Abstract Abstract
Pulmonary endarterectomy (PEA) is the standard therapy for chronic thromboembolic pulmonary hypertension (CTEPH). Balloon pulmonary angioplasty (BPA) is an alternative therapy for such patients. Here we report the case of a 60-year-old woman who presented with severe CTEPH resulting in low cardiac output and liver failure. Her clinical status rapidly deteriorated after she developed a respiratory infection that was refractory to antibiotic treatment. PEA was risky because of her unstable hemodynamics, uncontrolled infection, and liver failure with jaundice. We thus performed rescue BPA. After 3 BPA procedures, her cardiac symptoms improved from World Health Organization functional class IV to II, and her jaundice resolved. The day after her final BPA procedure, her hemodynamics dramatically improved, and she continued to show improvement 3 months later. We thus suggest that BPA is a good treatment option in CTEPH patients with rapidly deteriorating heart failure and uncontrolled comorbidities.
Keywords: chronic thromboembolic pulmonary hypertension (CTEPH), balloon pulmonary angioplasty, right heart failure, liver failure
Chronic thromboembolic pulmonary hypertension (CTEPH) is defined as a mean pulmonary pressure greater than 25 mmHg in the presence of multiple chronic pulmonary thrombi after at least 3 months of anticoagulation. The current gold standard therapy for CTEPH is pulmonary endarterectomy (PEA). The selection of patients for PEA depends on the extent and location of the organized thrombi in relation to the degree of pulmonary hypertension, taking into consideration age and comorbidities.1 Balloon pulmonary angioplasty (BPA) is an alternative therapy in such patients. To our knowledge, this is the first report of the use of BPA to support a critically ill patient with inoperable CTEPH and multiple comorbidities.
Case description
In June 2012, a 60-year-old woman with a 10-year history of CTEPH presented with progressive dyspnea on exertion and leg edema. Her home medications at the time of hospital admission included the following: warfarin, 1.5 mg daily; beraprost sodium, 60 μg twice a day; ambrisentan, 5 mg daily; furosemide, 20 mg twice a day; spironolactone, 25 mg daily; and famotidine, 20 mg daily. At the time of admission she had severe right-sided heart failure with orthopnea, classified as World Health Organization (WHO) functional class IV, and liver failure with jaundice. On physical examination, her blood pressure was 86/64 mmHg, her heart rate was 92 bpm and regular, and her oxygen saturation was 91% on 1 L/min nasal cannula. She had severe leg edema and dilated jugular veins, and her second heart sound included a loud pulmonic component. A chest radiograph demonstrated the presence of severe cardiomegaly (Fig. 1A). The electrocardiogram showed sinus rhythm, right axis deviation, and T wave inversions in leads V1–V4. Echocardiography demonstrated a dilated right atrium and right ventricle, a flattened interventricular septum (Fig. 2A), dilation of the inferior vena cava without respiratory changes, and tricuspid valve regurgitation with a velocity of 3.1 m/s (estimated pressure gradient between the right ventricle and atrium was 37 mmHg). Abdominal echocardiography demonstrated hepatomegaly with a small amount of ascites. The results of a peripheral blood cell analysis were as follows: white blood cell count, 3,600 cells/μL; hemoglobin, 14.7 g/dL; hematocrit, 42.6%; and platelet count, 98,000 cells/μL. Blood chemistry results were as follows: total bilirubin, 3.3 mg/dL; direct bilirubin, 2.1 mg/dL; aspartate aminotransferase, 34 U/L; alanine aminotransferase, 11 U/L; lactate dehydrogenase, 356 U/L; alkaline phosphatase, 834 U/L; C-reactive protein, 0.35 mg/dL; serum urea nitrogen, 21 mg/dL; creatinine, 0.76 mg/dL; and brain natriuretic peptide (BNP), 643.9 pg/dL. Chest computed tomographic angiography revealed massive eccentric thrombotic material located primarily from the right intermediate artery to the right lower lobe artery (Fig. 3). The results of right heart catheterization were as follows: pulmonary arterial pressure (PAP), 54/32 mmHg (mean, 38); right atrial pressure, 19 mmHg; pulmonary capillary wedge pressure, 10 mmHg; cardiac index, 1.08 L/min/m2; pulmonary vascular resistance, 1,318 dyn s cm−5; and systemic vascular resistance, 2,635 dyn s cm−5. Mixed venous oxygen saturation was 49% on 4 L/min oxygen. The hemodynamic data and the results of chest computed tomography suggested the presence of severe right-sided heart failure causing low cardiac output in this patient with proximal CTEPH. We thus planned to perform PEA after hemodynamic stabilization. However, the patient’s condition rapidly deteriorated after she developed a respiratory infection that was refractory to antibiotic treatment. The infection exacerbated both her right heart failure (BNP, 1,527 pg/dL) and liver dysfunction (total bilirubin, 6.1 mg/dL). Given the comorbidities of uncontrolled infection and liver failure as well as her rapidly deteriorating hemodynamics, the operative risk of PEA was deemed too high. As a consequence of the discussions with experienced cardiovascular surgeons, we decided to perform immediate BPA for rescue. We first performed the procedure in the right intermediate artery, middle lobe artery (A4), and lower lobe artery (A6) with the semicompliant balloon catheters of appropriate size (2 and 4 mm, Ikazuchi Pad, Kaneka, Osaka, Japan; 6 mm, Sterling Monorail, Boston Scientific, Natick, MA) under noninvasive positive pressure ventilation 26 days after admission (Fig. 4). After 7 days we repeated the procedure on the right pulmonary posterobasal segmental artery (A10) and then on the left lower lobe segmental artery (A8, A9, A10) with 2- and 4-mm balloon catheters (Fig. 5). Reperfusion edema was not observed after any of the procedures, and the patient did not require invasive ventilation. On the day after the final BPA procedure, right heart catheterization revealed a PAP of 42/16 mmHg (mean, 21), a right atrial pressure of 0 mmHg, and a cardiac index of 2.1 L/min/m2. Mixed venous oxygen saturation was 68% on 3 L/min oxygen. BNP decreased to 81.4 pg/dL, and total bilirubin decreased to 1.4 mg/dL. The patient’s leg edema resolved and her WHO functional class improved from IV to II. Chest radiography showed a dramatic reduction in the previously observed cardiomegaly (Fig. 1B), and echocardiography revealed improvement of the right atrial and ventricular dilation and improvement of the flattened interventricular septum (Fig. 2B). Several months later, we performed additional BPA because the cardiac index was low at 1.68 L/min/m2 (mean PAP was 25 mmHg), resulting in further improvements in PAP (36/6 mmHg; mean, 16) and cardiac index (2.4 L/min/m2). Echocardiography at that time revealed complete resolution of the patient’s right heart dilation and flattened interventricular septum (Fig. 2C). One year after BPA, her hemodynamic status was stable, with a PAP of 42/15 mmHg (mean, 25) and a cardiac index of 1.85 L/min/m2. Pulmonary angiography revealed no restenosis (Fig. 6). Chest computed tomographic angiography revealed a decrease of central laminated thrombus in the right pulmonary artery (Fig. 7). She is now well, with WHO functional class II.
Figure 1.
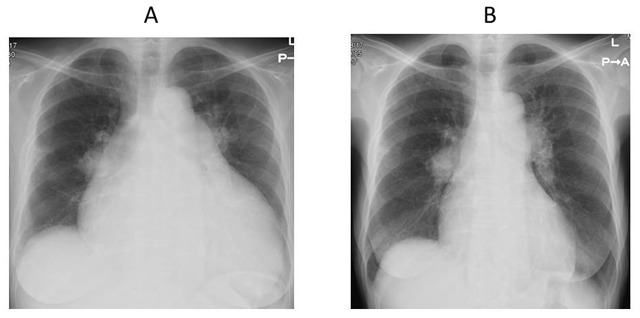
A, Chest radiograph demonstrating the presence of severe cardiomegaly on admission. B, Chest radiograph demonstrating improvement of cardiomegaly after balloon pulmonary angioplasty.
Figure 2.
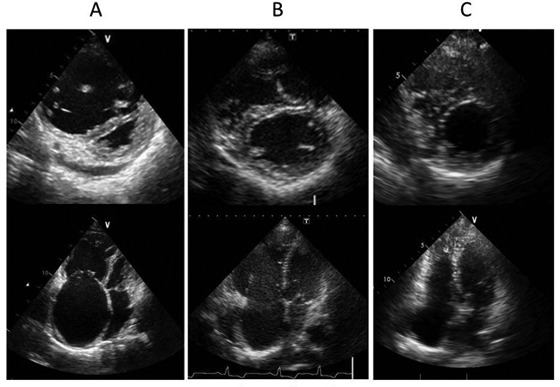
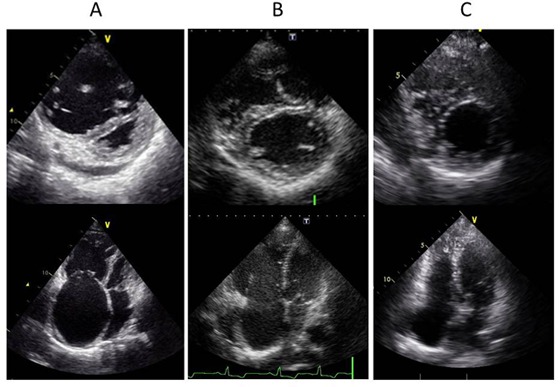
A, Echocardiography demonstrating a dilated right ventricle and flattened interventricular septum on admission. B, Echocardiography demonstrating improvement in these two conditions after balloon pulmonary angioplasty (BPA). C, Echocardiography demonstrating resolution of these conditions after additional BPA.
Figure 3.
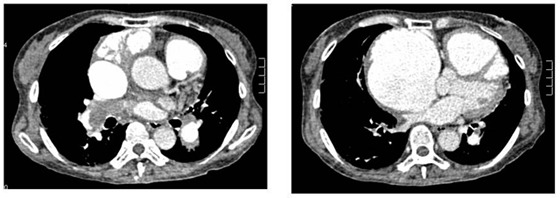
Chest computed tomographic angiography revealing massive thrombotic material located primarily from the right intermediate artery to the right lower lobe artery.
Figure 4.
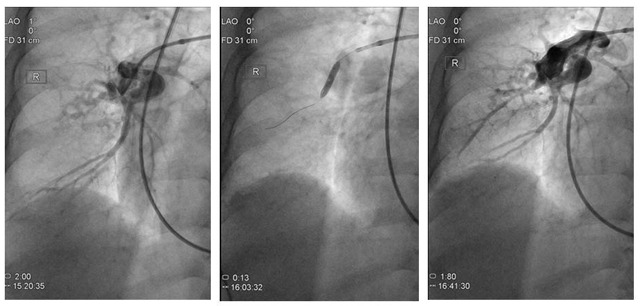
A, Preintervention pulmonary angiography showing total occlusion of the right middle lobe artery (A4). B, Balloon pulmonary angioplasty on the right middle artery (A4). C, Postintervention pulmonary angiography showing recanalization of the right middle lobe artery (A4).
Figure 5.
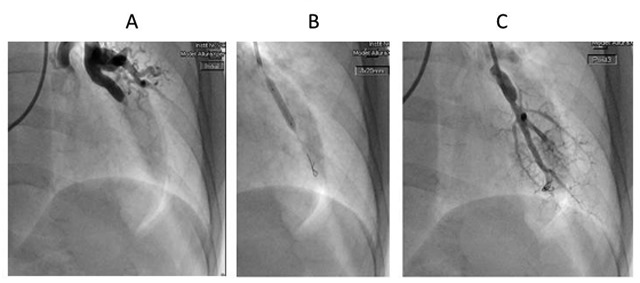
A, Preintervention pulmonary angiography showing total occlusion of the left lower lobe artery. B, Balloon pulmonary angioplasty on the left lower lobe artery. C, Postintervention pulmonary angiography showing recanalization of the left lower lobe artery.
Figure 6.
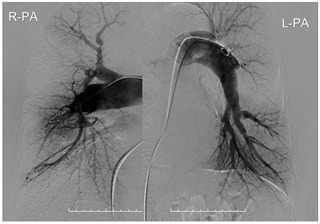
Pulmonary angiography after 1 year showing no restenosis of the balloon pulmonary angioplasty site.
Figure 7.
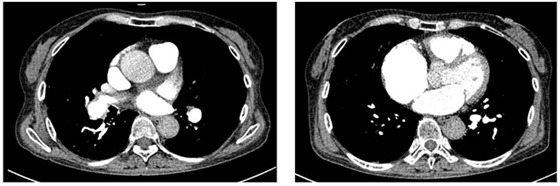
Chest computed tomographic angiography 1 year after balloon pulmonary angioplasty showing the decrease in central laminated thrombus in the right pulmonary artery.
Discussion
CTEPH is a rare disease, occurring in about 1% of patients after acute pulmonary embolism.2 The current gold standard therapy for CTEPH is PEA.3 The 30-day mortality rate for PEA ranges from less than 5% in the most experienced centers to 10% elsewhere.4-6 Advanced medical therapy may be effective in patients with inoperable CTEPH7,8 but is not a replacement for PEA in patients with operable disease. PEA is associated with a low in-hospital mortality rate, improvement in hemodynamics and exercise capacity, and a favorable prognosis.4,9,10 Thistlethwaite et al.11 showed that CTEPH patients with distal lesions have higher postoperative pulmonary artery systolic pressure, greater postoperative pulmonary resistance, and higher perioperative mortality compared with patients with proximal lesions. Hence, current consensus holds that indication for PEA depends on the extent and location of the organized thrombi in relation to the degree of pulmonary hypertension. Comorbidities must also be considered, as they increase the risk of PEA.12 Moreover, it is risky to perform PEA in decompensated CTEPH patients.13
BPA is an alternative therapy in select CTEPH patients who are deemed inoperable because of surgical inaccessibility of thrombi or persistent or recurrent pulmonary hypertension after thromboendarterectomy.12 Feinstein et al.14 reported that, in CTEPH patients who are not surgical candidates, BPA improves pulmonary arterial hypertension, New York Heart Association class, and 6-minute walk distances. Similar results were recently published by two Japanese groups.15,16 These data suggest that BPA is an effective treatment option for patients with inoperable CTEPH. In our report, BPA rescued a deteriorating CTEPH patient who had severe morbidities. No previous reports have demonstrated the efficacy of rescue BPA in deteriorating CTEPH patients with surgically accessible disease but multiple comorbidities. In this patient, BPA was remarkably efficacious. One reason for this might be that, despite the proximal lesions observed on computed tomography, she also had distal lesions, which are treatable by BPA. Moreover, BPA is less invasive than PEA and was thus preferable in our patient. BPA might be the only procedure that can help a deteriorating, inoperable patient. We never consider BPA to be superior to PEA surgery in patients with operable CTEPH. However, the indications for BPA need to be clarified, and a more detailed understanding of the pathogenesis of CTEPH and the efficacy of BPA is required.
To the best of our knowledge, this is the first case report to describe successful emergency BPA for deteriorating right heart failure in a patient with surgically accessible CTEPH who was deemed inoperable because of comorbidities.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Galiè N, Hoeper MM, Humbert M, et al. Guideline for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493–2537. [DOI] [PubMed]
- 2.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006;130:172–175. [DOI] [PubMed]
- 3.Keogh AM, Mayer E, Benza RL, et al. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol 2009;54(suppl.):S67–S77. [DOI] [PubMed]
- 4.Matsuda H, Ogino H, Minatoya K, et al. Long-term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg 2006;82:1338–1343. [DOI] [PubMed]
- 5.Piovella F, D’Armini AM, Barone M, Tapson VF. Chronic thromboembolic pulmonary hypertension. Semin Thromb Hemost 2006;32:848–855. [DOI] [PubMed]
- 6.Corsico AG, D’Armini AM, Cerveri I, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med 2008;178:419–424. [DOI] [PubMed]
- 7.Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronic Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008;52:2127–2134. [DOI] [PubMed]
- 8.Reichenberger R, Voswinckel R, Enke B, et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J 2007;30:922–927. [DOI] [PubMed]
- 9.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141:702–710. [DOI] [PubMed]
- 10.Ogino H, Ando M, Matsuda H, et al. Japanese single-center experience of surgery for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg 2006;82:630–636. [DOI] [PubMed]
- 11.Thistlethwaite PA, Mo M, Madani MM, et al. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2002;124:1203–1211. [DOI] [PubMed]
- 12.Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med 2011;364:351–360. [DOI] [PubMed]
- 13.Daily PO, Dembitsky WP, Iversen S, Moser KM, Auger W. Risk factors for pulmonary thromboendarterectomy. J Thorac Cardiovasc Surg 1990;99:670–678. [PubMed]
- 14.Feinstein JA, Goldhabar SZ, Lock JE, Fernandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001;103:10–13. [DOI] [PubMed]
- 15.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:748–755. [DOI] [PubMed]
- 16.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:756–762. [DOI] [PubMed]


