Abstract Abstract
Particulates from air pollution are implicated in causing or exacerbating respiratory and systemic cardiovascular diseases and are thought to be among the leading causes of morbidity and mortality. However, the contribution of ambient particulate matter to diseases affecting the pulmonary circulation, the right heart, and especially pulmonary hypertension is much less documented. Our own work and that of other groups has demonstrated that prolonged exposure to antigens via the airways can cause severe pulmonary arterial remodeling. In addition, vascular changes have been well documented in a typical disease of the airways, asthma. These experimental and clinical findings link responses in the airways with responses in the lung’s vasculature. It follows that particulate air pollution could cause, or exacerbate, diseases in the pulmonary circulation and associated pulmonary hypertension. This perspective details the literature for support of this concept. Data regarding the health effects of particulate matter from air pollution on the lung’s vasculature, with emphasis on the lung’s inflammatory responses to particulate matter deposition and pulmonary hypertension, are discussed. A deeper understanding of the health implications of exposure to ambient particulate matter will improve our knowledge of how to improve the management of lung diseases, including diseases of the pulmonary circulation. As man-made ambient particulate air pollution is typically linked to economic growth, a better understanding of the health effects of exposure to particulate air pollution is expected to integrate the global goal of achieving healthy living for all.
Keywords: particulate matter, pulmonary immune responses, pulmonary hypertension, cardiovascular, airways, pulmonary circulation
This perspective proposes that ambient air pollution could have a significant clinical impact on pulmonary hypertension. This topic is understudied: a current PubMed search for “pulmonary hypertension, air pollution, excluding cigarette or tobacco” returned 36 entries, and only one was specific to the topic of this review. In contrast, a PubMed search for “asthma, air pollution, excluding cigarette or tobacco” returned 2,968 entries, and a search for “cardiovascular, air pollution, excluding cigarette or tobacco” returned 1,679 entries. This perspective provides a summary of the global impact of air pollution on health in general and discusses deposition of particulate air pollutants in the airways and resulting effects in the airways, the systemic circulation, and in the pulmonary circulation. The latter aspect is strengthened by our own new experimental data showing that air pollution can exacerbate pulmonary arterial remodeling and lead to a significant increase in the pressure in the pulmonary circulation in mice.
Urban fog of particulate air pollution
Particulate air pollution has been of great health concern since the increased mortality reported as a the result of the “London Fog” in 1952.1 Similarly, incidents in Belgium in 1930 (the Meuse fog)2 and in Pennsylvania in 1948 (the Donora fog)3 showed that air pollution can be deadly. In these episodes, the particulate matter (soot/polluted dust) was thought to confer a large proportion of the observed morbidity and mortality.4 These disastrous incidents prompted government and policy interventions that resulted first in the Air Pollution Control Act and then in the Clean Air Act.3
Since the 1950s, great strides have been made to reduce particulate air pollution. The first step has been the measurement of air pollution levels. Today, this information is freely available on the internet for many regions: the American Lung Association for the United States (State of the Air app), CiteAir II for many European cities (http://www.airqualitynow.eu/), and the Embassy of the United States or the Chinese government for Beijing. For measurements of particulate matter (PM), levels are fractionated according to size, which determines their ability to be retained in the lungs:5-8 PM10 (particles up to 10 μm in aerodynamic diameter) deposit in the nasal passages or larger airways; PM2.5 (particles smaller than 2.5 μm in aerodynamic diameter) can reach the alveoli. Pressure by concerned residents has succeeded in convincing public officials to measure and publish air pollution levels9 or to achieve reduction in air pollution.10 Lowering emissions has improved health outcomes.11 In contrast to the successful efforts by established industrialized countries to reduce the effects of air pollution,11 record-breaking pollution levels have been measured in the winter of 2013 in Beijing, China.9 High levels of particulate air pollution are also a major problem in many other countries throughout the world,11,12 e.g., Mongolia,13 Iran,14 and India.15 Depending on the geographical location and weather conditions, small cities can also suffer from air pollution that significantly surpasses guideline values year-round or during specific seasons.6,16
Health effects of particulate air pollution
The World Health Organization has published a review of the most recent scientific data concerning the adverse health effects of PM2.5 exposure.17 PM2.5 levels have been linked to many diseases, including cardiovascular and respiratory diseases, diabetes, and neurodevelopment and cognitive impairment.17,18 Surprisingly, the most prominent detrimental health effects of ambient PM2.5 air pollution for hospital admissions and mortality have been observed in the cardiovascular system.11,19,20
Urban particulate air pollution and the airways
The idea that air pollution with high PM2.5 levels could precipitate inflammatory and remodeling changes in the lungs,6 thus exacerbating chronic conditions such as asthma and increasing asthma incidence, has long been proposed. Experimental studies suggest that the deposition of PM on epithelial cells that line the airways activates inflammatory signaling cascades.21-23 In addition, ambient PM pollution may alter systemic immunologic and systemic inflammatory responses.24-26
The inflammatory response is thought to predispose and exacerbate the asthmatic response to inhaled allergens, thereby precipitating the signs of asthma.21-23 In mice, instillation of PM 2.5, in a dose-dependent manner, increases the production of T helper 2 (Th2)– and T helper 1 (Th1)–related cytokines and respective transcription factors, upregulates toll-like receptors on alveolar macrophages,27 and activates complement28 and T cell responses.29 In keeping with the different response patterns triggered by ambient PM, atopic and nonatopic children have shown exacerbations of asthma that were correlated with exposure to indoor PM.30 A clinical association between outdoor air pollution and asthma has documented the contribution of road traffic pollution to chronic asthma, particularly in children.31-34 For example, a recent study showed that levels of nitrogen dioxide (NO2), a traffic-related air pollutant, and PM10 were linked with increased risk of developing asthma later in life.34 However, on a wider geographical level—for example, between countries—the levels of particulate air pollution are not correlated with asthma or respiratory allergies.35,36
First-responder immune cell types, such as alveolar macrophages and dendritic cells, are stressed and activated by air pollutants, such as diesel exhaust particles.25,27,37,38 Furthermore, particle-induced inflammation responses of the first-responder cells in the lungs result in stimulation of the bone marrow, maturation and release of progenitor cells, and recruitment of monocytes via the vasculature to the alveoli.25,38-41 This inflammatory response to ambient PM also affects the developing adaptive immune response by changing the molecular profiles in both alveolar macrophages and dendritic cells. The most pronounced changes in the behavior of antigen-presenting cells occur as result of the communication between airway epithelial cells and dendritic cells.42-47 For example, CCL20 (chemokine CC ligand 20), a chemoattractant for immature dendritic cells and T cells (in particular Th17 cells), TSLP (thymic stromal lymphopoietin), and GM-CSF (granulocyte-macrophage colony-stimulating factor) activate airway dendritic cells and enhance the ability of these cells to present antigen. This molecular interaction also includes the regulation of specific microRNA expression, in particular that of miR375.48
Interestingly, new reports show widespread vascular responses in asthma, indicating a coordinated response of the airways and the lung’s vasculature to allergen exposure.49-51 These types of observations and data from their own experimental studies52 prompted Dr. Said and his colleagues53 to propose the hypothesis that asthma and pulmonary hypertension share a key pathogenic mechanism.
Urban particulate air pollution and the cardiovascular system
The adverse effects of ambient PM pollution on the cardiovascular system are a major contributor to mortality.11,18,19,54 Because of exposure to ambient PM pollution, cardiovascular and circulatory diseases surpassed exacerbation of infections and other respiratory disorders as the major burden of disease in 2010.11 This outcome strongly suggests that PM deposition in the airway or alveolar epithelium has effects beyond the airspaces. Numerous mechanisms have been proposed to explain the mechanisms by which PM exerts cardiovascular effects. The ability of PM to activate autonomic neuronal reflexes in the lungs, resulting in functional cardiac changes and vasoconstriction, has been hypothesized.18,55 But this process alone is not sufficient to account for the structural changes in the cardiovascular system, including atherosclerosis, ischemic heart disease, and stroke, that are attributed to ambient PM exposure.18 Several additional mechanisms have been proposed to explain the cardiovascular structural changes induced by exposure to ambient PM2.5 (Fig. 1),5,18,56,57 including the following. (1) Ambient PM deposited in the airspaces translocates into the blood stream, directly via adhesion and diffusion into the adjacent blood vessels or indirectly via lymph fluid movement through the lymph node.57-60 This process has been experimentally documented for ultrafine (nano-)particles that have an aerodynamic diameter in the nanometer range. (2) Deposited ambient particles disintegrate and dissolve partially (or fully) and release harmful chemicals (metals, organic compounds) that translocate directly into adjacent blood vessels or indirectly, via the lymph through the lymph node, into the blood stream.5,56 (3) Particles are phagocytosed in the airspaces, resulting in inflammation and migration of inflammatory cells (macrophages, dendritic cells) to the lymph nodes, followed by transport of the particles in the blood stream.61 (4) Deposits of ambient PM initiate inflammation in the airspaces with the release of cytokines and other inflammatory mediators into the vasculature and transfer via the cardiovascular system.18
Figure 1.
Routes of transfer of particulate matter (PM) from the airways to peripheral organs and inflammatory response. PM effects in the heart and other organs involve dissemination of PM via blood vessels (red arrows) or drainage via the lymphatics (gray line) to the lymph node, followed by dissemination via blood vessels (red arrow). Translocated materials include: (a) the PM itself (nanoparticles), (b) components of disintegrated PM, (c) phagocytic immune cells that have phagocytosed PM and that migrate via the lymphatics to the lymph nodes, and (d) inflammatory mediators (e.g., cytokines) produced in the lungs in response to PM deposits.
The lung’s vasculature, pulmonary hypertension, and cigarette smoke exposure
To optimize gas exchange, the architecture of the vasculature in the lungs is intricately linked to the air-carrying airways and alveoli. The pulmonary artery carries the blood from the right heart to the capillary bed of the alveoli and respiratory bronchi. Constriction, inflammation, thickening (i.e., remodeling) and loss of branches of the pulmonary arteries lead to pulmonary hypertension.62,63
Cigarette smoking is one of the toxic environmental exposures that cause respiratory diseases, including chronic obstructive pulmonary disease (COPD) and lung cancer.64-67 In addition, cigarette smoke exposure may be a risk factor for individuals who have pulmonary hypertension, as suggested by clinical data68 and animal studies.69,70 Dissecting the molecular mechanism of COPD-associated pulmonary hypertension induced by cigarette smoke exposure in mice, Dr. Weissmann and colleagues70 showed that the pathway to cigarette smoke–induced pulmonary hypertension involved oxidative and nitrosative stress followed by accumulation of proteins that are rendered less functional by nitrosylation. Genetic susceptibility factors are additional determinants of the extent and severity of pulmonary arterial remodeling and right heart hypertrophy in response to tobacco smoke exposure, as shown in studies of different mouse strains from Dr. Gordon’s laboratory.69 Tobacco smoke can also trigger pulmonary vascular remodeling in smokers who have not yet developed COPD.71-73 The fine PM in secondhand smoke is similar to the PM of air pollution, and secondhand smoke can induce inflammation and cause cardiovascular disease.74
Pulmonary arterial remodeling triggered by immune responses elicited in the airways
In experimental animals—mice and rats—there is clear evidence from several different groups that prolonged antigen exposure via the airways can induce severe pulmonary arterial remodeling, with thickening of the wall, disorganization of the smooth muscle cell layer, and smooth muscle cell proliferation.75-79 Particulate75 and soluble76-79 antigens can elicit this remodeling response. Our own work76 has shown that this remodeling response is dependent on the presence of CD4+ T cells and Th2 cytokines (interleukin: IL-4, IL-13), mediators known for their critical role in asthma.80 The role of IL-13 in pulmonary arterial remodeling has been supported by studies of transgenic mice overexpressing IL-13 in the airways.81 In addition, mice that have IL-25, a major inducer of IL-13, elicited in the airways by antigen exposure or transgenic expression also demonstrate pulmonary arterial remodeling.82 BMPR2, a receptor known for its central role in the development of pulmonary hypertension when present at a hypomorphic state, with decreased activity, has been shown to be a regulator of the asthmatic airway hyperreactivity response.83 Our own data have shown that antigen exposure via the airways efficiently triggers the pulmonary hypertension phenotype in mice that carry a hypomorphic BMPR2.84 Taken together, these findings strengthen the notion53 that responses in the airways and in the pulmonary vasculature can be linked and that this may occur via coordinated molecular signals.
Ambient particulate air pollution and pulmonary vascular changes
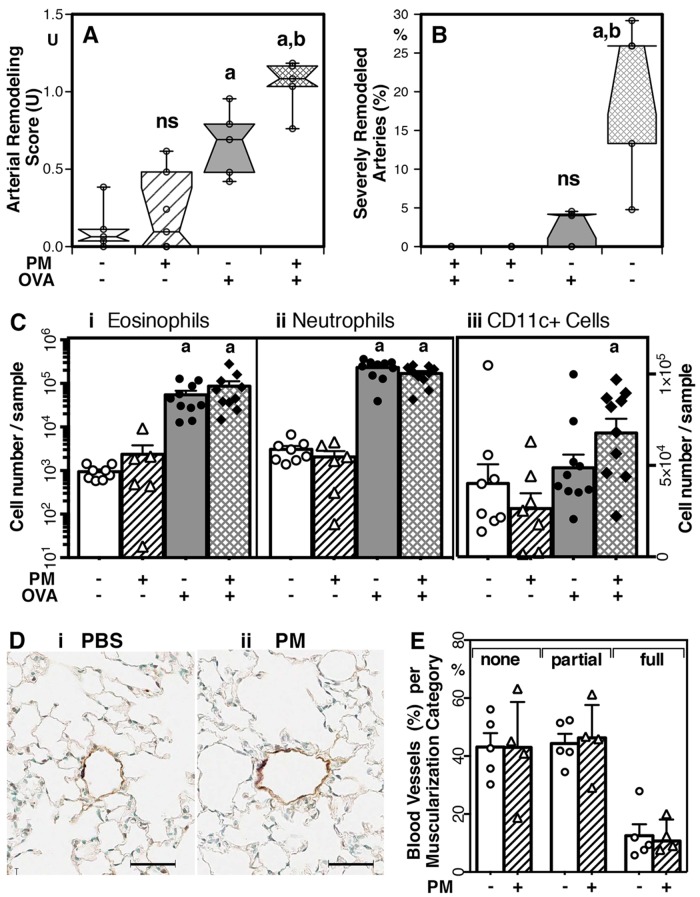
Figure 2 shows the results from our study designed to test the hypothesis that PM2.5 would exacerbate antigen-induced pulmonary arterial remodeling in mice. We found that when given PM2.5 alone at a dose of 25 μg per instillation, the equivalent of 1.25 mg/kg body weight, mice did not show arterial remodeling, even when we used a sensitive immunohistochemistry method coupled with computer analysis (Fig. 2). In keeping with the published literature using PM2.5 sampled in Beijing,27 New York City,85 or Baltimore,28,29,86 the dose of PM2.5 that we used did not elicit significant airway inflammation, as determined by bronchoalveolar lavage cellularity (Fig. 2). In order to focus on the exacerbating role of PM2.5, we used a low dose, approximately half the concentration of PM2.5 that has been reported to elicit a significant inflammatory response,27,85,86 and an antigen, ovalbumin (OVA), that in nonimmunized mice has very mild proinflammatory activity.87 Our data clearly show that immunized mice that were challenged with PM2.5 given together with the mild antigen had significantly exacerbated pulmonary arterial remodeling when compared with immunized mice challenged with the antigen alone (Fig. 2).
Figure 2.
Significant exacerbation of pulmonary arterial remodeling due to combined exposure to antigen and urban PM2.5 (particulate matter <2.5 μm in aerodynamic diameter). A, B, Arterial remodeling as average remodeling score per lung (A) and as percentage of severely remodeled arteries in the lungs (B); box plots with individual data points, n = 4–10. C, Numbers of eosinophils (i), neutrophils (ii), and CD11c+ cells (iii; note linear scale) in the bronchoalveolar lavage samples; bar graphs show means, standard error of the means (SEM), and individual data points (n = 6–10). D, E, Blood vessel muscularization. D, Representative histological sections from a phosphate-buffered-saline (PBS)-exposed mouse (i) and a PM-exposed mouse (ii) stained with anti–smooth muscle actin (dark; scale bars = 50 μm). E, Quantitative analysis of muscularization as percentage of blood vessels that were in each of the following categories: none (<30% of the circumference positive for smooth muscle actin staining), partial (30%–80% of the circumference positive), or full (>80% of the circumference positive); bar graphs show means, SEM, and individual data points (n = 4–5). In A–C, significant differences (P < 0.05, 2-tailed, unpaired Mann-Whitney test) relative to groups of mice exposed to saline (a) or ovalbumin (OVA; b) are indicated; ns: not significant. The study was performed in two independent experiments using C57BL/6 wild-type mice that were purchased from Jackson Labs and housed in specific pathogen-free conditions under the supervision of the Institutional Animal Care and Use Committee at New York University Medical Center as described.41,84 The mice were immunized by intraperitoneal injection with OVA complexed to alum and given intranasal challenges in a 50-μl volume per dose using the described schedule.41,76,84 PM2.5 was collected in New York City by Dr. Gordon’s group, as described,85 and diluted in saline to a concentration of 0.5 mg/mL. The intranasal dose of OVA was 100 μg, and for the OVA-PM instillations, OVA and PM2.5 were mixed to achieve final concentrations of 100 and 25 μg per instillation, respectively. Bronchoalveolar lavage,76,84 arterial remodeling,76,84 and blood vessel muscularization84 were analyzed as published. The percentage of severely remodeled arteries was calculated as a fraction of arteries given the score 1.5 or 2 (severely thickened arterial wall with irregularity of wall thickening and cellular disorganization).76 In comparison, a score of 0 represented a normal artery, and a score of 1 was given for mild, circular thickening of the artery wall. A color version of this figure is available online.
Ambient particulate air pollution and pulmonary hypertension
Figure 3 shows the results from our study designed to test the hypothesis that PM2.5 would trigger pulmonary hypertension induced by an antigen in mice. Right ventricular systolic pressures were measured by heart catheterization via the jugular vein.88 Immunized mice that were challenged with the combination of PM2.5 and an antigen demonstrated significantly increased right ventricular pressures, but mice challenged with the antigen or with PM2.5 alone did not (Fig. 3). The significant increase in the right ventricular pressures in the group of mice challenged with PM2.5 and antigen was detected with two analysis methods, comparisons of group medians (Fig. 3A) and contingency table analysis (Fig. 3B).
Figure 3.

Exposure to the combination of antigen (ovalbumin [OVA]) and urban ambient particulate matter (PM) causes increased pressures in the pulmonary circulation. Right ventricular systolic pressure (RVSP) is shown by notched box plots and circles showing individual data points (A) and by a bar graph showing the number of observations of RVSP less or greater than 26 mmHg (B). Data were pooled from two independent experiments; n = 4–10. The letter a indicates P < 0.05 compared to group of mice exposed to saline by 2-tailed, unpaired Mann-Whitney test (A) or by χ2 test and 2-tailed Fisher’s exact test (B); ns: not significant.
The data shown in Figures 2 and 3 represent a hypothesis-generating finding, suggesting that even low-dose PM2.5 can have clinical significance for pulmonary hypertension if PM2.5 exposure is added to another inflammatory condition, such as inflammation induced by exogenous (infectious or inhaled) or endogenous (autoimmunity) antigens. Furthermore, high-dose PM2.5 exposure may even further increase the risk for developing pulmonary arterial remodeling and pulmonary hypertension. Barriers to obtaining clinical data to test this hypothesis are expected to be overcome in the near future by noninvasive diagnostic techniques, such as echocardiography, computed tomography, and magnetic resonance imaging (MRI), that will complement the current gold standard in diagnosing pulmonary hypertension, invasive right heart catheterization.
A recent study, presented at the American Thoracic Society meeting in 2013, showed that living in proximity to roadways, and therefore being exposed to high levels of PM2.5 and nitrogen oxides, was associated with greater right ventricular mass and changes in right ventricular function, as detected by cardiac MRI.89 Right ventricular mass is a measure of strain, which could be caused by molecular changes in the right ventricle or changes in the pulmonary circulation, in turn causing limitations in the blood flow.89 While this study needs further mechanistic exploration and confirmation, it is in keeping with our observations, as shown in Figures 2 and 3, and indicates that air pollution can exacerbate parameters of the pulmonary hypertension phenotype. Further support for this notion comes from clinical studies in humans exposed to indoor90 or outdoor91 air pollution and from experimental studies in animals.92-94 Residents of low- and middle-income countries often are exposed to high indoor PM levels because of biomass fuel use. In this setting, indoor PM is thought to be a significant risk factor for developing pulmonary hypertension and right heart failure.90 In children living in Mexico, outdoor exposure levels of PM2.5 were associated with increased pulmonary arterial pressures (measured by echocardiography) and with elevated plasma endothelin-1 levels.91 Exposure of mice to urban PM resulted in increased production of endothelin-1 in the lungs.92 Endothelin-1 is a potent biological mediator of arterial constriction and has an important role in pulmonary hypertension.95,96 Exposure of rats to concentrated ambient PM impaired the relaxation response of pulmonary arteries to nitric oxide93 and induced pulmonary hypertension.94 Batalha and colleagues94 also observed a decrease in the ratio of lumen or wall thickness of the pulmonary arteries, an indicator of vascular remodeling, which was associated with the content of silica in the ambient PM.
This latter finding may hint toward a generalizable biological effect of silica on the pulmonary vasculature. Individuals exposed to silica-containing dust, e.g., miners of coal, stone, or gold, can develop a condition called silicosis/pneumoconiosis. Silicosis has been recognized as an occupational disease caused by air pollution exposure in the coal mines since the mid-1840s;97 the original articles from that time period are freely available on the Scottish Mining Website. Silicosis is characterized by chronic lung inflammation and fibrosis and includes other comorbidities, such as pulmonary hypertension and chronic cor pulmonale (right heart failure).98-101 Silicosis, despite some decline,11 is still the most prevalent occupational lung disease, occurring globally, for example, in the United States, China, South America, and Africa.102 Because of the severity of the condition and the lack of an effective treatment or a cure, silicosis has been placed on a list of diseases targeted for elimination by the World Health Organization.103 Interestingly, construction and glass dust containing silica/silicates/silicon were also constituents of the World Trade Center dust,104,105 and pulmonary arterial thickening has been reported as part of the pathological findings in a small group of exposed individuals who developed chronic, progressive lung disease.106 Exposure to silica crystals, and thus the potential for pulmonary vascular effects, can occur in many environments; a constituent of cement and glass, silica can be released into urban ambient air during construction activities.107 Moreover, silica, a main constituent of soil, is a component of desert dust storms and volcanic eruptions and can reach urban and rural areas.108 A large amount of silica-containing dust is also expected to be produced by the sand mining needed for hydraulic fracturing of oil and gas.109
Summary
More research is needed to understand the effects of ambient PM on the pulmonary circulation. PM constituents that translocate from the airspaces to the lung’s vasculature, by one of the molecular processes (Fig. 1)18,56 thought to cause systemic left heart cardiovascular morbidity and mortality, may also cause right heart morbidity and mortality because of changes in the pulmonary vasculature. Studies in experimental animals show that exposure to ambient PM can cause significant changes in the pulmonary vasculature, on the morphological, functional, and molecular levels,92,94 and that PM significantly exacerbates the pulmonary vascular response to antigen (Figs. 2, 3). These data suggest that exposure to ambient PM could exacerbate comorbidities, causing pulmonary hypertension and contributing to right heart failure. This could mirror the documented effects of ambient PM with respect to the left heart.11,18,19 Exposure to silica-containing PM mixed with chemicals from coal or fossil fuels (miner’s cor pulmonale) is perhaps the best-documented cause of air pollution exposure–induced pulmonary hypertension.98-101 However, silica-containing ambient PM is also generated by natural and man-made phenomena. In addition to silica, other components of ambient outdoor PM (specifically, urban PM) and indoor PM may also increase the risk for developing pulmonary arterial remodeling and pulmonary hypertension (Fig. 2).89 A deeper knowledge of the networked responses to ambient PM by the lung’s immune and vascular cells and how these relate to cardiovascular function will improve our ability to manage vascular diseases of the lungs and pulmonary hypertension.
Acknowledgments
These ideas were presented in part as a poster at the DACH Symposium for pulmonary hypertension, Thoraxklinik, University of Heidelberg, Heidelberg, Germany, October, 18–20, 2012; as an oral presentation at the symposium “From the Outside In: Sustainable Futures for Global Cities and Suburbs,” Hofstra University, Hempstead, New York, March 7–9, 2013; and as an oral presentation at the international meeting of the American Thoracic Society, Philadelphia, Pennsylvania, May 17–22, 2013.
Source of support: The work was funded in part by the National Institutes of Health award 1R21HL092370–01 (GG), 1R01 HL095764–01 (GG); the American Heart Association, Founders affiliate (0855943D, GG); the Stony Wold–Herbert Fund, New York (S-HP); and a National Institute of Environmental Health Sciences center grant (ES00260 for PM collection).
Conflicts of Interest: None declared.
References
- 1.Bell ML, Davis DL, Fletcher T. A retrospective assessment of mortality from the London smog episode of 1952: the role of influenza and pollution. Environ Health Perspect 2004;112:6–8. [DOI] [PMC free article] [PubMed]
- 2.Nemery B, Hoet PH, Nemmar A. The Meuse Valley fog of 1930: an air pollution disaster. Lancet 2001;357:704–708. [DOI] [PubMed]
- 3.Helfand WH, Lazarus J, Theerman P. Donora, Pennsylvania: an environmental disaster of the 20th century. Am J Public Health 2001;91:553. [DOI] [PMC free article] [PubMed]
- 4.Hunt A, Abraham JL, Judson B, Berry CL. Toxicologic and epidemiologic clues from the characterization of the 1952 London smog fine particulate matter in archival autopsy lung tissues. Environ Health Perspect 2003;111:1209–1214. [DOI] [PMC free article] [PubMed]
- 5.Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med 1980;37:337–362. [DOI] [PMC free article] [PubMed]
- 6.Pinkerton KE, Green FH, Saiki C, Vallyathan V, Plopper CG, Gopal V, Hung D, et al. Distribution of particulate matter and tissue remodeling in the human lung. Environ Health Perspect 2000;108:1063–1069. [DOI] [PMC free article] [PubMed]
- 7.Brauer M, Avila-Casado C, Fortoul TI, Vedal S, Stevens B, Churg A. Air pollution and retained particles in the lung. Environ Health Perspect 2001;109:1039–1043. [DOI] [PMC free article] [PubMed]
- 8.Churg A, Brauer M. Human lung parenchyma retains PM2.5. Am J Respir Crit Care Med 1997;155:2109–2111. [DOI] [PubMed]
- 9.Stein R. China’s air pollution linked to millions of early deaths. National Public Radio online 2013. http://www.npr.org/blogs/health/2013/04/02/176017887/chinas-air-pollution-linked-to-millions-of-early-deaths. Published April 2, 2013.
- 10.Mouawad J. Shell settles air pollution accusations. New York Times. April 24, 2009, page B9.
- 11.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–2260. [DOI] [PMC free article] [PubMed]
- 12.World Health Organization. Tackling the global clean air challenge. http://www.who.int/mediacentre/news/releases/2011/air_pollution_20110926/en/. Published September 26, 2011.
- 13.Allen RW, Gombojav E, Barkhasragchaa B, Byambaa T, Lkhasuren O, Amram O, Takaro TK, Janes CR. An assessment of air pollution and its attributable mortality in Ulaanbaatar, Mongolia. Air Qual Atmos Health 2013;6:137–150. [DOI] [PMC free article] [PubMed]
- 14.Naddafi K, Sowlat M, Safari M. Integrated assessment of air pollution in Tehran, over the period from September 2008 to September 2009. Iran J Public Health 2012;41:77–86. [PMC free article] [PubMed]
- 15.Rizwan S, Nongkynrih B, Gupta SK. Air pollution in Delhi: its magnitude and effects on health. Indian J Comm Med 2013;38:4–8. [DOI] [PMC free article] [PubMed]
- 16.Almbauer R, Pucher K, Sturm PJ. Air quality modeling for the city of Graz. Meteorol Atmos Phys 1995;57:31–42.
- 17.Anderson HR, Brunekreef B, Cohen A, Katsouyanni K, Krewski D, Kreyling WG, Künzli N, et al. Review of evidence on health aspects of air pollution—REVIHAAP: first results. Copenhagen: World Health Organization, 2013.
- 18.Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378. [DOI] [PubMed]
- 19.Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004;109:71–77. [DOI] [PubMed]
- 20.Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, Martin RV, et al. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med 2013;188:593–599. [DOI] [PMC free article] [PubMed]
- 21.Hao M, Comier S, Wang M, Lee JJ, Nel A. Diesel exhaust particles exert acute effects on airway inflammation and function in murine allergen provocation models. J Allergy Clin Immunol 2003;112:905–914. [DOI] [PubMed]
- 22.Jin C, Shelburne CP, Li G, Potts EN, Riebe KJ, Sempowski GD, Foster WM, Abraham SN. Particulate allergens potentiate allergic asthma in mice through sustained IgE-mediated mast cell activation. J Clin Invest 2011;121:941–955. [DOI] [PMC free article] [PubMed] [Research Misconduct Found]
- 23.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, Ning Z, Kleinman MT, Sioutas C, Nel AE. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol 2010;299:L374–L383. [DOI] [PMC free article] [PubMed]
- 24.van Eeden SF, Hogg JC. Systemic inflammatory response induced by particulate matter air pollution: the importance of bone-marrow stimulation. J Toxicol Environ Health A 2002;65:1597–1613. [DOI] [PubMed]
- 25.Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa T, Goto Y, Vincent R, van Eeden SF. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J Respir Cell Mol Biol 2002;27:34–41. [DOI] [PubMed]
- 26.Zhao J, Gao Z, Tian Z, Xie Y, Xin F, Jiang R, Kan H, Song W. The biological effects of individual-level PM2.5 exposure on systemic immunity and inflammatory response in traffic policemen. Occup Environ Med 2013;70:426–431. [DOI] [PubMed]
- 27.Zhao C, Liao J, Chu W, Wang S, Yang T, Tao Y, Wang G. Involvement of TLR2 and TLR4 and Th1/Th2 shift in inflammatory responses induced by fine ambient particulate matter in mice. Inhal Toxicol 2012;24:918–927. [DOI] [PubMed]
- 28.Walters DM, Breysse PN, Schofield B, Wills-Karp M. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol 2002;27:413–418. [DOI] [PubMed]
- 29.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect 2010;118:640–646. [DOI] [PMC free article] [PubMed]
- 30.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Peng RD, Curtin-Brosnan J, Williams DL, Wills-Karp M, Diette GB. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann Allergy Asthma Immunol 2011;106:308–315. [DOI] [PMC free article] [PubMed]
- 31.Spira-Cohen A, Chen LC, Kendall M, Sheesley R, Thurston GD. Personal exposures to traffic-related particle pollution among children with asthma in the South Bronx, NY. J Exposure Sci Environ Epidemiol 2010;20:446–456. [DOI] [PMC free article] [PubMed]
- 32.Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res 2012;117:36–45. [DOI] [PubMed]
- 33.Brandt SJ, Perez L, Künzli N, Lurmann F, McConnell R. Costs of childhood asthma due to traffic-related pollution in two California communities. Eur Respir J 2012;40:363–370. [DOI] [PMC free article] [PubMed]
- 34.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, Thyne S, et al. Early-life air pollution and asthma risk in minority children: the GALA II and SAGE II studies. Am J Respir Crit Care Med 2013;188:309–318. [DOI] [PMC free article] [PubMed]
- 35.von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann HH. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med 1994;149:358–364. [DOI] [PubMed]
- 36.Anderson HR, Butland BK, van Donkelaar A, Brauer M, Strachan DP, Clayton T, van Dingenen R, et al. Satellite-based estimates of ambient air pollution and global variations in childhood asthma prevalence. Environ Health Perspect 2012;120:1333–1339. [DOI] [PMC free article] [PubMed]
- 37.Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol 1999;163:5582–5591. [PubMed]
- 38.Suwa T, Hogg JC, Vincent R, Mukae H, Fujii T, van Eeden SF. Ambient air particulates stimulate alveolar macrophages of smokers to promote differentiation of myeloid precursor cells. Exp Lung Res 2002;28:1–18. [DOI] [PubMed]
- 39.Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods 2006;312:12–19. [DOI] [PubMed]
- 40.Rani R, Smulian AG, Greaves DR, Hogan SP, Herbert DR. TGF-β limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol 2011;41:2000–2009. [DOI] [PMC free article] [PubMed]
- 41.Hoffman C, Park SH, Daley E, Emson C, Louten J, Sisco M, de Waal Malefyt R, Grunig G. Interleukin-19: a constituent of the regulome that controls antigen presenting cells in the lungs and airway responses to microbial products. PLOS ONE 2011;6:e27629. [DOI] [PMC free article] [PubMed]
- 42.Peden D, Reed CE. Environmental and occupational allergies. J Allergy Clin Immunol 2010;125:S150–S160. [DOI] [PubMed]
- 43.Loh MM, Levy JI, Spengler JD, Houseman EA, Bennett DH. Ranking cancer risks of organic hazardous air pollutants in the United States. Environ Health Perspect 2007;115:1160–1168. [DOI] [PMC free article] [PubMed]
- 44.Liu J, Ballaney M, Al-alem U, Quan C, Jin X, Perera F, Chen L, Mille RL. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci 2008;102:76–81. [DOI] [PMC free article] [PubMed]
- 45.Niedzwiecki M, Zhu H, Corson L, Grunig G, Factor PH, Chu S, Jiang H, Miller RL. Prenatal exposure to allergen, DNA methylation, and allergy in grandoffspring mice. Allergy 2012;67:904–910. [DOI] [PMC free article] [PubMed]
- 46.Ng S, Yoshida K, Zelikoff JT. Tumor challenges in immunotoxicity testing. Methods Mol Biol 2010;598:143–155. [DOI] [PubMed]
- 47.Ng SP, Zelikoff JT. The effects of prenatal exposure of mice to cigarette smoke on offspring immune parameters. J Toxicol Environ Health A 2008;71:445–453. [DOI] [PubMed]
- 48.Bleck B, Grunig G, Chiu A, Liu M, Gordon T, Kazeros A, Reibman J. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol 2013;190:3757–3763. [DOI] [PMC free article] [PubMed]
- 49.Johansson MW, Kruger SJ, Schiebler ML, Evans MD, Sorkness RL, Denlinger LC, Busse WW, et al. Markers of vascular perturbation correlate with airway structural change in asthma. Am J Respir Crit Care Med 2013;188:167–178. [DOI] [PMC free article] [PubMed]
- 50.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax 2001;56:902–906. [DOI] [PMC free article] [PubMed]
- 51.Green FH, Butt JC, James AL, Carroll NG. Abnormalities of the bronchial arteries in asthma. Chest 2006;130:1025–1033. [DOI] [PubMed]
- 52.Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang Y, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 2007;115:1260–1268. [DOI] [PubMed]
- 53.Said SI, Hamidi SA, Gonzalez Bosc L. Asthma and pulmonary arterial hypertension: do they share a key mechanism of pathogenesis? Eur Respir J 2010;35:730–734. [DOI] [PMC free article] [PubMed]
- 54.Johnson RL Jr. Relative effects of air pollution on lungs and heart. Circulation 2004;109:5–7. [DOI] [PubMed]
- 55.Nadziejko C, Fang K, Narciso S, Zhong M, Su WC, Gordon T, Nádas A, Chen LC. Effect of particulate and gaseous pollutants on spontaneous arrhythmias in aged rats. Inhal Toxicol 2004;16:373–380. [DOI] [PubMed]
- 56.Kreyling WG, Semmler-Behnke M, Takenaka S, Moller W. Differences in the biokinetics of inhaled nano- versus micrometer-sized particles. Acc Chem Res 2012. [DOI] [PMC free article] [PubMed]
- 57.Geiser M, Rothen-Rutishauser B, Kapp N, Schürch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect 2005;113:1555–1560. [DOI] [PMC free article] [PubMed]
- 58.Muhlfeld C, Geiser M, Kapp N, Gehr P, Rothen-Rutishauser B. Re-evaluation of pulmonary titanium dioxide nanoparticle distribution using the “relative deposition index”: evidence for clearance through microvasculature. Part Fibre Toxicol 2007;4:7. [DOI] [PMC free article] [PubMed]
- 59.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002;105:411–414. [DOI] [PubMed]
- 60.Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, Tsuda A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol 2010;28:1300–1303. [DOI] [PMC free article] [PubMed]
- 61.Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 2005;106:3234–3241. [DOI] [PMC free article] [PubMed]
- 62.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54:S10–S19. [DOI] [PubMed]
- 63.Erzurum S, Rounds SI, Stevens T, Aldred M, Aliotta J, Archer SL, Asosingh K, et al. Strategic plan for lung vascular research: an NHLBI-ORDR workshop report. Am J Respir Crit Care Med 2010;182:1554–1562. [DOI] [PMC free article] [PubMed]
- 64.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann NY Acad Sci 1993;686:12–28. [DOI] [PubMed]
- 65.Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol 2008;59(suppl 6):19–34. [PubMed]
- 66.Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol 2009;9:375–383. [DOI] [PMC free article] [PubMed]
- 67.Taylor JD. COPD and the response of the lung to tobacco smoke exposure. Pulm Pharmacol Ther 2010;23:376–383. [DOI] [PubMed]
- 68.Schiess R, Senn O, Fischler M, Huber LC, Vatandaslar S, Speich R, Ulrich S. Tobacco smoke: a risk factor for pulmonary arterial hypertension? a case-control study. Chest 2010;138:1086–1092. [DOI] [PubMed]
- 69.Nadziejko C, Fang K, Bravo A, Gordon T. Susceptibility to pulmonary hypertension in inbred strains of mice exposed to cigarette smoke. J Appl Physiol 2007;102:1780–1785. [DOI] [PubMed]
- 70.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 2011;147:293–305. [DOI] [PubMed]
- 71.Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, Barberà JA. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J 2002;19:632–638. [DOI] [PubMed]
- 72.Weissmann N, Grimminger F, Seeger W. Smoking: is it a risk factor for pulmonary vascular diseases? Pulm Circ 2012;2:395–396. [DOI] [PMC free article] [PubMed]
- 73.Grau M, Barr RG, Lima JA, Hoffman EA, Bluemke DA, Carr JJ, Chahal, H, et al. Percent emphysema and right ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis-Lung and Multi-Ethnic Study of Atherosclerosis-Right Ventricle studies. Chest 2013;144:136–144. [DOI] [PMC free article] [PubMed]
- 74.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 2005;111:2684–2698. [DOI] [PubMed]
- 75.Curtis JL, Warnock ML, Arraj SM, Kaltreider HB. Histologic analysis of an immune response in the lung parenchyma of mice: angiopathy accompanies inflammatory cell influx. Am J Pathol 1990;137:689–699. [PMC free article] [PubMed]
- 76.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 2008;205:361–372. [DOI] [PMC free article] [PubMed]
- 77.Tormanen KR, Uller L, Persson CG, Erjefalt JS. Allergen exposure of mouse airways evokes remodeling of both bronchi and large pulmonary vessels. Am J Respir Crit Care Med 2005;171:19–25. [DOI] [PubMed]
- 78.Tigani B, Cannet C, Karmouty-Quintana H, Blé F-X, Zurbruegg S, Schaeublin E, Fozard JR, Beckmann N. Lung inflammation and vascular remodeling after repeated allergen challenge detected noninvasively by MRI. Am J Physiol Lung Cell Mol Physiol 2007;292:L644–L653. [DOI] [PubMed]
- 79.Rydell-Tormanen K, Uller L, Erjefalt JS. Allergic airway inflammation initiates long-term vascular remodeling of the pulmonary circulation. Int Arch Allergy Immunol 2009;149:251–258. [DOI] [PubMed]
- 80.Grunig G, Corry DB, Reibman J, Wills-Karp M. Interleukin 13 and the evolution of asthma therapy. Am J Clin Exp Immunol 2012;1:20–27. [PMC free article] [PubMed]
- 81.Cho W, Chen N, Tang C, Elias JA, Lee C. IL-13-induces vascular remodeling and pulmonary arterial hypertension via arginase 2-dependent pathway. Am J Respir Crit Care Med 2010;181(conf abstr):A6323.
- 82.Kawashima S, Hirose K, Takahashi K, Tamachi T, Ikeda K, Tokoyoda K, Nakayama T, Nakajima H. Interleukin-25 induces pulmonary arterial remodeling via natural killer T cell-dependent mechanisms. Int Arch Allergy Immunol 2013;161(suppl 2):118–124. [DOI] [PubMed]
- 83.Mushaben EM, Hershey GK, Pauciulo MW, Nichols WC, Le Cras TD. Chronic allergic inflammation causes vascular remodeling and pulmonary hypertension in BMPR2 hypomorph and wild-type mice. PLOS ONE 2012;7:e32468. [DOI] [PMC free article] [PubMed]
- 84.Park SH, Chen WC, Hoffman C, Marsh LM, West J, Grunig G. Modification of hemodynamic and immune responses to exposure with a weak antigen by the expression of a hypomorphic BMPR2 gene. PLOS ONE 2013;8:e55180. [DOI] [PMC free article] [PubMed]
- 85.Gilmour MI, McGee J, Duvall RM, Dailey L, Daniels M, Boykin E, Cho S-H, Doerfler D, Gordon T, Devlin RB. Comparative toxicity of size-fractionated airborne particulate matter obtained from different cities in the United States. Inhalation Toxicol 2007;19(suppl 1):7–16. [DOI] [PubMed]
- 86.Walters DM, Breysse PN, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med 2001;164:1438–1443. [DOI] [PubMed]
- 87.Padilla J, Daley E, Chow A, Robinson K, Parthasarathi K, McKenzie ANJ, Tschernig T, Kurup VP, Donaldson DD, Grunig G. IL-13 regulates the immune response to inhaled antigens. J Immunol 2005;174:8097–8105. [DOI] [PubMed]
- 88.Chen WC, Park SH, Hoffman C, Philip C, Robinson L, West J, Grunig G. Right ventricular systolic pressure measurements in combination with harvest of lung and immune tissue samples in mice. J Vis Exp 2013;71:e50023. doi:10.3791/50023. [DOI] [PMC free article] [PubMed]
- 89.Leary PJ, Barr RG, Bluemke DA, Hough CL, Kaufman JD, Szpiro AA, Kawut SM, Van Hee VC. The relationship of roadway proximity and NOx with right ventricular structure and function: the MESA-Right Ventricle and MESA-Air studies. Am J Respir Crit Care Med 2013;187(conf abstr):A3976.
- 90.Bloomfield GS, Lagat DK, Akwanalo OC, Carter EJ, Lugogo N, Vedanthan R, Velazquez EJ, Kimaiyo S, Sherman CB. Conditions that predispose to pulmonary hypertension and right heart failure in persons exposed to household air pollution in LMIC. Global Heart 2012;7:249–259. [DOI] [PMC free article] [PubMed]
- 91.Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Henríquez-Roldán C, Barragán-Mejía G, Garrido-García L, et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect 2007;115:1248–1253. [DOI] [PMC free article] [PubMed]
- 92.Thomson EM, Williams A, Yauk CL, Vincent R. Toxicogenomic analysis of susceptibility to inhaled urban particulate matter in mice with chronic lung inflammation. Part Fibre Toxicol 2009;6:6. [DOI] [PMC free article] [PubMed]
- 93.Courtois A, Andujar P, Ladeiro Y, Baudrimont I, Delannoy E, Leblais V, Begueret H, et al. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: comparison of urban particulate matter and manufactured nanoparticles. Environ Health Perspect 2008;116:1294–1299. [DOI] [PMC free article] [PubMed]
- 94.Batalha JR, Saldiva PH, Clarke RW, Coull BA, Stearns RC, Lawrence J, Krishna Murthy GG, Koutrakis P, Godleski JJ. Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ Health Perspect 2002;110:1191–1197. [DOI] [PMC free article] [PubMed]
- 95.Rubin LJ. Endothelin receptor antagonists for the treatment of pulmonary artery hypertension. Life Sci 2012;91:517–521. [DOI] [PubMed]
- 96.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, Sitbon O, Tapson VF, Galiè N. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54(suppl):S78–S84. [DOI] [PMC free article] [PubMed]
- 97.Makellar A. An investigation into the nature of black phthisis; or ulceration induced by carbonaceous accumulation in lungs of coal miners and other operatives. Scottish Mining Website. http://www.scottishmining.co.uk/500.html. Originally published in 1846.
- 98.Samuelson S. Chronic cor pulmonale in silicosis. Acta Med Scand Suppl 1952;266:875–885. [PubMed]
- 99.Rosenman KD, Zhu Z. Pneumoconiosis and associated medical conditions. Am J Ind Med 1995;27:107–113. [DOI] [PubMed]
- 100.Murray J, Reid G, Kielkowski D, de Beer M. Cor pulmonale and silicosis: a necropsy based case-control study. Br J Ind Med 1993;50:544–548. [DOI] [PMC free article] [PubMed]
- 101.Herget J, Kuncova M, Havrankova J, Palecek F. Pulmonary hypertension in silicotic rats. Arch Environ Health 1979;34:320–324. [DOI] [PubMed]
- 102.Leung CC, Yu IT, Chen W. Silicosis. Lancet 2012;379:2008–2018. [DOI] [PubMed]
- 103.World Health Organization. Silicosis. Fact sheet 238. http://www.who.int/inf-fs/en/fact238.html. Published May 2000.
- 104.Lioy PJ, Weisel CP, Millette JR, Eisenreich S, Vallero D, Offenberg J, Buckley B, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect 2002;110:703–714. [DOI] [PMC free article] [PubMed]
- 105.Yiin LM, Millette JR, Vette A, Ilacqua V, Quan C, Gorczynski J, Kendall M, et al. Comparisons of the dust/smoke particulate that settled inside the surrounding buildings and outside on the streets of southern New York City after the collapse of the World Trade Center, September 11, 2001. J Air Waste Manag Assoc 2004;54:515–528. [DOI] [PubMed]
- 106.Caplan-Shaw CE, Yee H, Rogers L, Abraham JL, Parsia SS, Naidich DP, Borczuk A, et al. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J Occup Environ Med 2011;53:981–991. [DOI] [PubMed]
- 107.Beaudry C, Lavoué J, Sauvé JF, Bégin D, Senhaji Rhazi M, Perrault G, Dion C, Gérin M. Occupational exposure to silica in construction workers: a literature-based exposure database. J Occup Environ Hyg 2013;10:71–77. [DOI] [PubMed]
- 108.Cook AG, Weinstein P, Centeno JA. Health effects of natural dust: role of trace elements and compounds. Biol Trace Elem Res 2005;103:1–15. [DOI] [PubMed]
- 109.Chalupka S. Occupational silica exposure in hydraulic fracturing. Workplace Health Safe 2012;60:460. [DOI] [PubMed]