Abstract
Despite decades of research, the roles of climate and humans in driving the dramatic extinctions of large-bodied mammals during the Late Quaternary remain contentious. We use ancient DNA, species distribution models and the human fossil record to elucidate how climate and humans shaped the demographic history of woolly rhinoceros, woolly mammoth, wild horse, reindeer, bison and musk ox. We show that climate has been a major driver of population change over the past 50,000 years. However, each species responds differently to the effects of climatic shifts, habitat redistribution and human encroachment. Although climate change alone can explain the extinction of some species, such as Eurasian musk ox and woolly rhinoceros, a combination of climatic and anthropogenic effects appears to be responsible for the extinction of others, including Eurasian steppe bison and wild horse. We find no genetic signature or any distinctive range dynamics distinguishing extinct from surviving species, underscoring the challenges associated with predicting future responses of extant mammals to climate and human-mediated habitat change.
Toward the end of the Late Quaternary, beginning c. 50,000 years ago, Eurasia and North America lost c. 36% and 72% of their large-bodied mammalian genera (megafauna), respectively1. The debate surrounding the potential causes of these extinctions has focused primarily on the relative roles of climate and humans2,3,4,5. In general, the proportion of species that went extinct was greatest on continents that experienced the most dramatic climatic changes6, implying a major role of climate in species loss. However, the continental pattern of megafaunal extinctions in North America approximately coincides with the first appearance of humans, suggesting a potential anthropogenic contribution to species extinctions3,5.
Demographic trajectories of different taxa vary widely and depend on the geographic scale and methodological approaches used3,5,7. For example, genetic diversity in bison8,9, musk ox10 and European cave bear11 declines gradually from c. 50–30,000 calendar years ago (ka BP). In contrast, sudden losses of genetic diversity are observed in woolly mammoth12,13 and cave lion14 long before their extinction, followed by genetic stability until the extinction events. It remains unresolved whether the Late Quaternary extinctions were a cross-taxa response to widespread climatic or anthropogenic stressors, or were a species-specific response to one or both factors15,16. Additionally, it is unclear whether distinctive genetic signatures or geographic range-size dynamics characterise extinct or surviving species—questions of particular importance to the conservation of extant species.
To disentangle the processes underlying population dynamics and extinction, we investigate the demographic histories of six megafauna herbivores of the Late Quaternary: woolly rhinoceros (Coelodonta antiquitatis), woolly mammoth (Mammuthus primigenius), horse (wild Equus ferus and living domestic Equus caballus), reindeer/caribou (Rangifer tarandus), bison (Bison priscus/Bison bison) and musk ox (Ovibos moschatus). These taxa were characteristic of Late Quaternary Eurasia and/or North America and represent both extinct and extant species. Our analyses are based on 846 radiocarbon-dated mitochondrial DNA (mtDNA) control region sequences, 1,439 directly-dated megafaunal remains, and 6,291 radiocarbon determinations associated with Upper Palaeolithic human occupations in Eurasia. We reconstruct the demographic histories of the megafauna herbivores from ancient DNA data, model past species distributions and determine the geographic overlap between humans and megafauna over the last 50,000 years. We use these data to investigate how climate change and anthropogenic impacts affected species dynamics at continental and global scales, and contributed to in the extinction of some species and the survival of others.
Effects of climate change differ across species and continents
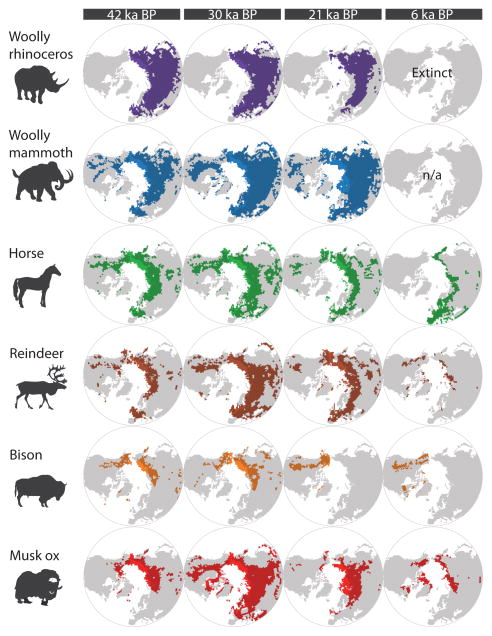
The direct link between climate change, population size and species extinctions is difficult to document10. However, population size is likely controlled by the amount of available habitat and is indicated by the geographic range of a species17,18. We assessed the role of climate using species distribution models, dated megafauna fossil remains and palaeoclimatic data on temperature and precipitation. We estimated species range sizes at the time periods of 42, 30, 21 and 6 ka BP as a proxy for habitat availability (Fig. 1; Supplementary Information section S1). Range size dynamics were then compared to demographic histories inferred from ancient DNA using three distinct analyses (Supplementary Information section S3): (i) coalescent-based estimation of changes in effective population size through time (Bayesian skyride19), which allows detection of changes in global genetic diversity; (ii) serial coalescent simulation followed by Approximate Bayesian Computation, which selects among different models describing continental population dynamics; and (iii) isolation-by-distance analysis, which estimates potential population structure and connectivity within continents. If climate was a major factor driving species population sizes, we would expect expansion and contraction of a species’ geographic range to mirror population increase and decline, respectively.
Figure 1.
Modelled potential ranges of megafauna species at 42, 30, 21 and 6 ka BP. Ranges were modelled using palaeoclimatic data for temperature and precipitation and the megafauna fossil record. Range measurements were restricted to the regions for which fossils were used to build the models, rather than all potentially suitable Holarctic area.
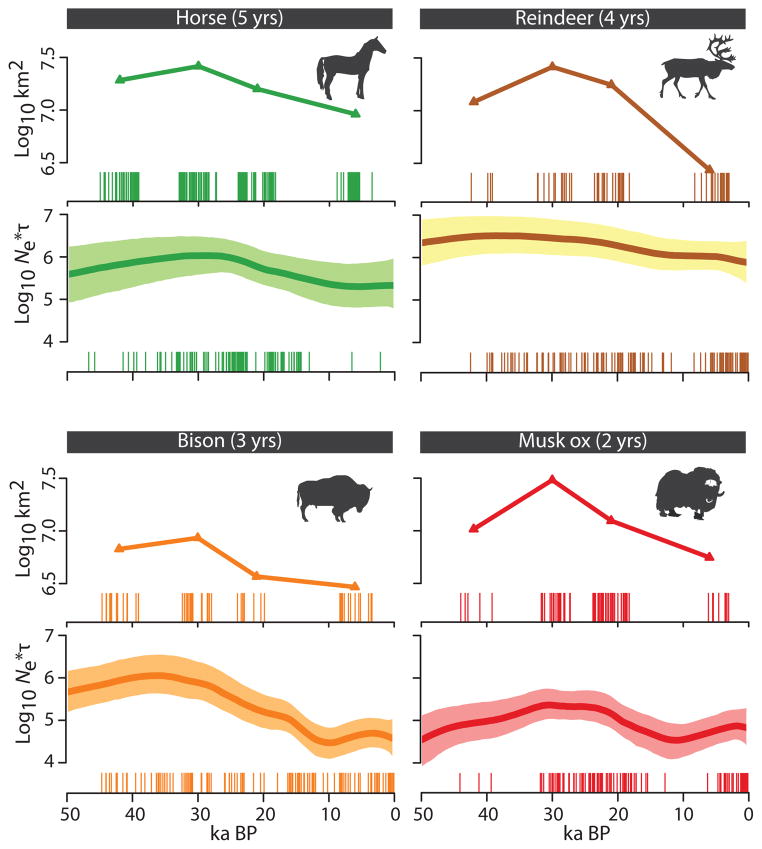
We find a positive correlation between changes in the size of available habitat and genetic diversity for the four species—horse, reindeer, bison and musk ox—for which we have range estimates spanning all four time-points (the correlation is not statistically significant for reindeer: p = 0.101) (Fig. 2; Supplementary Information section S4). Hence, species distribution modelling based on fossil distributions and climate data are congruent with estimates of effective population size based on ancient DNA data, even in species with very different life-history traits. We conclude that climate has been a major driving force in megafauna population changes over the past 50,000 years. It is noteworthy that both estimated modelled ranges and genetic data are derived from a subset of the entire fossil record (Supplementary Information sections S1 and S3). Thus, changes in effective population size and range size may change with the addition of more data, especially from outside the geographical regions covered by the present study. However, we expect that the reported positive correlation will prevail when congruent data are compared.
Figure 2.
Temporal changes in global genetic diversity and range size in horse, bison, reindeer and musk ox. X-axis is in calendar years; y-axis is the product of effective population size and generation time (Ne*τ). Comparable estimates of associated range sizes (km2) are from Figure 1. The temporal span of the radiocarbon-dated samples used in each approach is shown as vertical lines below each panel and each line represents one dated individual.
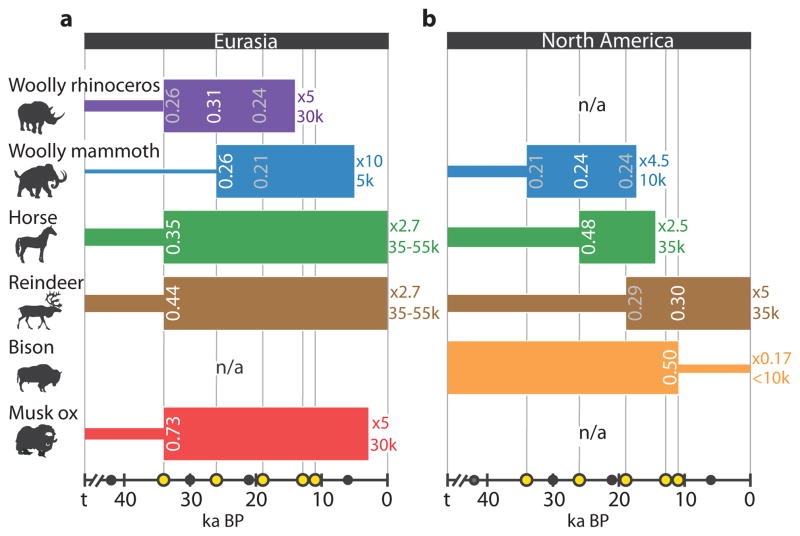
The best-supported models of changes in effective population size in North America and Eurasia during periods of dramatic climatic change during the past 50,000 years are those in which populations increase in size (Fig. 3, Supplementary Information section S3). This is true for all taxa except bison. However, the timing is not synchronous across populations. Specifically, we find highest support for population increase beginning c. 34 ka BP in Eurasian horse, reindeer and musk ox (Fig. 3a). Eurasian mammoth and North American horse increase prior to the Last Glacial Maximum (LGM) c. 26 ka BP. Models of population increase in woolly rhinoceros and North American mammoth fit equally well before and after the LGM, and North American reindeer populations increase later still. Only North American bison shows a population decline (Fig. 3b), the intensity of which likely swamps the signal of global population increase starting at c. 35 ka BP identified in the skyride plot (Fig. 2a).
Figure 3.
Best-supported demographic models inferred by ABC model-selection for (a) Eurasia and (b) North America. Grey dots on the time axis indicate periods with range size estimates. Yellow dots indicate the periods of demographic increase or decline, which were tested against each other in the approach. White values inside coloured bars reflect support for the best-supported model (e.g. Eurasian woolly mammoth, increase at 26 ka BP). The intensity of increase or decline (e.g. x5) and effective population size at the time of the youngest sample (e.g. 10k individuals) are shown. We indicate in grey cases where multiple models received similar levels of support.
These increases in effective population size likely reflect responses to climate change. By 34 ka BP, the relatively warmer Marine Isotope Stage (MIS) 3 interstadial marked the transition to cold, arid full-glacial conditions of MIS 2, which began c. 30 ka BP20,21. Although the pre-LGM density of humans in Siberia remains uncertain, Pleistocene archaeological sites in the Siberian far north are scarce22 and humans were presumably absent from North America prior to at least 15 ka BP23. These point to climate, rather than humans, as the key driver of these species-specific and, in some cases, continent-specific demographic changes. This conclusion is supported by the significant correlations between modelled range sizes and effective population sizes (Fig. 2).
Modes of extinction
Both woolly rhinoceros and woolly mammoth suffered global extinctions during the Late Quaternary. Neither shows evidence of a decline in genetic diversity leading to their extinction at either continental or global scales (Supplementary Figs S3.2 and S3.6). However, the fossil records of the two species differ; woolly rhinoceros remains widely distributed across Eurasia until it disappears from the fossil record c. 14 ka BP (Supplementary Fig. S2.2), whereas the woolly mammoth range retreats northward during its last millennia (Supplementary Figs S2.3, S5.2c,d). We find increased isolation-by-distance preceding extinction (Supplementary Fig. S3.1; Supplementary Information section 3) suggesting that populations of both species became increasingly fragmented, although the results are not statistically significant for mammoth. The high and sustained levels of genetic diversity in these species may reflect the fixation of multiple distinct haplotypes in increasingly isolated and diminishing subpopulations. For mammoth, this scenario is also supported by fossil evidence24.
Our data suggest similar scenarios of increased isolation-by-distance prior to the extinctions of musk ox in Eurasia (c. 2.5 ka BP25,26) and of steppe bison in the north of the North American plains, which potentially survived until only a few hundred years ago8 (Supplementary Fig. S3.1). Such fragmentation is commonly observed in wide-ranging species undergoing population decline, due to populations aggregating in patches of high-quality habitat27. In contrast, we find low levels of isolation-by-distance in wild horse and in Eurasian and North American reindeer, suggesting these populations remained relatively panmictic over time.
Disentangling the roles of climate and humans
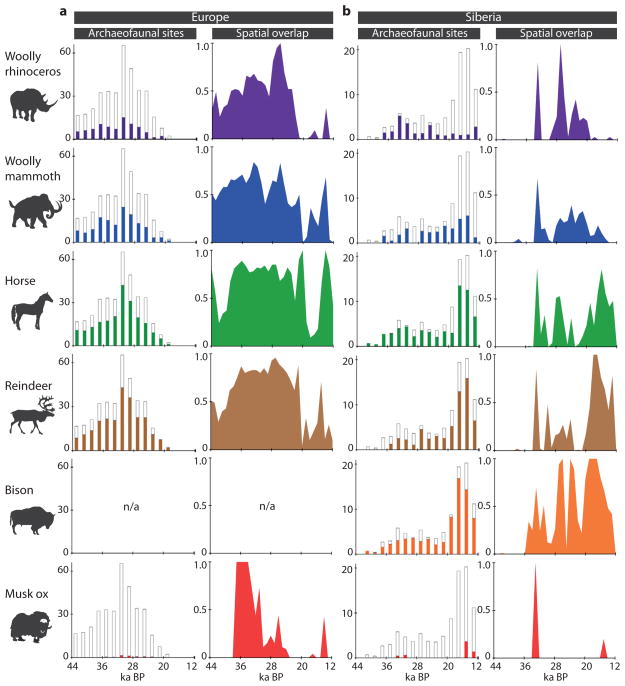
To evaluate the potential role of humans in the local and global megafauna extinctions, we measured: (i) the spatial overlap between the modelled range of each megafauna species and the Eurasian Palaeolithic archaeological record at 42, 30 and 21 ka BP; (ii) the presence of megafauna remains in Palaeolithic archaeological assemblages from Europe (48–18 ka BP) and Siberia (41–12 ka BP); and (iii) variation in fossil abundance and the temporal and spatial distributions of known Palaeolithic archaeological sites and the Eurasian megafauna fossil record at 1,000-year intervals. For the latter, we added 1,557 indirectly-dated megafaunal remains to the 1,439 directly-dated specimens to increase sample sizes. Although associated with greater age-estimate uncertainties, the integrity of each of the indirectly dated samples was evaluated prior to inclusion following the guidelines listed in Supplementary Information section S5.
Woolly rhinoceros and Eurasian woolly mammoth experience a five- to ten-fold increase in effective population size between 34 ka BP and 19 ka BP (Fig. 3), at least 10,000 years after first human contact as inferred from the overlap between estimated ranges and archaeological sites (Supplementary Figs S1.2, S1.5). This result directly contradicts models of population collapse from human overkill (blitzkrieg)2 or infectious diseases following the first human contact (hyperdisease)28.
We find no evidence that Palaeolithic humans greatly impacted musk ox populations, in agreement with previous conclusions that humans were not responsible for the extinction of musk ox in Eurasia10. Musk ox remains are found in only 1% of European archaeological sites and 6% of Siberian sites, and do not overlap noticeably in range with Palaeolithic humans in either Europe or Siberia (Fig. 4). However, the decline in the potential range of musk ox by 60% between 21 and 6 ka BP (Fig. 1), the increase in isolation-by-distance at 19 ka BP (Supplementary Fig. S3.1, Supplementary Table S3.3), and the positive correlation between climate-driven range size and genetic diversity (Fig. 2b) all point towards climate as the main driver of musk ox population dynamics, including the decrease in genetic diversity after the LGM (Fig. 2a). The importance of climate is further supported by the physiology of musk ox, which may be a more sensitive indicator of environmental warming than the other species. Musk ox has extreme temperature sensitivity and is unable to tolerate high summer temperatures; the 10°C summer isotherm approximates the southern limit of its present-day range29.
Figure 4.
Spatial and temporal association between megafauna and Upper Palaeolithic humans in (a) Europe and (b) Siberia. Column graphs represent all known cultural occupations containing ≥ one of the six species, averaged over 2,000-year time bins. Open bars indicate the number of archaeofaunal sites, closed bars represent the frequency of each species in the binned assemblages. Area graphs show the fraction of megafauna surface area shared with humans at 1,000-year intervals, calculated from mean ± 1sd of latitude and longitude; data represented in Supplementary Figure S5.2. Graphs use coordinates of data associated with both direct and indirect dates.
We find little regional overlap between Palaeolithic humans and woolly rhinoceros in Siberia after the LGM (i.e., < 20 ka BP); the species is found in fewer than 11% of Siberian archaeological sites during this time (Fig. 4). This suggests that woolly rhinoceros was not a common prey species for humans, and that overhunting is an unlikely explanation for their extinction in Siberia. However, we note that geographic overlap existed between humans and woolly rhinoceros in Europe during the two millennia preceding extinction (Fig. 4), and therefore cannot exclude the hypothesis that humans influenced the final collapse of the species in this region. The continued presence of woolly rhinoceros in the fossil record throughout Siberia and parts of Europe up until the species extinction event (Supplementary Fig. S2.2) suggests that the final collapse of the species was synchronous across its range.
The data from woolly mammoth are inconclusive regarding the causes of extinction. We find that the range of Eurasian woolly mammoth overlaps continuously with humans throughout the Palaeolithic (Fig. 4), in agreement with previous results based on a more limited dataset30. Mammoth remains are found in 40% and 35% of all European and Siberian Palaeolithic sites, respectively, and mammoth subsistence hunting by Clovis peoples in North America has been documented31. However, the prevalence of mammoth in Siberian sites declines after the LGM (43% of sites before 19 ka BP versus 30% after; Fig. 4). This decline could indicate a northward range shift of mammoth ahead of humans30 (Fig. S5.2c,d), an increasing scarcity of mammoths in southern Siberia, or an increasing human preference for other prey species.
In wild horse, the large mid-Holocene range of over 9 million km2 (Fig. 1, Supplementary Table S1.3) suggests the potential for a large Eurasian population at this time, and is not consistent with climate driving the final disappearance of the species in the wild. Rather, the decline in genetic diversity observed after the LGM in horse and bison, and to a lesser degree in reindeer (Fig. 2), may reflect the impact of expanding human populations in Europe and Asia. The presence of the three species in the archaeological record suggests that their populations are more likely to have been influenced by humans. Bison and horse are the most common megafauna herbivores found in archaeological sites (Fig. 4), with horse present in 58% and 66% of European and Siberian sites, respectively. Furthermore, horse shows extensive geographic overlap with humans in both Europe and Siberia after the LGM, although large population sizes may have insulated horses to some extent from the effects of selective hunting by humans.
In bison, the pre-human decline in genetic diversity starting c. 35 ka BP and the strong correlation between range size and genetic diversity indicate climate as a main driver of demographic change (Fig. 2). This conclusion is supported by the five-fold decline in effective population size (Fig. 3) and increased isolation-by-distance c. 11 ka BP in North America (Supplementary Fig. S3.1, Supplementary Table S3.3). The timing of these demographic changes coincide with the pronounced climatic shifts associated with the Pleistocene/Holocene transition32, although they also coincide with fossil evidence of growing populations of potential competitors such as Alces and Cervus33. The accelerated rate of decline in genetic diversity after c. 16 ka BP (Fig. 2) is coincident with the earliest known human expansion in the Americas23, and the significant presence of bison in 77% of the Siberian archaeological assemblages points to their popularity as a prey species (Fig. 4).
Reindeer are the most abundant of the six taxa today. As with horse, reindeer show continuous geographic overlap with Palaeolithic humans in Eurasia (Fig. 4). Reindeer are common in both European and Siberian Palaeolithic assemblages, are found in 67% of Siberian sites after the LGM and were an important prey species for humans in both Eurasia and North America34. Unlike bison and horse, the potential range of reindeer declines by 84% between 21 and 6 ka BP (Fig. 1, Supplementary Table S1.3). Despite the apparently detrimental influences of both humans and climate change, wild and domestic reindeer currently number in the millions across the Holarctic35. Although individual populations are affected by changing climate36, the species is not currently under threat of extinction. The success of reindeer may be explained by high fecundity37 and ecological flexibility38. In addition, continued low levels of isolation-by-distance suggest high mobility and near-panmixia of populations over millennia (Supplementary Fig. S3.1, Supplementary Table S3.3).
Conclusions
We find that neither the effects of climate nor human occupation alone can explain the megafauna extinctions of the Late Quaternary. Rather, our results demonstrate that changes in megafauna abundance are idiosyncratic, with each species (and even continental populations within species) responding differently to the effects of climate change, habitat redistribution and human encroachment. While reindeer remain relatively unaffected by any of these factors on a global scale, climate change alone explains the extinction of Eurasian musk ox and woolly rhinoceros, and a combination of climatic and anthropogenic effects appears to be responsible for the demise of wild horse and steppe bison. The causes underlying the extinction of woolly mammoth remain elusive.
We have shown that changes in habitat distribution and population size are intrinsically linked over evolutionary time, supporting the view that populations of many species will decline in the future due to climate change and habitat loss.
Intriguingly however, we find no distinguishing characteristics in the rate or pattern of decline in those species that went extinct versus those that have survived. Our study demonstrates the importance of incorporating lessons from the past into rational, data-driven strategies for the future to address our most pressing environmental challenges: the ongoing global mass-extinction of species and the impacts of global climate change and humans on the biodiversity that remains.
METHODS (ONLINE-ONLY)
Data
Mitochondrial DNA sequences and accelerator mass spectrometry (AMS) radiocarbon dates were collected from the past and present geographic ranges of six megafauna herbivores from Eurasia and North America: woolly rhinoceros (Coelodonta antiquitatis), woolly mammoth (Mammuthus primigenius), horse (wild Equus ferus and living domestic Equus caballus), reindeer/caribou (Rangifer tarandus), bison (Bison priscus/Bison bison) and musk ox (Ovibos moschatus) (Supplementary Fig. S2.1; Supplementary Information sections S2 and S3). Our data comprise 846 radiocarbon-dated ancient mitochondrial DNA sequences (274 of which are new), 1,439 directly-dated megafauna specimens (357 of which are new) and 6,291 dated remains associated with Upper Palaeolithic humans in Eurasia. In one analysis of the spatial and temporal association between humans and megafauna detailed below, we included an additional 1,557 indirectly-dated megafaunal remains.
Species distribution modelling
We assessed changes in potential range size over the past 50 thousand calendar years (ka BP) using 829 radiocarbon-dated megafauna fossils calibrated with the IntCal09 calibration curve39 and palaeoclimatic estimates of precipitation and temperature40. Potential ranges were estimated for the four periods for which palaeoclimatic data are available, 42, 30, 21 and 6 ka BP, using only contemporaneous fossils (±3 ka) for each period (Supplementary Fig. S1.2). We compared temporal changes in potential range size (from species distribution models) and genetic diversity (from Bayesian skyrides20) during the past 50 ka BP to assess the relationship between these independent proxies of population size. If climate were a major driver of changes in population size, we would expect these two measures to be positively correlated. Estimating past ranges using species distribution models can be affected by an incomplete or biased fossil record as well as inaccuracies in the palaeoclimate simulations used in the models; uncertainties associated with these issues are depicted in our estimates of range size and how it correlates to genetic diversity (Supplementary Fig. S4.3). Range measurements were restricted to regions for which fossils were used to build the models, rather than all potentially suitable Holarctic areas. Fossil localities represent a subset, rather than an exhaustive search, of the literature available, and modelled ranges consequently represent a subset of the entire past distribution of the species. Too few fossils were available to estimate the potential ranges of woolly rhinoceros and mammoth at 6 ka BP, as the former was extinct and the latter was restricted to two island populations. Thus, too few periods with range estimates for these two species precluded statistical comparison with the genetic data, which spanned 50,000 years. For further details see Supplementary Information sections S1 and S4.
Ancient genetic analysis
We used three analytical approaches capable of incorporating serially sampled data to reconstruct the past population dynamics of each megafauna herbivore species: (i) The Bayesian skyride approach20 estimates changes in genetic diversity through time as a proxy for effective population size, and was used to estimate the global demographic trajectory of each species. Because these data sets comprise samples from both a broad temporal and geographic extent, it is likely that they violate, at least during some of their evolutionary history, the assumption of panmixia made by the coalescent models currently implemented in BEAST. However, the skyride makes the least stringent prior assumptions among these coalescent models, and therefore is the most likely to accommodate the temporal changes in structure that may characterise each of these species; (ii) Serial-coalescent simulations and the Approximate Bayesian Computation (ABC) model-selection approach41 were used to test for demographic change in the continental subpopulations (Eurasia and North America) and in the global dataset. Time points were chosen to represent midpoints between the four periods (42, 30, 21 and 6 ka BP) for which we modelled potential megafauna ranges, and periods of dramatic climatic changes: the beginning (26 ka BP) and end (19 ka BP) of the Last Glacial Maximum, the onset of the Younger Dryas (12.9 ka BP) and the beginning of the Holocene (11 ka BP); (iii) Isolation-by-distance was used to test for changes in population structure over time in the continental subpopulations. Note that as with the species distribution models, the demographic events inferred from the ancient DNA data are conditional upon the samples included in the analysis. Hence, although we use the broad geographic terms of Eurasia and North America, the regions are limited to the localities covered by the sequenced samples (Supplementary Fig. S2.1). For further details on the genetics data see Supplementary Information section S2. For further details on the statistical analysis see Supplementary Information section S3.
Spatial association between megafauna and Palaeolithic humans
The presence of humans within the range of a species may directly or indirectly influence the capacity of the species to occupy that habitat. As a proxy for human impact, we assessed the spatial and temporal association between humans and megafauna using three approaches: (i) we compiled the human Upper Palaeolithic fossil record (50–12 ka BP), including 6,291 radiocarbon determinations associated with human occupations in Europe and Siberia. We analysed variations in fossil abundance and spatial and temporal overlap at 1,000-year intervals between humans and the megafauna fossil record; (ii) we inferred the area of overlap between the archaeological record from (i) and the megafauna ranges at 42, 30 and 21 ka BP estimated using species distribution models; and (iii) we assembled a list of 380 cultural occupations in Europe (48–18 ka BP) and 98 sites in Siberia (41–12 ka BP) with megafauna presence, to determine which taxa were directly associated with Palaeolithic humans. For further details see Supplementary Information sections S5.
Supplementary Material
Acknowledgments
This paper is in the memory of our friend and colleague Dr. Andrei Sher, who was a major contributor of this study. Dr Sher died unexpectedly, but his major contributions to the field of Quaternary science will be remembered and appreciated for many years to come. We are grateful to Dr. Adrian Lister and Dr. Tony Stuart for guides and discussions. Thanks to Tina B. Brandt, Dr. Bryan Hockett and Alice Telka for laboratory help and samples and to L. Malik R. Thrane for his work on the megafauna locality database. Data taken from the Stage 3 project was partly funded by Grant #F/757/A from the Leverhulme Trust, together with a grant from the McDonald Grants and Awards Fund. We acknowledge the Danish National Research Foundation, the Lundbeck Foundation, the Danish Council for Independent Research and the US National Science Foundation for financial support.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author contributions
E.W. initially conceived and headed the overall project. C.R. headed the species distribution modelling and range measurements. E.D.L. and J.T.S. extracted, amplified and sequenced the reindeer DNA sequences. J.B. extracted, amplified and sequenced the woolly rhinoceros DNA sequences; M.H. generated part of the woolly rhinoceros data. J.W., K-P.K., J.L. and R.K.W. generated the horse DNA sequences; A.C. generated part of the horse data. L.O., E.D.L. and B.S. analysed the genetic data, with input from R.N., K.M., M.A.S. and S.Y.W.H. Palaeoclimate simulations were provided by P.B., A.M.H, J.S.S. and P.J.V. The directly-dated spatial LAT/LON megafauna locality information was collected by E.D.L., K.A.M., D.N.-B., D.B. and A.U.; K.A.M. and D.N-B performed the species distribution modelling and range measurements. M.B. carried out the gene-climate correlation. A.U. and D.B. assembled the human Upper Palaeolithic sites from Eurasia. T.G. and K.E.G. assembled the archaeofaunal assemblages from Siberia. A.U. analysed the spatial overlap of humans and megafauna and the archaeofaunal assemblages. E.D.L., L.O., B.S., K.A.M., D.N.-B., M.K.B., A.U., T.G. and K.E.G. wrote the Supplementary Information. D.F., G.Z., T.W.S., K.A-S., G.B., J.A.B., D.L.J., P.K., T.K., X.L., L.D.M., H.G.M., D.M., M.M., E.S., M.S., R.S.S., T.S., E.S., A.T., R.W., A.C. provided the megafauna samples used for ancient DNA analysis. E.D.L. made the figures. E.D.L, L.O. and E.W. wrote the majority of the manuscript, with critical input from B.S., M.H., K.A.M., M.T.P.G., C.R., R.K.W, A.U. and the remaining authors.
Mitochondrial DNA sequences have been deposited in GenBank under accession numbers JN570760-JN571033.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306:70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 2.Martin PS. In: Quaternary Extinctions: A Prehistoric Revolution. Martin PS, Klein RG, editors. Univ. Arizona Press; Tucson: 1984. pp. 364–403. [Google Scholar]
- 3.Alroy JA. multispecies overkill simulation of the end-Pleistocene megafaunal mass extinction. Science. 2001;292:1893–1896. doi: 10.1126/science.1059342. [DOI] [PubMed] [Google Scholar]
- 4.Stuart AJ, Kosintsev PA, Higham TFG, Lister AM. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature. 2004;431:684–689. doi: 10.1038/nature02890. [DOI] [PubMed] [Google Scholar]
- 5.Koch PL, Barnosky AD. Late Quaternary extinctions: state of the debate. Ann Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 6.Nogués-Bravo D, Ohlemüller R, Batra P, Araújo MB. Climate predictors of late Quaternary extinctions. Evolution. 2010;64:2442–2449. doi: 10.1111/j.1558-5646.2010.01009.x. [DOI] [PubMed] [Google Scholar]
- 7.Haile J, et al. Ancient DNA reveals late survival of mammoth and horse in interior Alaska. P Nat Acad Sci. 2009;106:22363–22368. doi: 10.1073/pnas.0912510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro B, et al. Rise and fall of the Beringian steppe bison. Science. 2004;306:1561–1565. doi: 10.1126/science.1101074. [DOI] [PubMed] [Google Scholar]
- 9.Drummond AJ, Rambaut A, Shapiro B, Pybus OG. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol Biol Evol. 2005;22:1185–1192. doi: 10.1093/molbev/msi103. [DOI] [PubMed] [Google Scholar]
- 10.Campos PF, et al. Ancient DNA analyses exclude humans as the driving force behind late Pleistocene musk ox (Ovibos moschatus) population dynamics. P Nat Acad Sci. 2010;107:5675–5680. doi: 10.1073/pnas.0907189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiller M, et al. Withering away—25,000 years of genetic decline preceded cave bear extinction. Mol Biol Evol. 2010;27:975–978. doi: 10.1093/molbev/msq083. [DOI] [PubMed] [Google Scholar]
- 12.Barnes I, et al. Genetic structure and extinction of the woolly mammoth, Mammuthus primigenius. Curr Biol. 2007;17:1–4. doi: 10.1016/j.cub.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Debruyne R, et al. Out of America: ancient DNA evidence for a new world origin of late Quaternary woolly mammoths. Curr Biol. 2008;18:1320–1326. doi: 10.1016/j.cub.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 14.Barnett R, Yamaguchi N, Barnes I, Cooper A. The origin, current diversity, and future conservation of the modern lion (Panthera leo) Proc R Soc Lond B. 2006;273:2159–2168. doi: 10.1098/rspb.2006.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthrie RD. Rapid body size decline in Alaskan Pleistocene horses before extinction. Nature. 2003;426:169–171. doi: 10.1038/nature02098. [DOI] [PubMed] [Google Scholar]
- 16.Grayson DK. Deciphering North American Pleistocene extinctions. J Anthropol Res. 2007;63:185–214. [Google Scholar]
- 17.Andrewartha HG, Birch LC. The Distribution and Abundance of Animals. Univ. of Chicago Press; Chicago: 1954. [Google Scholar]
- 18.Borregaard MK, Rahbek C. Causality in the relationship between geographic distribution and species abundance. Q Rev Biol. 2010;85:3–25. doi: 10.1086/650265. [DOI] [PubMed] [Google Scholar]
- 19.Minin VN, Bloomquist EW, Suchard MA. Smooth skyride through a rough skyline: Bayesian coalescent-based inference of population dynamics. Mol Biol Evol. 2008;25:1459–1471. doi: 10.1093/molbev/msn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zazula GD, et al. Ice age steppe vegetation in east Beringia. Nature. 2003;423:603. doi: 10.1038/423603a. [DOI] [PubMed] [Google Scholar]
- 21.Zazula GD, Froese DG, Elias SA, Kuzmina S, Mathewes RW. Arctic ground squirrels of the mammoth-steppe: paleoecology of Late Pleistocene middens (~24 000-29 450 14C yr BP), Yukon Territory, Canada. Quat Sci Rev. 2007;26:979–1003. [Google Scholar]
- 22.Pitulko VV, et al. The Yana RHS site: humans in the Arctic before the last glacial maximum. Science. 2004;303:52–56. doi: 10.1126/science.1085219. [DOI] [PubMed] [Google Scholar]
- 23.Goebel T, Waters MR, O’Rourke DH. The late Pleistocene dispersal of modern humans in the Americas. Science. 2008;319:1497. doi: 10.1126/science.1153569. [DOI] [PubMed] [Google Scholar]
- 24.Stuart AJ, Sulerzhitsky LD, Orlova LA, Kuzmin YV, Lister AM. The latest woolly mammoths (Mammuthus primigenius Blumenbach) in Europe Asia: a review of the current evidence. Quaternary Sci Rev. 2002;21:1559–1569. [Google Scholar]
- 25.Vereshchagin NK. Prehistoric hunting and the extinction of Pleistocene mammals in the USSR. Proceedings ZIN. 1971;69:200–232. [Google Scholar]
- 26.Kuznetsova TV, Sulerzhitsky LD, Siegert C, Schirrmeister L. New data on the ‘mammoth’ fauna of the Laptev Shelf Land (Arctic Siberia). In: Cavarretta G, Giola P, Mussi M, Palombo MR, editors. La Terra degli Elefanti/The World of Elephants; Proc. 1st Intl. Congress; Rome. 16–20 October 2001.2001. pp. 289–292. [Google Scholar]
- 27.Wilson RJ, Thomas CD, Fox R, Roy DB, Kunin WE. Spatial patterns in species distributions reveal biodiversity change. Nature. 2004;432:393–396. doi: 10.1038/nature03031. [DOI] [PubMed] [Google Scholar]
- 28.MacPhee RDE, Marx PA. The 40,000-year plague: Humans, hyperdisease, and first-contact extinctions. In: Goodman SM, Patterson BD, editors. Natural Change and Human Impact in Madagascar. Washington, D.C: Smithsonian Institution Press; 1997. pp. 169–217. [Google Scholar]
- 29.Tener JS. Muskoxen in Canada: a biological and taxonomic review. Canadian Wildlife Service Monograph Series No 2. 1965 [Google Scholar]
- 30.Ugan A, Byers D. A global perspective on the spatiotemporal pattern of the Late Pleistocene human and woolly mammoth radiocarbon record. Quatern Int. 2008;191:69–81. [Google Scholar]
- 31.Surovell TA, Waguespack NM. How many elephant kills are 14? Clovis mammoth and mastodon kills in context. Quatern Int. 2008;191:82–97. [Google Scholar]
- 32.Zielinski GA, Mershon GR. Paleoenvironmental implications of the insoluble microparticle record in the GISP2 (Greenland) ice core during the rapidly changing climate of the Pleistocene-Holocene transition. Geol Soc Am Bull. 1997;109:547–559. [Google Scholar]
- 33.Guthrie RD. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature. 2006;441:207–209. doi: 10.1038/nature04604. [DOI] [PubMed] [Google Scholar]
- 34.Farnell R, et al. Multidisciplinary investigations of alpine ice patches in southwest Yukon, Canada: paleoenvironmental and paleobiological investigations. Arctic. 2004;57:247–259. [Google Scholar]
- 35.Williams TM, Heard DC. World status of wild Rangifer tarandus population. Rangifer Spec Issue. 1986;1:19–28. [Google Scholar]
- 36.Joly K, Klein DR, Verbyla DL, Rupp TS, Chapin FS. Linkages between large-scale climate patterns and the dynamics of Arctic caribou populations. Ecography. 2011;34:345–352. [Google Scholar]
- 37.Skogland T. The effects of density-dependent resource limitation on the demography of wild reindeer. J Anim Ecol. 1985;54:359–374. doi: 10.1007/BF00379517. [DOI] [PubMed] [Google Scholar]
- 38.Leader-Williams N. Reindeer on South Georgia. Cambridge, UK: Cambridge University Press; 1988. [Google Scholar]
- 39.Reimer PJ, et al. IntCal09 and Marine09 radiocarbon age calibration curves, 0–50,000 years cal BP. Radiocarbon. 2009;51:1111–1150. [Google Scholar]
- 40.Nogués-Bravo D, Rodríguez J, Hortal J, Batra P, Araújo MB. Climate change, humans, and the extinction of the woolly mammoth. PLOS Biol. 2008;6:e79. doi: 10.1371/journal.pbio.0060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaumont M. Joint determination of topology, divergence time and immigration in population trees. In: Matsumura S, Forster P, Renfrew C, editors. Simulations, Genetics and Human Prehistory. Cambridge: McDonald Institute for Archaeological Research; 2008. pp. 134–154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.