Abstract Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by remodeling and vasoconstriction of the pulmonary vasculature, ultimately leading to right ventricular (RV) failure and death. Recent developments in echocardiography, cardiovascular magnetic resonance imaging, computed tomography, and positron emission tomography allow advanced, noninvasive, in vivo assessment of the RV and have contributed to the identification of risk factors, prognostic factors, and monitoring of therapeutic responses in patients with PAH. Although far from reaching its future potential, these techniques have not only provided global RV assessment but also allowed evaluation of changes in cellular and molecular tissue processes, such as metabolism, oxygen balance and ischemia, angiogenesis, and apoptosis. Integrated application of these techniques could provide full insights into the different pathophysiological aspects of a failing RV in the setting of PAH. Recent advances in hybrid imaging have implemented simultaneous measurements of myocardial and vascular interactions and will be one of the most important potential future developments.
Keywords: pulmonary arterial hypertension, right ventricle, cardiovascular magnetic resonance imaging, echocardiography, positron emission tomography
Introduction
Historically, the role of the right ventricle (RV) was considered of minor relevance in the maintenance of adequate blood flow and therefore has been largely understudied.1 Currently, the RV is receiving increased attention, because the prognostic significance of RV dysfunction is now recognized in pulmonary hypertension (PH), left ventricular (LV) failure, acute respiratory distress syndrome, and sepsis.2 In pulmonary arterial hypertension (PAH), a progressive disease characterized by pulmonary vascular remodeling leading to chronic pressure overload, RV failure is often the cause of death.3,4 However, RV dysfunction is not simply determined by a chronically elevated afterload.5-7 It has been recognized that patients with PAH differ in their tendency to develop RV failure, with some patients developing early RV failure and others showing an adapted RV that tolerates the same hemodynamics on a long-term basis.8-10 The underlying mechanisms behind the transition from RV adaptation to decompensation are only partially understood. Remarkably, a patient with RV failure can completely recover after lung transplantation.11 Insights into the pathophysiology of RV failure and its relation to the pulmonary circulation are relevant for diagnosis, risk stratification, and management of patients with PAH.
Over the previous decades, advanced, noninvasive imaging techniques have emerged. Recent developments in echocardiography, cardiovascular magnetic resonance imaging (CMR), computed tomography (CT), and positron emission tomography (PET) not only allow for global RV assessment but can also detect changes on a regional and molecular level. These techniques may help considerably to better understand the contributing pathophysiological factors in the development of RV failure. Here, we provide an overview of advanced, noninvasive assessment of the RV, review new imaging modalities, and assess the potential value of combining imaging techniques to improve our insights into the mechanisms of RV adaptation and failure in PAH.
Morphological and functional imaging
RV hypertrophy and dilatation
CMR has become the gold standard for quantification of RV mass, volumes, and function and provides high-resolution imaging without requirement of geometric assumptions and ionizing radiation.12 Integrated assessment of multiple CMR parameters may provide insights into RV remodeling processes. Using combined measurements of RV pressures and CMR analyses of RV mass and volumes, ventricular wall stress can be calculated according to the law of Laplace (i.e., wall stress = intraluminal pressure times chamber internal radius, divided by wall thickness).13 Elevated pressures result in increased ventricular wall stress, which is detrimental for the RV in the long run.14,15 RV hypertrophy is initially considered a favorable remodeling mechanism by reducing wall stress and improving pumping effectiveness. In PAH, RV mass, wall thickness, and the ventricular mass index (i.e., ratio of RV mass to LV mass) measured by CMR or multidetector computed tomography (MDCT) were increased and moderately related to pulmonary pressures.16-19 However, in PAH, RV mass is, on average, 2.5-fold increased, which might be insufficient to compensate for an often fourfold increase in pulmonary pressure. In line with these findings, Simon et al.20 demonstrated regional heterogeneity in RV structural remodeling, and it was shown that, although RV wall thickness increases during the course of disease, it remained insufficient to normalize the RV wall stress. These results might explain the limited value of RV mass for predicting mortality in patients with idiopathic PAH.21 Moreover, in patients with scleroderma and PAH, elevated RV mass was associated with increased mortality.17 An increase in RV mass is therefore not only a favorable physiologic adaptation mechanism and may be associated with maladaptive changes.
Although RV dilatation may aid in sustaining cardiac output via the Frank-Starling mechanism, at the same time, it contributes to elevated wall stress. An increased RV end-diastolic volume and progressive dilatation during follow-up assessed by CMR were among the strongest predictors of mortality in PAH.21,22
Although readily available in clinical practice, volumetric assessment can be less reliably performed by two-dimensional echocardiography because of the difficulty in visualizing the RV anterior wall and infundibulum. This explains the variable correlations between volumetric echocardiographic and CMR measures.2,23,24 Three-dimensional echocardiography is a promising technique and showed better agreement with CMR compared with a two-dimensional assessment; nevertheless, it resulted in underestimations of RV volumes and function, particularly in a dilated RV.25 Future advances can be expected to allow a better image acquisition of wide-angle windows to obtain data from the entire RV with higher temporal and spatial resolution.
Global RV function
Stroke volume and RV ejection fraction measured by CMR are the most commonly used parameters to evaluate global systolic RV function2 and contain therapeutic and prognostic information in PAH.6,21,22,26 Only a few studies have assessed RV diastolic dysfunction, and few data exist on their accuracy.27,28 Although these functional parameters are dependent on preload and afterload and therefore do not fully characterize intrinsic RV function,15,29 load-independent measures can provide new insights on intrinsic cardiac properties or direct cardiac treatment effects. RV end-systolic elastance (Ees) is accepted as a load-independent measure of intrinsic myocardial contractility. Although the classical determination of Ees requires multiple pressure-volume loops and dangerous, invasive interventions, a single-beat method has been validated that enables estimation of Ees using a simpler approach that can be obtained by right heart catheterization and assessment of a RV pressure-volume relationship during a single heartbeat, without invasive mediations. Using this method, arterial elastance (Ea) as a measure of RV afterload can also be determined.30-32 The ratio of Ees to Ea represents efficiency of the ventriculo-arterial coupling. In addition, CMR approaches have been developed to estimate Ees noninvasively.33,34 Paradoxically, it has been found that RV contractility is increased in patients with PAH, whereas global measures of RV systolic function, such as RV ejection fraction and stroke volume, are decreased.35 These findings might be explained by the fact that, although the RV compensates for the increased afterload by hypercontractility, this was insufficient, leading to ventriculo-arterial uncoupling. Future studies are required to validate and evaluate the clinical relevance of contractility measures. Recently, Rain and coworkers35 have applied and validated the single-beat method to determine load-independent RV diastolic stiffness, which was found to be significantly increased in PAH and was related to disease severity.
Regional RV function and strain
The normal RV consists of transverse-oriented superficial muscle fibers and more longitudinally aligned deep muscle fibers, resulting in a primarily bellows-like or peristaltic contraction pattern.36-39 RV longitudinal shortening (tricuspid annular plane excursion [TAPSE]) and transverse shortening provide clinical relevant measures that are correlated with global RV function.22,40,41 Although the determination of global measures of RV function is relative time consuming, these measures are simpler to obtain. Mauritz et al.22 showed that both transverse and longitudinal shortening were valuable parameters and that, in particular, a progressive decrease in RV transverse wall motion is associated with end-stage RV disease.
Regional RV function, as reflected by local myocardial deformation, can be estimated by segmental strain (relative amount of deformation) and strain rate (velocity of deformation) using echocardiographic tissue Doppler velocity imaging (TDI) or two-dimensional speckle tracking. TDI measures do not rely on geometric assumptions but are one-dimensional and angle dependent.42 Speckle tracking is a more recently developed method that can reliably determine myocardial deformation. However, it has limited reproducibility and loss of speckles due to motion outside the imaging plane.43 According to Hooke’s law (i.e., forces and deformation are linked to elasticity44), myocardial deformation is mainly influenced by intrinsic contractility (active forces), cavity pressures, geometry and neighboring segment interaction (passive forces or wall stress), and tissue elasticity (fiber structure and fibrosis), of which contractility is the most important.45 In healthy animals, Jamal et al.46 showed by TDI that longitudinal strain and strain rate provided complementary information in which strain is a reflection of stroke volume and strain rate is a reflection of load-independent contractility. Myocardial acceleration during isovolumic contraction is another measure of load-independent RV function in healthy animals, but validation in PAH is required.47 In patients with PAH, a reduced longitudinal strain has been demonstrated, with the most severe impairments in patients with RV failure.48-54 Regional heterogeneity was an important feature of a dysfunctional RV in which the apical region is the most affected.53 Furthermore, RV longitudinal strain and strain rate are independent predictors of survival.48,55 However, longitudinal strain describes only one aspect of myocardial deformation and does not allow estimation in the complex three-dimensional RV contraction pattern. Circumferential and radial strain are more difficult to study by two-dimensional speckle tracking and TDI, respectively. Recent three-dimensional speckle tracking methods have been developed that allow a more complete and accurate assessment of myocardial deformation in the LV.56 Studies have not yet been performed in the RV. Intramyocardial motion can also be determined by CMR techniques in which the myocardium is initially “tagged” by a line or grid pattern at end-diastole that deforms during contraction.57 The deformation of these “tagging” lines can be quantified. CMR tagging techniques are currently considered the reference for RV strain analysis and allow assessment in multiple directions, but their clinical application is limited because of complex and time-consuming analyses. It has been found in patients with PH that both longitudinal and circumferential shortening were reduced to a similar level.58 Moreover, it was recently illustrated that, although global RV function was intact, regional longitudinal wall deformation was already impaired, implying that this could be a sensitive measure to detect early RV dysfunction in PAH.59 In a systemic RV, circumferential exceeds longitudinal wall deformation, which is comparable to the normal LV.60
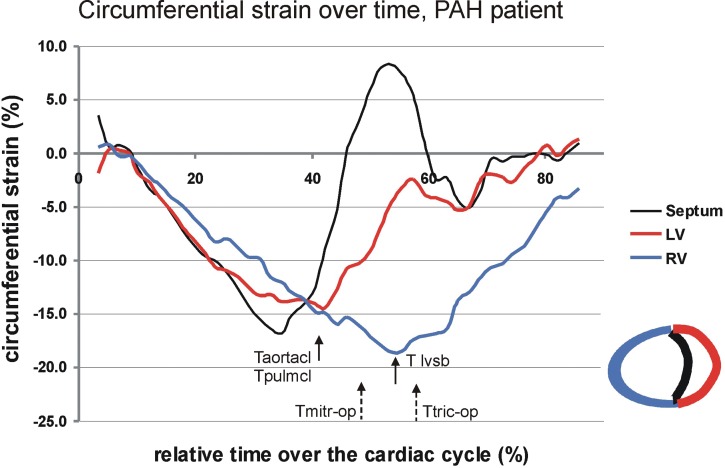
In healthy hearts, a significant proportion of RV output is attributable to the interventricular septum (IVS)61,62 and LV contraction.63-65 Therefore, both intra- and interventricular dyssynchrony are important aspects of RV function. Echocardiography demonstrated a longer delay in the time to peak strain between the IVS and RV lateral wall and a delay in time to peak strain between the LV and RV lateral walls in patients with PAH compared with controls.66,67 The delay in LV-RV peak shortening is the result of a prolonged RV contraction time that even continues after pulmonary valve closure and is related to leftward IVS bulging, LV underfilling, and a lowered stroke volume (Fig. 1).68,69
Figure 1.
The presence of ventricular dyssynchrony illustrated in a patient with pulmonary arterial hypertension (PAH) measured by cardiac magnetic resonance imaging (CMR) strain analysis. Circumferential strain curves reflect myocardial shortening (negative strain) and stretching (positive stain) over time of the cardiac cycle for the right ventricular (RV) free wall (blue), left ventricular (LV) free wall (red), and interventricular septum (black). The LV, RV, and septum start simultaneously with shortening. However, RV peak shortening occurs later than LV peak shortening in the cardiac cycle. The aortic and pulmonary valves close (Taortacl and Tpulmcl) at the time of LV peak shortening. The time of maximal leftward septal bowing (Tlvsb) occurs coincidently with septal stretching and with peak shortening of the RV. Tmitr-op and Ttric-op indicate the opening times of the mitral and tricuspid valves and indicate the onset of LV and RV filling.
Metabolic imaging
In patients with a hypertrophic or dilated LV cardiomyopathy, PET imaging has shown that the predominant metabolic substrate of the LV has switched from fatty acids to glucose metabolism, thereby preserving the adenosine triphosphate supply for force generation.70-72 In line with these findings, single-photon emission tomography (SPECT) imaging has demonstrated that, in the hypertrophied RV of patients with PH, myocardial fatty acid uptake was decreased.73 Although it has been shown that measuring myocardial glucose metabolism using PET with 18F-2-deoxy-2-fluoro-D-glucose (18FDG) tracers is feasible in patients with PAH,74 inconsistent results have been reported. Some studies have shown that the ratio of RV glucose uptake to LV uptake is increased in PAH and is associated with disease severity.75-77 However, it remains uncertain whether the increased ratio is due to increased RV glucose uptake78-80 or decreased LV glucose uptake.77 In addition, other studies have shown that the changes in the ratio of RV to LV glucose uptake did not correlate with disease severity.81 These conflicting results may be caused by differences in patient populations, scanning protocols, and data analysis. Other limitations could be that 18FDG uptake is not a direct reflection of glycolysis, because 18FDG uptake is also influenced by other factors, reflects the uptake of the overall myocardial tissue instead of the cellular uptake, and cannot appropriately be metabolized through all the glycolysis steps because of its chemical structure.82 At this moment, clinical PET studies cannot provide firm answers about an eventual “metabolic switch” in the failing RV of patients with PAH, whereas this metabolic switch has been previously demonstrated in animal models.83-85
Perfusion imaging and oxygen balance
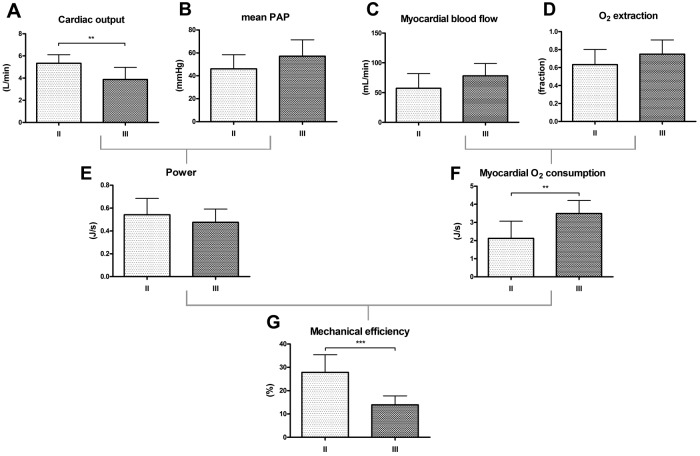
Using PET with 11C-acetate or a combination of H215O−, 15O2−, C15O− tracers, myocardial oxygen (O2) consumption can be accurately measured. In PAH, it has been shown by PET imaging that the myocardial O2 demand was increased, which was primarily determined by elevated pulmonary pressures and heart rate.81,86 An increased myocardial O2 demand can be balanced by 2 mechanisms, (1) coronary flow reserve and (2) myocardial O2 extraction fraction (OEF) reserve. Although there are no normal human resting values of OEF, the resting value of OEF in dogs is 45%–50%.87 This implies a significant OEF reserve in the normal RV and resembles the primary source for an augmented O2 demand during exercise.88 In patients with PAH, the resting OEF is significantly increased ( ˜70%),81 which infers that the OEF reserve in PAH is already limited at rest, and it has been suggested that the RV becomes more dependent on its coronary flow (like the LV).89 However, despite a higher O2 demand, RV myocardial blood flow remained unchanged. In addition, the elevated O2 demand was related to impaired mechanical efficiency, which is a characteristic for a deteriorating RV (Fig. 2).81 Similarly, it has been shown by CMR that right coronary blood flow is impaired in patients with PAH and was related to the amount of RV hypertrophy.90 In addition, it has been demonstrated by PET that exercise-induced RV O2 supply is limited.91 In line with these findings, adenosine stress perfusion CMR in patients with PAH revealed that the perfusion reserve is restricted, not only in the RV, but also in the LV, which suggests that a systemic component plays a role in the development of RV dysfunction (Fig. 3).92 These results imply that the increased RV O2 demand in patients with PAH cannot be adequately balanced by an increase in O2 supply. The possible consequences of ischemia have been illustrated by Gomez et al.,93 who demonstrated by stress-induced SPECT RV wall ischemia at specific IVS insertion points that delayed contrast enhancement (DCE) was related to RV dysfunction. It has been repeatedly observed, using gadolinium contrast-enhanced CMR, that DCE appears as a unique pattern at the same IVS insertion points and is related to RV remodeling and dysfunction.94-97 McCann et al.96 showed that focal fibrosis could be the cause of DCE.
Figure 2.
Impaired right ventricular (RV) mechanical efficiency in patients with idiopathic pulmonary arterial hypertension (IPAH) is primarily determined by increased myocardial oxygen consumption (MVO2). Dark bars = New York Heart Association (NYHA) functional class III patients; light gray bars = NYHA functional class II patients. Cardiac output (CO) measured by cardiovascular magnetic resonance imaging (CMR) is higher in NYHA II patients than NYHA III patients (A), and mean pulmonary artery pressure (PAP) was similar in both groups (B). RV myocardial blood flow measured by positron emission tomography (PET) with H215O tracers (C) and oxygen (O2) extraction fraction estimated by PET using 15O2 tracers (D) were not statistically higher in NYHA III patients compared with NYHA II patients. RV power output (i.e., product of CO and mean PAP) was comparable in both groups (E). There was a significantly higher MVO2 per gram of myocardial tissue in NYHA III patients compared with NYHA II patients (F). A similar RV power output but higher MVO2 led to a significant reduction in RV mechanical efficiency (i.e., ratio of RV power output and MVO2) of ˜50% in NYHA III patients compared with NYHA II patients (G). Reprinted with permission from Wong et al.81
Figure 3.
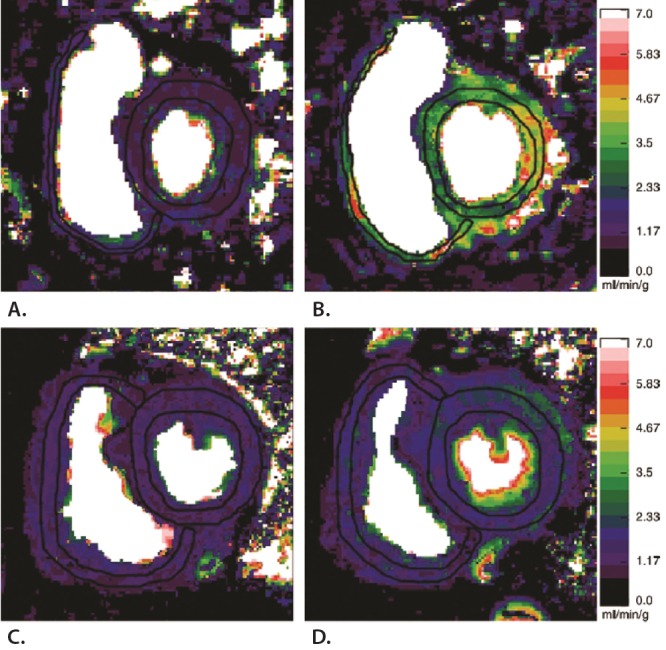
Representative perfusion maps showing that biventricular perfusion reserve was diminished in patients with pulmonary arterial hypertension (PAH) compared with controls. Cardiovascular magnetic resonance imaging perfusion maps of the right ventricle (RV) and left ventricle (LV) were obtained under resting conditions and after adenosine induced stress. Perfusion scales are on the right. Myocardial perfusion at rest in a healthy control subject (A) is significantly increased during adenosine stress (B). C, Resting myocardial perfusion in the hypertrophic RV of a patient with PAH. D, After adenosine stress, myocardial perfusion does not increase in both the LV and RV, illustrating that the perfusion reserve is limited in patients with PAH compared with controls. Reprinted with permission from Vogel-Claussen et al.92
Angiogenic imaging
Preclinical studies in PAH animal models have shown that RV ischemia and fibrosis may result from insufficient angiogenesis in the setting of rapid myocardial hypertrophy.83,98 Perfusion and metabolic imaging provide indirect evaluation of angiogenic processes. Novel imaging strategies have been developed that can directly detect angiogenic molecular events. Vascular endothelial growth factor (VEGF) is the major contributor to angiogenesis, and adhesion molecules, such as integrins, are other important angiogenic modulators. In animal models of PH, capillary rarefaction and reduced VEGF expression have been demonstrated both in the RV and in the lungs.98-101 Although not yet quantified in the lungs, PET imaging with 64CU-labeled VEGF121 or 18F arginine-glycine-aspartic acid peptide (RGD) with affinity for the 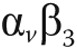 integrins have enabled measurements of angiogenic myocardial repair processes in rat models with myocardial infarction.102-104 Other tissue processes, such as apoptosis, can be localized and quantified using in vivo CMR annexin imaging.105 These imaging techniques might be important to study pathophysiological pathways of myocardial disease processes in vivo and can provide longitudinal monitoring to detect therapeutic responses.
integrins have enabled measurements of angiogenic myocardial repair processes in rat models with myocardial infarction.102-104 Other tissue processes, such as apoptosis, can be localized and quantified using in vivo CMR annexin imaging.105 These imaging techniques might be important to study pathophysiological pathways of myocardial disease processes in vivo and can provide longitudinal monitoring to detect therapeutic responses.
Hybrid imaging
Hybrid imaging provides optimal in vivo evaluation of RV hemodynamic consequences by integration of anatomical information with physiological and molecular imaging in the same setting. It allows molecular imaging per anatomic region or per gram of myocardial tissue. PET/CT has become commonplace in clinical practice and research settings and has significantly contributed to our insights in pathophysiological processes in cardiovascular disease.106 Hybrid PET/magnetic resonance imaging (MRI) is an innovative, fast-upcoming technique and not only allows simultaneous combination of PET with anatomic imaging but also provides functional imaging, perfusion, tissue characterization, and flow imaging, thereby improving clinical application and stratification of patients with RV failure. Figure 4 demonstrates CT/PET 18F-Galakto-RGD imaging of integrin expression in a patient 2 weeks after myocardial infarction.107 Although measured sequentially, CMR delayed enhancement showed the exact anatomical localization of tracer retention in the infarcted area. In addition, the first case reports and small patient series using cardiac hybrid PET/MRI imaging have been published.108-110 However, one limitation of hybrid systems with MRI is that it does not provide information required for attenuation correction of nuclear images. Although it remains particularly challenging to measure density variations in the lungs, this has become an active area of research.111,112
Figure 4.
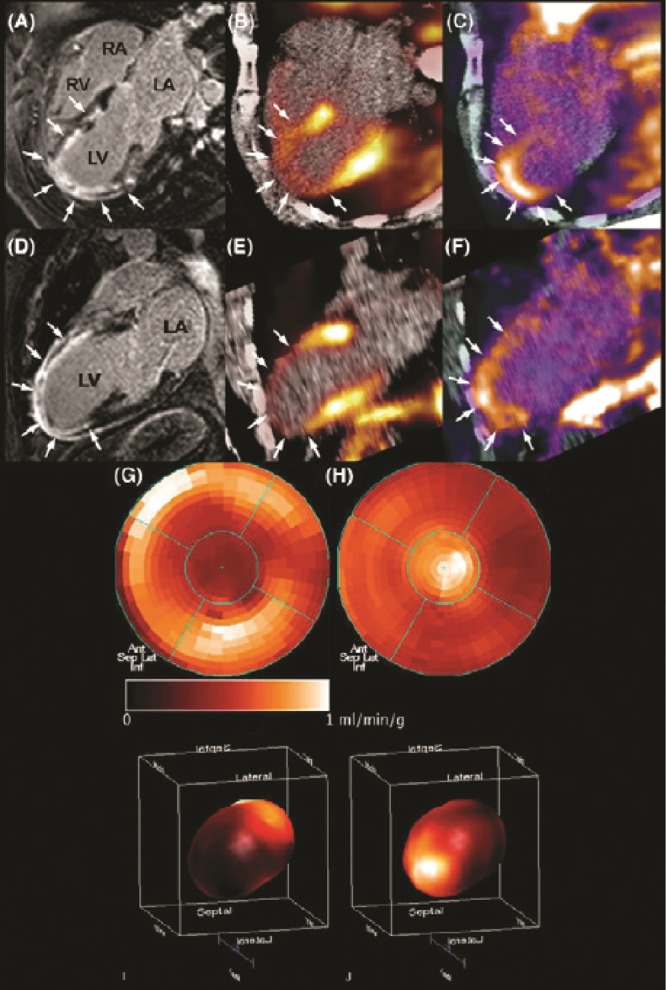
In vivo molecular imaging of angiogenesis in a patient after acute myocardial infarction. Two weeks after acute myocardial infarction and percutaneous coronary intervention, a patient underwent cardiovascular magnetic resonance imaging (CMR) and positron emission tomography (PET)/computed tomography (CT). CMR gadolinium delayed contrast enhancement (DCE) was observed almost transmural in the anterior, anteriorseptal, anteriorlateral, and apical left ventricle wall (A, D). PET imaging with 13N-ammonia revealed impaired myocardial blood flow in correspondence with the regions of DCE (arrows; B, E). PET with the agent 18F arginine-glycine-aspartic acid peptide (18F-RGD), with affinity for the 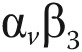 integrins, demonstrated focal signal areas in the infarcted area (C, F). This signal may reflect angiogenesis within the healing area (arrows). Polar maps of myocardial blood flow assessed by PET with 13N-ammonia (G, I) show severely reduced blood flow in the distal left anterior descending coronary artery perfusion region. Co-localized 18F-RGD signal corresponded to the regions of reduced blood flow (H, J), demonstrating the extent of the
integrins, demonstrated focal signal areas in the infarcted area (C, F). This signal may reflect angiogenesis within the healing area (arrows). Polar maps of myocardial blood flow assessed by PET with 13N-ammonia (G, I) show severely reduced blood flow in the distal left anterior descending coronary artery perfusion region. Co-localized 18F-RGD signal corresponded to the regions of reduced blood flow (H, J), demonstrating the extent of the 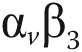 integrin expression in the infarcted area. Reprinted with permission from Makowski et al.107
integrin expression in the infarcted area. Reprinted with permission from Makowski et al.107
Imaging in clinical practice
RV imaging parameters might provide important information for the diagnosis and risk stratification of patients with PAH and could also be helpful in therapeutic decision making. Current PAH targeted medical therapies (i.e., prostacyclins, endothelin receptor antagonists, and phosphodiesterase 5 inhibitors) can affect the RV directly or indirectly through lowering of load. Although CT is considered part of the diagnostic work-up of patients with PH, the clinical relevance of RV assessment by CT has not been demonstrated.113 Echocardiography is readily available in clinical practice and can be helpful in the detection of the cause of PH. Measures of pericardial effusion and TAPSE are considered of prognostic relevance and as treatment parameters during follow-up.113,114 Although serial assessment of echocardiographic RV longitudinal strain and strain rate could provide therapeutic information,115,116 the clinical role of these newer echocardiographic modalities has to be determined. At present, the clinical value of CMR has been clearly established,12 and clinical decision making increasingly relies on measurements of RV volumes and function and their trends during follow-up. It has been suggested that considering RV imaging parameters as end points in clinical trials could be of clinical importance,117 but only a few studies have evaluated cardiac parameters as therapeutic outcomes, and most studies included small patient populations. Recently, the EURO-MR study has shown that changes in global RV function measures after medical treatment reflect changes in functional class and survival in patients with PAH.118 RV mass has been shown to be a valuable end point in clinical trials, but a clinically relevant change has not been determined.119,120 Stroke volume obtained by CMR is the only imaging parameter that has been validated to monitor therapeutic effects, and a clinically relevant change was found to be greater than 10 mL.26 The role of PET imaging in PH has been primarily restricted to investigational imaging, and its clinical applicability is currently limited. However, PET techniques allow assessment of pathophysiological pathways of myocardial disease processes in vivo and therefore have great potential to monitor therapeutic responses longitudinally in the near future. One study has demonstrated that PET RV 18FDG uptake might be of clinical relevance after epoprostenol therapy.80 Future studies are required to validate RV imaging parameters to assess therapeutic effects.
Summary
The RV determines symptoms and survival in patients with PAH. Therefore, insights in the pathophysiology of RV failure and the identification of risk factors, prognostic factors, and indicators to measure treatment response on a regular basis associated with the RV are required, and it is preferred that these be determined noninvasively. Advanced imaging techniques have evolved that provide noninvasive, in vivo information about the RV in the setting of chronic pressure overload. These techniques provide complementary RV information: CT provides morphological imaging, CMR provides global volumetric and functional analyses, echocardiography provides the assessment of regional RV function, and PET provides perfusion and molecular imaging. Therefore, integrated application of these techniques could provide full insights into the different pathophysiological aspects of a failing RV in patients with PAH. Recent developments in hybrid imaging will implement simultaneous measurements of myocardial and vascular interactions and will be one of the most important potential future developments.
Source of Support: This work was funded by a VIDI grant from the Netherlands Organization for Scientific Research (329 917.96.306).
Conflict of Interest: None declared.
References
- 1.Starr I, Jeffers WA, Meade RH. The absence of conspicuous increments of venous pressure after severe damage to the RV of the dog, with discussion of the relation between clinical congestive heart failure and heart disease. Am Heart J 1943;26:291–301.
- 2.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008;117(13):1717–1731. [DOI] [PubMed]
- 3.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 2006;114(13):1417–1431. [DOI] [PubMed]
- 4.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006;114(17):1883–1891. [DOI] [PubMed]
- 5.Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging 2012;5(4):378–387. [DOI] [PubMed]
- 6.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011;58(24):2511–2519. [DOI] [PubMed]
- 7.Bristow MR, Zisman LS, Lowes BD, Abraham WT, Badesch DB, Groves BM, et al. The pressure-overloaded right ventricle in pulmonary hypertension. Chest 1998;114(1 suppl):101S–106S. [DOI] [PubMed]
- 8.Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M, et al. Survival in primary pulmonary hypertension: validation of a prognostic equation. Circulation 1994;89(4):1733–1744. [DOI] [PubMed]
- 9.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115(5):343–349. [DOI] [PubMed]
- 10.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 1984;70(4):580–587. [DOI] [PubMed]
- 11.Pasque MK, Trulock EP, Cooper JD, Triantafillou AN, Huddleston CB, Rosenbloom M, et al. Single lung transplantation for pulmonary hypertension: single institution experience in 34 patients. Circulation 1995;92(8):2252–2258. [DOI] [PubMed]
- 12.Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Eur Heart J 2004;25(21):1940–1965. [DOI] [PubMed]
- 13.Mauritz GJ, Vonk-Noordegraaf A, Kind T, Surie S, Kloek JJ, Bresser P, et al. Pulmonary endarterectomy normalizes interventricular dyssynchrony and right ventricular systolic wall stress. J Cardiovasc Magn Reson 2012;14:5. [DOI] [PMC free article] [PubMed]
- 14.Handoko ML, de Man FS, Allaart CP, Paulus WJ, Westerhof N, Vonk-Noordegraaf A. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: lessons from the left heart. Eur Respir Rev 2010;19(115):72–82. [DOI] [PMC free article] [PubMed]
- 15.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135(3):794–804. [DOI] [PubMed]
- 16.Saba TS, Foster J, Cockburn M, Cowan M, Peacock AJ. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J 2002;20(6):1519–1524. [DOI] [PubMed]
- 17.Hagger D, Condliffe R, Woodhouse N, Elliot CA, Armstrong IJ, Davies C, et al. Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford) 2009;48(9):1137–1142. [DOI] [PubMed]
- 18.Chan AL, Juarez MM, Shelton DK, MacDonald T, Li CS, Lin TC, et al. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging 2011;11:7. [DOI] [PMC free article] [PubMed]
- 19.Roeleveld RJ, Marcus JT, Boonstra A, Postmus PE, Marques KM, Bronzwaer JG, et al. A comparison of noninvasive MRI-based methods of estimating pulmonary artery pressure in pulmonary hypertension. J Magn Reson Imaging 2005;22(1):67–72. [DOI] [PubMed]
- 20.Simon MA, Deible C, Mathier MA, Lacomis J, Goitein O, Shroff SG, et al. Phenotyping the right ventricle in patients with pulmonary hypertension. Clin Transl Sci 2009;2(4):294–299. [DOI] [PMC free article] [PubMed]
- 21.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007;28(10):1250–1257. [DOI] [PubMed]
- 22.Mauritz GJ, Kind T, Marcus JT, Bogaard HJ, van de Veerdonk M, Postmus PE, et al. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 2012;141(4):935–943. [DOI] [PubMed]
- 23.Morikawa T, Murata M, Okuda S, Tsuruta H, Iwanaga S, Murata M, et al. Quantitative analysis of right ventricular function in patients with pulmonary hypertension using three-dimensional echocardiography and a two-dimensional summation method compared to magnetic resonance imaging. Am J Cardiol 2011;107(3):484–489. [DOI] [PubMed]
- 24.Kjaergaard J, Petersen CL, Kjaer A, Schaadt BK, Oh JK, Hassager C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr 2006;7(6):430–438. [DOI] [PubMed]
- 25.Shimada YJ, Shiota M, Siegel RJ, Shiota T. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr 2010;23(9):943–953. [DOI] [PubMed]
- 26.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, Jacobs W, Marcus JT, Marques KM, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest 2011;139(5):1003–1009. [DOI] [PubMed]
- 27.Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 2010;11(2):81–96. [DOI] [PubMed]
- 28.Gan CT, Holverda S, Marcus JT, Paulus WJ, Marques KM, Bronzwaer JG, et al. Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest 2007;132(1):11–17. [DOI] [PubMed]
- 29.Guihaire J, Haddad F, Boulate D, Decante B, Denault AY, Wu J, et al. Non-invasive indices of right ventricular function are markers of ventricular-arterial coupling rather than ventricular contractility: insights from a porcine model of chronic pressure overload. Eur Heart J Cardiovasc Imaging 2013;14(12):1140–1149. [DOI] [PubMed]
- 30.Trip P, Kind T, van de Veerdonk MC, Marcus JT, de Man FS, Westerhof N, et al. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant 2013;32(1):50–55. [DOI] [PubMed]
- 31.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, et al. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol 2003;284(5):H1625–H1630. [DOI] [PubMed]
- 32.Sunagawa K, Yamada A, Senda Y, Kikuchi Y, Nakamura M, Shibahara T, et al. Estimation of the hydromotive source pressure from ejecting beats of the left ventricle. IEEE Trans Biomed Eng 1980;27(6):299–305. [DOI] [PubMed]
- 33.Sanz J, Garcia-Alvarez A, Fernandez-Friera L, Nair A, Mirelis JG, Sawit ST, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 2012;98(3):238–243. [DOI] [PubMed]
- 34.Kuehne T, Yilmaz S, Steendijk P, Moore P, Groenink M, Saaed M, et al. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation 2004;110(14):2010–2016. [DOI] [PubMed]
- 35.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013;128(18):2016–2025. [DOI] [PubMed]
- 36.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008;117(11):1436–1448. [DOI] [PubMed]
- 37.Petitjean C, Rougon N, Cluzel P. Assessment of myocardial function: a review of quantification methods and results using tagged MRI. J Cardiovasc Magn Reson 2005;7(2):501–516. [DOI] [PubMed]
- 38.Dell’Italia LJ. The right ventricle: anatomy, physiology, and clinical importance. Curr Prob Cardiol 1991;16(10):653–720. [DOI] [PubMed]
- 39.Armour JA, Randall WC. Structural basis for cardiac function. Am J Physiol 1970;218(6):1517–1523. [DOI] [PubMed]
- 40.Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson 2010;12:35. [DOI] [PMC free article] [PubMed]
- 41.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Resp Crit Care Med 2006;174(9):1034–1041. [DOI] [PubMed]
- 42.Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, et al. Two-dimensional strain—a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr 2005;18(12):1247–1253. [DOI] [PubMed]
- 43.Bansal M, Cho GY, Chan J, Leano R, Haluska BA, Marwick TH. Feasibility and accuracy of different techniques of two-dimensional speckle based strain and validation with harmonic phase magnetic resonance imaging. J Am Soc Echocardiogr 2008;21(12):1318–1325. [DOI] [PubMed]
- 44.Westerhof N, Stergiopulos N, Noble M. Snapshots of hemodynamics: an aid for clinical research and graduate education. Berlin: Springer, 2010.
- 45.Bijnens BH, Cikes M, Claus P, Sutherland GR. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur J Echocardiogr 2009;10(2):216–226. [DOI] [PubMed]
- 46.Jamal F, Bergerot C, Argaud L, Loufouat J, Ovize M. Longitudinal strain quantitates regional right ventricular contractile function. Am J Physiol 2003;285(6):H2842–H2847. [DOI] [PubMed]
- 47.Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, et al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation 2002;105(14):1693–1699. [DOI] [PubMed]
- 48.Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, et al. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging 2012;5(5):628–636. [DOI] [PubMed]
- 49.Fukuda Y, Tanaka H, Sugiyama D, Ryo K, Onishi T, Fukuya H, et al. Utility of right ventricular free wall speckle-tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. J Am Soc Echocardiogr 2011;24(10):1101–1108. [DOI] [PubMed]
- 50.Puwanant S, Park M, Popovic ZB, Tang WH, Farha S, George D, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 2010;121(2):259–266. [DOI] [PMC free article] [PubMed]
- 51.Giusca S, Dambrauskaite V, Scheurwegs C, D’Hooge J, Claus P, Herbots L, et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart 2010;96(4):281–288. [DOI] [PubMed]
- 52.Simon MA, Rajagopalan N, Mathier MA, Shroff SG, Pinsky MR, Lopez-Candales A. Tissue Doppler imaging of right ventricular decompensation in pulmonary hypertension. Congest Heart Fail 2009;15(6):271–276. [DOI] [PMC free article] [PubMed]
- 53.Dambrauskaite V, Delcroix M, Claus P, Herbots L, D’Hooge J, Bijnens B, et al. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr 2007;20(10):1172–1180. [DOI] [PubMed]
- 54.Pirat B, McCulloch ML, Zoghbi WA. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol 2006;98(5):699–704. [DOI] [PubMed]
- 55.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011;139(6):1299–1309. [DOI] [PubMed]
- 56.Urbano-Moral JA, Patel AR, Maron MS, Arias-Godinez JA, Pandian NG. Three-dimensional speckle-tracking echocardiography: methodological aspects and clinical potential. Echocardiography (Mount Kisco, NY) 2012;29(8):997–1010. [DOI] [PubMed]
- 57.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology 1989;171(3):841–845. [DOI] [PubMed]
- 58.Fayad ZA, Ferrari VA, Kraitchman DL, Young AA, Palevsky HI, Bloomgarden DC, et al. Right ventricular regional function using MR tagging: normals versus chronic pulmonary hypertension. Magn Reson Med 1998;39(1):116–123. [DOI] [PubMed]
- 59.Shehata ML, Harouni AA, Skrok J, Basha TA, Boyce D, Lechtzin N, et al. Regional and global biventricular function in pulmonary arterial hypertension: a cardiac MR imaging study. Radiology 2013;266(1):114–122. [DOI] [PMC free article] [PubMed]
- 60.Pettersen E, Helle-Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, et al. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol 2007;49(25):2450–2456. [DOI] [PubMed]
- 61.Klima UP, Lee MY, Guerrero JL, Laraia PJ, Levine RA, Vlahakes GJ. Determinants of maximal right ventricular function: role of septal shift. J Thorac Cardiovasc Surg 2002;123(1):72–80. [DOI] [PubMed]
- 62.Goldstein JA, Harada A, Yagi Y, Barzilai B, Cox JL. Hemodynamic importance of systolic ventricular interaction, augmented right atrial contractility and atrioventricular synchrony in acute right ventricular dysfunction. J Am Coll Cardiol 1990;16(1):181–189. [DOI] [PubMed]
- 63.Santamore WP, Dell’Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 1998;40(4):289–308. [DOI] [PubMed]
- 64.Feneley MP, Gavaghan TP, Baron DW, Branson JA, Roy PR, Morgan JJ. Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation 1985;71(3):473–480. [DOI] [PubMed]
- 65.Taylor RR, Covell JW, Sonnenblick EH, Ross J Jr. Dependence of ventricular distensibility on filling of the opposite ventricle. Am J Physiol 1967;213(3):711–718. [DOI] [PubMed]
- 66.Lopez-Candales A, Dohi K, Bazaz R, Edelman K. Relation of right ventricular free wall mechanical delay to right ventricular dysfunction as determined by tissue Doppler imaging. Am J Cardiol 2005;96(4):602–606. [DOI] [PubMed]
- 67.Lopez-Candales A, Dohi K, Rajagopalan N, Suffoletto M, Murali S, Gorcsan J, et al. Right ventricular dyssynchrony in patients with pulmonary hypertension is associated with disease severity and functional class. Cardiovasc Ultrasound 2005;3:23. [DOI] [PMC free article] [PubMed]
- 68.Mauritz GJ, Marcus JT, Westerhof N, Postmus PE, Vonk-Noordegraaf A. Prolonged right ventricular post-systolic isovolumic period in pulmonary arterial hypertension is not a reflection of diastolic dysfunction. Heart 2011;97(6):473–478. [DOI] [PubMed]
- 69.Marcus JT, Gan CT, Zwanenburg JJ, Boonstra A, Allaart CP, Gotte MJ, et al. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol 2008;51(7):750–757. [DOI] [PubMed]
- 70.Neubauer S. The failing heart: an engine out of fuel. New Engl J Med 2007;356(11):1140–1151. [DOI] [PubMed]
- 71.de las Fuentes L, Herrero P, Peterson LR, Kelly DP, Gropler RJ, Davila-Roman VG. Myocardial fatty acid metabolism: independent predictor of left ventricular mass in hypertensive heart disease. Hypertension 2003;41(1):83–87. [DOI] [PubMed]
- 72.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40(2):271–277. [DOI] [PubMed]
- 73.Nagaya N, Goto Y, Satoh T, Uematsu M, Hamada S, Kuribayashi S, et al. Impaired regional fatty acid uptake and systolic dysfunction in hypertrophied right ventricle. J Nucl Med 1998;39(10):1676–1680. [PubMed]
- 74.Bokhari S, Raina A, Rosenweig EB, Schulze PC, Bokhari J, Einstein AJ, et al. PET imaging may provide a novel biomarker and understanding of right ventricular dysfunction in patients with idiopathic pulmonary arterial hypertension. Circ Cardiovasc Imaging 2011;4(6):641–647. [DOI] [PubMed]
- 75.Can MM, Kaymaz C, Tanboga IH, Tokgoz HC, Canpolat N, Turkyilmaz E, et al. Increased right ventricular glucose metabolism in patients with pulmonary arterial hypertension. Clin Nucl Med 2011;36(9):743–748. [DOI] [PubMed]
- 76.Fang W, Zhao L, Xiong CM, Ni XH, He ZX, He JG, et al. Comparison of 18F-FDG uptake by right ventricular myocardium in idiopathic pulmonary arterial hypertension and pulmonary arterial hypertension associated with congenital heart disease. Pulm Circ 2012;2(3):365–372. [DOI] [PMC free article] [PubMed]
- 77.Kluge R, Barthel H, Pankau H, Seese A, Schauer J, Wirtz H, et al. Different mechanisms for changes in glucose uptake of the right and left ventricular myocardium in pulmonary hypertension. J Nucl Med 2005;46(1):25–31. [PubMed]
- 78.Lundgrin EL, Park MM, Sharp J, Tang WH, Thomas JD, Asosingh K, et al. Fasting 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Am Thorac Soc 2013;10(1):1–9. [DOI] [PMC free article] [PubMed]
- 79.Hagan G, Southwood M, Treacy C, Ross RM, Soon E, Coulson J, et al. (18)FDG PET imaging can quantify increased cellular metabolism in pulmonary arterial hypertension: a proof-of-principle study. Pulm Circ 2011;1(4):448–455. [DOI] [PMC free article] [PubMed]
- 80.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol 2005;45(11):1849–1855. [DOI] [PubMed]
- 81.Wong YY, Ruiter G, Lubberink M, Raijmakers PG, Knaapen P, Marcus JT, et al. Right ventricular failure in idiopathic pulmonary arterial hypertension is associated with inefficient myocardial oxygen utilization. Circ Heart Fail 2011;4(6):700–706. [DOI] [PubMed]
- 82.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Euro Resp J 2012;40(6):1555–1565. [DOI] [PMC free article] [PubMed]
- 83.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, et al. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013;91(11):1315–1327. [DOI] [PubMed]
- 84.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, et al. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Resp Cell Mol Biol 2011;45(6):1239–1247. [DOI] [PMC free article] [PubMed]
- 85.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med (Berl) 2013;88(10):1011–1020. [DOI] [PMC free article] [PubMed]
- 86.Wong YY, Westerhof N, Ruiter G, Lubberink M, Raijmakers P, Knaapen P, et al. Systolic pulmonary artery pressure and heart rate are main determinants of oxygen consumption in the right ventricular myocardium of patients with idiopathic pulmonary arterial hypertension. Eur J Heart Fail 2011;13(12):1290–1295. [DOI] [PubMed]
- 87.Kusachi S, Nishiyama O, Yasuhara K, Saito D, Haraoka S, Nagashima H. Right and left ventricular oxygen metabolism in open-chest dogs. Am J Physiol 1982;243(5):H761–H766. [DOI] [PubMed]
- 88.Hart BJ, Bian X, Gwirtz PA, Setty S, Downey HF. Right ventricular oxygen supply/demand balance in exercising dogs. Am J Physiol 2001;281(2):H823–H830. [DOI] [PubMed]
- 89.Saito D, Tani H, Kusachi S, Uchida S, Ohbayashi N, Marutani M, et al. Oxygen metabolism of the hypertrophic right ventricle in open chest dogs. Cardiovasc Res 1991;25(9):731–739. [DOI] [PubMed]
- 90.van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J 2008;29(1):120–127. [DOI] [PubMed]
- 91.Wong YY, Raijmakers PG, Knaapen P, Lubberink M, Ruiter G, Marcus JT, et al. Supine-exercise-induced oxygen supply to the right myocardium is attenuated in patients with severe idiopathic pulmonary arterial hypertension. Heart 2011;97(24):2069–2074. [DOI] [PubMed]
- 92.Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology 2011;258(1):119–127. [DOI] [PMC free article] [PubMed]
- 93.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, et al. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol 2001;38(4):1137–1142. [DOI] [PubMed]
- 94.Blyth KG, Groenning BA, Martin TN, Foster JE, Mark PB, Dargie HJ, et al. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur Heart J 2005;26(19):1993–1999. [DOI] [PubMed]
- 95.Sanz J, Dellegrottaglie S, Kariisa M, Sulica R, Poon M, O’Donnell TP, et al. Prevalence and correlates of septal delayed contrast enhancement in patients with pulmonary hypertension. Am J Cardiol 2007;100(4):731–735. [DOI] [PubMed]
- 96.McCann GP, Gan CT, Beek AM, Niessen HW, Vonk Noordegraaf A, van Rossum AC. Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension. AJR Am J Roentgenol 2007;188(2):349–355. [DOI] [PubMed]
- 97.McCann GP, Beek AM, Vonk-Noordegraaf A, van Rossum AC. Delayed contrast-enhanced magnetic resonance imaging in pulmonary arterial hypertension. Circulation 2005;112(16):e268. [DOI] [PubMed]
- 98.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009;120(20):1951–1960. [DOI] [PubMed]
- 99.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, et al. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol 1998;275(6 Pt 2):H1948–H1956. [DOI] [PubMed]
- 100.Ruiter G, Ying Wong Y, de Man FS, Louis Handoko M, Jaspers RT, Postmus PE, et al. Right ventricular oxygen supply parameters are decreased in human and experimental pulmonary hypertension. J Heart Lung Transplant 2013;32(2):231–240. [DOI] [PubMed]
- 101.Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJ, Westerhof N, et al. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009;120(1):42–49. [DOI] [PubMed]
- 102.Rodriguez-Porcel M, Cai W, Gheysens O, Willmann JK, Chen K, Wang H, et al. Imaging of VEGF receptor in a rat myocardial infarction model using PET. J Nucl Med 2008;49(4):667–673. [DOI] [PMC free article] [PubMed]
- 103.Higuchi T, Bengel FM, Seidl S, Watzlowik P, Kessler H, Hegenloh R, et al. Assessment of alphavbeta3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res 2008;78(2):395–403. [DOI] [PubMed]
- 104.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest 2004;113(12):1684–1691. [DOI] [PMC free article] [PubMed]
- 105.Sosnovik DE, Nahrendorf M, Panizzi P, Matsui T, Aikawa E, Dai G, et al. Molecular MRI detects low levels of cardiomyocyte apoptosis in a transgenic model of chronic heart failure. Circ Cardiovasc Imaging 2009;2(6):468–475. [DOI] [PMC free article] [PubMed]
- 106.Kaufmann PA, Di Carli MF. Hybrid SPECT/CT and PET/CT imaging: the next step in noninvasive cardiac imaging. Semin Nucl Med 2009;39(5):341–347. [DOI] [PubMed]
- 107.Makowski MR, Ebersberger U, Nekolla S, Schwaiger M. In vivo molecular imaging of angiogenesis, targeting alphavbeta3 integrin expression, in a patient after acute myocardial infarction. Eur Heart J 2008;29(18):2201. [DOI] [PubMed]
- 108.Nensa F, Poeppel TD, Beiderwellen K, Schelhorn J, Mahabadi AA, Erbel R, et al. Hybrid PET/MR imaging of the heart: feasibility and initial results. Radiology 2013;268(2):366–373. [DOI] [PubMed]
- 109.Schlosser T, Nensa F, Mahabadi AA, Poeppel TD. Hybrid MRI/PET of the heart: a new complementary imaging technique for simultaneous acquisition of MRI and PET data. Heart 2013;99(5):351–352. [DOI] [PubMed]
- 110.Ibrahim T, Nekolla SG, Langwieser N, Rischpler C, Groha P, Laugwitz KL, et al. Simultaneous positron emission tomography/magnetic resonance imaging identifies sustained regional abnormalities in cardiac metabolism and function in stress-induced transient midventricular ballooning syndrome: a variant of Takotsubo cardiomyopathy. Circulation 2012;126(21):e324–e326. [DOI] [PubMed]
- 111.Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, et al. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med 2008;49(11):1875–1883. [DOI] [PubMed]
- 112.Zaidi H. Is MR-guided attenuation correction a viable option for dual-modality PET/MR imaging? Radiology 2007;244(3):639–642. [DOI] [PubMed]
- 113.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30(20):2493–2537. [DOI] [PubMed]
- 114.Vonk Noordegraaf A, Galie N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev 2011;20(122):243–253. [DOI] [PMC free article] [PubMed]
- 115.Giusca S, Jurcut R, Coman IM, Ghiorghiu I, Catrina D, Popescu BA, et al. Right ventricular function predicts clinical response to specific vasodilator therapy in patients with pulmonary hypertension. Echocardiography 2013;30(1):17–26. [DOI] [PubMed]
- 116.Hardegree EL, Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Kushwaha SS, et al. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am J Cardiol 2013;111(1):143–148. [DOI] [PubMed]
- 117.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53(17):1573–1619. [DOI] [PubMed]
- 118.Peacock AJ, Crawley S, McLure L. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging 2014;7(1):107–114. [DOI] [PubMed]
- 119.Wilkins MR, Ali O, Bradlow W, Wharton J, Taegtmeyer A, Rhodes CJ, et al. Simvastatin as a treatment for pulmonary hypertension trial (SiPHT). Am J Resp Crit Care Med 2010;181(10):1106–1113. [DOI] [PMC free article] [PubMed]
- 120.Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin-Sing W, Banya WA, et al. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Resp Crit Care Med 2005;171(11):1292–1297. [DOI] [PubMed]