Abstract Abstract
Despite currently available treatments, the prognoses of pulmonary arterial hypertension (PAH) and pulmonary capillary hemangiomatosis (PCH) remain poor. Platelet-derived growth factor and its receptor (PDGFR) have been implicated in the pathogenesis of pulmonary hypertension in PAH and PCH. Imatinib, a PDGFR antagonist, may be beneficial in the treatment of both conditions because of its potent antiproliferative effect. We report two cases that demonstrate the potential for safe and efficacious use of imatinib in PAH and PCH.
Pulmonary arterial hypertension (PAH) is a life-threatening condition characterized by a marked and sustained elevation in pulmonary artery pressure, which leads to progressive increases in pulmonary vascular resistance, right-sided heart failure, and death.1 Current therapeutic approaches mainly provide symptomatic benefit but do not substantially reduce mortality rates.2
Pulmonary capillary hemangiomatosis (PCH) is a rare cause of pulmonary hypertension characterized by an uncontrolled proliferation of pulmonary capillaries infiltrating vascular, bronchial, and interstitial pulmonary structures.3 Pharmacological treatment of PCH has had very limited success, and the prognosis remains poor.4
Platelet-derived growth factor (PDGF) and its receptor (PDGFR) have been implicated in the abnormal proliferation and migration of smooth muscle cells in the development of pulmonary hypertension in PAH and PCH.5 Imatinib is a PDGFR antagonist and may be efficacious in the treatment of PAH and PCH because of its potent antiproliferative effect.5 We review the effect of imatinib in 2 patients, one with PCH (case 1) and the other with PAH (case 2), and we discuss the potential role of imatinib in the treatment of these conditions.
Case Description
Case 1
A previously active 63-year-old woman presented with a 2-week history of progressive dyspnea with no associated symptoms. Her background medical history included the limited cutaneous form of systemic sclerosis (i.e., CREST syndrome) diagnosed 9 years earlier. She had no cardiovascular or respiratory medical history and was a nonsmoker. Her family history was not significant. Her cardiorespiratory examination findings were entirely unremarkable.
Full blood count, blood chemistry, arterial blood gases, and electrocardiogram findings were normal. Chest radiographs showed cardiomegaly and diffuse interstitial lung markings. Pulmonary function testing revealed low diffusion capacity, carbon monoxide (DLCO), corrected for alveolar volume (52% of the predicted value) and normal FEV1/FVC (78%). Ventilation perfusion scan indicated low probability of pulmonary embolism. High-resolution computed tomography showed bilateral pleural effusions, ground glass shadows, and interstitial lung markings with a mosaic pattern. Transthoracic echocardiogram indicated pulmonary hypertension (pulmonary artery systolic pressure, 59 mmHg) and severe right ventricular dilatation and impaired systolic function. Left ventricular size and systolic function were normal. Right heart catheterization revealed pulmonary artery pressure of 70/38 mmHg, right atrial pressure of 14 mmHg, wedge pressure of 3 mmHg, pulmonary vascular resistance of 1,213 dyn s cm−5, and cardiac index of 2.1 L/min/m2. The patient’s 6-minute walk test distance was 291 m.
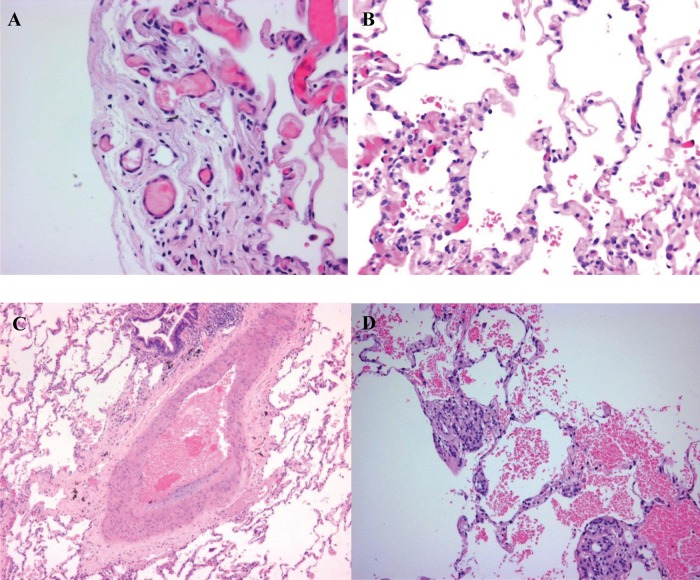
The patient subsequently initiated therapy with bosentan (125 mg bd) and sildenafil (25 mg tds). However, over the next 6 months, she experienced worsening dyspnea (New York Heart Association [NYHA] class III) and right-side heart failure that required hospitalization. Echocardiogram indicated increased pulmonary artery systolic pressure (83 mmHg), and 6-minute walk distance decreased to 90 m. A lung biopsy was performed, which showed definite features of PCH with secondary pulmonary arterial changes (Fig. 1).
Figure 1.
Lung biopsy showing abnormal proliferation of capillaries and intimal thickening of pulmonary vasculature (hematoxylin-eosin stain, original magnification ×200).
The patient was unsuitable for lung transplantation because of age, severity of underlying illness, gastroesophageal dysmotility, and poor nutritional state. She initiated therapy with doxycycline (100 mg bd), but her condition deteriorated significantly over the following weeks. Intravenous epoprostenol (4 ng/kg/min) was initiated; however, this caused acute pulmonary edema and was ceased. In this situation, treatment with imatinib (100 mg daily) was initiated on December 3, 2010, in conjunction with the patient’s existing medications. The patient tolerated imatinib well and improved symptomatically over the next 2 days (from functional class IV to III).
Over the next 6 months, she continued to take imatinib. There was no recurrence of signs of right-sided heart failure. Her dyspnoea improved from NYHA class III to class II. She was not reliant on home oxygen, and she was able to complete activities of daily living, including shopping, cooking, and washing, independently. Six-minute walk distance increased to 295 m. Echocardiogram indicated decreased pulmonary artery systolic pressure (45 mmHg).
She subsequently required 2 hospital admissions for right-sided heart failure and fluid overload. Her last echocardiogram showed an increase in pulmonary artery systolic pressure (69 mmHg), and she required home oxygen 24 hours a day. In August 2012, she died due to right-sided heart failure.
Case 2
A 43-year-old woman presented with worsening exertional dyspnea in 1995. She received a diagnosis of idiopathic PAH on the basis of echocardiogram findings and right heart catheterization (pulmonary artery pressure, 100/59 mmHg).
Between 1996 and 2001, the patient was treated with inhaled iloprost (50 μg daily in 5 split doses), which was initially quite effective. Over time, however, she developed mild ascites and hepatomegaly. She then commenced therapy with bosentan (125 mg bd) in 2002. This provided a clinical improvement in terms of dyspnea and signs of right-sided heart failure, but pulmonary pressures (pulmonary artery systolic pressure, 106 mmHg), 6-minute walk test distance (320 m), and oxygen saturation levels (96%) did not improve considerably.
In November 2008, the patient’s condition deteriorated significantly. She was hemodynamically unstable and had type 1 respiratory failure with severe dyspnea. She also developed polycythemia secondary to hypoxia and experienced multiorgan failure.
She initiated therapy with imatinib (200 mg daily) on November 15, 2008, in conjunction with her other medications. She tolerated it well, and her dyspnea improved. She reported having more energy and decreased reliance on home oxygen. However, there was no considerable improvement in terms of her pulmonary pressures (pulmonary artery systolic pressure, 110 mmHg), 6-minute walk test distance (275 m), and oxygen saturation levels (95%).
In January 2011, imatinib therapy was stopped, because the patient developed severe cellulitis-like reaction of the lower body. Two weeks later, she was admitted to the hospital with a sustained deterioration of her pulmonary hypertension. The deterioration was precipitated by atrial flutter. Later in the year, she required 2 hospital admissions for fluid drainage, and she died in July 2011 due to a chest infection.
Discussion
These cases describe the rapid clinical benefit achieved and the potential for safe and efficacious longer-term use in 2 patients with refractory PAH and PCH. To our knowledge, this is the only reported case of imatinib use in the treatment of PCH.
PAH is a life-threatening disease characterized by increased pulmonary vascular resistance caused by narrowing of the pulmonary vascular bed, vascular remodeling, and thrombosis.5 PCH is a rare cause of pulmonary hypertension characterized by an uncontrolled proliferation of pulmonary capillaries infiltrating vascular, bronchial, and interstitial pulmonary structures.3 There is also secondary intimal thickening and medial hypertrophy of small muscular pulmonary arteries, which results in increased pulmonary vascular resistance.6
Smooth muscle and fibroblast proliferation is an important pathological feature contributing to increased pulmonary vascular resistance in both PAH and PCH.4,5 Several growth factors, including PDGF, have been implicated in the abnormal proliferation and migration of smooth muscle cells.1 PDGF acts as a potent mitogen and chemoattractant for smooth muscle cells, activating signal transduction pathways associated with smooth muscle hyperplasia.7
Recent studies have shown upregulation of PDGF and PDGFR in tissue samples of PAH and PCH. PDGFR-α and PDGFR-β protein levels were upregulated in an ovine model of chronic intrauterine pulmonary hypertension. Lung biopsies of patients with PAH showed expression of PDGFR-β,1 and both PDGFR-α and PDGFR-β8 levels were increased compared with those in healthy donor lungs. Furthermore, PDGF-B and PDGFR-β genes were overexpressed in patients with PCH.4,9
Imatinib is a tyrosine kinase inhibitor that inhibits PDGFR-α and PDGFR-β as well as other kinases, including c-KIT and BCR-ABL.10,11 It is currently used to treat chronic myelogenous leukemia12 and gastrointestinal stromal tumors.13 The rationale for imatinib use in PAH and PCH is its potential to reverse pulmonary vascular remodeling.14
In 2 animal models of pulmonary hypertension, imatinib reversed the increased expression and phosphorylation of PDGFR-β and suppressed activation of downstream signaling pathways.1 This resulted in reversal of pulmonary hypertension and pulmonary vessel proliferation, reduction in right heart hypertrophy, and improved survival.1
Clinical case reports have also described the clinical and hemodynamic improvements (pulmonary vascular resistance and cardiac output) after treatment with imatinib in patients with refractory PAH.2,14,15 A case series by Hatano et al.16 found a significant decrease in plasma PDGF-BB and increase in DLCO but not in hemodynamic parameters. In the 2 cases we have presented, right heart catheterization was not performed after the institution of imatinib therapy, because it could not alter the therapies available to the patients.
A Phase II trial by Ghofrani et al.5 investigating the use of imatinib in PAH found that imatinib seemed safe and well tolerated over a 6-month period and demonstrated significant improvement in pulmonary vascular resistance and cardiac output, but not 6-minute walk distance, compared with placebo.5
By contrast, a case series by Hernández et al.17 reports less favorable results from the use of imatinib in 4 patients with severe PAH (functional class IV), 3 of whom died 1 month after starting therapy due to right-sided heart failure and 1 of whom died within 5 months due to hepatotoxicity in which imatinib may have played a role. Other important reported adverse reactions include potential cardiotoxicity5 and cutaneous adverse effects of varying severity.18
Conclusion
These cases have demonstrated the potential for safe and efficacious use of imatinib in occasional cases of PAH and PCH.
Presentation at a Meeting: Pulmonary Hypertension Society of Australia and New Zealand; Sydney, Australia; November 2011.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 2005;115(10):2811–2821. [DOI] [PMC free article] [PubMed]
- 2.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med 2006;145(2):152–153. [DOI] [PubMed]
- 3.Wagenvoort CA, Beetstra A, Spijker J. Capillary haemangiomatosis of the lungs. Histopathology 1978;2(6):401–406. [DOI] [PubMed]
- 4.Assaad AM, Kawut SM, Arcasoy SM, Rosenzweig EB, Wilt JS, Sonett JR, Borczuk AC, et al. Platelet-derived growth factor is increased in pulmonary capillary hemangiomatosis. Chest 2007;131(3):850–855. [DOI] [PubMed]
- 5.Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, Shapiro S. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 2010;182(9):1171–1177. [DOI] [PMC free article] [PubMed]
- 6.Tron V, Magee F, Wright JL, Colby T, Churg A. Pulmonary capillary hemangiomatosis. Hum Pathol 1986;17(11):1144–1150. [DOI] [PubMed]
- 7.Heldin C-H, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999;79(4):1283–1316. [DOI] [PubMed]
- 8.Perros F, Montani D, Dorfmüller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008;178(1):81–88. [DOI] [PubMed]
- 9.Kawut SM, Assaad AM, Arcasoy SM, Rosenzweig EB, Sonett JR, Borczuk AC. Pulmonary capillary hemangiomatosis: results of gene expression analysis. Chest 2005;128(6 suppl):575S–576S. [DOI] [PubMed]
- 10.de Kogel CE, Schellens JHM. Imatinib. Oncologist 2007;12(12):1390–1394. [DOI] [PubMed]
- 11.Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, Walker C, Jarai G. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol 2008;599(1–3):44–53. [DOI] [PubMed]
- 12.Cohen MH, Williams G, Johnson JR, Duan J, Gobburu J, Rahman A, Benson K, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res 2002;8(5):935–942. [PubMed]
- 13.Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, Rahman A, et al. Approval summary. Clin Cancer Res 2002;8(10):3034–3038. [PubMed]
- 14.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. New Engl J Med 2005;353(13):1412–1413. [DOI] [PubMed]
- 15.Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax 2006;61(8):736. [DOI] [PMC free article] [PubMed]
- 16.Hatano M, Yao A, Shiga T, Kinugawa K, Hirata Y, Nagai R. Imatinib mesylate has the potential to exert its efficacy by down-regulating the plasma concentration of platelet-derived growth factor in patients with pulmonary arterial hypertension. Int Heart J 2010;51(4):272–276. [DOI] [PubMed]
- 17.Hernández FJG, Palma MJC, León RC, Rasco RG, Medina CO, Román JS. Experience with imatinib to treat pulmonary arterial hypertension. Arch Bronconeumol 2008;44(12):3. [DOI] [PubMed]
- 18.Robert C, Soria J-C, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 2005;6(7):491–500. [DOI] [PubMed]