130. doi: 10.1016/0012-1606(75)90318-8. [DOI] [PubMed] [Google Scholar]
- Noden DM. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev Biol. 1978;67:296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103(Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Raible DW, Eisen JS. Restriction of neural crest cell fate in the trunk of the embryonic zebrafish. Development. 1994;120:495–503. doi: 10.1242/dev.120.3.495. [DOI] [PubMed] [Google Scholar]
- Raible DW, Wood A, Hodsdon W, Henion PD, Weston JA, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev Dyn. 1992;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev Biol. 1998a;200:234–246. doi: 10.1006/dbio.1998.8963. [DOI] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA. Specification and migration of melanoblasts at the vagal level and in hyperpigmented Silkie chickens. Dev Dyn. 1998b;213:476–485. doi: 10.1002/(SICI)1097-0177(199812)213:4<476::AID-AJA12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Fawcett JW, Keynes RJ. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J Embryol Exp Morphol. 1985;90:437–455. [PubMed] [Google Scholar]
- Rosenquist TH, Beall AC. Elastogenic cells in the developing cardiovascular system. Smooth muscle, nonmuscle, and cardiac neural crest. Ann N Y Acad Sci. 1990;588:106–119. doi: 10.1111/j.1749-6632.1990.tb13201.x. [DOI] [PubMed] [Google Scholar]
- Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- Sato A, Scholl AM, Kuhn EB, Stadt HA, Decker JR, Pegram K, Hutson MR, Kirby ML. FGF8 signaling is chemotactic for cardiac neural crest cells. Dev Biol. 2011;354:18–30. doi: 10.1016/j.ydbio.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Aizawa S, Kuratani S. Overlapping origins of pharyngeal arch crest cells on the postotic hind-brain. Development Growth & Differentiation. 1995;37:733–746. doi: 10.1046/j.1440-169X.1995.t01-4-00011.x. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M. Cardiac neural crest stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:34–42. doi: 10.1002/ar.a.10132. [DOI] [PubMed] [Google Scholar]
- Suzuki HR, Kirby ML. Absence of neural crest cell regeneration from the postotic neural tube. Dev Biol. 1997;184:222–233. doi: 10.1006/dbio.1997.8529. [DOI] [PubMed] [Google Scholar]
- Teillet MA, Kalcheim C, Le Douarin NM. Formation of the dorsal root ganglia in the avian embryo: segmental origin and migratory behavior of neural crest progenitor cells. Dev Biol. 1987;120:329–347. doi: 10.1016/0012-1606(87)90236-3. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Erickson CA. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development. 2009;136:1849–1858. doi: 10.1242/dev.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H, Kumanogoh A, Kikutani H. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol. 2008;321:251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Ciment G, Thiery JP. Pathways of avian neural crest cell migration in the developing gut. Dev Biol. 1986;116:439–450. doi: 10.1016/0012-1606(86)90145-4. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Delarue M, Zada S, Boucaut JC, Thiery JP. Expression of the HNK-1/NC-1 epitope in early vertebrate neurogenesis. Cell Tissue Res. 1988;251:457–465. doi: 10.1007/BF00215855. [DOI] [PubMed] [Google Scholar]
- Wendling O, Dennefeld C, Chambon P, Mark M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development. 2000;127:1553–1562. doi: 10.1242/dev.127.8.1553. [DOI] [PubMed] [Google Scholar]
- Weston JA. Sequential Segregation and Fate of Developmentally Restricted Intermediate Cell-Populations in the Neural Crest Lineage. Current Topics in Developmental Biology. 1991;25:133–153. doi: 10.1016/s0070-2153(08)60414-7. [DOI] [PubMed] [Google Scholar]
- Yao KM, Samson ML, Reeves R, White K. Gene Elav of Drosophila-Melanogaster - a Prototype for Neuronal-Specific Rna-Binding Protein Gene Family That Is Conserved in Flies and Humans. Journal of Neurobiology. 1993;24:723–739. doi: 10.1002/neu.480240604. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Youn YH, Feng J, Tessarollo L, Ito K, Sieber-Blum M. Neural crest stem cell and cardiac endothelium defects in the TrkC null mouse. Mol Cell Neurosci. 2003;24:160–170. doi: 10.1016/s1044-7431(03)00125-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.