Abstract Abstract
Pulmonary endothelium displays considerable heterogeneity along the vascular axis, from arteries to capillaries to veins. Griffonia simplicifolia is a lectin that recognizes pulmonary microvascular endothelium with preference over extra-alveolar endothelium in both arteries and veins, yet the precise vascular location where this phenotypic shift occurs is poorly resolved. We gelatin-filled the circulation and agarose-loaded the airways and then labeled the lung with Griffonia lectin to enable visualization of the endothelial transition zone. Endothelium in vessels with internal diameters less than 38 μm were uniformly Griffonia positive, whereas vessels with internal diameters greater than 60 μm were always Griffonia negative. Two populations of endothelium were identified in vessels ranging from 38 to 60 μm in diameter, including some that were positive and others that were negative for binding to G. simplicifolia. To better resolve this endothelial transition zone, we performed morphology studies to measure the distribution of Weibel-Palade bodies (WPbs), since WPbs are present in conduit vessel endothelium and absent in capillary endothelium. WPbs were found in endothelium with vascular dimensions as small as 18 μm in diameter but were not found in capillaries. Thus, we identify with precision that the endothelial phenotype transition from a cell that does not interact with Griffonia lectin to one that does occurs in blood vessels with internal diameters of approximately 38 μm, and we reveal an unappreciated vascular zone, between 18 and 38 μm in diameter, where endothelium both is Griffonia positive and possesses WPbs.
Keywords: lectin, vascular, heterogeneity, von Willebrand factor, P-selectin
Introduction
A discrete cellular layer lines the lumens of blood and lymphatic vessels. This cell layer is now widely recognized as endothelium, although in terms of medical history, the appreciation for the structure and function of endothelium is a recent advance.1 Three distinctive types of endothelium are described in anatomic terms: continuous, discontinuous, and fenestrated cells.2,3 The continuous subtype of endothelium lines all of the pulmonary circulation.2 These cells reside on a basement membrane that defines the margins of the tunica intima, they possess cell-cell borders, and they lack fenestrae, or openings through the cytoplasm. While pulmonary endothelium is of the continuous subtype, the structure and function of endothelial cells differ starkly as the circulation branches from large pulmonary arteries to progressively smaller arterioles and into the capillary plexus.4-7 Similar diversity is seen in cells arising from capillaries and feeding into the venules and veins. Analyzing the molecular and biochemical basis for heterogeneity along the pulmonary vascular axis has given insight into the physiological role of endothelium in anatomically restricted segments.
There are now many examples of the distinctive endothelial physiology that arises along the pulmonary vascular axis, but of these examples, barrier function and inflammation have been among the most extensively studied.4-9 Endothelium forms a semipermeable barrier to water, solutes, and macromolecules, and the restrictive nature of this barrier varies along the vascular tree. Permeability is higher in extra-alveolar endothelium than it is in alveolar endothelium, both in vivo and in vitro. Moreover, the permeability response to inflammatory agonists differs by vascular segment. Gq-linked agonists, such as thrombin and protease-activated receptor ligands,10 and oxidants11 have greater permeability-evoking actions on extra-alveolar endothelium than on alveolar endothelium. However, 5,6- and 14,15-epoxyeicosatrienoic acids (EETs) increase permeability in the capillary segment and not in extra-alveolar vessels.12-14 The action of these EETs is mimicked by 4αPDD, because both classes of agonists activate vanilloid transient receptor potential protein 4 (TRPV4) channels that are expressed within lung capillary endothelium. Calcium entry through the TRPV4 channel causes endothelial sloughing and increased permeability yet does not promote P-selectin surface translocation.15 Interestingly, the expression of another endothelial calcium channel, the α1G T type calcium channel (Cav3.1), is limited to lung capillaries.16 Activation of this channel does not increase permeability but is responsible for P-selectin surface expression.15,17 It is increasingly clear that therapeutic approaches seeking to improve barrier function or prevent airway neutrophil recruitment must be directed toward very specific molecular targets within discrete vascular locations.
Such insight into the heterogeneity of pulmonary endothelium has prompted more rigorous investigation into the vascular location where a phenotypic transition occurs. Prior studies utilized Griffonia simplicifolia and Helix pomatia lectins to resolve the transition site.5,7 Griffonia simplicifolia preferentially recognizes microvascular endothelial cells, whereas H. pomatia preferentially recognizes macrovascular cells, and while this demarcation is stark, only a range of vascular diameters (25–100 μm) could be resolved in the transition zone. We labeled endothelium with the Griffonia lectin and then utilized a gelatin-agarose-filled lung to reconstruct vascular dimensions. This lectin-binding analysis was coupled with morphological evaluation of Weibel-Palade body (WPb) distribution, because WPbs are present in extra-alveolar endothelium and absent in the capillary endothelium.18,19 From the comparison of G. simplicifolia labeling with WPb distribution, we identify with clarity this previously undetermined endothelial phenotype transition zone, and we identify a highly selective endothelial niche characterized by both the presence of WPbs and Griffonia binding.
Methods
Animals
Mice of either sex ranging 8–12 weeks in age and 18–35 g in weight were used in the study. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of South Alabama.
Isolated lung preparation and experiment protocol
Lungs were isolated for in situ perfusion as previously described.15,20 Briefly, mice were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg body weight). After tracheotomy, the trachea was cannulated, and the mice were ventilated with a gas mixture of 20% O2, 5% CO2, and 75% N2 by use of a mouse ventilator (MiniVent, Type 845; Hugo Sachs Elektronik, March, Germany). The tidal volume was adjusted to obtain a peak inspiratory pressure of 9 cmH2O at a respiratory rate of 60 breaths/min, with a positive end-expiratory pressure of 1.5 cmH2O. The chest was opened, 100 IU of heparin sodium was injected into the right ventricle, and a suture was placed around the pulmonary artery and aorta. A plastic cannula (0.86-mm internal diameter, 1.27-mm outside diameter) connected to a reservoir was advanced into the pulmonary artery via an incision in the right ventricular free wall, and the cannula was secured with the suture. Next, a second plastic cannula of the same size was advanced into the left atrium via an incision in the apex of the left ventricle. Both cannulas were secured by a cotton thread tied around the ventricles. All lungs were perfused with Earle’s buffer salt solution (EBSS) containing 4% bovine serum albumin (BSA; 4%BSA/EBSS), via a roller pump (Reglo Digital compact cassette pump; ISMATEC, Labortechnik-Analytik, Glattbrugg, Switzerland), at a constant flow rate of 1 mL/min in a recirculating system with a total volume of 10 mL. The venous outflow was collected in a reservoir, the height of which was adjusted to a fixed venous pressure (5 cmH2O, zeroed at the mid-lung level).
For in situ confocal fluorescence microscopy, lung slices were prepared with the method reported by Perez and Sanderson.21 In brief, following a 10-minute vascular perfusion with 4%BSA/EBSS, the pulmonary vessels were infused with 6% gelatin and the airway was intratracheally infused with 2% agarose (type VII-A, low gelling temperature; Sigma, St. Louis, MO) in Hank’s Buffered Salt Solution at 37°C. The lungs were immediately placed at 4°C for 30 minutes to allow the tissue to stiffen, as liquid gelatin and agarose gelled at the reduced temperature, and were then sectioned into approximately 200-μm slices with a Vibratome 1000Plus manual tissue-sectioning system (Technical Products International, St. Louis, MO). Lung slices were incubated with Alexa Fluor 594-conjugated Isolectin GS-IB4 from G. simplicifolia (200 nmol/L, in 4%BSA/EBSS) at 37°C for 10 min in Dulbecco’s modified Eagle medium (DMEM) and then rinsed with DMEM 3 times. Lung slices were mounted in an Attofluor cell chamber (Molecular Probes, Eugene, OR), moisturized with a drop of 4% BSA/EBSS, and examined with a Nikon A1 confocal laser microscope system. A ×40 and a ×60 (0.75 and 1.20 numerical aperture, respectively) water immersion objective were used, along with the following filter settings: for Alexa Fluor 594, 590-nm excitation and 617-nm emission; for 4′,6-diamidino-2-phenylindole (DAPI), 345-nm excitation and 455-nm emission. A set of serial optical sections in both fluorescence and differential interference contrast modes (Z-stacks) was taken at 0.2–0.3-μm intervals.
Transmission electron microscopy and sample preparation
Lungs were isolated from anesthetized adult mice (n = 5), fixed in 3% glutaraldehyde in cacodylate buffer by vascular perfusion, and then immersed in fresh fixative. The specimens were rinsed in cacodylate buffer, postfixed for 1 hour with 1% aqueous osmium tetroxide, dehydrated with a graded alcohol series, and embedded in PolyBed 812 epoxy resin (Polysciences, Warrington, PA). Thick (1-μm) sections were cut with glass knives, stained with 1% toluidine blue, and examined via light microscopy for “structure orientation.” Thin (80-nm) sections cut from the same block with diamond knives were stained with uranyl acetate and Reynolds’s lead citrate and examined and photographed with a Philips CM 100 transmission electron microscope (FEI, Hillsboro, OR), and measurements were made from the micrographs.
Results and Discussion
Griffonia simplicifolia is a plant lectin that has been used successfully to discriminate between extra-alveolar and alveolar endothelium.4-7 This lectin interacts with pulmonary microvascular endothelial cells, but it does not readily interact with pulmonary arterial endothelial cells, both in vivo and in vitro. This distinctive binding pattern, along with other endothelial marker characteristics, has been successfully utilized to enrich cell populations in vitro that possess functional behaviors characteristic of those seen in vivo.22 By adhering to these guidelines, investigators have found that pulmonary microvascular endothelial cells differ from their conduit cell counterparts in many ways, including semipermeable barrier function, junctional protein expression, flow alignment, proliferation, migration, angiogenesis, cell shape, organelle distribution and function, caveolae dynamics, resting membrane potential, ion channel expression and function, and signal transduction networks (for review, see works by Stevens,4,6 Gebb and Stevens,5 King et al.,7 and Ochoa et al.8,9). The location where endothelial cells begin to interact with Griffonia has been estimated to be in blood vessels with internal diameters between 25 and 100 μm. This size estimate has mostly been made from histological sections and in situ immunofluorescence images, along with estimates from methyl methacrylate casts. We sought to specifically identify the vascular site where endothelial cells have the ability to interact with G. simplicifolia.
To address this issue, gelatin was delivered through the circulation and agarose was loaded into the airways under physiologically appropriate pressures. Lungs were then cooled for 30 minutes and cut into 200-μm sections, and lung slices were exposed to G. simplicifolia. Figure 1 illustrates the prominent interaction between pulmonary microvascular endothelial cells and G. simplicifolia. Consistent with previous reports, endothelial cells leading into the capillary plexus did not interact with Griffonia lectin. Summary data from high-magnification images reveal an exact estimation: 38-μm diameter blood vessels represent the mean vascular diameter where endothelium interacts with G. simplicifolia (Fig. 2). From the scatter of these data, we see that endothelium in blood vessels between 38 and 60 μm may or may not interact with G. simplicifolia, suggesting that these are vessels of diverse structure and function. The exact nature of these vessels is not addressed here. In the future, it will be important to determine whether endothelium interacts with G. simplicifolia in similar or distinctive ways in conventional and supernumerary branches, and it will be of interest to determine whether the presence or absence of adjoining smooth muscle is a determinant of lectin binding. Nonetheless, our analysis clearly reveals that endothelium in blood vessels less than 38 μm in diameter uniformly binds to G. simplicifolia, whereas that in vessels greater than 60 μm in diameter does not.
Figure 1.
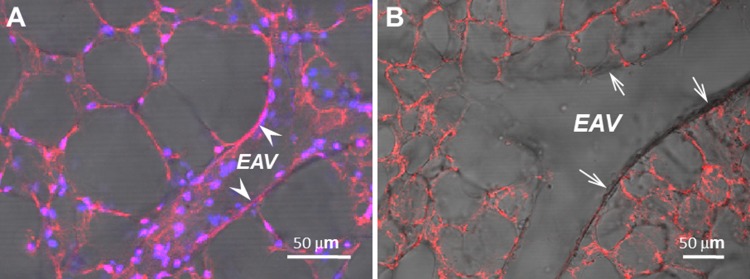
Alveolar capillary and extra-alveolar microvessel endothelium consistently exhibit positive surface binding of Griffonia simplicifolia. Fluorescence imaging of endothelial surface staining of Alexa Fluor 568–conjugated G. simplicifolia in alveolar capillaries and extra-alveolar microvessels. Representative projected images of all parts of Z-stacks, combining both fluorescence and differential interference contrast modes, taken at 0.2-μm intervals in <200-μm viable slices of a mouse lung. Note the appearance of Alexa Fluor 568 fluorescence on the alveolar septal capillary network as well as on the luminal surface of a small extra-alveolar vessel (EAV) in A (arrowheads), but not on the surface of a large EAV in B (arrows). Lung cell nuclei were visualized using 4′,6-diamidino-2-phenylindole (DAPI) staining (blue). Images were acquired at 21°C on a Nikon A1 confocal laser microscope with a ×60, 1.20 numerical aperture water immersion objective (replicated 3 times). Scale bars = 50 μm.
Figure 2.
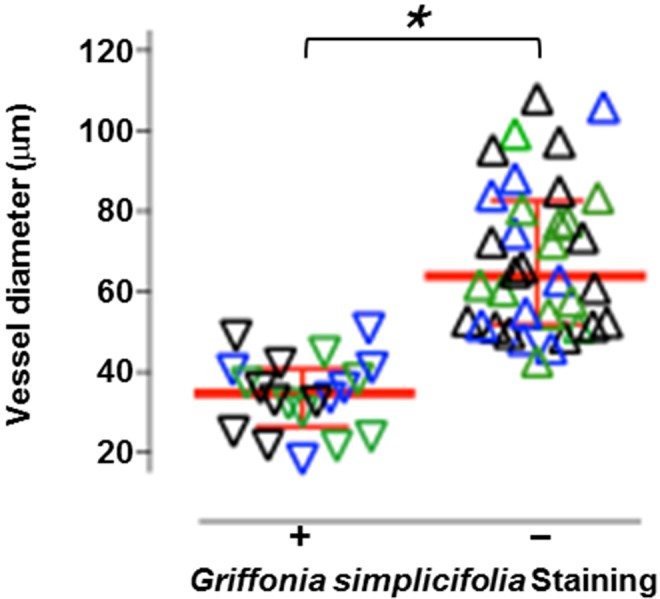
Summarized data from a total of 56 measurements of the diameter of the vessels where the binding of Griffonia simplicifolia was positive (plus sign; n = 20) or negative (minus sign; n = 36), obtained from mouse lungs (n = 3). Symbols, scatter plot (note that data obtained from each individual mouse are identified by different colors, i.e., green, blue, and black); red lines with error bars indicate median and interquartile range. The asterisk indicates P < 0.0001, per Student’s t test.
In 1964, Weibel and Palade18,23 observed a novel “rod-shaped cytoplasmic component” that later became known as the WPb. This organelle was localized to endothelium, and even in the initial observations it was shown to be abundant in the pulmonary artery. WPbs are not just in lung endothelium, as they are readily visible in endothelium of the systemic circulation. However, WPbs are not found in capillaries.18,19 This marked distinction between the presence and absence of WPbs in lung arteries and capillaries, respectively, represents an important example of endothelial heterogeneity.
The exact vascular location where WPbs cease to be prominent is not known. We utilized an electron microscopic approach to visualize WPbs in perfusion-fixed lung tissue, focusing specifically on the arterial-to-capillary border. As seen in Figure 3, a small arteriolar endothelial cell possesses WPbs, while the adjoining capillary endothelium does not. The averaged data reveal that endothelium lining blood vessels that are approximately 25 μm in diameter possesses WPbs, whereas endothelium in capillaries less than approximately 10 μm in diameter do not possess WPbs (Fig. 4). These data therefore illustrate the precise location where WPbs cease to be present.
Figure 3.
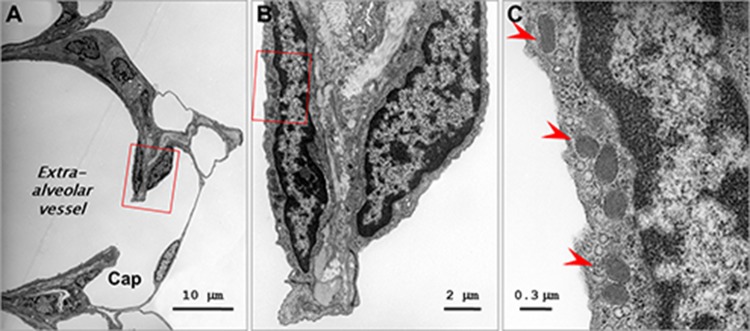
Heterogeneity of Weibel-Palade body (WPb) distribution in pulmonary vascular endothelium. Cross-section transmission electron micrographs of an extra-alveolar vessel and adjacent alveolar capillaries in a mouse lung. A, Low-magnification micrograph taken at the junction where an extra-alveolar vessel connects to an alveolar capillary (Cap). B, C, Higher powers of magnification of the boxed area in A, demonstrating a cluster of WPbs (arrowheads in C). Note that the endothelial cell exhibiting WPbs is at the extra-alveolar side of the junction.
Figure 4.
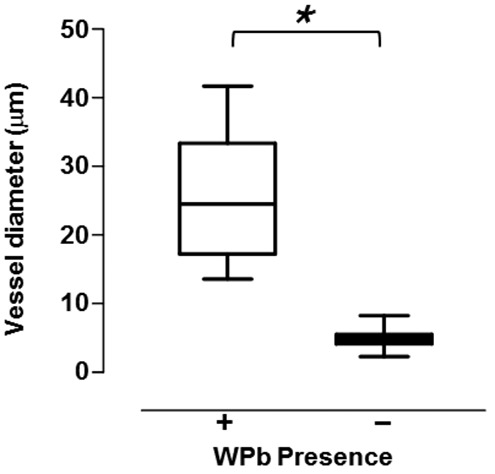
Summary data displayed as a box-and-whisker diagram from a total of 90 measurements of the diameter of the vessels where the WPbs were (plus sign) or were not (minus sign) detected, obtained from mouse lungs (n = 5). Asterisk indicates P < 0.0001, per Student’s t test.
By comparing the results from these two different anatomical analyses—one analyzing the endothelial interaction with Griffonia lectin and another analyzing the presence or absence of WPbs—we see that the presence of WPbs is not solely a characteristic of conduit or resistance arterial endothelium; it also includes precapillary arteriolar endothelium. Vessels in an approximately 22-μm range in vascular diameters—18–40 μm—possess both the ability to interact with Griffonia lectin and WPbs. Interestingly, von Willebrand factor expression is sufficient to drive WPb formation,24 yet capillary endothelium expresses von Willebrand factor while lacking WPbs.18,19 Hence, phenotypically distinct cellular mechanisms must be utilized among these different endothelial cells in control of von Willebrand packaging for its stimulated secretion, even though the different cell phenotypes can be immediately adjacent. In vitro evidence suggests that while full-length von Willebrand factor is sufficient to promote WPb formation,25 splicing of the D′-D3 N-terminal sequence renders von Willebrand factor unable to generate the organelle (see Ochoa et al.9 and references therein). Whether or not the D′-D3 region is spliced out of lung capillary endothelium is not currently known and is an important area of future study.
Von Willebrand factor is an important regulator of hemostasis, yet its stimulated secretion in extra-alveolar versus alveolar endothelium is poorly understood. Lung capillaries possess a stored pool of von Willebrand factor that can be secreted. They also possess a stored pool of P-selectin that can be stimulated to move to the plasma membrane. The intracellular location of von Willebrand factor and P-selectin are distinct in lung capillaries, and so these endothelial cells differ from those in larger vessels, not only because they lack WPbs but also because the intracellular pools of von Willebrand factor and P-selectin are detected in different sites.15,17 The physiological significance of this heterogeneity requires additional study.
In summary, our studies resolve the endothelial cell transition site for Griffonia binding to be at vessels with internal diameters of 38 μm. We identify a unique pulmonary microvascular endothelial cell niche that can be defined by its binding to Griffonia lectin and the presence of WPbs, specifically at the precapillary border. This niche is small, constituting only blood vessels with internal diameters that range from 18 to 40 μm. These studies suggest that WPbs per se are not a defining feature of either the macro- or the microvascular endothelial cell specification. Resolving the physiological significance of the WPb- and Griffonia lectin–positive cell phenotype awaits further study.
Source of Support: This work was supported by National Institutes of Health grants HL66299 (SW and TS) and HL60024 (TS). We thank Drs. Ivan F. McMurtry and Masahiko Oka and also Kaori Oshima, for their discussions of this manuscript.
Conflict of Interest: None declared.
References
- 1.Laubichler MD, Aird WC, Maienschein J. The endothelium in history. In: Aird WC, ed. Endothelial biomedicine. Cambridge: Cambridge University Press, 2007:5–19.
- 2.Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res 2007;100:174–190. [DOI] [PubMed]
- 3.Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res 2007;100:158–173. [DOI] [PubMed]
- 4.Stevens T. Microheterogeneity of the lung endothelium. In: Aird WC, ed. Endothelial biomedicine. Cambridge: Cambridge University Press; 2007:1161–1170.
- 5.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res 2004;68:1–12. [DOI] [PubMed]
- 6.Stevens T. Molecular and cellular determinants of lung endothelial cell heterogeneity. Chest 2005;128:558S–564S. [DOI] [PubMed]
- 7.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 2004;67:139–151. [DOI] [PubMed]
- 8.Ochoa CD, Stevens T. Studies on the cell biology of interendothelial cell gaps. Am J Physiol Lung Cell Mol Physiol 2012;302:L275–L286. [DOI] [PMC free article] [PubMed]
- 9.Ochoa CD, Wu S, Stevens T. New developments in lung endothelial heterogeneity: von Willebrand factor, P-selectin, and the Weibel-Palade body. Semin Thromb Hemost 2010;36:301–308. [DOI] [PMC free article] [PubMed]
- 10.Troyanovsky B, Alvarez DF, King JA, Schaphorst KL. Thrombin enhances the barrier function of rat microvascular endothelium in a PAR-1-dependent manner. Am J Physiol Lung Cell Mol Physiol 2008;294:L266–L275. [DOI] [PubMed]
- 11.Khimenko PL, Taylor AE. Segmental microvascular permeability in ischemia-reperfusion injury in rat lung. Am J Physiol Lung Cell Mol Physiol 1999;276:L958–L960. [DOI] [PubMed]
- 12.Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol 2004;286:L445–L451. [DOI] [PubMed]
- 13.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4–mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 2006;99:988–995. [DOI] [PMC free article] [PubMed]
- 14.Townsley MI, King JA, Alvarez DF. Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation 2006;13:725–739. [DOI] [PubMed]
- 15.Wu S, Jian MY, Xu YC, Zhou C, Al-Mehdi A-B, Liedtke W, Shin HS, Townsley MI. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 2009;297:L650–L657. [DOI] [PMC free article] [PubMed]
- 16.Wu S, Haynes J Jr., Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. Cav3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res 2003;93:346–353. [DOI] [PubMed]
- 17.Zhou C, Chen H, King JA, Sellak H, Kuebler WM, Yin J, Townsley MI, Shin H-S, Wu S. α1G T-type calcium channel selectively regulates P-selectin surface expression in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 2010;299:L86–L97. [DOI] [PMC free article] [PubMed]
- 18.Weibel ER. Fifty years of Weibel-Palade bodies: the discovery and early history of an enigmatic organelle of endothelial cells. J Thromb Haemost 2012;10:979–984. [DOI] [PubMed]
- 19.Fuchs A, Weibel ER. Morphometric study of the distribution of a specific cytoplasmatic organoid in the rat’s endothelial cells. Z Zellforsch Mikrosk Anat 1966;73:1–9 [in German]. [PubMed]
- 20.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 2007;293:L923–L932. [DOI] [PubMed]
- 21.Perez JF, Sanderson MJ. The contraction of smooth muscle cells of intrapulmonary arterioles is determined by the frequency of Ca2+ oscillations induced by 5-HT and KCl. J Gen Physiol 2005;125:555–567. [DOI] [PMC free article] [PubMed]
- 22.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 2008;294:L419–L430. [DOI] [PubMed]
- 23.Weibel ER, Knight BW. A morphometric study on the thickness of the pulmonary air-blood barrier. J Cell Biol 1964;21:367–396. [DOI] [PMC free article] [PubMed]
- 24.Hop C, Guilliatt A, Daly M, de Leeuw HP, Brinkman H-JM, Peake IR, van Mourik JA, Pannekoek H. Assembly of multimeric von Willebrand factor directs sorting of P-selectin. Arterioscler Thromb Vasc Biol 2000;20:1763–1768. [DOI] [PubMed]
- 25.Wagner DD, Saffaripour S, Bonfanti R, Sadler JE, Cramer EM, Chapman B, Mayadas TN. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell 1991;64:403–413. [DOI] [PubMed]


