Introduction
Adolescence is a highly unique neurodevelopmental period, characterized by increases in risk-taking behaviors (1). Importantly, during this time the regions of the brain most able to weigh consequences and judge the costs and benefits of one's actions are still very much in development (2). Cannabis abuse during adolescence has been associated with both immediate and longer term negative outcomes, including poorer academic performance, increased risk of school dropout (3), and broad-based declines in working memory, attention, and executive function during adulthood (4,5).
These effects are due in large part to the effects of cannabis use on endogenous cannabinoid receptors in the brain. Because the discovery of these cannabinoid receptors was quite recent (6,7), the mechanisms and consequences of cannabis use are not well understood. However, cannabis use in adolescence clearly precedes alterations in cognitive and emotional function in adulthood (8). Cannabinoid receptor density in both gray and white matter is greater in children and adolescents, declining into adulthood (9–12). Reductions in gray matter volume (13) and axonal connectivity (14) in heavy cannabis users suggest that adolescence, the modal developmental stage for cannabis initiation, is also a period during which the brain is more sensitive to cannabis exposure.
Further complexity is added by the finding that cannabis users differ from non-users even before they have ever used cannabis (15). Differences attributed to cannabis may be due to premorbid neuropsychological differences (16), or to other risk factors (17). Despite this uncertainty, understanding how cannabis use and dependence affect the developing brain is vital to developing effective interventions. As more states in the U.S. move to decriminalize or legalize medical or recreational cannabis use it becomes critical to understand the potential impact of cannabis use in order to guide policy as well as treatment.
Interventions to reduce cannabis use for adolescents have generally produced mixed results (e.g., (18–20)). This variability in treatment outcomes may be due, in part, to our limited understanding of how psychotherapy works (21), especially for adolescents (22,23). Evaluations of basic biological factors hold great promise for deconstructing adolescents' patterns of intervention response (24–26). The measurement of these factors, such as resting brain activity, may ultimately yield better-targeted interventions.
Analysis of the brain at rest has a relatively long history in neuroimaging research, beginning in the early 20th century with electroencephalographic recordings (e.g., (27)), and subsequently with magnetoencephalography (28) and functional magnetic resonance imaging (fMRI) (29). Resting scans provide information about what the brain is doing when it is not doing anything in particular. By measuring task-independent activity, resting scans provide a glimpse of the baseline state of brain activity. This is particularly useful in the context of functional connectivity; that is, the correlation between functional units of the brain (30).
One common technique for assessing functional connectivity in resting fMRI is independent component analysis (ICA), a method of separating signal mixtures such as those produced by fMRI into statistically unrelated (i.e., independent) components (31,32). In fMRI, ICA is used to detect temporally coherent, spatially independent components. Each spatial component is a weighted network – that is, it defines a spatial pattern of regions that tended to co-activate (or de-activate) during the resting scan, independent of other spatial patterns. These components are also known as intrinsic connectivity networks (ICN). Estimation also produces a corresponding set of loading parameters for each participant that indicate the degree to which the participant contributes to each component. These participant-specific loading parameters may be compared between groups, or correlated with other measures. ICNs include not only the default mode network (33,34) but also networks for attention (35,36) as well as salience and executive control (37). ICNs have also been linked to task performance (38,39). ICNs can also be measured reliably in young samples (40), an important finding given the greater variability seen in the BOLD response seen in these age groups (41).
ICNs directly address critiques that fMRI is only an incremental improvement to phrenology (42) (43). More than merely showing how the brain “lights up”, measures of functional connectivity like ICA demonstrate how brain regions interact, and how this interaction may vary between populations. ICNs may then be characterized further using complementary imaging modalities, or modulated using targeted behavioral, psychopharmacological, or other interventions. Disease states including depression (44), psychosis (45), schizophrenia (46), autism (47), and substance use (48) (49) (50) have been characterized using these techniques (for a review, see (51)).
Structural analyses in adult cannabis-abusing populations have revealed lower gray matter volume and density for users than controls, particularly around bilateral hippocampus (13,52). In addition to these structural differences, several functional neuroimaging studies have observed effects of cannabis use on the resting brain. Using electroencephalography (EEG), Böcker and colleagues found that after cannabis administration, adult male cannabis users showed a dose-related decrease in power in the theta band (53), a marker of working memory. While localization of the decrease was not possible due to low electrode density, theta oscillations have been associated with activity in mesial temporal lobe during working memory performance (54).
Several studies using positron emission tomography (PET) have observed effects of adult cannabis use on the brain at rest. One found that severity of cannabis use is correlated with reduced metabolism in bilateral dorsolateral prefrontal cortex (48), while another observed that even after a period of abstinence cannabis users show increased metabolism in temporal regions (49). Another group observed that cannabis-using adults showed greater blood volume in right frontal, left temporal, and cerebellum while at rest than did controls (55).
Similar relationships have been observed using fMRI. Effects of substance dependence on an executive control network have been detected, with substance dependent adults showing greater activity than did controls (56). While that sample included cannabis dependent adults, the participants were also dependent upon a number of other substances including stimulants and club drugs, limiting the specificity of the finding. Assessments of these effects in adolescent samples have been sparse; one recent review found no studies of resting brain activity in adolescent cannabis users (57). To our knowledge only one very recent study had applied fMRI to directly assess resting brain activity in adolescent cannabis users. Behan and colleagues found greater connectivity in adolescent cannabis users than controls between blood oxygen level dependent (BOLD) time series in specific regions of interest (ROIs) including bilateral parietal lobe and cerebellum (58). They also observed a correlation between this connectivity and higher self-reported recent cannabis use, although this correlation was not corrected for multiple comparisons.
Relationships between resting brain activity and cannabis use have not been well-studied with youth. This is particularly important given the dearth of literature on functional networks in this age range, and the potential importance of identifying factors that may improve treatment response. Thus, the goal of the present study was to analyze the associations between cannabis use (as measured by the Marijuana Use Scale (59)) and resting brain activity in a sample of high-risk adolescents, with the hypothesis that resting brain activity would be correlated with cannabis use.
Method
All procedures were approved by the local institutional review board and were protected by a federal Certificate of Confidentiality. Eighty-two non-treatment-seeking high-risk youth (M age 16.15 years (SD 1.07), 33.3% female) were recruited from juvenile justice programs in the Southwest as part of an ongoing study (NINR R01 NRxxxxx). This parent study involves a behavioral intervention to reduce risky sexual behavior that does not include treatment for drug or alcohol use. To recruit potential participants, trained research staff introduced the project at juvenile justice programs (e.g., diversion programs), informing youth that study participation was voluntary and would not affect their experience within the juvenile justice system. All youth provided informed assent (written) and parent/guardian consent (audiorecorded). To participate, youth needed to be age 14-18, fluent in English, and participating in a juvenile justice day program. This day program does not include treatment for drug or alcohol use; instead, it provides an alternative to secure detention, incorporating structured programs that promote, encourage and reinforce proactive and acceptable social behaviors. Exclusion criteria included antipsychotics/anticonvulsants, MRI contra-indications, and TBI with loss of consciousness ≥ 6 min. Eligible participants could earn $150. Descriptive statistics are given in Table 1. Youth are contacted for behavioral follow-up at three, six, and twelve months. As data collection is ongoing, only baseline data is assessed in the present analysis.
Table 1. Sample demographics.
| Measure | Mean/N | SD/% |
|---|---|---|
| Age | 16.1 | 1.07 |
| Gender | ||
| Male | 46 | 66.7% |
| Female | 23 | 33.3% |
| Ethnicity* | ||
| White | 14 | 20.3% |
| African American | 6 | 8.7% |
| Hispanic | 50 | 72.5% |
| American Indian/Alaska Native | 8 | 11.6% |
| Asian or Pacific Islander | 1 | 1.4% |
| Other | 5 | 7.2% |
| Age at cannabis onset | 11.8 | 2.4 |
| Marijuana Dependence Scale | 2.3 | 2.3 |
| Marijuana Use Scale | 25.3 | 8.6 |
| Weekday cannabis use | ||
| Yes | 37 | 53.6% |
| No | 32 | 46.4% |
| Cannabis use days (of 30) | 8.3 | 11.0 |
| Age at alcohol onset | 12.2 | 2.3 |
| AUDIT | 6.5 | 7.0 |
| Alcohol Use Scale | 22.6 | 8.3 |
| Weekday alcohol use | ||
| Yes | 26 | 37.7% |
| No | 43 | 62.3% |
| Alcohol use days (of 30) | 2.0 | 3.2 |
| Framewise displacement (mm) | 0.32 | 0.42 |
| ImpSS | ||
| Impulsivity | 4.2 | 2.1 |
| Sensation-seeking | 6.9 | 2.5 |
| Total | 11.0 | 4.0 |
| DERS | ||
| Nonacceptance of emotional responses | 11.2 | 5.3 |
| Difficulties engaging in goal-directed behavior | 13.0 | 4.9 |
| Impulse control difficulties | 14.2 | 5.5 |
| Lack of emotional awareness | 18.1 | 6.0 |
| Limited access to emotion regulation strategies | 16.7 | 6.8 |
| Lack of emotional clarity | 11.2 | 3.8 |
| Total | 84.3 | 21.9 |
| WISC | ||
| Digit span scaled score | 7.6 | 3.1 |
| Letter numbering scaled score | 7.7 | 2.7 |
| WAIS | ||
| Digit span scaled score | 8.8 | 1.9 |
| Letter numbering scaled score | 8.8 | 3.4 |
This item may not total to 100% due to selection of multiple responses.
Note. ImpSS = Impulsivity and Sensation Seeking. DERS = Difficulties in Emotion Regulation Scale. WAIS = Wechsler Adult Intelligence Scale. WISC = Wechsler Intelligence Scale for Children. Framewise displacement = mean head motion in mm.
At baseline, all youth completed a brief neurocognitive battery including the Wechsler Intelligence Scale for Children (for youth up to 16 years of age), the Wechsler Adult Intelligence Scale (for youth over 16 years of age), as well as assessments of current cannabis use and dependence (see Table 1). All youth were also evaluated for basic literacy, as assessed by demonstrated comprehension of the written assent form. Quantity and frequency of cannabis use and alcohol use during the prior 30 days were assessed using a structured timeline follow-back interview (TLFB; 60). This assessment also queried weekday (i.e., Monday-Friday) cannabis use, which may indicate greater risk of dependence. Cannabis use during the prior 3 months was assessed using the Marijuana Use Scale, a 6-item measure derived from a previous substance use study (61). The scale has a possible range of 1-37. The range in the present sample was 2-36, with acceptable internal consistency (alpha = 0.706). Cannabis dependence was assessed using the Marijuana Dependence Scale (59), a 10-item measure with a possible range of 1-10. The range in the present study was 1-10 with acceptable internal consistency (alpha = .742). Scores of 4 and higher suggest dependence (59), a criterion that applied to 18 participants (26.1%) in the present study. Alcohol use during the prior 3 months was measured using the Alcohol Use Scale, a 7-item measure derived from a previous substance use study (61), with a possible range of 1-42. The range in the present study was 4-38, with adequate internal consistency (alpha = .715). Alcohol dependence was measured using the reliable and valid 10-item Alcohol Use Disorders Identification Test (AUDIT) scale (62), which has a possible range of 0-40. The range in the present study was 0-27, with good internal consistency (alpha = .867). Scores of 4 and higher on the AUDIT suggest alcohol dependence (63), a criterion that applied to 4 participants (5.8%) in the present study. Impulsivity was measured using the Impulsive Sensation Seeking (ImpSS) scale (64), a 19-item measure with strong evidence of reliability and validity (65). The possible range of the ImpSS total scale is 0-19; the range in the present study was 2-19, with questionable internal consistency (alpha = .678). Emotion regulation was evaluated using the 36-item Difficulties in Emotion Regulation Scale (66), a reliable and valid measure of emotion regulation that has a possible range of 37-185 on its total scale. The range in the present study was 43-147, with excellent internal consistency (alpha = .907). Also performed at baseline was a 5-minute resting MRI scan (Siemens 3T Trio, TR = 2000 ms, TE = 29 ms, FOV 240 mm, voxel size 3.5 × 3.5 × 3.5 mm, 64 × 64 acquisition matrix, 33 slices ascending, 165 images). For 95.7% of participants, this fMRI scan session on the day of the baseline assessment. For the remaining four participants, the mean time between the baseline assessment and the scan was 10 days (SD 18.8).
Because motion is diagnostic of attention deficit hyperactive disorder (67), which has a high prevalence (≈20%) among justice-involved youth (68,69), we have also included descriptive statistics on the mean framewise displacement (i.e., the head motion per fMRI image in the x, y, and z directions, as well as pitch, yaw, and roll) (70) in mm during the resting scans. Due to this potential confound, head motion was not included as a regressor of no interest during preprocessing; instead, participants with head motion greater than two standard deviations above the mean were excluded from the group analysis. Thirteen participants were excluded on this criterion. Results and demographics are reported only for the 69 retained participants. The excluded participants did not significantly differ from included participants on measures of age or substance use (all p's > .15).
Image preprocessing was performed using AFNI (71), including slice timing correction, de-spiking, normalization to Talairach space, and smoothing with a 6 mm full-width half-maximum Gaussian kernel. The initial image of each run was dropped to allow magnetization to reach steady state. Preprocessed data was entered into a group spatial independent component analysis (72) using the GIFT toolbox (http://mialab.mrn.org/software/gift/index.html). The Infomax ICA algorithm was repeated 20 times in ICASSO (73). In order to avoid false positive components (74) a moderate model order of 30 components was selected. Aggregate spatial maps were estimated as the modes of the component clusters. Subject-specific spatial maps and time courses were estimated using the GICA1 back-reconstruction method based on PCA compression and projection (72,75). Components were evaluated based on anatomical constraints including that ICNs should exhibit peak activations in grey matter, low spatial overlap with known artifacts, and should have timecourses dominated by low-frequency fluctuations (76). Relationships between component timecourses were assessed using the MANCOVAN utility in the GIFT toolbox. From the surviving components, the component that followed the hypothesized spatial pattern was retained for further analysis. The relationship between ICN activity and cannabis use was assessed by forming groups of high use (youth with scores of 21-47 on the Marijuana Use Scale) and low use (youth with scores of 1-20 on the Marijuana Use Scale), and then assessing brain network activity using an independent-samples t-test in AFNI with alcohol use (as measured by the Alcohol Use Scale) entered as a covariate, and the results converted to z-scores.
Results
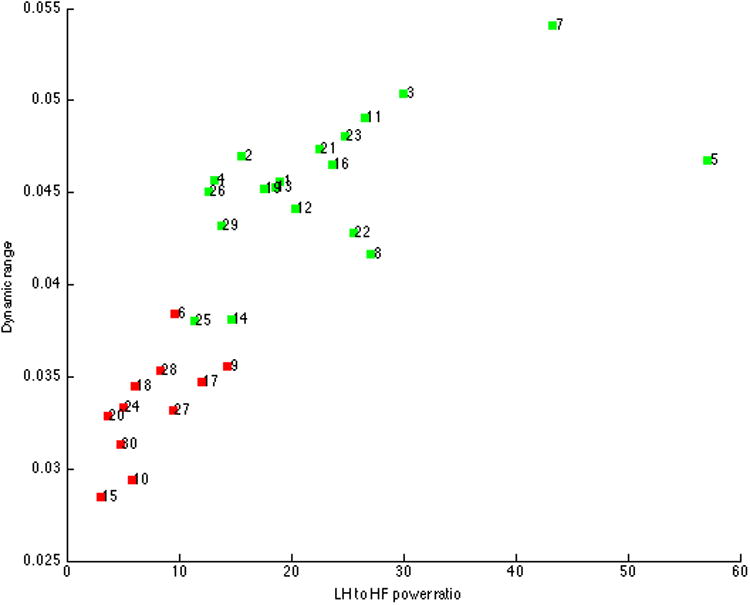
Of the 30 components extracted, 11 were discarded due to low dynamic range, low ratio of low frequency to high frequency power (LH:HF), or anatomical constraints. Figure 1 displays the spread of dynamic range and LF:HF for all 30 components. As in previous work (77), approximately 1/3 of the extracted components did not meet quality criteria. Components were then sorted in GIFT based on their spatial correlation with a resting state template. Of the three components most highly related to the resting state template, one matched the executive control network template (77) included in GIFT and was selected for further analysis based upon its spatial pattern.
1.
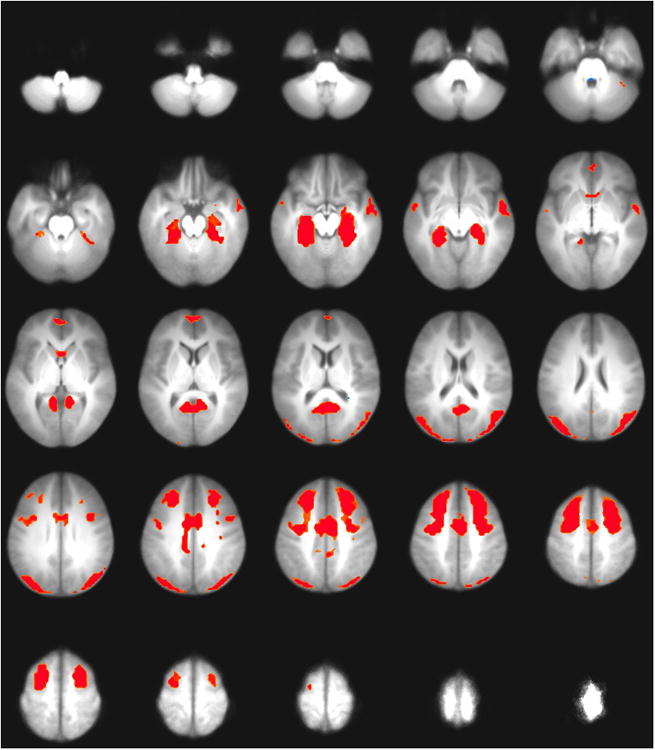
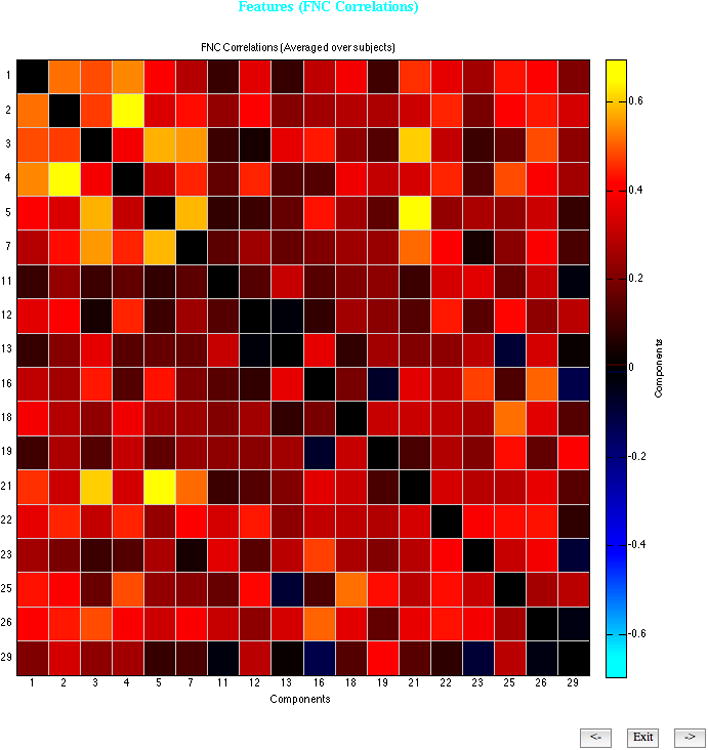
Spatially, this component was primarily characterized by bilateral activation in mesial temporal lobe including hippocampus, middle frontal gyrus, and superior occipital gyrus (see Figure 3), and is referred to subsequently as the fronto-temporal network. Figure 2 indicates the correlations between all component timecourses. The timecourse for the front-temporal network was only moderately correlated with the timecourses of other components.
3.
2.
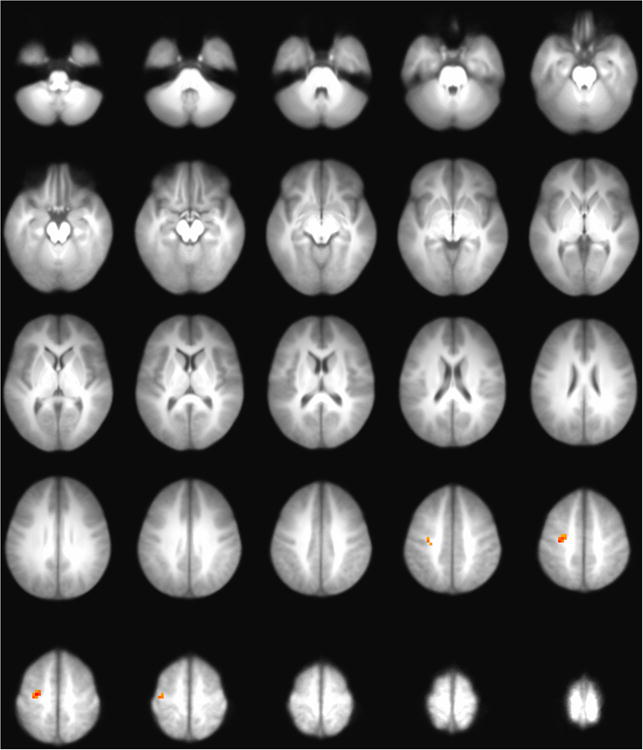
An independent samples t-test of high and low cannabis users with alcohol use entered as a covariate revealed two elements correlated with cannabis use within this network (see Figure 4): one positively correlated source in middle frontal gyrus (z = 3.51, Talariach = −23, 15, 52), and one negatively correlated source in middle temporal gyrus (z = −3.34, Talairach = −44, 62, 26). The alcohol use covariate did not explain any significant variance in ICN activity. All p values were corrected for multiple comparisons using an uncorrected p of .005 and a cluster size threshold of 1200 mm3 (78). Only the correlation in middle frontal gyrus survived this correction. Note that the ICN described represents a weighted network that tended to co-activate during rest. While ICNs extracted from scans conducted during a behavioral task might be expected to show widespread voxelwise correlations with related behaviors (e.g., correlations between response-inhibition networks measured during a Go/NoGo task and baseline alcohol use), it would be unexpected for a substantial fraction of a resting ICN to show widespread voxelwise correlations with behavioral measures (39).
4.
Discussion
In an effort to identify brain networks that might be important to intervention response, the present study used independent component analysis (ICA) to reveal distinct cortical networks in resting brain activity among high-risk adolescents. Consistent with measurements in adults (48,49), greater cannabis use was correlated with activity in a fronto-temporal network: positively in middle frontal gyrus and negatively in middle temporal gyrus. Only the correlation with network activity in middle frontal gyrus survived correction for multiple comparisons. To our knowledge the present study is among the first to apply independent component analysis to the assessment of brain networks in adolescent cannabis users. One study with adolescents observed relationships between higher cannabis use and increased functional connectivity in parietal lobes and cerebellum (58), but not in frontal or temporal regions. Another found that higher cannabis use was correlated with reduced cerebral blood flow in frontal regions, but also that increased time since last cannabis use was associated with lower blood flow in medial frontal lobe (80). Both studies were limited by small samples (n < 25) of cannabis users.
Previous adult studies of the brain at rest have observed activity more similar to the present findings. Sneider and colleagues found that after 4 weeks of supervised abstinence, cannabis-using adults showed increased blood volume in temporal, but not frontal regions (49). Following administration of δ9 tetrahydrocannabinol to current cannabis users, Klumpers and colleagues (79) found significant increases in resting state functional connectivity in regions including dorsal medial prefrontal cortex and precentral gyrus, and decreases in regions including the dorsal visual stream network.
Similar patterns of activity have also been observed in analysis of task-related data in adolescents. Tapert and colleagues (81) found significantly more activity in cannabis users than nonusers in dorsolateral prefrontal and medial frontal regions during response inhibition. Stanger and colleagues found that high delay discounting teens showed lower network engagement than did low delay discounting teens in two networks: an executive control network including dorsolateral prefrontal cortex as well as reward valuation network including hippocampus and insula (82). Using a virtual navigation task (a test of spatial working memory, see (83)), Sneider found that although chronic cannabis using teens and non-using teens performed similarly during training, during probe trials current cannabis users showed less activity in mesial temporal and cingulate regions than did nonusing teens (84). Norman and colleagues (85) observed that less activity in dorsolateral prefrontal cortex, middle frontal gyrus, and middle temporal gyrus during a response inhibition task predicted the age of onset for subsequent substance use in adolescents.
Ultimately, these studies highlight the relevance of investigating the adolescent cannabis user's brain at rest, and further the role of middle frontal and mesial temporal (including hippocampal) areas for adolescent cannabis users. This is highly important because these regions, including the medial frontal gyrus and the insula, have been particularly responsive to the purported active ingredients in interventions such as motivational interviewing in cannabis-using adolescents (10). In terms of potential treatment implications, this study continues to build upon the prior body of work to indicate the relevance of fronto-temporal systems. More specifically, the results from this study suggest treatment approaches that are attentive to processes relevant to the frontal networks (such as executive functions) and temporal networks (such as reward valuation and working memory) may have a greater a greater impact for high-risk youth. Interventions that may be particularly responsive to these processes include (but are not limited to) motivational interviewing, contingency management, and behavioral skills training. Ultimately, these findings reflect the importance of continuing to assess and evaluate developmentally salient factors, in order to create more efficacious intervention programming for these high-risk, high-need youth.
Limitations
We recommend that the observed findings be interpreted in light of the following limitations. While the sample in the present study is representative of justice-involved adolescents, it may not be representative of the broader adolescent population, who typically use cannabis at somewhat lower rates. The sample was also predominantly male, with a relatively small sample size (n=69) that precluded adequately-powered assessment of gender effects. Because the study reports assessments of adolescents from only one time, at baseline, it is not possible to make strong claims about relationships between the observed brain activity and cannabis use. Future studies should consider incorporating a follow-up resting scan some time after treatment. Future studies would also benefit from the inclusion of a comparison group, such as a sample of alcohol users (86,87); this would help determine whether the observed patterns may be attributable to a particular substance or are generally related to substance use. In addition, although the parent study includes both a behavioral intervention at baseline and behavioral follow-ups at 3, 6, and 12 months, at present it is too early in the study to link baseline brain responses to patterns of subsequent substance use.
Conclusion
This study joins with the extant research to suggest a consistent pattern of coherent frontal and temporal activity related to adult and adolescent cannabis use, both at rest and during task performance. Fronto-temporal regions have been broadly linked to working memory and attention (88–90), functions that are essential to academic and vocational performance, and also shown to be suppressed among adults who are heavy users of cannabis (91,92). In addition, psychosocial interventions for a range of problem health behaviors have been shown to modulate activity in mesial temporal lobe (24,93,94), which may suggest a role for this region in treatment response. Given the effects of cannabis use in early adolescence on cognitive function (4,5), it remains possible that the relationship between cannabis dependence and activity in the fronto-temporal network observed in the present study reflects the near-term sequelae of cannabis use. Alternatively, this relationship may indicate an underlying neuropsychological effect or a risk factor for cannabis use. Taken together, these preliminary results suggest that prevention and intervention strategies that address fronto-temporal functioning may be particularly helpful in this population.
Table 2. Sample demographics for low (n=33) and high (n=36) cannabis use groups.
| Measure | Low cannabis use | High cannabis use | ||
|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | |
| Age | 16.3 | 0.9 | 16.0 | 1.1 |
| Gender | ||||
| Male | 23 | 69.7% | 23 | 63.9% |
| Female | 10 | 30.3% | 13 | 36.1% |
| Ethnicity* | ||||
| White | 5 | 15.2% | 9 | 25.0% |
| African American | 3 | 9.1% | 3 | 8.3% |
| Hispanic | 27 | 81.8% | 23 | 63.9% |
| American Indian/Alaska Native | 2 | 6.1% | 6 | 16.7% |
| Asian or Pacific Islander | 1 | 3.0% | 0 | 0.0% |
| Other | 4 | 12.1% | 1 | 2.8% |
| Age at cannabis onset | 12.4 | 2.5 | 11.2 | 2.2 |
| Marijuana Dependence Scale | 1.5 | 2.0 | 3.1 | 2.3 |
| Marijuana Use Scale | 18.2 | 6.6 | 31.8 | 3.8 |
| Weekday cannabis use | ||||
| Yes | 6 | 18.2% | 31 | 86.1% |
| No | 27 | 81.8% | 5 | 13.9% |
| Cannabis use days (of 30) | 0.8 | 2.0 | 15.3 | 11.3 |
| Age at alcohol onset | 12.1 | 2.8 | 12.3 | 1.7 |
| AUDIT | 3.5 | 5.7 | 9.2 | 7.0 |
| Alcohol Use Scale | 19.2 | 7.0 | 25.7 | 8.3 |
| Weekday alcohol use | ||||
| Yes | 7 | 21.2% | 19 | 52.8% |
| No | 26 | 78.8% | 17 | 47.2% |
| Alcohol use days (of 30) | 0.6 | 1.6 | 3.3 | 3.7 |
| Framewise displacement (mm) | 0.36 | 0.55 | 0.28 | 0.25 |
| ImpSS | ||||
| Impulsivity | 3.8 | 2.2 | 4.5 | 2.0 |
| Sensation-seeking | 6.7 | 2.6 | 7.0 | 2.4 |
| Total | 10.5 | 4.4 | 11.5 | 3.7 |
| DERS | ||||
| Nonacceptance of emotional responses | 11.3 | 5.6 | 11.1 | 5.0 |
| Difficulties engaging in goal-directed behavior | 11.9 | 4.1 | 14.0 | 5.3 |
| Impulse control difficulties | 13.2 | 5.5 | 15.1 | 5.4 |
| Lack of emotional awareness | 17.9 | 6.1 | 18.4 | 6.1 |
| Limited access to emotion regulation strategies | 16.7 | 7.0 | 16.6 | 6.8 |
| Lack of emotional clarity | 10.1 | 3.7 | 11.5 | 3.9 |
| Total | 81.9 | 21.1 | 87.0 | 22.7 |
| WISC | ||||
| Digit span scaled score | 7.7 | 3.0 | 7.5 | 3.2 |
| Letter numbering scaled score | 8.0 | 2.5 | 7.4 | 2.8 |
| WAIS | ||||
| Digit span scaled score | 8.7 | 1.9 | 8.9 | 2.1 |
| Letter numbering scaled score | 8.6 | 2.7 | 8.9 | 4.0 |
This item may not total to 100% due to selection of multiple responses.
Note. ImpSS = Impulsivity and Sensation Seeking. DERS = Difficulties in Emotion Regulation Scale. WAIS = Wechsler Adult Intelligence Scale. WISC = Wechsler Intelligence Scale for Children. Framewise displacement = mean head motion in mm.
Acknowledgments
This research was supported by NINR R01 NR013332, NIAAA K01AA021431, and NIDA L30 DA034353.
Contributor Information
Jon M. Houck, Mind Research Network
Angela D. Bryan, University of Colorado
Sarah W. Feldstein Ewing, University of New Mexico
References
- 1.Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52(3):216–24. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 2.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition. 2010 Feb;72(1):101–13. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95(11):1621–30. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 4.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. [cited 2013 Feb 26];PNAS. 2012 Aug 27; doi: 10.1073/pnas.1206820109. Internet. Available from: http://www.pnas.org/content/early/2012/08/22/1206820109. [DOI] [PMC free article] [PubMed]
- 5.Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012 Sep;26(3):496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988 Nov;34(5):605–13. [PubMed] [Google Scholar]
- 7.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 8.Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacological Research. 2009 Aug;60(2):132–8. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Glass M, Faull RL, Dragunow M. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997 Feb 21;77(2):299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 10.Molina-Holgado E, Vela JM, Arévalo-Martín A, Almazán G, Molina-Holgado F, Borrell J, et al. Cannabinoids Promote Oligodendrocyte Progenitor Survival: Involvement of Cannabinoid Receptors and Phosphatidylinositol-3 Kinase/Akt Signaling. J Neurosci. 2002 Nov 15;22(22):9742–53. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, et al. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997 Jul;26(3):317–23. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. European Journal of Neuroscience. 2003 May;17(9):1747–54. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- 13.Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005 Jan 7;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Zalesky A, Solowij N, Yücel M, Lubman DI, Takagi M, Harding IH, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012 Jul 1;135(7):2245–55. doi: 10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]
- 15.Swift W, Coffey C, Carlin JB, Degenhardt L, Patton GC. Adolescent cannabis users at 24 years: trajectories to regular weekly use and dependence in young adulthood. Addiction. 2008;103(8):1361–70. doi: 10.1111/j.1360-0443.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- 16.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007 Sep;13(5):807–20. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008 Dec;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker DD, Roffman RA, Stephens RS, Berghuis J, Kim W. Motivational Enhancement Therapy for Adolescent Marijuana Users: A Preliminary Randomized Controlled Trial. J Consult Clin Psychol. 2006 Jun;74(3):628–32. doi: 10.1037/0022-006X.74.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCambridge J, Slym RL, Strang J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction. 2008 Nov 1;103(11):1809–18. doi: 10.1111/j.1360-0443.2008.02331.x. [DOI] [PubMed] [Google Scholar]
- 20.Martin G, Copeland J. The adolescent cannabis check-up: Randomized trial of a brief intervention for young cannabis users. Journal of Substance Abuse Treatment. 2008 Jun;34(4):407–14. doi: 10.1016/j.jsat.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Hutchison KE. Substance Use Disorders: Realizing the Promise of Pharmacogenomics and Personalized Medicine. Annual Review of Clinical Psychology. 2010;6(1):577–89. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- 22.Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: Emerging translational approaches that bridge biology and behavior. Psychology of Addictive Behaviors. 2013 Jun;27(2):329–35. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldstein Ewing SW, Mead HK, Yezhuvath U, DeWitt S, Hutchison KE, Filbey FM. A preliminary examination of how serotonergic polymorphisms influence brain response following an adolescent cannabis intervention. Psychiatry Research: Neuroimaging. 2012 Nov 30;204(2–3):112–6. doi: 10.1016/j.pscychresns.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating Brain and Behavior: Evaluating Adolescents' Response to a Cannabis Intervention. Psychology of Addictive Behaviors. 2012 Aug 27; doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldstein-Ewing S, Filbey F, Hendershot C, McEachern AD, Hutchison KE. Proposed model of the neurobiological mechanisms underlying psychosocial alcohol interventions: the example of motivational interviewing. Journal of studies on alcohol and drugs. 2011;72(6):903–903. 16. doi: 10.15288/jsad.2011.72.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houck JM, Moyers TB, Tesche CD. Through a Glass Darkly: Some Insights on Change Talk via Magnetoencephalography. Psychology of Addictive Behaviors. 2013 Jun;27(2):489–500. doi: 10.1037/a0029896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill D, Rowntree D. The spontaneous variability of the EEG in some schizophrenics. Electroencephalography & Clinical Neurophysiology. 1949;1 [Google Scholar]
- 28.Reeve A, Knight J, Maclin E, Lewine J, Orrison W. Abstracts. International Congress for Schizophrenia Research; Colorado Springs: 1993. Resting-state magnetoencephalography in schizophrenia. [Google Scholar]
- 29.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic Resonance in Medicine. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 30.Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapping. 1994;2(1-2):56–78. [Google Scholar]
- 31.Bell AJ, Sejnowski TJ. An Information-Maximization Approach to Blind Separation and Blind Deconvolution. Neural Computation. 1995 Nov 1;7(6):1129–59. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 32.Comon P. Independent component analysis, A new concept? Signal Processing. 1994 Apr;36(3):287–314. [Google Scholar]
- 33.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A Default Mode of Brain Function. PNAS. 2001 Jan 16;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain's Default Network. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 35.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002 Mar;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 36.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J Neurophysiol. 2008 Dec 1;100(6):3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. The Journal of Neuroscience. 2007 Feb 28;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. [cited 2013 Mar 1];PNAS. 2009 Jul 20; doi: 10.1073/pnas.0905267106. Internet. Available from: http://www.pnas.org/content/early/2009/07/17/0905267106. [DOI] [PMC free article] [PubMed]
- 39.Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping. 2008;29(7):828–38. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomason ME, Dennis EL, Joshi AA, Joshi SH, Dinov ID, Chang C, et al. Resting-state fMRI can reliably map neural networks in children. NeuroImage. 2011 Mar 1;55(1):165–75. doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomason ME, Burrows BE, Gabrieli JDE, Glover GH. Breath holding reveals differences in fMRI BOLD signal in children and adults. NeuroImage. 2005 Apr 15;25(3):824–37. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 42.Gonsalves BD, Cohen NJ. Brain Imaging, Cognitive Processes, and Brain Networks. Perspectives on Psychological Science. 2010 Nov 1;5(6):744–52. doi: 10.1177/1745691610388776. [DOI] [PubMed] [Google Scholar]
- 43.Uttal WR. The New Phrenology: The Limits of Localizing Cognitive Processes in the Brain. A Bradford Book; 2003. [Google Scholar]
- 44.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry. 2007 Sep 1;62(5):429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DEJ. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophrenia Research. 2010 Mar;117(1):21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29(11):1265–75. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage. 2010 Oct 15;53(1):247–56. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno-López L, Stamatakis EA, Fernández-Serrano MJ, Gómez-Río M, Rodríguez-Fernández A, Pérez-García M, et al. Neural Correlates of the Severity of Cocaine, Heroin, Alcohol, MDMA and Cannabis Use in Polysubstance Abusers: A Resting-PET Brain Metabolism Study. PLoS ONE. 2012 Jun 29;7(6):e39830. doi: 10.1371/journal.pone.0039830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sneider JT, Pope HG, Jr, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. European Neuropsychopharmacology. 2008 Aug;18(8):612–9. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang G, Qiu Y, Zhang X, Han LJ, Lv XF, Li L, et al. Amplitude low-frequency oscillation abnormalities in the heroin users: A resting state fMRI study. NeuroImage. 2011 Jul 1;57(1):149–54. doi: 10.1016/j.neuroimage.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews. 2009 Mar;33(3):279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Yücel M, Pantelis C. REgional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008 Jun 2;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 53.Böcker KBE, Hunault CC, Gerritsen J, Kruidenier M, Mensinga TT, Kenemans JL. Cannabinoid Modulations of Resting State EEG Theta Power and Working Memory Are Correlated in Humans. Journal of Cognitive Neuroscience. 2009 Oct 5;22(9):1906–16. doi: 10.1162/jocn.2009.21355. [DOI] [PubMed] [Google Scholar]
- 54.Tesche CD, Karhu J. Theta Oscillations Index Human Hippocampal Activation During a Working Memory Task. PNAS. 2000 Jan 18;97(2):919–24. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sneider JT, Pope HGJ, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Altered regional blood volume in chronic cannabis smokers. Experimental and Clinical Psychopharmacology. 2006 Nov;14(4):422–8. doi: 10.1037/1064-1297.14.4.422. [DOI] [PubMed] [Google Scholar]
- 56.Krmpotich TD, Tregellas JR, Thompson LL, Banich MT, Klenk AM, Tanabe JL. Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug and Alcohol Dependence. 2013 Apr 1;129(1–2):1–7. doi: 10.1016/j.drugalcdep.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, et al. Structural and Functional Imaging Studies in Chronic Cannabis Users: A Systematic Review of Adolescent and Adult Findings. PLoS ONE. 2013 Feb 4;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, et al. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. [cited 2013 Aug 14];Neuropharmacology. doi: 10.1016/j.neuropharm.2013.05.027. Internet. Available from: http://www.sciencedirect.com/science/article/pii/S0028390813002414. [DOI] [PubMed]
- 59.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000 Oct;68(5):898–908. [PubMed] [Google Scholar]
- 60.Sobell LC, Sobell MB. Validity of self-reports in three populations of alcoholics. Journal of Consulting and Clinical Psychology. 1978 Oct;46(5):901–7. doi: 10.1037//0022-006x.46.5.901. [DOI] [PubMed] [Google Scholar]
- 61.White HR, Labouvie EW. Towards the Assessment of Adolescent Problem Drinking. Journal of Studies on Alcohol and Drugs. 1989 Jan 1;50(01):30. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- 62.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Pimrary Care. World Health Organization; 2001. [Google Scholar]
- 63.Chung T, Colby SM, Barnett NP, Rohsenow DJ, Spirito A, Monti PM. Screening Adolescents for Problem Drinking: Performance of Brief Screens against DSM-IV Alcohol Diagnoses. Journal of Studies on Alcohol and Drugs. 2000 Jun 1;61(4):579. doi: 10.15288/jsa.2000.61.579. [DOI] [PubMed] [Google Scholar]
- 64.Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The Big Three, the Big Five, and the Alternative Five. Journal of Personality and Social Psychology. 1993 Oct;65(4):757–68. [Google Scholar]
- 65.McDaniel SR, Mahan JE., III An examination of the ImpSS scale as a valid and reliable alternative to the SSS-V in optimum stimulation level research. Personality and Individual Differences. 2008 May;44(7):1528–38. [Google Scholar]
- 66.Gratz KL, Roemer L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2004 Mar 1;26(1):41–54. [Google Scholar]
- 67.Teicher MH, Ito Y, Glod CA, Barber NI. Objective Measurement of Hyperactivity and Attentional Problems in ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 1996 Mar;35(3):334–42. doi: 10.1097/00004583-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 68.Fazel S, Doll H, Långström N. Mental Disorders Among Adolescents in Juvenile Detention and Correctional Facilities: A Systematic Review and Metaregression Analysis of 25 Surveys. Journal of the American Academy of Child & Adolescent Psychiatry. 2008 Sep;47(9):1010–9. doi: 10.1097/CHI.ObO13e31817eecf3. [DOI] [PubMed] [Google Scholar]
- 69.Gordon JA, Moore PM. ADHD among incarcerated youth: An investigation on the congruency with ADHD prevalence and correlates among the general population. Am J Crim Just. 2005 Sep 1;30(1):87–97. [Google Scholar]
- 70.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012 Feb 1;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996 Jun;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 72.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14(3):140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Himberg J, Hyvarinen A. Icasso: software for investigating the reliability of ICA estimates by clustering and visualization. 2003 IEEE 13th Workshop on Neural Networks for Signal Processing, 2003 NNSP'03. 2003:259–268. [Google Scholar]
- 74.Elseoud AA, Littow H, Remes J, Starck T, Nikkinen J, Tervonen O, et al. Group-ICA model order highlights patterns of functional brain connectivity. Front Syst Neurosci. 2011;5:37. doi: 10.3389/fnsys.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping. 2011;32(12):2075–95. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies Contributing to Functional Connectivity in the Cerebral Cortex in “Resting-state” Data. AJNR Am J Neuroradiol. 2001 Aug 1;22(7):1326–33. [PMC free article] [PubMed] [Google Scholar]
- 77.Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold. Magnetic Resonance in Medicine. 1995 May 1;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 79.Klumpers LE, Cole DM, Khalili-Mahani N, Soeter RP, te Beek ET, Rombouts SARB, et al. Manipulating brain connectivity with δ9-tetrahydrocannabinol: A pharmacological resting state FMRI study. NeuroImage. 2012 Nov 15;63(3):1701–11. doi: 10.1016/j.neuroimage.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 80.Jacobus J, Goldenberg D, Wierenga C, Tolentino N, Liu T, Tapert S. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology. 2012;222(4):675–84. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tapert S, Schweinsburg A, Drummond S, Paulus M, Brown S, Yang T, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanger C, Elton A, Ryan SR, James A, Budney A, Kilts C. Neuroeconomics and Adolescent Substance Abuse: Individual Differences in Neural Networks and Delay Discounting. [cited 2013 Jun 3];Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1016/j.jaac.2013.04.013. Internet. Available from: http://www.jaacap.com/article/S0890-8567(13)00263-3/abstract. [DOI] [PMC free article] [PubMed]
- 83.Hanlon FM, Houck JM, Klimaj Sd, Caprihan A, Mayer AR, Weisend MP, et al. Frontotemporal anatomical connectivity and working-relational memory performance predict everyday functioning in schizophrenia. Psychophysiology. 2012;49(10):1340–52. doi: 10.1111/j.1469-8986.2012.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sneider JT, Gruber SA, Rogowska J, Silveri MM, Yurgelun-Todd DA. A Preliminary Study of Functional Brain Activation among Marijuana Users during Performance of a Virtual Water Maze Task. Journal of Addiction. 2013;2013:1–12. doi: 10.1155/2013/461029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011 Dec 15;119(3):216–23. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF. Adolescents' fMRI Activation to a Response Inhibition Task Predicts Future Substance Use. [cited 2012 Sep 18];Addictive Behaviors. doi: 10.1016/j.addbeh.2012.07.012. Internet. Available from: http://www.sciencedirect.com/science/article/pii/S0306460312002912?v=s5. [DOI] [PMC free article] [PubMed]
- 87.Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106(3):564–73. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. PNAS. 1993 Feb 1;90(3):878–82. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997 Apr 10;386(6625):604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 90.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task∷ A large and reliable sex difference. Behavioural Brain Research. 1998 Jun;93(1-2):185–90. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 91.Jager G, Kahn R, Van Den Brink W, Van Ree J, Ramsey N. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology. 2006;185(3):358–68. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- 92.Solowij N, Michie PT, Fox AM. Effects of long-term cannabis use on selective attention: An event-related potential study. Pharmacology Biochemistry and Behavior. 1991 Nov;40(3):683–8. doi: 10.1016/0091-3057(91)90382-c. [DOI] [PubMed] [Google Scholar]
- 93.Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WHR. Effects of cognitive-behavioral therapy on brain activation in specific phobia. NeuroImage. 2006 Jan 1;29(1):125–35. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Vocks S, Busch M, Schulte D, Grönermeyer D, Herpertz S, Suchan B. Effects of body image therapy on the activation of the extrastriate body area in anorexia nervosa: An fMRI study. Psychiatry Research: Neuroimaging. 2010 Aug 30;183(2):114–8. doi: 10.1016/j.pscychresns.2010.05.011. [DOI] [PubMed] [Google Scholar]


