Abstract
Some bacteria utilize (semi)conductive iron-oxide minerals as conduits for extracellular electron transfer (EET) to distant, insoluble electron acceptors. A previous study demonstrated that microbe/mineral conductive networks are constructed in soil ecosystems, in which Geobacter spp. share dominant populations. In order to examine how (semi)conductive iron-oxide minerals affect EET paths of Geobacter spp., the present study grew five representative Geobacter strains on electrodes as the sole electron acceptors in the absence or presence of (semi)conductive iron oxides. It was found that iron-oxide minerals enhanced current generation by three Geobacter strains, while no effect was observed in another strain. Geobacter sulfurreducens was the only strain that generated substantial amounts of currents both in the presence and absence of the iron oxides. Microscopic, electrochemical and transcriptomic analyses of G. sulfurreducens disclosed that this strain constructed two distinct types of EET path; in the absence of iron-oxide minerals, bacterial biofilms rich in extracellular polymeric substances were constructed, while composite networks made of mineral particles and microbial cells (without polymeric substances) were developed in the presence of iron oxides. It was also found that uncharacterized c-type cytochromes were up-regulated in the presence of iron oxides that were different from those found in conductive biofilms. These results suggest the possibility that natural (semi)conductive minerals confer energetic and ecological advantages on Geobacter, facilitating their growth and survival in the natural environment.
Keywords: extracellular electron transfer, energy metabolism, microbial fuel cell, Geobacter, iron oxide
Some dissimilatory metal-reducing bacteria (DMRBs) are able to utilize extracellular insoluble electron acceptors (e.g., metal oxides and electrodes) for their respiration (7, 19, 49). This is termed extracellular electron transfer (EET) and recognized as important energy-conservation processes in natural and engineered environments (e.g., global carbon/metal cycling, bioremediation of toxic metals, and microbial fuel/electrolysis cells) (18–21, 55, 57).
Three distinct mechanisms for microbial EET have previously been proposed (7, 21, 51). The first mechanism is direct electron transfer (ET) mediated by outer-membrane (OM) c-type cytochromes (c-Cyts) (17, 25, 33, 38, 45). Microorganisms using this mechanism have to directly attach to solid materials, resulting in a limitation in the number of microbial cells that participate in this EET mechanism. The second is EET via self-secreted, naturally-occurring and/or artificially-supplemented soluble electron shuttles (28, 40, 54, 56). In this mechanism, microbial cells are able to perform long-range ET without the necessity of direct contact to solids, whereas their ET is limited by the diffusion of electron shuttles (51). The third is EET via conductive biofilms that are synthesized by microorganisms themselves. The molecular mechanisms conferring conductivity on biological matrices have been tentatively studied on a DMRB, Geobacter sulfurreducens, and it has been speculated that extracellular c-Cyts (30, 37, 46), extracellular polysaccharides (48), and conductive pili (3, 6, 27, 42, 43) are essential for the functions. EET based on conductive biofilms is considered to be the most efficient among the three mechanisms (51), since a large number of microbial cells in thick biofilms are able to respire distant solid electron acceptors without diffusion resistance (4, 43). Different DMRB may utilize different EET mechanisms, while each may also be able to utilize different mechanisms depending on environmental settings.
We have recently proposed another possibility for long-range ET, which is mediated by microbe/mineral conductive networks. In the presence of (semi)conductive iron-oxide nanoparticles, Shewanella strains formed microbe/mineral conductive networks and performed efficient EET (34). It was also demonstrated that microbe/mineral conductive networks were generated by soil microbes, in which Geobacter spp. (e.g., those closely related to Geobacter bremensis and Geobacter pelophilus) grew abundantly (14). Since (semi)conductive iron-oxide minerals are abundantly present in the natural environment (47, 57) and often biologically produced by DMRB themselves (2, 5, 24, 53), such EET is considered to largely contribute to the establishment and functioning of microbial communities. In addition, it has been found that Geobacter strains utilize (semi)conductive iron oxides for interspecies electron transfer, facilitating “electric syntrophy” with a nitrate reducer (16) or methanogenic archaea (15). These studies provide new insights into the ecological roles of Geobacter spp., whereas information is limited as to how (semi)conductive iron oxides affect the physiology of Geobacter populations.
The present study investigated respiratory interactions of Geobacter spp. with iron-oxide minerals in axenic cultures. We analyzed five Geobacter spp. (listed in Table 1) for their abilities to form microbe/mineral conductive networks. Furthermore, microscopic, electrochemical and transcriptomic analyses of G. sulfurreducens were performed to clarify the EET paths used in the absence or presence of (semi)conductive iron oxides, since this was the only strain that formed two different types of EET paths, i.e., conductive biofilm and microbe/mineral network.
Table 1.
Geobacter spp. used in this study
| Strain name | Clade in the family Geobacteraceae | Ferrihydrite reduction | Ability for EETa | |
|---|---|---|---|---|
|
| ||||
| Non-Fe | + Fe oxides | |||
| G. sulfurreducens | G. metallireducens | + | + | + |
| G. metallireducens | G. metallireducens | + | − | + |
| G. bremensis | Subsurface 1 | + | − | + |
| G. bemidjensis | Subsurface 1 | + | − | − |
| G. pelophilus | Subsurface 2 | + | − | + |
Current production (>10 μA cm−2) was observed or not (see Fig. 1).
Materials and Methods
Bacterial strains and culture conditions
G. sulfurreducens (DSM12127T), Geobacter metallireducens (DSM7210T), G. bremensis (DSM12179T), Geobacter bemidjensis (DSM16622T) and G. pelophilus (DSM12255T) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkukturen GmbH (Braunschweig, Germany). The Geobacter strains were routinely cultivated in PSN medium with acetate (10 mM) and fumarate (40 mM) as the electron donor and acceptor, respectively, with the exception of nitrate (20 mM) as an electron acceptor for G. metallireducens. PSN medium is composed of 10 mM NH4Cl, 1 mM KH2PO4, 0.1 mM MgCl2, 0.1 mM CaCl2, 0.04 mM MgSO4, 20 mM NaHCO3, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 0.01% Bacto yeast extract, and 1 mL L−1 each of vitamin solution, trace element solution and Se/W solution (9). Cultivation was conducted at 30°C under an atmosphere of (N2/CO2 80:20 [v/v]) without shaking. Ferrihydrite was prepared as described elsewhere (22). Concentrations of HCl-extractable Fe(II) were determined by the ferrozine method as described elsewhere (23).
Electrochemical cultivation
Electrochemical cultivation was conducted as described previously (14). A tin-doped In2O3 (ITO) glass electrode, an Ag/AgCl (KCl sat.) electrode and a platinum wire were used as working, reference and counter electrodes, respectively. After sterilization, the electrochemical cell was filled with the sterilized PSN medium without the vitamin solution. A sterilized, oxygen-free 1 M stock solution of sodium acetate was injected into the cell to give a final concentration of 10 mM. Nanoparticles of hematite and magnetite were synthesized according to previously described methods (11, 32), and injected into the cell with a final concentration of 10 mM as Fe atoms. After removing oxygen from the medium by bubbling with N2/CO2 gas, Geobacter cells grown as described above up to an early stationary phase (optical density at 600 nm [OD600] of approximately 0.3) were inoculated to give a final OD600 of 0.01. The working electrode was set at +0.2 V (vs. Ag/AgCl) throughout incubation using a HA-1510 potentiostat (Hokuto Denko), and the current was recorded. Cyclic voltammetry (CV) analysis was conducted using the potentiostat HSV-110 (Hokuto Denko) at a scan rate of 10 mV s−1. Photo-irradiation was conducted using a xenon lamp SX-UI501XQ (Ushio) as described previously (34). A cut-off (wavelength of >420 nm) glass filter was used to remove UV light from the light source.
Scanning electron microscopy (SEM) analysis
G. sulfurreducens cells precipitated on the ITO electrode were gently washed with HEPES buffer (0.1 M of HEPES, pH 7) and fixed with 2.5% glutaraldehyde in HEPES buffer for 3 hours. Fixed cells were washed three times with HEPES buffer, dehydrated using a graded series of ethanol solutions (30, 50, 75, 90, 95% [v/v]), and dried with a freeze-drying device VFD-21S (Vacuum device) according to the manufacturer’s instructions. The dried samples were coated with platinum and imaged using a VE-9800 microscope (Keyence).
Microarray analysis
Bacterial cells were collected from precipitates on an ITO electrode, when current density reached approximately 70% of the maximum. Total RNA was isolated using TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions. The quality of RNA was evaluated using an Agilent 2100 Bioanalyzer with RNA 6000 Pico reagents and RNA Pico Chips (Agilent Technologies) according to the manufacturer’s instructions. Specific oligonucleotides (60 mer) were designed for 3,402 genes (corresponding to 99.2% of the total predicted genes in the annotated genome of G. sulfurreducens [31]) by the eArray protocol (Agilent Technologies) and fabricated on slide glasses by SurePrint technology (Agilent Technologies). For each gene, 4 spots (with 4 different specific sequences) were printed in one array. A mean value of normalized signal intensities of 4 spots was used for expression analyses. Fluorescence labeling of cDNA, hybridization and scanning of a hybridized array were performed according to the method of two-color microarray-based gene-expression analysis as described previously (12, 13). To minimize dye biases, dyes (Cy3 and Cy5) were swapped for each replicate. RNA isolated from G. sulfurreducens cells under non-Fe conditions was used as the reference sample. Fluorescence-dye labeled cDNA for either +Hematite or +Magnetite cultures was mixed with oppositely labeled cDNA as the reference and hybridized with the probes on a microarray. RNA samples from three independent cultures were analyzed for each condition as biological replicates. Microarray data analysis was conducted by the methods described previously (12, 13) using GeneSpring GX ver. 10 (Agilent Technologies). The transcriptome data have been deposited in the CIBEX (Center for Information Biology gene EXpression) database under accession number CBX206.
Quantitative reverse transcription PCR (qRT-PCR) analysis
PCR primers used for qRT-PCR were designed by Nihon Gene Research Laboratories, and listed in Table S1. Quantitative gene expression analysis was performed by real-time RT-PCR using a LightCycler system and a LightCycler RNA Master SYBR Green I kit (Roche Applied Science) as described previously (12). Data of qRT-PCR analysis of the 16S rRNA gene were used as a reference for normalization of the gene expression values.
Results and Discussion
EET by Geobacter spp
Five Geobacter strains listed in Table 1 were grown in anaerobic bottles to compare their EET properties. G. sulfurreducens and G. metallireducens are members of the G. metallireducens clade in the family Geobacteraceae and have been extensively studied for their physiology and genetics as model DMRBs (1, 26, 31). G. bremensis and G. bemidjensis are affiliated with subsurface clade 1 in the family Geobacteraceae, while G. pelophilus is affiliated with the subsurface clade 2 (36, 50). While Geobacter spp. in the subsurface clades have often been detected as major members of various natural communities and enrichment cultures (8, 14), only limited information is available for their physiology.
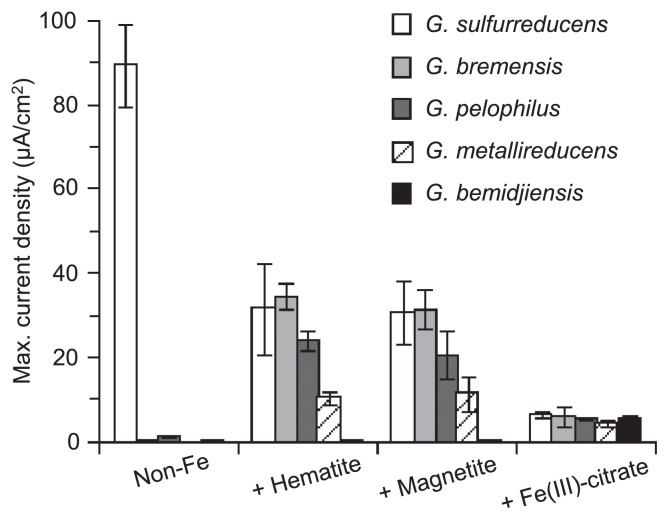
As the initial step in our investigation, their abilities to reduce insoluble, poorly crystalline iron oxides (ferrihydrite) were checked (Fig. S1). All reduced ferrihydrite as reported (19), confirming that they have abilities for EET. Next, in order to examine EET to electrodes (i.e., electric current generation), each strain was cultivated in an electrochemical cell with acetate (10 mM) as the sole electron donor and an ITO electrode (poised at +0.2 V vs. Ag/AgCl) as the sole electron acceptor. Maximum current densities were determined from current versus time curves (Fig. S2) and are summarized in Fig. 1. Under this condition (non-Fe control), G. sulfurreducens achieved a large current density (up to 90 μA cm−2), while the others showed much lower current values (0.4 to 1.2 μA cm−2). The ability of G. sulfurreducens to generate such a large current has frequently been reported and appears to be conferred by the production of thick conductive biofilm (27, 37, 43).
Fig. 1.
Current generation by the five Geobacter strains in electrochemical cells in the absence and presence of iron-oxide minerals. Maximum current densities were determined from current versus time curves shown in Fig. S2. Data are presented as the means of three independent cultures, and error bars represent standard deviations.
Supplementation with 10 mM ferric ion (chelated with citrate) affected current generation by the Geobacter strains (+Fe(III)-citrate samples in Fig. 1). Different from the results of non-Fe control cultures, the five Geobacter strains achieved similar current densities (4.6 to 6.6 μA cm−2). Under this condition, ferric/ferrous ions could work as electron shuttles; Geobacter reduces Fe3+ to Fe2+, Fe2+ diffuses to electrode surfaces and is oxidized back to Fe3+ by releasing electrons, resulting in the current generation. It is noteworthy that supplementation with Fe ions resulted in a marked decrease in current density in the G. sulfurreducens culture, implying that Fe ions suppressed the production of biological conductive matrices by G. sulfurreducens. This result is consistent with previous findings; G. sulfurreducens biofilms grown on electrodes in the presence of a soluble electron acceptor (e.g., fumarate) showed neither active current generation nor electrical conductance (27, 37).
Effects of (semi)conductive iron oxides on EET by Geobacter spp
Electrochemical cultures were supplemented with either hematite (semiconductor) or magnetite (conductor) nanoparticles (10 mM as Fe atom) (+Hematite or +Magnetite in Fig. 1). Supplementation with hematite or magnetite similarly affected current production by G. bremensis, G. pelophilus and G. metallireducens. These Geobacter strains generated larger currents in the (semi)conductive mineral-amended cultures (11 to 35 μA cm−2) than in the non-Fe and +Fe(III)-citrate cultures. The results indicate that these Geobacter strains are able to construct microbe/mineral conductive networks as observed in Shewanella spp. (34). In contrast, current generation by G. bemidjensis was not affected by supplementation with the (semi)conductive minerals, suggesting that the ability to construct microbe/mineral conductive networks is not a universal feature in the genus Geobacter.
In the case of G. sulfurreducens, current densities in hematite- or magnetite-supplemented cultures were comparable to those in G. bremensis and G. pelophilus cultures, while current densities were smaller than in the non-Fe G. sulfurreducens culture (Fig. 1). Two plausible explanations were possible for these results, namely, (1) G. sulfurreducens produced conductive biofilm matrices, while iron-oxide nanoparticles inhibited long-range ET via conductive biofilms; (2) G. sulfurreducens generated currents via microbe/mineral conductive networks without producing conductive biofilms.
EET paths of G. sulfurreducens
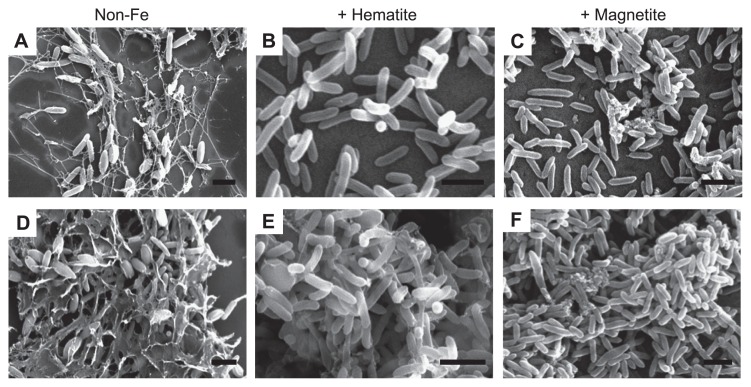
In order to compare EET paths of G. sulfurreducens in the presence and absence of iron-oxide nanoparticles, microscopic and (photo-)electrochemical analyses were conducted for G. sulfurreducens cells grown on the electrodes. It has been reported that G. sulfurreducens produces thick biofilm structures on electrode surfaces under current-generating conditions (39, 43), and such biofilms have been observed with SEM (10, 48). During the preparation of SEM samples, iron-oxide nanoparticles that were precipitated and loosely attached to cell surfaces were removed. In the non-Fe sample, G. sulfurreducens produced thick biofilms, in which cells were surrounded by extracellular polymeric substances (EPSs) (Fig. 2A and D), as has been observed in previous studies (10, 48). It has been shown that EPSs are crucial for long-range ET in conductive biofilms, in which they function as nets to trap extracellular c-Cyts (48). In contrast, EPS-like matrices were not observed around G. sulfurreducens cells when they grew in the presence of hematite (Fig. 2B and E) or magnetite (Fig. 2C and F). These observations indicate that the production of biological conductive matrices was suppressed by supplementation of hematite or magnetite. This may be an important physiological response for G. sulfurreducens to proliferate on solid electron acceptors, and future studies will direct qualitative and quantitative identification of this response.
Fig. 2.
SEM images of G. sulfurreducens cells growing on ITO electrodes. Cells were sampled during current generation from non-Fe (A, D), +Hematite (B, E), and +Magnetite (C, F) cultures. Images show cells when current was increased (A–C) and those after current densities reached the maximum values (D–F). Bars are 2 μm.
A photo-electrochemical technique was used to confirm the construction of a microbe/mineral conductive network by G. sulfurreducens. Photo-irradiation of semiconductive iron oxides is known to induce the excitation of electrons from the valence band to the conduction band and generate a current (photo-current) when microbe/mineral conductive networks are formed on electrodes (34). We performed this analysis and found that the G. sulfurreducens/hematite mixture also generated photo-current (Trace A in Fig. 3), indicating that microbe/mineral conductive networks were formed by G. sulfurreducens. Photo-current densities reached 4 to 5 μA cm−2, which is comparable to those from Shewanella/hematite biofilms formed in similar experimental settings (34). Control samples without G. sulfurreducens cells or hematite nanoparticles (Trace B or C in Fig. 3, respectively) generated low photo-current (<0.2 μA cm−2), suggesting that both bacterial cells and hematite nanoparticles are necessary for conductive networks.
Fig. 3.

Photo-induced currents from hematite-amended G. sulfurreducens cultures. Light (>420 nm) was irradiated for 30 sec at the time points indicated by arrows. Trace A, G. sulfurreducens cells in the presence of hematite nanoparticles; B, a control without G. sulfurreducens cells; C, a control without hematite nanoparticles.
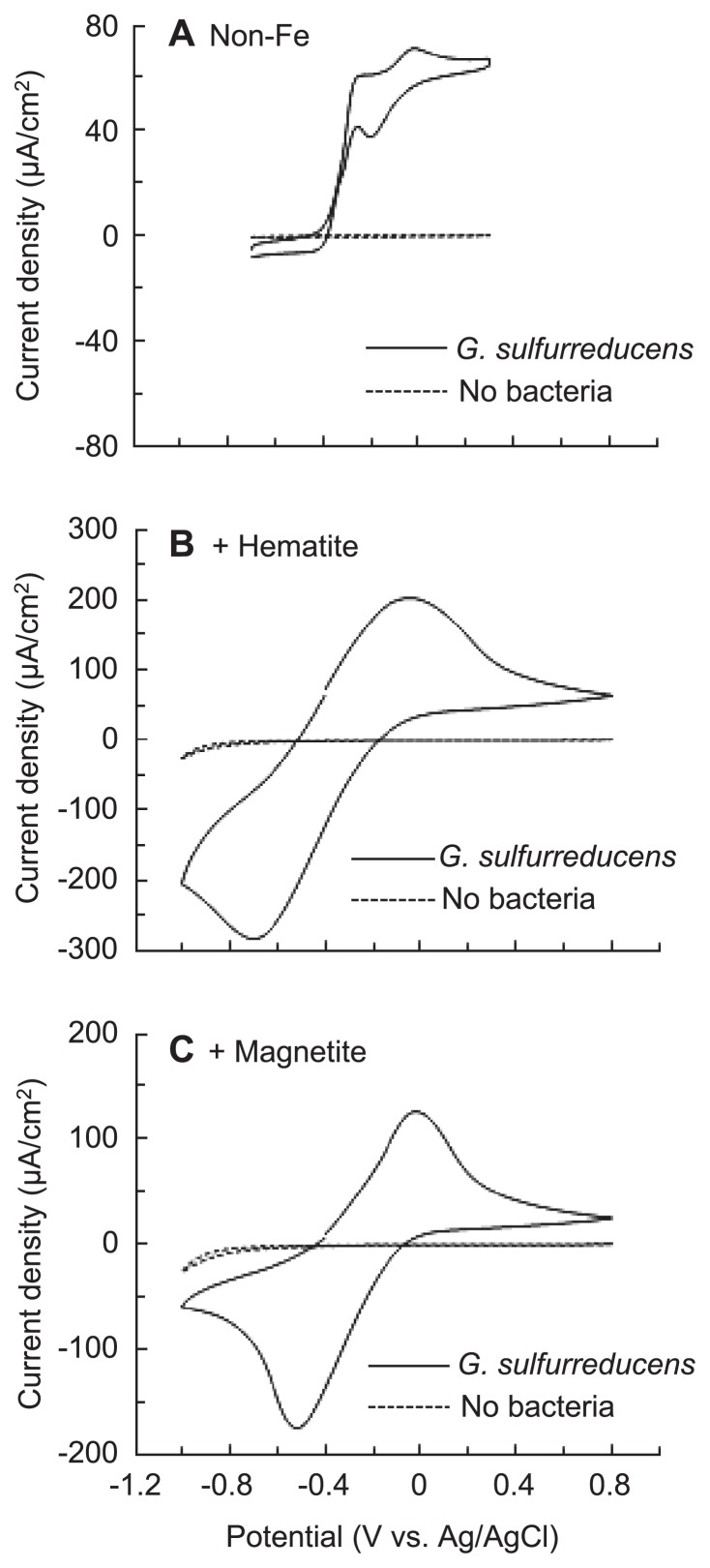
EET paths of G. sulfurreducens were also analyzed by CV under current-generating conditions (Fig. 4). G. sulfurreducens biofilm formed under non-Fe conditions showed a sigmoidal CV curve (Fig. 4A) typical of conductive biofilm of this strain (29, 30, 46). Supplementation with hematite and magnetite markedly altered its CV pattern (Fig. 4B and 4C, respectively); symmetric redox couples appeared and peak currents were largely increased. Similar symmetric CV patterns have been reported for Shewanella cultures amended with hematite nanoparticles (34); in that report, the redox peaks were attributed to OM c-Cyts that were interconnected via hematite nanoparticles. We therefore consider that the CV data shown in Fig. 4B and 4C support the idea that microbe/mineral conductive networks (rather than conductive biofilms) were formed in the presence of hematite and magnetite nanoparticles. Similar symmetric CV patterns were also observed for G. bremensis (Fig. S3), a representative of Geobacter strains that do not form conductive biofilms.
Fig. 4.
CVs of G. sulfurreducens electrochemical cultures (solid lines) in the absence of iron oxides (A), in the presence of hematite (B), and in the presence of magnetite (C). CVs for control samples (without G. sulfurreducens cells) are also presented (broken lines).
The microscopic, photo-electrochemical and CV data consistently suggested that, when iron-oxide nanoparticles were present, G. sulfurreducens utilized microbe/mineral conductive networks for EET as a substitute for conductive biofilms. The ability to utilize microbe/mineral networks was also found in other Geobacter strains, including G. bremensis, G. pelophilus and G. metallireducens. Among them, G. bremensis is a member of subsurface clade 1, whose closely related sequences have been detected abundantly from various anaerobic ecosystems (8, 14), suggesting that these Geobacter populations occurred in the environment using microbe/mineral conductive networks.
Effects of (semi)conductive iron oxides on gene expression in G. sulfurreducens
The above experiments showed the morphological and electrochemical differences in G. sulfurreducens EET paths in the absence and presence of (semi)conductive iron oxides (i.e., conductive biofilm and microbe/mineral network, respectively). In order to gain insights into the molecular mechanisms underlying these differences in its EET path, gene-expression analyses were performed for current-generating G. sulfurreducens cells in the absence and presence of iron oxides. To date, studies have been performed to identify molecular components that constitute the conductive biofilm of G. sulfurreducens (20, 21, 27, 37, 48), while no information has been obtained for the microbe/mineral networks. It has been suggested that many c-Cyts play crucial roles in EET; for instance, electrons are transferred from inner-membrane (IM) c-Cyts to OM c-Cyt complexes (e.g., OmcB and OmcS) via soluble periplasmic c-Cyts (e.g., PpcA). Electrons are further transferred to extracellular electron acceptors directly from the OM c-Cyts or via conductive biofilm. It is speculated that the conductivity of the biofilm is conferred by extracellular c-Cyts (e.g., OmcZ, OmcB and OmcS) and conductive pili (27, 30, 37, 43, 46). In the present study, we conducted microarray analyses to investigate differential gene expression in G. sulfurreducens grown under non-Fe, +Hematite and +Magnetite conditions. The microarray system for G. sulfurreducens was customized, and was validated by comparing expression-fold data for several genes obtained by the microarray system and those obtained by qRT-PCR (Fig. S4).
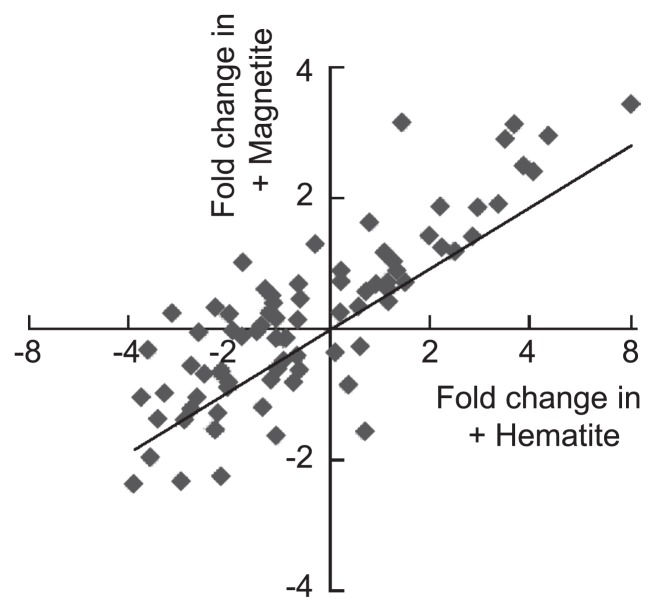
Microarray analysis revealed that the expression of genes for IM and periplasmic c-Cyts (macA and ppcA) was not significantly different between non-Fe and (semi)conductive mineral-amended conditions (data not shown). In addition, genes for OM and extracellular c-Cyts (omcB, omcS and omcZ) and a gene for the main component of pili (pilA) (42) were mostly down-regulated in the (semi)conductive mineral-amended samples (Table 2). In contrast, we also found that some c-Cyt genes were up-regulated in the presence of iron oxides (Table 2). Comparisons of the expression patterns of c-Cyt genes in the G. sulfurreducens genome under +Hematite and +Magnetite conditions are presented in Fig. 5, showing that they were correlated well. Among the over 100 c-Cyt genes present in the G. sulfurreducens genome (31), twelve c-Cyt genes were found to be highly expressed under +Hematite and +Magnetite conditions (Table 2). These c-Cyts are likely ET components constituting microbe/mineral conductive networks, although these c-Cyts (except for PgcA [52]) have not yet been analyzed in molecular and physiological studies. It is also noteworthy that 7 c-Cyts are encoded in a gene cluster (GSU3216–3233) which contains two sets of two-component regulatory systems (GSU3216–3217 and GSU3229–3230). The genes for these 7 c-Cyts have typical signal-peptide sequences, supporting the idea that they are components of EET paths in microbe/mineral conductive networks. It is therefore suggested that c-Cyts used in the conductive biofilm and in the microbe/mineral network are different. Further molecular and physiological analyses are necessary to elucidate their exact roles in conductive networks. In addition, comparative genomics for the five Geobacter strains may provide fruitful information as to the c-Cyts necessary for microbe/mineral networks, for which it is desirable to determine genome sequences of G. pelophilus and G. bremensis.
Table 2.
Differentially expressed c-Cyt and related genes in +Hematite and +Magnetite cultures
| Gene ID | Annotation (gene name) | +Hematite | +Magnetite | ||
|---|---|---|---|---|---|
|
|
|
||||
| Fold changea | p value | Fold changea | p value | ||
| Up-regulated in + Hematite and +Magnetite | |||||
| GSU0701 | cytochrome c family protein (OmcJ) | 4.08 | 2E-4 | 2.13 | 4E-3 |
| GSU1771 | cytochrome c family protein (PgcA) | 2.17 | 6E-6 | 1.55 | 1E-3 |
| GSU2203 | cytochrome c family protein (OmcK) | 2.68 | 1E-3 | 1.65 | 5E-3 |
| GSU2204 | cytochrome c family protein, putative | 2.36 | 2E-3 | 1.53 | 2E-3 |
| GSU2299 | cytochrome c family protein | 3.20 | 7E-6 | 1.96 | 1E-5 |
| GSU3218 | cytochrome c family protein | 4.51 | 8E-4 | 2.81 | 1E-3 |
| GSU3221 | cytochrome c family protein | 3.37 | 4E-5 | 2.77 | 2E-4 |
| GSU3223 | cytochrome c family protein | 3.82 | 4E-5 | 2.40 | 8E-4 |
| GSU3226 | cytochrome c family protein | 2.80 | 7E-4 | 1.92 | 4E-3 |
| GSU3228 | cytochrome c family protein | 8.09 | 8E-6 | 3.33 | 5E-4 |
| GSU3232 | cytochrome c family protein | 3.58 | 3E-4 | 2.98 | 9E-4 |
| GSU3233 | cytochrome c family protein | 2.15 | 2E-4 | 1.93 | 7E-3 |
| Down-regulated in +Hematite and +Magnetite | |||||
| GSU0274 | cytochrome c family protein | −3.91 | 3E-5 | −2.28 | 4E-5 |
| GSU0592 | cytochrome c family protein (OmcQ) | −3.30 | 8E-5 | −1.60 | 5E-5 |
| GSU2737 | cytochrome c family protein (OmcB) | −2.99 | 2E-4 | −1.09b | 2E-1 |
| GSU0670 | cytochrome c family protein (OmcX) | −2.63 | 2E-3 | −1.52 | 4E-3 |
| GSU1760 | Cyd-5, cytochrome c3 (PpcE) | −2.20 | 3E-5 | −1.70 | 4E-3 |
| GSU1996 | cytochrome c family protein | −3.48 | 1E-6 | −1.97 | 2E-4 |
| GSU2076 | cytochrome c family protein (OmcZ) | −2.15 | 1E-3 | −2.18 | 3E-3 |
| GSU2201 | cytochrome c family protein | −2.25 | 6E-5 | −1.70 | 5E-3 |
| GSU3259 | cytochrome c family protein | −2.82 | 3E-4 | −2.23 | 2E-3 |
| GSU1496 | pilin domain-containing protein (PilA) | −1.68 | 7E-3 | −2.18 | 3E-3 |
| GSU2504 | cytochrome c family protein (OmcS) | −1.45b | 1E-1 | −1.76 | 7E-4 |
Gene expressions under +Hematite and +Magnetite conditions are expressed as positive (up-regulated) or negative (down-regulated) fold changes against their expressions under non-Fe condition.
Not statistically significant.
Fig. 5.
Expression profiles of 82 c-Cyt genes in the G. sulfurreducens genome. Expression fold changes in hematite- and magnetite-amended conditions against the control (non-Fe) were calculated from the microarray data. An approximation curve (y=0.50x + 0.20, r=0.84) derived from the least-square method is shown.
Implications
The present study demonstrates that Geobacter strains have the ability to construct microbe/mineral conductive networks in the presence of (semi)conductive iron-oxide nanoparticles. In addition to this ability, G. sulfurreducens has the unique ability to construct conductive biofilms, and this bacterium utilizes either of these long-range ET paths depending on the environmental setting. It is suggested that microbe/mineral conductive networks are widely used by bacteria in the genus Geobacter, while only limited members are able to form conductive biofilms.
When iron-oxide nanoparticles were present, G. sulfurreducens generated microbe/mineral networks for electrode respiration, even though the current density achieved with the microbe/mineral networks was much less than that with conductive biofilms (Fig. 1). This result may be relevant to our previous findings; in the absence of iron-oxide nanoparticles, electrochemical cultivation enriched bacteria closely related to G. sulfurreducens from rice paddy-field soil, while supplementation of the soil with iron oxides allowed Geobacteraceae populations affiliated with the subsurface clades to occur abundantly (14). These results indicate that the microbe/mineral network has ecological advantages over conductive biofilm. We propose that this finding can be explained by considering the cost of constructing long-range ET paths; namely, large amounts of EPSs and extracellular proteins (e.g., c-Cyts and pili) are necessary for constructing conductive biofilms, and Geobacter may consume substantial amounts of carbon and energy sources for this purpose. The use of environmental costless materials (e.g., conductive minerals) therefore confers ecological advantages upon Geobacter populations, resulting in the preferential use of these materials and the outgrowth of bacteria using these materials. It is likely that the conductive biofilm is the last resort of G. sulfurreducens and is synthesized only when there is no easily accessible electron acceptor.
It has recently been argued that electric currents play important roles in biogeochemical reactions in the environment (15, 35, 41, 44). The present study also supports this idea by showing that Geobacter strains preferentially use (semi)conductive minerals for their EET. Further investigation will shed light on the impact of (semi)conductive minerals on microbial physiology and evolution in natural ecosystems.
Supplementary Material
Acknowledgements
This work was supported by the Exploratory Research for Advanced Technology (ERATO) program of the Japanese Science and Technology Agency (JST). We thank Reiko Hirano for technical assistance.
References
- 1.Aklujkar M, Krushkal J, DiBartolo G, Lapidus A, Land ML, Lovley DR. The genome sequence of Geobacter metallireducens: features of metabolism, physiology and regulation common and dissimilar to Geobacter sulfurreducens. BMC Microbiol. 2009;9:109. doi: 10.1186/1471-2180-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coker VS, Bell AMT, Pearce CI, Pattrick RAD, van der Laan G, Lloyd JR. Time-resolved synchrotron powder X-ray diffraction study of magnetite formation by the Fe(III)-reducing bacterium Geobacter sulfurreducens. Am Mineral. 2008;93:540–547. [Google Scholar]
- 3.El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, Lau WM, Nealson KH, Gorby YA. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci USA. 2010;107:18127–18131. doi: 10.1073/pnas.1004880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franks AE, Nevin KP, Glaven RH, Lovley DR. Microtoming coupled to microarray analysis to evaluate the spatial metabolic status of Geobacter sulfurreducens biofilms. ISME J. 2009;4:509–519. doi: 10.1038/ismej.2009.137. [DOI] [PubMed] [Google Scholar]
- 5.Glasauer S, Weidler PG, Langley S, Beveridge TJ. Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochimi Cosmochimi Acta. 2003;67:1277–1288. [Google Scholar]
- 6.Gorby YA, Yanina S, McLean JS, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gralnick JA, Newman DK. Extracellular respiration. Mol Microbiol. 2007;65:1–11. doi: 10.1111/j.1365-2958.2007.05778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes DE, O’Neil RA, Vrionis HA, et al. Subsurface clade of Geobacteraceae that predominates in a diversity of Fe(III)-reducing subsurface environments. ISME J. 2007;1:663–677. doi: 10.1038/ismej.2007.85. [DOI] [PubMed] [Google Scholar]
- 9.Ishii S, Kosaka T, Hori K, Hotta Y, Watanabe K. Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl Environ Microbiol. 2005;71:7838–7845. doi: 10.1128/AEM.71.12.7838-7845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii S, Shimoyama T, Hotta Y, Watanabe K. Characterization of a filamentous biofilm community established in a cellulose-fed microbial fuel cell. BMC Microbiol. 2008;8:6. doi: 10.1186/1471-2180-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang YS, Risbud S, Rabolt JF, Stroeve P. Synthesis and characterization of nanometer-size Fe3O4and γ-Fe2O3particles. Chem Mater. 1996;8:2209–2211. [Google Scholar]
- 12.Kato S, Kosaka T, Watanabe K. Comparative transcriptome analysis of responses of Methanothermobacter thermautotrophicus to different environmental stimuli. Environ Microbiol. 2008;10:893–905. doi: 10.1111/j.1462-2920.2007.01508.x. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Kosaka T, Watanabe K. Substrate-dependent transcriptomic shifts in Pelotomaculum thermopropionicum grown in syntrophic co-culture with Methanothermobacter thermautotrophicus. Microbial Biotechnol. 2009;2:575–584. doi: 10.1111/j.1751-7915.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato S, Kai F, Nakamura R, Watanabe K, Hashimoto K. Respiratory interactions of soil bacteria with (semi)conductive iron-oxide minerals. Environ Microbiol. 2010;12:3114–3123. doi: 10.1111/j.1462-2920.2010.02284.x. [DOI] [PubMed] [Google Scholar]
- 15.Kato S, Hashimoto K, Watanabe K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ Microbiol. 2012;14:1646–1654. doi: 10.1111/j.1462-2920.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Hashimoto K, Watanabe K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc Natl Acad Sci USA. 2012;109:10042–10046. doi: 10.1073/pnas.1117592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Newton GJ, Nakamura R, Hashimoto K, Nakanishi S. Electrochemical characterization of a single electricity-producing bacterial cell of Shewanella by using optical tweezers. Angew Chem Int Ed. 2010;49:6596–6599. doi: 10.1002/anie.201000315. [DOI] [PubMed] [Google Scholar]
- 18.Lovley DR. Cleaning up with genomics: applying molecular biology to bioremediation. Nat Rev Microbiol. 2003;1:35–44. doi: 10.1038/nrmicro731. [DOI] [PubMed] [Google Scholar]
- 19.Lovley DR. Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microbial Physiol. 2004;49:219–286. doi: 10.1016/S0065-2911(04)49005-5. [DOI] [PubMed] [Google Scholar]
- 20.Lovley DR. Bug juice: harvesting electricity with micro-organisms. Nat Rev Microbiol. 2006;4:497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- 21.Lovley DR. The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol. 2008;19:564–571. doi: 10.1016/j.copbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Lovley DR, Phillips EJ. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovley DR, Phillips EJ. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol. 1987;53:1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovley DR, Stolz JF, Nord GLJ, Phillips EJP. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature. 1987;330:413–416. [Google Scholar]
- 25.Magnuson TS, Isoyama N, Hodges-Myerson AL, Davidson G, Maroney MJ, Geesey GG, Lovley DR. Isolation, characterization and gene sequence analysis of a membrane-associated 89 kDa Fe(III) reducing cytochrome c from Geobacter sulfurreducens. Biochem J. 2001;359:147–152. doi: 10.1042/0264-6021:3590147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahadevan R, Palsson BØ, Lovley DR. In situ to in silico and back: elucidating the physiology and ecology of Geobacter spp. using genome-scale modelling. Nat Rev Microbiol. 2011;9:39–50. doi: 10.1038/nrmicro2456. [DOI] [PubMed] [Google Scholar]
- 27.Malvankar NS, Vargas M, Nevin KP, et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol. 2011;6:573–579. doi: 10.1038/nnano.2011.119. [DOI] [PubMed] [Google Scholar]
- 28.Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA. 2008;105:3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsili E, Rollefson JB, Baron DB, Hozalski RM, Bond DR. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl Environ Microbiol. 2008;74:7329–7337. doi: 10.1128/AEM.00177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsili E, Sun J, Bond DR. Voltammetry and growth physiology of Geobacter sulfurreducens biofilms as a function of growth stage and imposed electrode potential. Electroanalysis. 2010;22:865–874. [Google Scholar]
- 31.Methé BA, Nelson KE, Eisen JA, et al. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science. 2003;302:1967–1969. doi: 10.1126/science.1088727. [DOI] [PubMed] [Google Scholar]
- 32.Mulvaney P, Cooper R, Grieser F, Meisel D. Charge trapping in the reductive dissolution of colloidal suspensions of iron(III) oxides. Langmuir. 1988;4:1206–1211. [Google Scholar]
- 33.Myers JM, Myers CR. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl Environ Microbiol. 2001;67:260–269. doi: 10.1128/AEM.67.1.260-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura R, Kai F, Okamoto A, Newton GJ, Hashimoto K. Self-constructed electrically conductive bacterial networks. Angew Chem Int Ed. 2009;48:508–511. doi: 10.1002/anie.200804750. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura R, Takashima T, Kato S, Takai K, Yamamoto M, Hashimoto K. Electrical current generation across a black smoker chimney. Angew Chem Int Ed. 2010;49:7692–7694. doi: 10.1002/anie.201003311. [DOI] [PubMed] [Google Scholar]
- 36.Nevin KP, Holmes DE, Woodard TL, Hinlein ES, Ostendorf DW, Lovley DR. Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates. Int J Syst Evol Microbiol. 2005;55:1667–1674. doi: 10.1099/ijs.0.63417-0. [DOI] [PubMed] [Google Scholar]
- 37.Nevin KP, Kim BC, Glaven RH, et al. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS One. 2009;20:e5628. doi: 10.1371/journal.pone.0005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevin KP, Lovley DR. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter. Appl Environ Microbiol. 2000;66:2248–2251. doi: 10.1128/aem.66.5.2248-2251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nevin KP, Richter H, Covalla SF, Johnson JP, Woodard TL, Orloff AL, Jia H, Zhang M, Lovley DR. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ Microbiol. 2008;10:2505–2514. doi: 10.1111/j.1462-2920.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 40.Newman DK, Kolter R. A role for excreted quinones in extracellular electron transfer. Nature. 2000;405:94–97. doi: 10.1038/35011098. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen LP, Risgaard-Petersen N, Fossing H, Christensen PB, Sayama M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature. 2010;463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- 42.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 43.Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol. 2006;72:7345–7348. doi: 10.1128/AEM.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revil A, Mendonca CA, Atekwana EA, Kulessa B, Hubbard SS, Bohlen KJ. Understanding biogeobatteries: where geophysics meets microbiology. J. Geophys. Res. 2010;115:G00G02. [Google Scholar]
- 45.Richter H, McCarthy K, Nevin KP, Johnson JP, Rotello VM, Lovley DR. Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir. 2008;24:4376–4379. doi: 10.1021/la703469y. [DOI] [PubMed] [Google Scholar]
- 46.Richter H, Nevin KP, Jia HF, Lwy DA, Lovley DR, Tender LM. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ Sci. 2009;2:506–516. [Google Scholar]
- 47.Roden EE, Urrutia MM. Influence of biogenic Fe(II) on bacterial crystalline Fe(III) oxide reduction. Geomicrobiol J. 2002;19:209–251. [Google Scholar]
- 48.Rollefson JB, Stephen CS, Tien M, Bond DR. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J Bacteriol. 2011;193:1023–1033. doi: 10.1128/JB.01092-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stams AJ, de Bok FA, Plugge CM, van Eekert MH, Dolfing J, Schraa G. Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol. 2006;8:371–382. doi: 10.1111/j.1462-2920.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 50.Straub KL, Buchholz-Cleven BE. Geobacter bremensis sp. nov. and Geobacter pelophilus sp. nov., two dissimilatory ferric-iron-reducing bacteria. Int J Syst Evol Microbiol. 2001;51:1805–1808. doi: 10.1099/00207713-51-5-1805. [DOI] [PubMed] [Google Scholar]
- 51.Torres CI, Marcus AK, Lee HS, Parameswaran P, Krajmalnik-Brown R, Rittmann BE. A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol Rev. 2010;34:3–17. doi: 10.1111/j.1574-6976.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- 52.Tremblay PL, Summers ZM, Glaven RH, Nevin KP, Zengler K, Barrett CL, Qiu Y, Palsson BO, Lovley DR. A c-type cytochrome and a transcriptional regulator responsible for enhanced extracellular electron transfer in Geobacter sulfurreducens revealed by adaptive evolution. Environ Microbiol. 2011;13:13–23. doi: 10.1111/j.1462-2920.2010.02302.x. [DOI] [PubMed] [Google Scholar]
- 53.Vali H, Weiss B, Li YL, Sears SK, Kim SS, Kirschvink JL, Zhang CL. Formation of tabular single-domain magnetite induced by Geobacter metallireducens GS-15. Proc Natl Acad Sci USA. 2004;101:16121–16126. doi: 10.1073/pnas.0404040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Von Canstein H, Ogawa J, Shimizu S, Lloyd JR. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol. 2008;74:615–623. doi: 10.1128/AEM.01387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J Biosci Bioeng. 2008;106:528–536. doi: 10.1263/jbb.106.528. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe K, Manefield M, Lee M, Kouzuma A. Electron shuttles in biotechnology. Curr Opin Biotechnol. 2009;20:633–641. doi: 10.1016/j.copbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Weber KA, Achenbach LA, Coates JD. Micro-organisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006;4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.