Abstract Abstract
The objective of this study was to assess the effect of pulmonary endarterectomy (PEA) on right ventricular (RV) reverse remodeling using magnetic resonance imaging (MRI) and to correlate MRI findings with clinical and hemodynamic outcomes postsurgery. We performed a retrospective analysis in 72 patients undergoing PEA surgery in whom MRI and right heart catheterization (RHC) were performed preoperation and 3 months postoperation. RV volumes and mass were assessed by MRI. Continuous variables were expressed as means, changes were compared with a paired t test, and associations between the variables were explored using Pearson correlation coefficients. The mean age was 57 years, and 51% were male. Both RV end-diastolic volume (EDV; 176–117 mL; P < 0.001) and RV end-systolic volume (ESV; 129–64 mL; P < 0.001) reduced significantly following PEA. Preoperative pulmonary artery pressure (PAP) correlated moderately with ESV (r = 0.46, P < 0.001). Postoperatively, PAP correlated with EDV (r = 0.45, P < 0.001) and ESV (r = 0.44, P < 0.001). Moderate correlation was present between hemodynamic parameters: PAP, pulmonary vascular resistance, and right atrial pressure with pre- and postoperation end-systolic and end-diastolic RV mass (P < 0.001). RHC and MRI measurements of cardiac output and RV volumes were significantly different (P < 0.001). In conclusion, RV reverse remodeling, as measured by improvement in RV volumes and mass by MRI, was observed for 3 months in patients who underwent PEA surgery. This is the largest series of patients with pre- and post-PEA MRI assessment so far reported. MRI detects changes in parameters reflecting cardiac remodeling and pulmonary clearance, but measurements are significantly different from those of RHC.
Keywords: pulmonary endarterectomy, magnetic resonance, chronic thromboembolic pulmonary hypertension (CTEPH)
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) results from incomplete resolution of the vascular obstruction caused by pulmonary thromboemboli—up to 3.8%.1 Long-term survival and functional status are dependent on the degree of pulmonary hypertension (PH) and pulmonary vascular resistance (PVR) at diagnosis,2 which are major determinants of right ventricular (RV) function. Advanced CTEPH leads to cardiac remodeling, involving RV dilatation and hypertrophy, tricuspid regurgitation, and leftward ventricular septal bowing, with consequent impact on left ventricular (LV) function. Pulmonary endarterectomy (PEA) represents the therapy of choice for patients with surgically accessible disease.3-7 PEA may be performed with a low mortality risk and results in clinical improvement and improved prognosis.8 Magnetic resonance imaging (MRI) is a tool to noninvasively quantify and follow up changes in biventricular volume and function. Several authors9-14 have used this approach in combination with pulmonary flow measurements and magnetic resonance angiography in small series of CTEPH patients before and after PEA. Real-time cine MRI can be applied to dynamically evaluate ventricular coupling in patients with acute or chronic RV overload.15 The aim of this study was to assess the effect of PEA on the right ventricle using pre- and postoperative MRI assessment and to correlate MRI parameters with clinical and hemodynamic variables assessed by right heart catheterization (RHC).
Methods
Patient population
The patient population consisted of 72 local patients who were available for pre- and post-MRI follow-up at Papworth Hospital out of the national cohort. Eligibility for surgery was defined by at least two of three imaging modalities: conventional pulmonary angiography, computed tomography pulmonary angiography, or magnetic resonance (MR) pulmonary angiography, taking into account hemodynamic data and comorbidities. All referred patients were discussed in a dedicated multidisciplinary team meeting. MR angiography and RHC were performed 3 months after PEA. Preoperative characteristics, operative procedures, hospital outcomes, and follow-up data were prospectively entered into a dedicated database (Table 1). The local research ethics committee approved the study; the requirement for written informed consent was waived as the study was retrospective and individual patients were not identified.
Table 1.
Summary of functional and magnetic resonance imaging variables in pulmonary endarterectomy (PEA) patients pre- and postoperation and change after operation
| Variable | Pre-PEA | 3 mo post-PEA | Difference (95% CI) and significance level, P |
|---|---|---|---|
| Mean age (SD) | 57 (15) | … | … |
| n male | 37 (51) | … | … |
| New York Heart Association classification | <0.001a | ||
| 1, % | 0 | 23 (33) | |
| 2, % | 2 (3) | 34 (47) | |
| 3, % | 50 (69) | 14 (19) | |
| 4, % | 20 (28) | 1 (1) | |
| 6-minute walk distance, m | 293 (137) | 370 (112) | 81 (55–108), <0.001 |
| Right heart catheter measurements | |||
| Cardiac output, L/min | 4.6 (1.2) | 4.7 (0.9) | 0.1 (−0.2–0.4), 0.53 |
| Cardiac index, L/min/m2 | 2.4 (0.6) | 2.5 (0.4) | 0.09 (−0.08–0.26), 0.30 |
| Mean right atrial pressure, mmHg | 9.7 (5.0) | 5.6 (3.3) | 4.3 (2.9–5.8), <0.001 |
| Pulmonary artery pressure, mmHg | 48 (13) | 26 (9) | −23 (−19 to −26), <0.001 |
| Pulmonary vascular resistance, dyn/s/cm−5 | 698 (279) | 265 (186) | −393 (−307 to −479), <0.001 |
| MRI measurements | |||
| Flow per beat, mL/beat | 24 (12) | 35 (13) | 11 (8–15), <0.001 |
| Flow per min, mL/min | 1,798 (923) | 2,679 (886) | 881 (635–1127), <0.001 |
| End-diastolic volume, mL | 176 (76) | 117 (48) | −58 (−46 to −71), <0.001 |
| End-systolic volume, mL | 129 (68) | 64 (40) | −65 (−53 to −76), <0.001 |
| Cardiac output, L/min | 3.0 (1.3) | 2.8 (1.2) | 0.1 (−0.3–0.4), 0.69 |
| End-diastolic volume, mL | 137 (59) | 93 (46) | 44 (35–53), <0.001 |
| End-systolic volume, mL | 103 (51) | 62 (37) | 41 (33–49), <0.001 |
| Fraction shortening, % | 28 (11) | 36 (10) | −8 (−11 to −5), <0.001 |
| Right mass diastolic, g | 145 (54) | 101 (31) | 45 (36–54), <0.001 |
| Right mass systolic, g | 125 (46) | 81 (25) | 44 (35–52), <0.001 |
| Stroke volume, mL/beat | 47 (19) | 53 (15) | −6 (−11 to −2), 0.005 |
| Ejection fraction, % | 29 (12) | 48 (11) | −19 (−22 to −16), <0.001 |
CI: confidence interval; MRI: magnetic resonance imaging; SD: standard deviation.
Bowker’s test of symmetry P value.
Functional status
Functional status was evaluated at baseline, preoperatively and at 3 months post-PEA by New York Heart Association (NYHA) classification and 6-minute walking distance (6MWD).
Right heart catheterization
The procedure was performed in the majority of the cases through the right internal jugular vein by using a flow-directed, balloon-tipped Swan-Ganz catheter (7.5F; Edwards Lifesciences, Irvine, CA). Measured parameters were cardiac output (CO), right atrial, RV, pulmonary artery (PA), and capillary wedge pressures. PVR was calculated as 80 × (mean pulmonary artery pressure [mPAP] − mean pulmonary wedge pressure)/CO (dyn/s/cm−5), and cardiac index (CI) was calculated as CO/body surface area (L/min/m2).
Magnetic resonance
MRI was performed on a 1.5-T cardiac-optimized system (2004–2007 GE Sigma CVi system; GE Healthcare, Milwaukee, WI; from 2007 onward, Siemens Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany). Images were acquired using a phased-array torso coil, with retrospective cardiac gating achieved with a vector-electrocardiography system. Multislice gradient–echo sequences were used to obtain horizontal and vertical long-axis images of the heart and axial volume stacks. Axial slices of the right ventricle from base to apex were planned from the horizontal and vertical long-axis images in the plane of the atrioventricular groove (short axis). All cine sequences with fast imaging employing steady state acquisition were acquired with 25 cardiac phases at 10-mm-thick slices with 128 × 256 matrices. Interleaved, velocity-encoded, phase-difference sequences were used to measure flow and area in the main PA. MRIs were stored on a Cambridge Computed Imaging (CCI, Cambridge, UK) picture archive and communication system for subsequent recall and analysis.
Images were reviewed by two observers using Medis medical imaging systems software (Leiden, Netherlands). Manual delineation was used to generate areas for RV endocardium and epicardium in end diastole and endocardium in end systole. These time points were defined by the largest and smallest endocardial areas. The method of disk summation was then employed to provide volumetric and mass data for the right ventricle.
Operative technique
The operative technique was based on that described by Jamieson et al.3 and reproduced in the United Kingdom in more than 970 patients.16 Although the vast majority of the patients had significant tricuspid regurgitation, tricuspid repair was not performed because the incontinence was always deemed functional, a finding consistent with that of other groups.3,17
Statistical analysis
Continuous variables were summarized at the 2 time points using the mean and standard deviation and categorical variables using frequencies and proportions. To assess changes between pre- and postoperation for continuous variables, the mean difference and its 95% confidence interval were calculated and the paired t test was used. For categorical variables, Bowker’s test of symmetry was used. To explore the association between functional cardiac studies and MRI measures, the data were initially analyzed separately for the 2 time points, pre- and postoperation. Scatterplots of each combination of clinical/cardiac function variables and MRI variables were examined. Pearson correlation coefficients and their associated P values were calculated. Correlation coefficients were interpreted according to Franzblau18 as follows: 0–0.19, none/negligible; 0.2–0.39 low; 0.4–0.59 moderate; 0.6–0.79 marked; 081–1 high. To further explore the clinical utility of MRI variables, we calculated the percentage of the variation of other outcomes that could be explained by MRI variables (100 × correlation coefficient2). For variables that could be measured by both RHC and MRI, we plotted the difference between the measurements against the average of the 2 measures for each patient (Bland-Altman plots) and calculated the average disagreement between RHC and MRI results, with 95% confidence intervals.
Results
Baseline and 3-month post-PEA RHC, 6MWD, and MRI results are reported in Table 1. All parameters studied, with the exception of estimated CO and CI were, on average, significantly different after the PEA operation compared to before.
Tables 2 and 3 show Pearson correlation coefficients and percentage of variation explained (pre- and post-PEA, respectively). Measurements of flow had statistically significant, low to moderate correlation with PVR, with differences in flow explaining at most 31% of the variation in PVR. Additionally, PVR and pulmonary artery pressure (PAP) were moderately correlated with volumes, accounting for between 10% and 21% of the variation observed.
Table 2.
Pearson’s correlation coefficients, P values for the correlation between functional and magnetic resonance imaging variables pre–pulmonary endarterectomy operation, and percentage of variation explained
| Variable | Flow per beat (%) | Flow per minute (%) | End-diastolic volume (%) | End-systolic volume (%) |
|---|---|---|---|---|
| Cardiac output | 0.21, 0.08 (4) | 0.18, 0.14 (3) | −0.09, 0.47 (1) | −0.11, 0.36 (1) |
| Cardiac index | 0.09, 0.48 (1) | 0.03, 0.79 (0) | −0.15, 0.24 (2) | −0.17, 0.17 (3) |
| 6-minute walk distance | 0.32, 0.009 (10) | 0.28, 0.02 (8) | −0.13, 0.30 (2) | −0.13, 0.31 (2) |
| Mean pulmonary artery pressure | −0.13, 0.27 (2) | −0.10, 0.40 (1) | 0.39, 0.001 (15) | 0.46, <0.001 (21) |
| Pulmonary vascular resistance | −0.30, 0.02 (9) | −0.27, 0.04 (7) | 0.31, 0.02 (10) | 0.38, 0.002 (14) |
Table 3.
Pearson’s correlation coefficients, P values for the correlation between functional and magnetic resonance imaging variables post–pulmonary endarterectomy operation, and percentage of variation explained
| Variable | Flow per beat (%) | Flow per minute (%) | End-diastolic volume (%) | End-systolic volume (%) |
|---|---|---|---|---|
| Cardiac output | 0.51, <0.001 (26) | 0.56, <0.001 (31) | 0.003, 0.98 (0) | −0.004, 0.97 (0) |
| Cardiac index | 0.30, 0.01 (9) | 0.30, 0.01 (9) | −0.12, 0.32 (10) | −0.13, 0.27 (2) |
| 6-minute walk distance | 0.24, 0.04 (6) | 0.26, 0.03 (7) | −0.10, 0.43 (1) | −0.11, 0.36 (2) |
| Mean pulmonary artery pressure | 0.002, 0.99 (0) | 0.03, 0.82 (0) | 0.45, <0.001 (20) | 0.44, <0.001 (19) |
| Pulmonary vascular resistance | −0.24, 0.08 (6) | −0.20, 0.15 (4) | 0.44, 0.001 (19) | 0.41, 0.002 (17) |
Tables 4 and 5 give Pearson correlation coefficients and percentage of variation explained for the relationship between functional/cardiac measures and RV mass changes pre- and post-PEA. PVR, PAP, and right atrial pressure had statistically significant, low to moderate correlation with RV mass.
Table 4.
Pearson’s correlation coefficients, P values for the correlation between functional, right heart catheterization, and magnetic resonance imaging variables pre– and post–pulmonary endarterectomy operation, and percentage of variation explained
| Variable | Right mass diastolic (%) | Right mass systolic (%) |
|---|---|---|
| Cardiac output | −0.12, 0.33 (1) | −0.15, 0.22 (2) |
| Cardiac index | −0.20, 0.10 (4) | −0.23, 0.06 (5) |
| 6-minute walk distance | −0.05, 0.68 (0) | 0.01, 0.92 (0) |
| Mean pulmonary artery pressure | 0.38, 0.001 (14) | 0.41, <0.001 (17) |
| Pulmonary vascular resistance | 0.31, 0.02 (10) | 0.40, 0.002 (16) |
| Right atrial pressure | 0.27, 0.04 (7) | 0.27, 0.03 (7) |
Table 5.
Pearson’s correlation coefficients, P values for the correlation between functional and magnetic resonance imaging variables post–pulmonary endarterectomy operation, and percentage of variation explained
| Variable | Right mass diastolic (%) | Right mass systolic (%) |
|---|---|---|
| Cardiac output | −0.02, 0.88 (0) | −0.03, 0.83 (0) |
| Cardiac index | −0.16, 0.21 (3) | −0.21, 0.10 (4) |
| 6-minute walk distance | 0.06, 0.66 (0) | 0.10, 0.42 (1) |
| Mean pulmonary artery pressure | 0.34, 0.005 (12) | 0.41, <0.001 (17) |
| Pulmonary vascular resistance | 0.34, 0.02 (12) | 0.44, 0.001 (19) |
| Right atrial pressure | 0.09, 0.45 (1) | 0.10, 0.41 (1) |
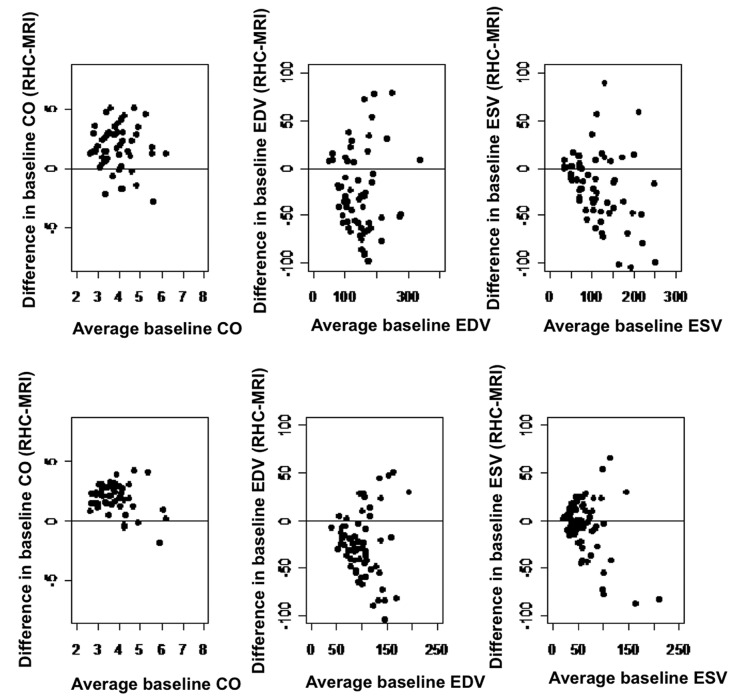
CO, end-diastolic volume (EDV), and end-systolic volume (ESV) were recorded using RHC and MRI. Table 6 shows the mean bias and 95% confidence intervals. With the exception of ESV at 3 months after the procedure, there was significant disagreement between the measurements. For CO, the average RHC measurements were approximately 2 L greater than those from MRI, while the difference between volumes ranged from 3 to 38 mL. Disagreements for individual patients are plotted in Figure 1, showing quite a large difference for some patients.
Table 6.
Mean (95% confidence interval [CI]) for the difference between right heart catheterization (RHC) and magnetic resonance imaging (MRI) measurements
| Measurement | Mean difference, RHC−MRI | SD | 95% CI, significance, P |
|---|---|---|---|
| Baseline cardiac output, L | 1.8 | 1.9 | 1.3–2.3, <0.001 |
| 3-month cardiac output | 2.0 | 1.2 | 1.6–2.3, <0.001 |
| Baseline end-diastolic volume, mL | 38 | 57 | 25–53, <0.001 |
| 3-month end-diastolic volume, mL | 25 | 43 | 15–36, <0.001 |
| Baseline end-systolic volume, mL | 25 | 43 | 14–35, <0.001 |
| 3-month end-systolic volume, mL | 3 | 33 | −5–11, 0.47 |
Figure 1.
Bland-Altman plots of disagreement between right heart catheterization and magnetic resonance imaging measurements against average for each patient. CO: cardiac output; EDV: end-diastolic volume; ESV: end-systolic volume; MRI: magnetic resonance imaging; RHC: right heart catheterization.
Discussion
PEA offers curative treatment for patients with CTEPH, with significant improvement in hemodynamics, symptoms, and survival.3,4 RHC is considered the reference technique for assessment of disease severity. However, this invasive technique is not suitable for repeated studies in order to monitor outcome in the postoperative episode and has significant limitations in assessment of CO when there is significant tricuspid regurgitation, which is commonly the case in the setting of PH. Comprehensive cardiac MRI can provide accurate information on biventricular function and volume, flow patterns, and surgical clearance of the pulmonary vascular bed, but this study showed, at best, moderate correlation with other clinical and hemodynamic variables. In addition there was statistically significant and clinically important disagreement between RHC and MRI measurements of CO, ESV, and EDV. CTEPH patients typically have abnormally dilated right ventricles with abnormal leftward bulging of the septum due to increased PAPs. After PEA surgery, reversed remodeling occurs in the majority of patients, consisting of a significant decrease in RV volumes and improvement of RV function. This study shows the decrease in RV volumes assessed by MRI and a reduction of pulmonary pressure and resistance assessed by RHC, within 3 months postsurgery. This is in line with the findings of other authors11,13 who demonstrated a remodeling of the right ventricle, mainly in the first month post-PEA followed by a much slower remodeling phase afterward.
This biphasic pattern matches well with the postoperative changes in systolic PAP (sPAP), with an early decrease followed by a plateau phase. We previously reported that reduction of mPAP had a sustained and positive impact on long-term outcome.7 D’Armini et al.10 showed this biphasic pattern on a 3-year follow-up post-PEA. Afterload reduction early post-PEA is most likely responsible for a fast reduction in RV volumes and subsequent improvement in RV contractility, with a progressive reduction of RV hypertrophy over time.
Reduction in RV mass was documented in our study as early as 3 months postsurgery. The mass reduction coincided with the immediate reduction of mPAP and PVR. This is in line with the results of Reesnik et al.,11 who demonstrated a similar reduction in RV mass post-PEA, although there were no further assessments in PAP and PA resistance at the time of repeated MRI in that study. Knowing that the pressures will continue to drop beyond 3 months, the remodeling process will continue with a reduction of RV hypertrophy, and improved flow and volume on the left side will stop the myocyte shrinkage and atrophic reduction of LV free wall mass.12,19
Our study showed that at 3 months post-PEA, there was significant increase in pulmonary flow but no evidence of an increase in CO assessed by RHC. The increase in pulmonary flow was in line with Kreitner’s9 group.
We showed in our study a low correlation between 6MWD and increased pulmonary flow after PEA. There was negligible correlation with other parameters, such as RV volumes and RV mass regression. It is possible that the 3-month assessment might have been too early in order to document significant correlation with 6MWD. This improvement of CMR (cardiac magnetic resonance)-derived stroke volume and 6MWD was documented by van Wolferen et al.20 over 1 year of follow-up.
The position and configuration of the ventricular septum are reliable indicators of the pressure difference between the left ventricle and the right ventricle and can be used to depict the RV pressure overload in patients with PH.13,20,21 Several studies have reported significant relationships between septal curvature derived from CMR images and PAPs22,23 or PA resistance.24 Alunni et al.24 confirmed the correlation between the interventricular septum (IVS) curvature and PAP and PA resistance. Relations between PH and left ventricle were demonstrated by several authors,24,25 strengthening the interdependence between the two ventricles. The concept was enhanced by several factors:24 (1) correlation between IVS, the ventricular area ratios, and invasive parameters; (2) correlation between the ratio of early to late ventricular filling velocities and sPAP and PVR; and (3) LVEDV related to LV diastolic function. Gan et al.26 demonstrated that reduction of LV stroke volume was related to reduced filling due to leftward IVS curvature. Sciancalepore et al.21 showed that 3-dimensional CMR provided quantitative information of the IVS and its patterns of changes throughout the cardiac cycle, reflecting on the extent of ventricular remodeling according to the severity of PH. Mauritz and Vonk-Noordgraaf27 showed in 13 patients post-PEA that at 6 months postsurgery the RV and LV peak strains are resynchronized. The reduction of left-right delay in time to first peak was more strongly associated with the reduction in RV wall stress (r = 0.69, P = 0.007) than with the reduction in sPAP (r = 0.53, P = 0.07).
Vogel-Claussen et al.28 looked into the relationship of RV and LV myocardial perfusion reserve indexes (MPRI) with ventricular function and PH by using adenosine perfusion CMR imaging. They found that RV and LV MPRIs were significantly correlated with increasing PAP and with measures of RV workload, systolic dysfunction, and remodeling (i.e., ventricular mass index [VMI]). Biventricular MPRI was inversely associated with mPAP and RV stroke index.
Vogel-Claussen et al.29 found a significant correlation between MR-derived measurement RV septomarginal trabeculation mass, VMI (VMI = RV mass/LV mass), and RHC-documented PH hemodynamics. Fernandez-Friera et al.30 showed a regional difference in RV function and volumes as a result of chronic PH. They found that apical RV dysfunction appeared to occur even in the presence of normal global RV ejection fraction (RVEF) in patients with PH, in association with an increase in apical RV ESV. In contrast, the contractility of basal segments remained unchanged or slightly increased, suggesting some degree of compensation to maintain normal global RVEF. The group noticed a similar apical dysfunction pattern in another group of patients with both PH and global RVEF.
In one study the CMR-derived PA distensability index consistently correlated with the RHC-derived PA stiffness index, PVR, and PA capacitance.31 They also showed that the CMR-derived PA distensability index could be used to predict functional class (FC) in patients with PAH. A cutoff for CMR-derived PA distensability of <20% predicted poor FC in 6MWD of <400 m with 82% sensitivity and 94% specificity. This is in line with our findings of direct correlation between 6MWD and flow to beat-derived MRI estimation.
MRI phase contrast velocity quantification was also significantly correlated with RHC-measured PH in our study, but the correlation was, at best, moderate. Helderman et al.32 found that a relative retrograde flow in midsystolic peak was significantly larger in PAH patients than in non-PAH patients (9% vs. 1%; P < 0.0001). Moreover, we found a significant increase in flow post-PEA, which was moderately correlated with RHC parameters and may be useful in clinical assessment post-PEA. Double inversion recovery “black blood” MRI suppresses the signal from flowing blood, and slow-flowing blood causes incomplete suppression, resulting in pulmonary blood flow artifact (PFA). Swift et al.33 looked into the diagnostic utility and prognostic value of a PFA scoring system in patients with PH by RHC. They found that PFA >1 demonstrated a high sensitivity (86%) and specificity (85%) for diagnosis of PH, with a good correlation with PVR, mPAP, and CI. Also, PFA was significantly associated with mortality over a mean follow-up of 19 months.
Kreitner et al.9 showed that the contrast-enhanced MR angiographic technique can delineate typical angiographic findings such as intraluminal webs and bands, abrupt vessel cutoffs, and abnormal proximal-to-distal tapering. Because it is cross-sectional imaging modality, contrast-enhanced MR angiography proved to be superior to selective digital subtraction angiography for assessment and delineation of the proximal extent of organized thrombotic material.
The most widely noninvasive tool used in clinical practice to study RV dysfunction is echocardiography. In CTEPH, echocardiographically assessed RV restoration was reported before.34-36 However, the usefulness of echocardiography in this context is limited because of the Doppler measurements of peak velocity of the tricuspid regurgitant jet, which are not always detected; therefore, the peak velocity of the jet may be difficult to measure in the presence of severe tricuspid regurgitation. There is also a need for an adequate acoustic window as well as the absence of a reliable mathematical assumption due to the complex geometry of the right ventricle.37 Nogami et al.38 demonstrated a higher correlation of flow and pressure between phase-contrast magnetic resonance and RHC than that between cardiac RHC and echocardiography in patients with PH.
There are several limitations for this study. (1) Two different MR systems were used during the follow-up study, making the patient group less homogenous. (2) Some of the studies presented in this review27-32 significantly correlating MR-derived parameters with RHC-measured hemodynamics and FC need to be validated in larger studies and enhanced experiences. (3) Inter- and intraobserver variability of MRI, 6MWD, and RHC was not assessed. (4) Flow measurements might be associated with errors and inaccuracies; an appropriate choice of velocity-encoding (VENC) gradient is crucial to avoid aliasing (VENC too low) and, on the other hand, to maintain high sensitivity, especially in low-flow states (VENC too high). Thus, an individual adjustment of the VENC is often necessary. Moreover, complex or turbulent flow may adversely affect flow measurement phase contrast MRI. (5) We expressed RV function in global terms, without quantifying regional myocardial function. (6) A large number of patients had tricuspid regurgitation secondary to RV dilatation and RV hypertension. Concomitant tricuspid regurgitation might explain the differences between RV systolic volume and pulmonary forward flow. (7) MRI findings as a prognostic indicator were not assessed, as this was outside the scope of our study. (8) The study population arose opportunistically, and the representativeness of our sample is not clear. (9) The MRI was performed at a 3-month follow-up after PEA. Numerous studies have shown progressive improvement in hemodynamics beyond 3 months, which might have led to better correlation between MRI and RHC parameters in a later stage. (10) 6MWD might not be fully representative at 3 months post-PEA, as some might still recover from surgery. However, this was recorded at 3 months post-PEA in order to have a snapshot concomitant with other investigations.
The main strength of the study was that this is the largest series to date of PEA patients, having all had RHC, 6MWD, NYHA, and CMR pre- and at 3 months post-PEA. In conclusion, we showed low to moderate correlation in the largest series of patients published to date who had PEA and who had MRI and RHC and clinical function assessed presurgery and at 3 months postsurgery. The major determinant of RV reverse remodeling is the restoration of physiologic PAP, a condition achieved by a successful PEA. MRI is noninvasive, does not require exposure to ionizing radiation, can be repeated, and gives morphological and functional information. There is abundant literature regarding MRI assessment of PH, including CTEPH. However, CO and volumetric measurements are significantly different between RHC and MRI. We conclude that a unified and agreed protocol of MRI would provide useful clinical information for assessment of PEA surgery outcome.
Acknowledgments
We acknowledge Carmen Treacy for help with prospective data collection and data processing. We are indebted to the contributions to patient care made by the referring pulmonary hypertension physicians in the United Kingdom, from Glasgow, London, Newcastle, Papworth, and Sheffield designated pulmonary hypertension hospitals. At Papworth we would like to acknowledge the contribution of our pulmonary endarterectomy anesthetists and intensive care physicians. This work was presented at the 31st International Society for Heart and Lung Transplantation meeting, San Diego, 2011.
Source of Support: L.S. was supported by the Medical Research Council (program no. U015232027).
Conflict of Interest: None declared.
References
- 1.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, et al. N Engl J Med 2004;350(22):2257–2264. [DOI] [PubMed]
- 2.Riedel M, Stanek V, Widimski J, Preroysky I. Longterm follow-up of patients with pulmonary thromboembolism, late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81(2):151–158. [DOI] [PubMed]
- 3.Jamieson SW, Kapelanski DP, Sakakibara N, Manacke GR, Thistlethwaite PA, Kerr KM, Channik RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: experience and lessons learned in 1500 cases. Ann Thorac Surg 2003;76:1457–1464. [DOI] [PubMed]
- 4.Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, Ilkjaer LB, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141(3):702–710. [DOI] [PubMed]
- 5.Vuylsteke A, Sharples L, Charman G, Kneeshaw J, Tsui S, Dunning J, Wheaton E, et al. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): a randomized controlled trial. Lancet 2011;378(9800):1379–1387. [DOI] [PubMed]
- 6.Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterecomy: recent changes in a single institution’s experience of more than 2700 patients. Ann Thorac Surg 2012;94:97–103. [DOI] [PubMed]
- 7.Freed DH, Thomson BM, Berman M, Tsui S, Dunning J, Sheares K, Pepke-Zaba J, Jenkins D. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg 2011;141(2):383–387. [DOI] [PubMed]
- 8.Kreitner KF, Ley S, Kauczor HU, Mayer E, Kramm T, Pitton MB, Krummenauer F, Thelen M. Chronic thromboembolic pulmonary hypertension: pre- and postoperative assessment with breath-hold MR imaging techniques. Radiology 2004:232(2):535–543. [DOI] [PubMed]
- 9.Kreitner KF, Kunz RF, Ley S, Oberholzer K, Neeb D, Gast KK, Heussel CP, et al. Chronic thromboembolic pulmonary hypertension: assessment by magnetic resonance imaging. Eur Radiol 2007;17:11–21. [DOI] [PubMed]
- 10.D’Armini AM, Zanotti G, Ghio S, Magrini G, Pozzi M, Scelsi L, Meloni G, Klersy C, Vigano M. Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2007:133(1):162–168. [DOI] [PubMed]
- 11.Reesink HJ, Marcus T, Tulevski II, Jamieson S, Kloek JJ, Noordegraaf AV, Bresser P. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg 2007:133(1):58–64. [DOI] [PubMed]
- 12.Iino M, Dymarkowski S, Chaothawee L, Delcroix M, Bogaert J. Time course of reversed cardiac remodeling after pulmonary endarterectomy in patients with chronic pulmonary thromboembolism. Eur Radiol 2008;18:792–799. [DOI] [PubMed]
- 13.Jenkins D, Mayer E, Screaton N, Madani M. State of the art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev 2012;21(123):32–39. [DOI] [PMC free article] [PubMed]
- 14.Hamilton-Craig C, Kermeen F, Dunning J, Slaughter RE. Cardiovascular magnetic resonance prior to surgical treatment of chronic thrombo-embolic pulmonary hypertension. Eur Heart J 2010;31(9):1040. [DOI] [PubMed]
- 15.Francone M, Dymarkowski S, Kalantzi M, Bogaert J. Real-time cine MRI of ventricular septal motion: a novel approach to assess ventricular coupling. J Magn Reson Imaging 2005;21:305–309. [DOI] [PubMed]
- 16.Berman M, Pavlushkov E, Abraham E, Dunning J, Tsui S, Hall R, Klein A, Jenkins D. Pulmonary endarterectomy: an example of right ventricular afterload failure. MMCTS 2009. doi:10.1510/mmcts.2008.003491. [DOI] [PubMed]
- 17.Menzel T, Kramm T, Wagner S, Mohr-Kahaly S, Mayer E, Meyer J. Improvement of tricuspid regurgitation after pulmonary thromboendarterectomy. Ann Thorac Surg 2002;73:756–761. [DOI] [PubMed]
- 18.Franzblau A. A primer of statistics for non-statisticians. New York: Harcourt Brace, 1958.
- 19.Hardziyenka M, Campian M, Reesnik HJ, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass. J Am Coll Cardiol 2011;57:921–928. [DOI] [PubMed]
- 20.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest 2010;139(5):1003–1009, doi:10.1378/chest.10-1066. [DOI] [PubMed]
- 21.Sciancalepore MA, Maffessanti F, Patel AR, et al. Three-dimensional analysis of interventricular septal curvature from cardiac magnetic resonance images for the evaluation of patients with pulmonary hypertension. Int J Cardiovasc Imaging 2012;28(5):1073–1085. [DOI] [PubMed]
- 22.Roeleveld RJ, Marcus JT, Faes TJ, et al. Interventricular septal configuration at MR imaging and pulmonary arterial pressure in pulmonary hypertension. Radiology 2005;234:710–717. [DOI] [PubMed]
- 23.Dellegrottaglie S, Sanz J, Poon M, et al. Pulmonary hypertension: accuracy of detection of left ventricular septal to free wall curvature ratio measured at cardiac MR. Radiology 2007;243:63–69. [DOI] [PubMed]
- 24.Alunni JP, Degano B, Arnaud C, et al. Cardiac MRI in pulmonary artery hypertension: correlations between morphological and functional parameters and invasive measurements. Eur Radiol 2010;20:1149–1159. [DOI] [PubMed]
- 25.Frank H, Globits S, Glogar D, et al. Detection and quantification of pulmonary hypertension with MR imaging: results in 23 patients. Am J Roentgenol 1993;161:27–31. [DOI] [PubMed]
- 26.Gan TJ, Lankhaar JW, Marcus JT, et al. Impaired left ventricular filling due to right to left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2006;290:H1528–H1533. [DOI] [PubMed]
- 27.Mauritz GJ, Vonk-Noordegraaf A, Kind T, et al. Pulmonary endarterectomy normalizes interventricular dysynchrony and right ventricular systolic wall stress. J Magn Reson Imaging 2012;14(5):1–9. [DOI] [PMC free article] [PubMed]
- 28.Vogel-Claussen J, Skrok J, Shehata ML, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology 2011;258:119–127. [DOI] [PMC free article] [PubMed]
- 29.Vogel-Claussen J, Shehata ML, Lossnitzer D, et al. Increased right ventricular septomarginal trabeculation mass is a novel marker for pulmonary hypertension. Invet Radiol 2011;46:567–575. [DOI] [PMC free article] [PubMed]
- 30.Fernandez-Friera L, Garcia-Alvarez A, Guzman G, et al. Apical right ventricular dysfunction in patients with pulmonary hypertension demonstrated with magnetic resonance. Heart 2011;97:1250–1256. [DOI] [PubMed]
- 31.Kang KW, Chang HJ, Kim YJ, et al. Cardiac magnetic resonance imaging derived pulmonary artery distensability index correlates with pulmonary artery stiffness and predicts functional capacity in patients with pulmonary arterial hypertension. Circ J 2011;75:2244–2251. [DOI] [PubMed]
- 32.Helderman F, Mauritz GJ, Andriga KE, et al. Early onset of retrograde flow in the main pulmonary artery is a characteristic of pulmonary arterial hypertension. J Magn Reson Imaging 2011;33:1362–1368. [DOI] [PubMed]
- 33.Swift AJ, Rajaram S, Marshall H, et al. Black blood MRI has diagnostic and prognostic value in the assessment of patients with pulmonary hypertension. Eur Radiol 2012;22(3):695–702. [DOI] [PubMed]
- 34.Menzel T, Wagner S, Kramm T, et al. Pathophysiology of impaired right and left ventricular function in chronic embolic pulmonary hypertension: changes after pulmonary thromboendarterectomy. Chest 2000;118:897–903. [DOI] [PubMed]
- 35.Menzel T, Kramm T, Bruckner A, Mohr-Kahaly S, Mayer E, Meyer J. Quantitative assessment of right ventricular volumes in severe chronic thromboembolic pulmonary hypertension using transthoracic three dimensional echocardiography: changes due to pulmonary thromboendarterectomy. Eur J Echocardiogr 2002;3:67–72. [DOI] [PubMed]
- 36.Menzel T, Kramm T, Mohr-Kahaly S, Mayer E, Oelert H, Meyer J. Assessment of cardiac performance using Tei indices in patients undergoing pulmonary thromboendarterectomy. Ann Thorac Surg 2002;73:762–766. [DOI] [PubMed]
- 37.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease. I. Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008;117:1436–1448. [DOI] [PubMed]
- 38.Nogami M, Ohno Y, Koyama H, et al. Utility of phase contrast MR imaging for assessment of pulmonary flow and pressure estimation in patients with pulmonary hypertension: comparison with right heart catheterization and echocardiography. J Magn Reson Imaging 2009;30:973–980. [DOI] [PubMed]