Abstract Abstract
The failure to translate positive results from preclinical studies into new clinical therapies is a major problem throughout medical research. Specifically, in pulmonary hypertension, numerous research studies have shown beneficial effects of new therapies in experimental models, but these have largely failed to translate into clinical benefit in human trials. This is undoubtedly due, at least in part, to inadequacies of the models, but while monogenic animal models will never fully recapitulate human disease, they do still provide the best platform on which to test novel therapeutic agents. In the postgenomic era, there is emphasis on a greater understanding of disease pathogenesis, which has subsequently led to the development of both new targets and new models in which to test them. The evolution of new technologies means that we are now better equipped to phenotype these models, but the level of detail provided varies dramatically throughout the literature. However, subtle variances in experimental methods can make comparing data/findings between research laboratories difficult and are a possible contributing factor to variance between preclinical and clinical data. The aim of this report was to capture information on current practice for use of the growing array of animal models, to help movement toward developing guidelines and standards for the “best” use of animal models of pulmonary hypertension.
Keywords: animal models, pulmonary hypertension, phenotyping
Introduction
Pulmonary hypertension (PH) describes a group of progressive conditions, each with a different origin but sharing a common hemodynamic diagnosis. The majority of research in the field is focused on pulmonary arterial hypertension (PAH), which is a rare but devastating disease characterized by sustained vasoconstriction and a progressive obliteration of small-resistance pulmonary arteries and arterioles through a process of medial thickening, intimal fibrosis, and the formation of angioproliferative (plexiform) lesions.1 Endothelial dysfunction and pulmonary artery endothelial cell (PA-EC) apoptosis/dysfunction are thought to play an important early role in disease pathogenesis. Subsequent proliferation and migration of medial cells, including smooth muscle cells, fibroblasts, and PA-EC,2,3 drive the pulmonary vascular remodeling. Current treatments target the sustained vasoconstriction via the prostacyclin, endothelin, or nitric oxide pathways4 in isolation or combination but do little to address the underlying proliferative vascular disease. Subsequently, there is still no curative treatment for PAH other than transplantation, and the 5-year survival for PAH remains low, between 57%5 and 20%6 in large United States– and United Kingdom–based registries.
The past 10–15 years have seen some major breakthroughs in our understanding of the pathobiology of PAH, and there are now well-established mechanistic insights into disease pathogenesis.2,3 Despite these important insights, the precise cell and molecular mechanisms leading to disease manifestation remain poorly understood. Dissecting the molecular mechanisms underlying PAH is therefore crucial if effective treatments for a condition that has a worse prognosis than many malignancies are to be developed.
Animal models are used globally and are an important research tool within medical research, and they serve multiple purposes. They can be used to identify therapeutic targets through studies examining aspects of disease pathogenesis, e.g., knockout and overexpression studies looking to establish causality of a particular gene, and as a preclinical platform for new therapies. Unfortunately for patients who suffer from PH, there are numerous reports in the literature of studies that have shown aspects of disease reversal, e.g., statins,7,8 but these findings have largely failed to translate fully to clinical benefit in human trials.9,10
This failure in clinical translation is in some part likely to be due to inherent limitations of the models, but investigators have significantly more models at their disposal today than they did even 5 years ago. It remains true, however, that no one single model is currently (or likely to be) a perfect substitution for the clinical manifestation of disease, particularly for a disease as heterogenous and complex as PAH. Methodological differences (strain, time courses, dosage, etc.) and local environmental conditions (e.g., health status, caging) can also make it difficult to compare research data and findings between labs. Despite these discrepancies and challenges, animal models do play a critical role and are fundamentally required to advance our scientific understanding and development of novel therapies for this disease. When used correctly, with full awareness of each model’s limitations, and in combination with other resources, such as human cells and tissues, they can lead to the identification of new altered molecules and pathways in PAH.
It is not the aim of this report to discuss the merits and limitations of individual models or to highlight emerging targets for translation; those topics have been discussed previously, with several excellent reviews already in the literature.2,3,10-14 Rather, this report aims to present a summary of the findings from a recent poll conducted through the Pulmonary Vascular Research Institute (PVRI). The aim of the poll was to examine the current use of, and phenotyping methods for, animal models for PAH across academia and industry. The ultimate goal was to establish a starting point from which to move toward establishing guidelines for the “best” use of currently available models and suggesting standards for animal phenotyping.
Methods
A questionnaire was designed to capture data on the current use of animal models for PH. The poll was open access and was hosted on the PVRI website from October 2012 until February 2013. A total of 36 responses were recorded from active researchers at all levels from PhD students through senior faculty, although the majority were from senior laboratory heads at major international research establishments (Fig. 1A), representing a global distribution similar to that for corresponding authors of articles published in the first 3 volumes of Pulmonary Circulation (Fig. 1B). The poll questions follow.
Figure 1.
Percentage breakdown of countries represented in the poll (A), in comparison to the breakdown of corresponding authors from the first 3 volumes of Pulmonary Circulation (B).
Section A: what models do you use, how do you phenotype them?
1. What models do you currently/have you previously used (a) for target identification/expression profile analysis; (b) to test a new intervention?
2. Do you use 1 model or multiple models, and what defines this?
3. What phenotyping tools do you have access to within your institution?
4. What parameters do you routinely record?
5. What aspects of a study determine what data you collect?
6. Do you blind yourself to groups when phenotyping?
7. What anesthesia do you use?
Section B: defining the “gold standards”
1. What platform do you think represents the best model on which to test a new potential therapeutic, i.e., target for validation?
2. What do you think is the “essential minimum” hemodynamic data that you think is required to accurately assess PH phenotype in a rodent?
3. What do you think would define the “gold-standard” hemodynamic data that you think is required to accurately assess PH phenotype in a rodent?
4. To increase transparency and reduce duplication, do you think there is a need for registering animal studies—akin to clinical trails, e.g., http://www.clinicaltrials.gov?
5. Would you be prepared to submit raw phenotyping data to a central repository for public access/data sharing, e.g., http://www.camarades.info?
Section C: current failings
In your opinion, why do so many drugs reverse experimental models of PH but not translate to humans?
Results and discussion
Frequency and number of models used to aid target identification
In response to the question about what model(s) were used to study disease mechanisms and help identify molecular targets, the most common responses were hypoxia (Hx) and Sugen5416 + Hypoxia (SuHx) in both rats and mice and the monocrotaline (Mct) rat (Fig. 2). Other responses reflected more specific research areas, e.g., schistosomiasis-associated PAH, models of left heart disease, or more direct focus on the right ventricle through the pulmonary-artery-banding model. The use of large-animal models was also acknowledged but was perhaps lower than expected, although this is no doubt due to the substantial increase in costs to both run these models and maintain the facilities to do so.
Figure 2.

Percentage of responders who use specific models routinely in the laboratory for target identification or basic studies on disease process. Hx: hypoxia; Mct: monocrotaline; SuHx: Sugen5416 + hypoxia; PAB: pulmonary artery banding; schisto: schistosomiasis; Tg: transgenic; FH Rat: fawn-hooded rat; ApoE: apolipoprotein E; Pyrol: pyrrole.
Of all responders, 19% used only 1 model, while most used at least 2 (Fig. 3A). A common free-text answer to this question was that investigators try to combine 1 mouse and 1 rat model. Where only 1 model was used, there was usually a specific scientific question to be answered (Fig. 3B), e.g., working on schistosomiasis. Overall, there was an obvious attempt to marry the relative strengths of individual models to provide a more robust platform for target discovery. Additional comments were also commonly made to state the increased confidence in data on new targets/pathways identified in these models, if these are matched by data from human samples/cells.
Figure 3.

Models used to identify novel targets or study basic disease mechanisms: A, percentage breakdown of responders by the number of different models used; B, percentage breakdown of models where only 1 model is used. Hx: hypoxia; Mct: monocrotaline; tg: transgenic.
Number and frequency of models used for preclinical intervention studies
One-third of poll responders stated that they either used only 1 model to test putative therapies or did not use models in this way (Fig. 4A). This left two-thirds of responders commonly testing for efficacy of target/pathway manipulation in 2 or more models. As with the target identification studies, this was almost entirely cross species. Of the responders who reported using only 1 model, 70% relied solely on the rat SuHx model (Fig. 4B), 15% were involved in schistosomiasis-based research and so utilized that model, and the remainder used only the Hx mouse model. The data demonstrate that the rat SuHx model is currently the most commonly used model by responders to this poll, closely followed by the Mct rat and the Hx rat/mouse (Fig. 5). There has undoubtedly been an increase in the use of the rat SuHx model, particularly since the publication of studies demonstrating complex angiogenic lesions,11 a commonly highlighted and perceived weakness of other models. The robustness of this model as a test bed for the next generation of translational therapies will be known only in time.
Figure 4.

Models to test the efficacy of novel preclinical interventions: A, percentage breakdown of responders by the number of different models used; B, percentage breakdown of models where only 1 model is used. SuHx: Sugen5416 + hypoxia; Hx: hypoxia; schisto: schistosomiasis.
Figure 5.

Percentage of poll responders who use each of the listed models in their preclinical tests. Abbreviations are as in Figure 2.
Experimental process and phenotyping
Only 17% of responders said that they were never blinded to their experimental groups, with 45% confirming that they were always blinded and 38% that they were sometimes blinded to treatment groups (Fig. 6A). Also, 47% of responders stated that they would be willing to submit their study design to a centralized register to help increase awareness of studies that have already been done and to help reduce unnecessary replication of studies, particularly negative ones (Fig. 6B). Encouragingly, 81% of responders to the poll said that they would be willing to submit phenotyping data to a central repository (Fig. 6C). With regard to phenotyping, most had access to either pressure or pressure-volume catheters for hemodynamic assessment of their models (Fig. 7). A similar number also reported access to preclinical ultrasound, and almost a third had access to either preclinical magnetic resonance imaging or computed tomography for noninvasive assessment of phenotype. Only a quarter of responders had access to telemetric pressure catheters for the continuous assessment of pressure before and after treatments (Fig. 7).
Figure 6.

Percentage of responders to the poll who are blinded while doing preclinical intervention studies (A), would be willing to submit study design to a central registry (B), and would be willing to submit data to a central repository (C).
Figure 7.

Percentage of responders who have access to the various equipment used to phenotype animals for pulmonary hypertension. Pressure cath: pressure catheter; PV-Cath: pressure-volume catheter; MRI: magnetic resonance imaging; CT: computed tomography; PET-CT: positron emission tomography–computed tomography; FMT: fluorescence-mediated tomography; Resp function: respiratory function.
Opinion on reason for failure to translate
Almost half of the responders to the poll thought that the failure to translate many successful preclinical studies into humans was either due to the models not being good enough (24%) or simply a reflection of the difference between animals, particularly rodents, and humans (22%; Fig. 8). Almost a third of responders believed that this failure was due to either poor phenotyping (16%) or generally poor experimentation (16%). Interestingly, 15% suggested that this was also a reflection of a lack of desire to translate toward a clinical therapy, e.g., that the main objective was to publish a manuscript (Fig. 8). This was perhaps surprising, but the difficulty in progressing down the translational route should not be underestimated.
Figure 8.
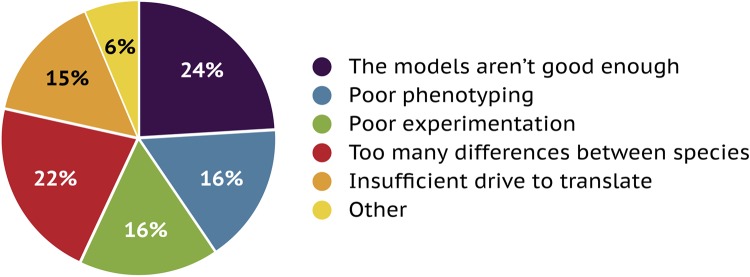
Percentage breakdown of responses from poll participants as to the reason for the current large percentage of failures to translate preclinical results into clinical benefits.
Phenotyping standards
On the request for suggestions for “minimum” and “gold” standards, the responses varied considerably, from a simple assessment of right ventricular systolic pressure and right ventricular hypertrophy (RVH) to very detailed assessments of right and left ventricular function, RVH/fibrosis, and detailed lung pathology. Interestingly, in some responses there was a substantial difference between the minimum and gold standards, while others had nearly identical parameters. There were also a few suggestions about including some commonly used clinical tests, such as pulmonary function and exercise tests. The inclusion of exercise is perhaps the most interesting finding, with some conflicting data in the literature about the effects of exercise in rodent models,12,13 although there will likely be significant differences between forced and voluntary exercise.
Summary and recommendations
Animal models can replicate phenotypic features of, share similar pathological features with, and display common dysregulated molecular pathways (by design or consequence) with clinical PAH. It remains the case that no animal model currently replicates all facets or the heterogeneity of the human disease. The inherent ethnic and genetic variability, along with possible environmental and epigenetic effects on specific genes and inflammatory responses, is unlikely to ever be replicated in a single inbred animal strain. However, this does not diminish the utility of carefully conducted experiments on the current vast array of models.
Use of models
On the basis of the data gathered in this report, studies looking to identify new targets or dysregulated pathways should ideally include 2 animal models to cross validate observations from human samples, or vice versa. Similarly, the efficacy of any treatment should be assessed in 2 models, albeit not necessarily interpreted as negative if effects vary or are seen in only 1 model. Human disease is heterogenous, and ongoing studies may identify new ways to stratify disease groups that may respond differently to new therapies.
Phenotyping
The recommendation on standards for phenotyping in PAH models is based on a combination of tallying the responses to the poll and considerations about access to the various phenotyping tools (shown in Fig. 7). The criteria for two recommended standards, minimum and gold, are provided in Table 1.
Table 1.
Suggested minimum and gold standards based on a tally of the responses from poll participants and most common access to phenotyping equipment
| “Minimum essential” phenotype data | “Gold standard” phenotype data | |
|---|---|---|
| Hemodynamic data | ||
| Right heart | Heart rate; RVSP (mouse); RV dP/dt; PAP (rat or larger) | Heart rate; RVSP (mouse); RVEDP; RAP; RV dP/dt; PAP (rat or larger) |
| Arterial/left heart | Systemic BP; LVEDP; cardiac output | Systemic BP; LVESP; LVEDP; RV dP/dt; cardiac output;a ejection fractiona |
| Noninvasive, e.g., echo/MRI/CT | Cardiac output;a ejection fraction;a PA-AT, PA-ET, or TAPSE | |
| Calculated hemodynamics | Estimated (mouse) or calculated (rat) PVR | Estimated (mouse) or calculated (rat) PVR and SVR |
| Right ventricular hypertrophy (RVH) | RVH by Fulton index | RVH by Fulton index; RV vs. LV nuclear density and fibrosis; RV ID and RV FWT (echo/MRI/CT) |
| Lung pathology | Score degree of thickening and percentage of muscular vessels | Score degree of thickening and percentage of muscular vessels |
| Additional considerations | Lung function tests, exercise tests |
RVSP: RV systolic pressure; RV: right ventricle/ventricular; dP/dt: pressure development over time; PAP: pulmonary arterial pressure; RVEDP: RV end-diastolic pressure; RAP: right atrial pressure; BP: blood pressure; LVEDP: LV end-diastolic pressure; LVESP: LV end-systolic pressure; LV: left ventricle/ventricular; echo: echocardiography; MRI: magnetic resonance imaging; CT: computed tomography; PA-AT: pulmonary artery acceleration time; PA-ET: pulmonary artery ejection time; TAPSE: tricuspid annular plane systolic excursion; ID: internal diameter; FWT: free-wall thickness.
Reversal studies should validate disease phenotype by noninvasive imaging, e.g., echo as a minimum or implanted telemetry catheters as a gold standard, before treatment.
Registry and repository
An established working group or task force should develop a repository for registering animal studies. Although just fewer than half the poll responders said that they would be willing to do this, that response may reflect an issue with when this would be done. The registration of clinical trials works largely because the costs involved are significant and therefore more difficult to replicate than those for preclinical studies. If registration of preclinical studies is to be done, it would have to be after the completion of the study or publication of the manuscript, to protect potential intellectual property. The value of this repository would be greater for agents where there is negligible efficacy, as studies of these are less likely to be published. Similarly, there was support to establish a central repository for phenotypic data. These data would be hugely beneficial for meta-analyses and physiological modeling.
Limitations and conclusions
This report is limited by the volume of responses submitted to the original online poll. Although there was broad diversity in the location of poll responders, the majority of responses were from major research establishments, which may skew the data obtained in favor of establishments that have access to more funding in order to use multiple animal models and/or costly imaging modalities, which may not be possible at smaller research centers. I have tried to balance this with regard to the future recommendations and conclusions highlighted below. The data collected from this study should be used as a starting point from which to move forward to establish specific standards for how we use animal models of PH to maximize reproducibility and implementation of the reduction, refinement, and replacement of animals.
PH is not the only disease for which there has been an attempt to make recommendations on how we utilize and improve our use of animal models. Representatives from the American Heart Association’s Council on Basic Cardiovascular Sciences, the Council on Clinical Cardiology, and the Council on Functional Genomics and Translational Biology made a similar statement with recommendations on animal models of heart failure in 2012.14 To generate such a comprehensive report as that would require the formation of an invested and committed working group, with representation from basic scientists and clinicians. Although there were responses to the poll from our industry colleagues as well as from academics, such a working group would undoubtedly benefit from representation of the former as well as input from regulatory bodies such as the US Food and Drug Administration and the European Medicines Agency, to help transition successful studies to the next stage on the translational pathway.
Acknowledgments
I would like to acknowledge the input on the creation of the poll from Martin Wilkins and Ghazwan Butrous and administrative support from Nikki Krol of the PVRI. I would also like to thank Christina Holt for supplying data of countries for Pulmonary Circulation authorship, and finally all those who took the time participate in this study.
This work was presented at the 7th PVRI Annual General Meeting and 6th Scientific Workshops and Debates, in Istanbul, Turkey, January 21–25, 2013.
Source of Support: Medical Research Council award G0800318.
Conflict of Interest: None declared.
References
- 1.Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG. Development and pathology of pulmonary hypertension. J Am Coll Cardiol 2009;54:S3–S9. [DOI] [PubMed]
- 2.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118:2372–2379. [DOI] [PMC free article] [PubMed]
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011;8:443–455. [DOI] [PMC free article] [PubMed]
- 4.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425–1436. [DOI] [PubMed]
- 5.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142:448–456. [DOI] [PubMed]
- 6.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012;39:945–955. [DOI] [PubMed]
- 7.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 2003;108:1640–1645. [DOI] [PubMed]
- 8.Zhao L, Sebkhi A, Ali O, Wojciak-Stothard B, Mamanova L, Yang Q, Wharton J, Wilkins MR. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. Eur Respir J 2009;34:948–957. [DOI] [PubMed]
- 9.Wilkins MR, Ali O, Bradlow W, Wharton J, Taegtmeyer A, Rhodes CJ, Ghofrani HA, et al. Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med 2010;181:1106–1113. [DOI] [PMC free article] [PubMed]
- 10.Zeng W-J, Xiong C-M, Zhao L, Shan G-L, Liu Z-H, Xue F, Gu Q, et al. Atorvastatin in pulmonary arterial hypertension (APATH) study. Eur Respir J 2012;40:67–74. [DOI] [PubMed]
- 11.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 2010;121:2747–2754. [DOI] [PubMed]
- 12.Souza-Rabbo MP, Silva LFF, Auzani JAS, Picoral M, Khaper N, Belló-Klein A. Effects of a chronic exercise training protocol on oxidative stress and right ventricular hypertrophy in monocrotaline-treated rats. Clin Exp Pharmacol Physiol 2008;35:944–948. [DOI] [PubMed]
- 13.Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJP, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009;120:42–49. [DOI] [PubMed]
- 14.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, et al. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 2012;111:131–150. [DOI] [PubMed]



