Abstract Abstract
Pulmonary hypertension (PH) is a chronic, complex, and progressive disease that eventuates in fatality. Research efforts over the past decades have resulted in therapeutic options that improve quality of life and prolong survival of patients, but they do not offer a cure. We propose a philosophical model that a disturbed balance of yin and yang results in pulmonary vascular remodeling, the hallmark of PH pathology. The model may be useful in exploring the wisdom of traditional Chinese medicine and incorporating it into mainstream PH research. In this context, the medicinal plant Rhodiola can be of profound interest owing to its variety of health-friendly attributes. Rhodiola has been shown to be beneficial in high-altitude-related symptoms and acute exacerbation of PH; moreover, improvement of PH has been demonstrated experimentally in chronically hypoxic rats. The beneficial effects of Rhodiola in PH may be attributable to its potential targeting of the signaling pathways, such as endothelin-1, nitric oxide, vascular endothelial growth factor, angiotensin-converting enzyme, nuclear factor κ-B, tumor necrosis factor α, and interleukin-6. Alterations in these mediators are implicated in PH pathogenesis, the characteristics of which include chronic pulmonary vasoconstriction, vasoproliferation, and vascular inflammation. Salidroside, one of the compounds extracted from Rhodiola, has been found to provide therapeutic benefits in experimental PH. As the data are limited and the field is in its infancy, further studies including in-depth analysis of the therapeutic effects on various animal models of PH are desirable. We believe that future PH research should place an adequate and special emphasis on exploring and promoting the potential of traditional Chinese medicine, and to this end, the medicinal plant Rhodiola offers a promising field on which to embark.
Keywords: Rhodiola, pulmonary hypertension, pulmonary vascular remodeling, Chinese medicine, salidroside
Introduction
Pulmonary hypertension (PH), a complex and heterogeneous group of diseases, represents a severe and life-threatening medical condition that in all of its clinical forms affects around 100 million people across the world.1,2 Although initial pathological events might differ between various PH groups, the molecular mechanisms, the progression of the disease, and different clinical manifestations are often shared.3 PH is characterized by a sustained increase of pulmonary artery pressure giving rise to an increased workload to the right ventricle, culminating in right heart failure and an inevitable outcome of death. The intense scientific studies on PH, especially during the past decade, have resulted in considerable in-depth understanding of the pathomechanisms and discoveries of potential therapeutics, such as phosphodiesterase-5 inhibitor, prostacyclin analogues, and endothelin-receptor antagonists.1,4 Despite the fact that these therapeutic approaches improved the quality of life and prolonged survival of the patients, novel clinical options and strategies to achieve the ultimate goal of PH as a curable disease are still in front of us.4,5 Recent years have witnessed an emergence of new therapeutic approaches, and others are expected in the years ahead, suggesting that the field of PH research has been receiving considerable focus. Nevertheless, there should be no incertitude that the search for new targets and drugs should adopt a comprehensive approach by keeping the doors to all alternative lines of research open. We underscore the notion that promotion and further exploration of the medical knowledge possessed by Eastern civilization may serve as one of the potential directions to be pursued, and the field of traditional Chinese medicine may offer a reasonable starting point. It is worth noting that the Chinese herb-derived compounds triptolide, a diterpenoid triepoxide from Tripterygium wilfordii Hook F., and ruscogenin, a major steroidal sapogenin from Radix Ophiopogon japonicus, have shown therapeutic potential in experimental models of PH.6-8 This encouraging evidence, although emerging gradually and at a slow pace, substantiates the rationale to explore the potential of Chinese medicine to the benefit of PH patients. In our review, we will discuss the current state of knowledge on the medical plant Rhodiola and its therapeutic potential in PH. Before embarking on a discussion of Rhodiola, we will try to generate a philosophical concept of pulmonary vascular remodeling as a consequence of the disturbed yin and yang balance.
The yin and yang concept and PH
The general philosophy of traditional Chinese medicine can be seen through the prism of the concept of the yin and the yang.9 The harmonic equilibrium between the yin and the yang, as two opposite forces, maintains a living system in a healthy state.9 The disease appears when the balance between these two forces is disturbed, and the general aim of the medicine would be to return to the lost equilibrium.9 The concept of the yin and the yang seems applicable to PH. The PH pathogenesis, which is characterized by sustained pulmonary vasoconstriction and vascular remodeling, is crucially linked to inappropriate regulation/functions of several molecular players. The sustained vasoconstriction occurs as a result of an imbalance between pulmonary vasodilators and vasoconstrictors.1,10 Moreover, the theory of imbalance between two kinds of forces holds true for pulmonary vascular remodeling, the pathological hallmark of PH.1 Pulmonary vascular remodeling is characterized by histomorphological changes involving all structural layers of the pulmonary arteries and is manifested as neomuscularization of nonmuscularized vessels and complex vascular features such as neointima formation and plexiform lesions.10-12 The homeostatic imbalance of proliferation, survival, and apoptosis of vascular cells has been implicated in the process of pulmonary vascular remodeling, and the altered vascular cell phenotype, in turn, is attributable to a disturbed balance among several molecular mediators (e.g., transforming growth factor-β, cytokines and chemokines, different growth factors, bone morphogenetic protein, matrix metalloproteinases, and Notch 3).1,13-15 When the literature is put together, the model of the yin and the yang seems able to explain the gist of molecular pathogenesis. Therefore, we would like to create a philosophical concept of pulmonary vascular remodeling as a sequel of disturbed balance of the yin and the yang, in order to connect the Chinese wisdom with the conventional research and development strategies in the Western world (Fig. 1). An imbalance between the yin and the yang (representing several known and yet-to-be identified molecules/mediators) will lead to a remodeling process and subsequent shift from a “healthy vessel” state to a “diseased vessel” state. A successful therapeutic strategy should therefore aim to restore the lost harmony between these two forces, thereby reversing the structural alteration of pulmonary vasculature to a normal physiological state (Fig. 1). Although one should be cautions to avoid oversimplification, we believe that our proposed model may be useful in exploring the wisdom of traditional Chinese medicine and incorporating it into the mainstream of modern medical science. Traditional Chinese medicine may complement contemporary knowledge and efforts and may offer alternative strategies for unmet medical needs. As already mentioned, we will discuss Rhodiola, a medical plant, and its potential therapeutic efficacy for PH.
Figure 1.
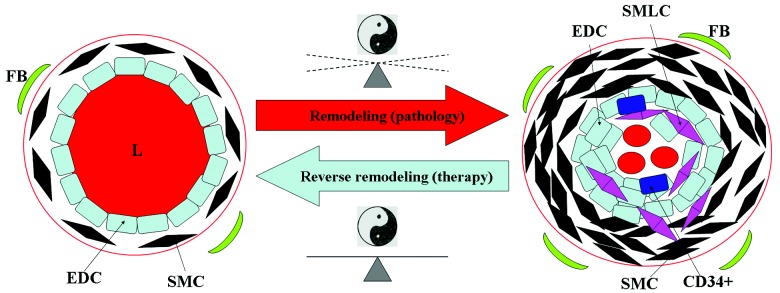
Yin and yang concept and pulmonary vascular remodeling. An imbalance between the yin and the yang will lead to the remodeling process, a hallmark of pulmonary hypertension pathology, and a subsequent shift from a healthy vessel (left) to a diseased vessel (right). The aim of the medicine is to restore the lost harmony between the yin and the yang and to reverse remodeling, with a consequent return to a healthystate. FB, fibroblast; SMC, smooth muscle cell; EDC, endothelial cell; L, lumen of pulmonary artery; CD34+, CD34+ precursor cell; SMLC, smooth muscle-like cell;47
 , yin-yang symbol.
, yin-yang symbol.
Rhodiola spp.: simple or “magic” medical plants?
A genus of Rhodiola covers different species that exist in the mountain regions of China and Siberia (Altai) and Arctic geographic locations in Siberia, Europe, and North America.9,16,17 The plant species of this genus, particularly Rhodiola rosea, have been used for thousands of years in China as part of traditional Chinese medicine for different therapeutic purposes.9 Interestingly, the plant has also been known to European scholars, prevalently during the eighteenth century.17 The famous naturalist, a father of the binomial nomenclature, Carl Linnaeus, in his work Materia Medica (1749), suggested the use of the Rhodiola root for the treatment of different medical conditions.17 The medicinal values of Rhodiola are attributable not only to its beneficial effects, including central nervous system stimulation, antidepression, headache mitigation, cardio- and hepatoprotection, but also to its activities as antioxidant, antifatigue, anticancer, adaptogen, and life span–augmenting agents.16-18 The whole list of health-friendly attributes of Rhodiola is suggestive of “magic” medicine. At the same time, however, several logical questions arise as to what makes this plant therapeutically beneficial for various diseases and conditions. The answer is not yet fully understood, and it requires profound studies in the future. It is noteworthy that several pharmacologically active compounds from the plant extract, such as salidroside/rhodioloside, p-tyrosol, rhodiolin, rosavin, rosarin, rosiridin, rosin, herbacetin glycosides, kaempferol, etc., have been identified.16,17,19 The list of compounds may be expected to expand, requiring more and more studies that will contribute to unwrapping the secret of the “magic.” In the following section we will discuss Rhodiola vis-à-vis potential therapeutic application in PH.
Rhodiola in PH: illusionary or promising?
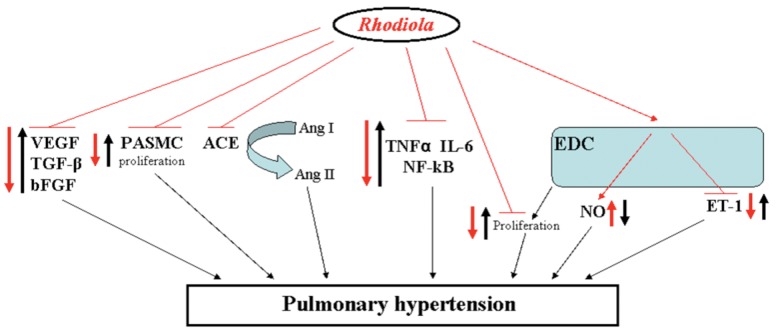
Most of the knowledge and traditional wisdom on medical values of Rhodiola are available in the Chinese literature, and very little can be found in modern medical science. This fact inhibits the visibility of the work in the scientific community worldwide and limits the true assessment of Rhodiola as a potential therapeutic strategy, particularly for PH. Nevertheless, the available data will be discussed in the context of the known molecular mechanisms of PH so as to evaluate whether the future of Rhodiola as a treatment approach for PH is illusionary or promising. Evidently, PH pathogenesis, which is characterized by vasoconstriction and vasoproliferation accompanied by vascular inflammation, is attributable to a disturbed balance of several molecular mediators, such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), basic fibroblast growth factor (bFGF), angiotensin-converting enzyme (ACE) and angiotensin II (Ang II), tumor necrosis factor α (TNFα), interleukin-6 (IL-6), nuclear factor κ-B (NF-κB), nitric oxide (NO), and endothelin-1 (ET-1).1,20-24 It is important to point out that many other pathological culprits play a role in the pathology of PH, in addition to these mentioned here; however, we would like to highlight only those that available literature used in our review suggests as potential targets for Rhodiola (Fig. 2).
Figure 2.
Potential and hypothetical therapeutic targets for Rhodiola in pathology of pulmonary hypertension. Some of the signaling pathways/cellular processes identified to be involved in the pathogenesis of pulmonary hypertension are schematically presented. The hypothetical therapeutic application of Rhodiola is also shown. Thick black arrows represent the changes that might lead to development of pulmonary hypertension (arrows directed upward denote upregulation; those directed downward indicate downregulation). The thick red arrows indicate the potential effect of Rhodiola, with the same interpretation of upward- and downward-oriented arrows. VEGF, vascular endothelial growth factor; ACE, angiotensin-converting enzyme; Ang, angiotensin; TNFα, tumor necrosis factor α; IL, interleukin; NF-κB, nuclear factor κ-B; TGF-β, transforming growth factor β; bFGF, basic fibroblast growth factor; EDC, endothelial cell; PASMC, pulmonary artery smooth muscle cell; NO, nitric oxide; ET, endothelin.
Bai et al.25 systematically investigated the effects of the Rhodiola herb on development of pulmonary vascular remodeling and VEGF expression in a rat model of high-altitude-induced PH. The authors demonstrated that an increase in mean pulmonary arterial pressure (mPAP) and right ventricular hypertrophy (RVH) in a high-altitude environment was significantly reduced in rats treated with Rhodiola.25 Furthermore, they analyzed the pulmonary vascular remodeling by electron microscopy and found a noticeable attenuation of remodeling in rats treated with Rhodiola, in comparison with a placebo group.25 Additionally, the authors showed that an increase of VEGF expression as a result of high altitude was lower after the application of the plant extract.25 Finally, Bai et al.25 concluded that the Rhodiola herb has a potential to attenuate high-altitude-induced PH and vascular remodeling and that VEGF inhibition might represent one of the mechanisms. Corroborating the findings, Shen et al.26 showed that Rhodiola inhibited the aortic remodeling due to atherosclerosis formation in rabbits, and the effect was associated with the decreased expression of VEGF in atherosclerotic plaque (Fig. 2). Rhodiola has also been able to inhibit the TGF-β expression and attenuate high-altitude-induced PH in rats.27
Huang et al.16,28 investigated the effects of salidroside, one of the extracts from Rhodiola, on chronic-hypoxia-induced PH in rats. The authors showed that increase in mPAP, RVH, and pulmonary vascular remodeling in chronically hypoxic rats was significantly attenuated by salidroside treatment, suggesting the promising therapeutic potency of this extract for PH.28 Notably, this extract could inhibit the proliferation of rabbit pulmonary artery smooth muscle cells under hypoxia,29 suggesting that the reduction of pulmonary vascular remodeling might be due to potential antiproliferative properties of salidroside. Moreover, salidroside might also possess anti-inflammatory properties, as observed by Guan et al.30 in their study on lipopolysaccharide (LPS)-induced lung injury in mice. They demonstrated that pretreatment with salidroside in LPS-induced acute lung injury (ALI) in mice resulted in reduction of inflammatory cells (such as neutrophils and macrophages) in the bronchoalveolar lavage fluid. Additionally, salidroside successfully inhibited the production of some inflammatory cytokines, such as TNFα and IL-6, and suppressed NF-kB DNA-binding activation,30 strongly implying the potential use of this compound to interfere with augmented inflammation (Fig. 2). In line with this finding, Zhang et al.31 suggested the protective effects of Rhodiola for patients with ALI/acute respiratory distress syndrome. Furthermore, Rhodiola root anti-inflammatory features have been demonstrated in different inflammatory conditions, such as carrageenan-induced paw edema, formaldehyde-induced arthritis, and nystatin-induced paw edema in a rat model.32 As inflammation is believed to play an important role in PH pathogenesis,33,34 Rhodiola extracts may exert beneficial effects on PH by interfering with the inflammatory process.
In addition to the inflammatory process, oxidative stress has been identified as an important pathological feature in PH patients, and there is corroborating evidence from experimental studies that implicate oxidative stress in PH pathogenesis.35,36 As mentioned above, herbacetin glycosides and kaempferol are among the compounds isolated from Rhodiola that possess antioxidant and anti-inflammatory properties.19 Moreover, Rhodiola extract contains other antioxidant compounds such as p-tyrosol, organic acids (gallic and caffeic acids), and flavonoids (catechins and proanthocyanidins).16,37,38 Indeed, there is experimental evidence that treatment of spontaneously hypertensive rats (SHR) with Rhodiola increased the superoxide dismutase activity.39 Clearly, the anti-inflammatory and antioxidant attributes of Rhodiola can be expected to yield therapeutic benefits in PH.16,18,19,35-38
Sui et al.40 noted that Rhodiola inhibited the growth of human endothelial cell line EVC-304. The findings therefore suggest that Rhodiola may exhibit an antiproliferative effect on vascular cells, in addition to the aforementioned promising anti-inflammatory activities. Moreover, Rhodiola extracts exerted ACE inhibitory activities,41 and it would be logical to conclude that ACE inhibition will subsequently reduce the production of potent vasoconstrictor Ang II, suggesting the possible vasodilatory effects of the plant. Indeed, the ACE inhibition has been shown to exert beneficial effects by modulating Ang II signaling through the type I receptor in experimental PH.21 The ACE inhibition resulted in attenuation of pulmonary vascular remodeling as shown by Jeffery and Wanstall;42 however, the authors observed significant influence on systemic circulation. Additionally, Wang et al.39 have recently studied the effects of different doses of Rhodiola on SHR and reported that the therapeutic benefits may be associated with its NO-releasing and antioxidant activities. Moreover, there is lack of data on the effects of Rhodiola on ACE2, the recently discovered homologue of ACE that has a beneficial role in the cardiopulmonary system.43 Therefore, future studies should meticulously evaluate any effects of Rhodiola on systemic circulation, especially focusing on its long-term use. Additionally, the beneficial effects of Rhodiola as reported in various disease conditions should be carefully considered and evaluated.
Finally, we would like briefly to discuss 3 clinical studies performed in China on Rhodiola.44-46 Shi et al.45 investigated the effects of Chinese herbal preparations, among them a Rhodiola rosea capsule, on de-adaptation to high altitude and found the noticeable reduction of high-altitude-related symptoms, such as fatigue, chest tightness, palpitations, vertigo, drowsiness, lack of attention, and memory loss. Yang et al.46 found that combination treatment with oxygen and Rhodiola in patients with chronic cor pulmonale at high-altitude areas effectively inhibited the serum bFGF levels, which was accompanied by markedly decreased mPAP values. Feng et al.44 studied the effects of Rhodiola on pulmonary arterial pressure in patients with chronic cor pulmonale during acute exacerbation in a high-altitude environment and possible mechanisms. The authors successfully demonstrated the significant improvement of hemodynamic and right ventricle hypertrophic parameters in the patients using Rhodiola, compared to the patients treated only with a routine approach.44 Finally, they suggested that these beneficial improvements might be explained mechanistically due to increased release of NO and reduction of ET-1 released from endothelial cells,44 implying Rhodiola as a hypothetical NO stimulator and ET-1 inhibitor. It is worth noting that the NO-releasing effects of the Rhodiola genus have been corroborated by subsequent study on SHR.39 These findings are of high interest and worth future systematic research.
Conclusion and future perspectives
We believe that future PH research should place an adequate and special emphasis on exploring and promoting the potential of traditional Chinese medicine, and to this end, the medicinal plant Rhodiola may provide a promising field on which to embark. Rhodiola possesses a variety of health-friendly attributes in addition to its beneficial effects on high-altitude-related symptoms and acute exacerbation of PH. On an experimental level, improvement of PH has been demonstrated in chronically hypoxic rats receiving Rhodiola. The beneficial effects may be attributable to its potential targeting of the signaling pathways, such as ET-1, NO, VEGF, ACE and Ang II, NF-κB, TNFα, and IL-6. Alterations in these mediators are believed to contribute to the PH pathogenesis. Moreover, salidroside that is derived from Rhodiola has been shown to provide therapeutic benefits in experimental PH. Although the previous studies are related to high-altitude/chronic-hypoxia-induced PH, Rhodiola may have potential benefits for other PH forms regardless of initiating stimuli or insults. Nevertheless, the field is obviously in its infancy, as also evident from the limited amount of relevant literature. It is therefore highly desirable that more studies in vitro as well as in vivo on various animal models of PH should be performed in order to understand the pharmacodynamics and pharmacokinetics of Rhodiola and its derivatives. The data will be valuable to envisage potential clinical studies. Rhodiola may be expected to offer a novel alternative therapeutic strategy for chronic illness such as PH, and, importantly, the therapy will be associated with benefits such as a low risk of side effects, wide availability, and lower cost.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011;8:443–455. [DOI] [PMC free article] [PubMed]
- 2.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:43–54. [DOI] [PubMed]
- 3.Jain S, Ventura H, deBoisblanc B. Pathophysiology of pulmonary arterial hypertension. Semin Cardiothorac Vasc Anesth 2007;11:104–109. [DOI] [PubMed]
- 4.Connell C, O’Callaghan DS, Gaine S. New drugs for pulmonary hypertension. Eur Respir Monogr 2012;57:233–246.
- 5.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163. [DOI] [PubMed]
- 6.Bi LQ, Zhu R, Kong H, et al. Ruscogenin attenuates monocrotaline-induced pulmonary hypertension in rats. Int Immunopharmacol 2013;16:7–16. [DOI] [PubMed]
- 7.Faul JL, Nishimura T, Berry GJ, Benson GV, Pearl RG, Kao PN. Triptolide attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 2000;162:2252–2258. [DOI] [PubMed]
- 8.Wei L, Liu T, Liu B, Wang XM, Zhao L, Zhou TF. Effect of triptolide on the development of monocrotaline-induced pulmonary hypertension in pneumonectomized rat [in Chinese]. Sichuan Da Xue Xue Bao Yi Xue Ban 2007;38:806–809. [PubMed]
- 9.Ip SP, Che CT, Leung PS. Association of free radicals and the tissue renin-angiotensin system: prospective effects of Rhodiola, a genus of Chinese herb, on hypoxia-induced pancreatic injury. J Pancreas 2001;2:16–25. [PubMed]
- 10.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118:2372–2379. [DOI] [PMC free article] [PubMed]
- 11.Nicod LP. The endothelium and genetics in pulmonary arterial hypertension. Swiss Med Wkly 2007;137:437–442. [DOI] [PubMed]
- 12.Yi ES, Kim H, Ahn H, et al. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension: a morphometric and immunohistochemical study. Am J Respir Crit Care Med 2000;162:1577–1586. [DOI] [PubMed]
- 13.Ghofrani HA, Barst RJ, Benza RL, et al. Future perspectives for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:108–117. [DOI] [PMC free article] [PubMed]
- 14.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:13–24. [DOI] [PubMed]
- 15.Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:20–31. [DOI] [PMC free article] [PubMed]
- 16.Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev 2001;6:293–302. [PubMed]
- 17.Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010;17:481–493. [DOI] [PubMed]
- 18.Chen CH, Chan HC, Chu YT, et al. Antioxidant activity of some plant extracts towards xanthine oxidase, lipoxygenase and tyrosinase. Molecules 2009;14:2947–2958. [DOI] [PMC free article] [PubMed]
- 19.Choe KI, Kwon JH, Park KH, et al. The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. Bor. Molecules 2012;17:11484–11494. [DOI] [PMC free article] [PubMed]
- 20.Furuya Y, Satoh T, Kuwana M. Interleukin-6 as a potential therapeutic target for pulmonary arterial hypertension. Int J Rheumatol 2010;2010:720305. [DOI] [PMC free article] [PubMed]
- 21.Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol 1995;269:1186–1194. [DOI] [PubMed]
- 22.Sawada H, Mitani Y, Maruyama J, et al. A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 2007;132:1265–1274. [DOI] [PubMed]
- 23.Wang Q, Zuo XR, Wang YY, Xie WP, Wang H, Zhang M. Monocrotaline-induced pulmonary arterial hypertension is attenuated by TNF-alpha antagonists via the suppression of TNF-alpha expression and NF-kappaB pathway in rats. Vasc Pharmacol 2013;58:71–77. [DOI] [PubMed]
- 24.Kosanovic D, Kojonazarov B, Luitel H, et al. Therapeutic efficacy of TBC3711 in monocrotaline-induced pulmonary hypertension. Respir Res 2011;12:87. [DOI] [PMC free article] [PubMed]
- 25.Bai MK, Guo Y, Bian BD, et al. Integripetal rhodiola herb attenuates high altitude-induced pulmonary arterial remodeling and expression of vascular endothelial growth factor in rats [in Chinese]. Sheng Li Xue Bao 2011;63:143–148. [PubMed]
- 26.Shen W, Fan WH, Shi HM. Effects of rhodiola on expression of vascular endothelial cell growth factor and angiogenesis in aortic atherosclerotic plaque of rabbits [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28:1022–1025. [PubMed]
- 27.Guo Y, Li WP, Bai MKZ, Cui CY. Effect of rhodiola on the expression of transforming growth factor-b1 in high-altitude environment-induced pulmonary hypertension rats. J Lanzhou Univ (Med Sci) 2011;37:1–9.
- 28.Huang XY, Fan R, Lu YY, Lin QD. The protective effect of salidroside on cor pulmonale rats induced by chronic hypoxia in normal pressure. Chin Arch Trad Chin Med 2011;29:1868–1871.
- 29.Lin SX, Liu YL, Zhao HL, Zhang SF. Inhibitory effect of salidroside on the proliferation of rabbit pulmonary artery smooth muscle cells under hypoxia. Chin J Pathophysiol 2001;17:968–970.
- 30.Guan S, Xiong Y, Song B, et al. Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol Immunotoxicol 2012;34:667–672. [DOI] [PubMed]
- 31.Zhang S, Gao W, Xu K, et al. Early use of Chinese drug rhodiola compound for patients with post-trauma and inflammation in prevention of ALI/ARDS [in Chinese]. Zhonghua Wai Ke Za Zhi 1999;37:238–240. [PubMed]
- 32.Pooja, Bawa AS, Khanum F. Anti-inflammatory activity of Rhodiola rosea: “a second-generation adaptogen.” Phytother Res 2009;23:1099–1102. [DOI] [PubMed]
- 33.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54:10–19. [DOI] [PubMed]
- 34.Tuder RM, Voelkel NF. Pulmonary hypertension and inflammation. J Lab Clin Med 1998;132:16–24. [DOI] [PubMed]
- 35.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med 2012;52:1970–1986. [DOI] [PMC free article] [PubMed]
- 36.Wong CM, Bansal G, Pavlickova L, Marcocci L, Suzuki YJ. Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid Redox Signal 2013;18:1789–1796. [DOI] [PMC free article] [PubMed]
- 37.Lee MW, Lee YA, Park HM, et al. Antioxidative phenolic compounds from the roots of Rhodiola sachalinensis A. Bor. Arch Pharm Res 2000;23:455–458. [DOI] [PubMed]
- 38.Ohsugi M, Fan W, Hase K, et al. Active-oxygen scavenging activity of traditional nourishing-tonic herbal medicines and active constituents of Rhodiola sacra. J Ethnopharmacol 1999;67:111–119. [DOI] [PubMed]
- 39.Wang XQ, Wang BH, Li YH, Zhao YL, Wang YY, Wang Y. Regulating effect and its mechanism of Tibet Rhodiola crenulata on blood pressure in spontaneous hypertension rat. Chin J Exp Trad Med Form 2012;18:150–154.
- 40.Sui XL, Yang F, Chen RH, et al. Inhibition of rhodiola on the growth of EVC-304 cell line [in Chinese]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2006;22:524–525. [PubMed]
- 41.Kwon YI, Jang HD, Shetty K. Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension. Asia Pac J Clin Nutr 2006;15:425–432. [PubMed]
- 42.Jeffery TK, Wanstall JC. Perindopril, an angiotensin converting enzyme inhibitor, in pulmonary hypertensive rats: comparative effects on pulmonary vascular structure and function. Br J Pharmacol 1999;128:1407–1418. [DOI] [PMC free article] [PubMed]
- 43.Shenoy V, Qi Y, Katovich MJ, Raizada MK. ACE2, a promising therapeutic target for pulmonary hypertension. Curr Opin Pharmacol 2011;11:150–155. [DOI] [PMC free article] [PubMed]
- 44.Feng EZ, Guo ZY, Yang SY, et al. Effects of rhodiola on pulmonary arterial pressure in patients with chronic cor pulmonale in acute exacerbation at high altitude areas and its mechanism. Chin J Health Care Med 2010;12:261–263.
- 45.Shi ZF, Zhou QQ, Xiang L, Ma SD, Yan CJ, Luo H. Three preparations of compound Chinese herbal medicines for de-adaptation to high altitude: a randomized, placebo-controlled trial [in Chinese]. Zhong Xi Yi Jie He Xue Bao 2011;9:395–401. [DOI] [PubMed]
- 46.Yang S, Shen J, Guo Z, et al. The relationship between change of serum basic fibroblast growth factor level and pulmonary arterial pressure and its intervention in patients with chronic cor pulmonale at high altitude areas. Clin Med J Chin 2008;15:47–49.
- 47.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 2009;10:95. [DOI] [PMC free article] [PubMed]