Abstract Abstract
Pulmonary arterial remodeling has been demonstrated in patients with severe chronic obstructive pulmonary disease (COPD), but it is not known whether lobar heterogeneity of remodeling occurs. Furthermore, the relationship between pulmonary hypertension (PH) and pulmonary arterial remodeling in COPD has not been established. Muscular pulmonary arterial remodeling in arteries 0.10–0.25 mm in diameter was assessed in COPD-explanted lungs and autopsy controls. Remodeling was quantified as the percentage wall thickness to vessel diameter (%WT) using digital image analysis. Repeat measures mixed-effects remodeling for %WT was performed according to lobar origin (upper and lower), muscular pulmonary arterial size (small, medium, and large), and echocardiography-based pulmonary arterial pressure (no PH, mild PH, and moderate-to-severe PH). Lobar perfusion and emphysema indices were determined from ventilation-perfusion and computed tomography scans, respectively. Overall, %WT was greater in 42 subjects with COPD than in 5 control subjects ( ). Within the COPD group, %WT was greater in the upper lobes (
). Within the COPD group, %WT was greater in the upper lobes ( ) and in the small muscular pulmonary arteries (
) and in the small muscular pulmonary arteries (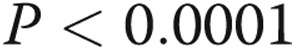 ). Lobar differences were most pronounced in medium and large arteries. Lobar emphysema index was not associated with arterial remodeling. However, there was a significant positive relationship between the lobar perfusion index and pulmonary arterial remodeling (
). Lobar differences were most pronounced in medium and large arteries. Lobar emphysema index was not associated with arterial remodeling. However, there was a significant positive relationship between the lobar perfusion index and pulmonary arterial remodeling ( ). The presence of PH on echocardiography showed only a trend to a small effect on lower lobe remodeling. The pattern of pulmonary arterial remodeling in COPD is complicated and lobe dependent. Differences in regional blood flow partially account for the lobar heterogeneity of pulmonary arterial remodeling in COPD.
). The presence of PH on echocardiography showed only a trend to a small effect on lower lobe remodeling. The pattern of pulmonary arterial remodeling in COPD is complicated and lobe dependent. Differences in regional blood flow partially account for the lobar heterogeneity of pulmonary arterial remodeling in COPD.
Keywords: emphysema, pathology, pulmonary circulation, pulmonary hypertension, regional blood flow.
Introduction
The presence of pulmonary hypertension (PH) in patients with chronic obstructive pulmonary disease (COPD) has been associated with increased mortality1,2 and morbidity.3,4 Thus far, studies using selective and nonselective pulmonary vasodilators in COPD have not translated into improvements in meaningful clinical outcomes.5-11 This has led to a renewed interest in the underlying pathogenesis of PH in COPD as a basis for determining the appropriateness of pursuing PH-specific therapies.12
The pathogenesis of COPD-associated PH is complex.12 Although hypoxia plays a key role, other factors, such as pulmonary arterial remodeling, have been implicated.13 Pulmonary arterial remodeling is an umbrella term that describes a range of pulmonary vascular changes observed in COPD, including medial hypertrophy, longitudinal muscle deposition, intimal hyperplasia, elastin and collagen deposition, and muscularization of the pulmonary arterioles.14 Pulmonary arterial remodeling has often been quantified anatomically as a measure of intimal and/or medial thickness relative to arterial size.15,16
Although pulmonary vascular changes have been demonstrated in subjects with COPD-associated PH,14 the relationship between PH and pulmonary arterial remodeling has been difficult to establish.14,16,17 This is due to (i) the difficulty in determining the natural progression of pulmonary arterial remodeling, (ii) the intersubject and intrasubject variability of pulmonary arterial changes, (iii) the inability to accurately assess pulmonary arterial pressure using noninvasive techniques, and (iv) the confounder of reciprocal causation (although changes due to pulmonary arterial remodeling may elevate pulmonary arterial pressures, PH itself may lead to pulmonary arterial remodeling).18 Because COPD is a disease of significant parenchymal and perfusion heterogeneity,19,20 this may partially account for the intrasubject variability of pulmonary arterial remodeling. However, it is unclear whether this heterogeneity is reflected in differences in arterial remodeling between the upper and lower lung lobes. This study primarily aims to determine whether subjects with severe COPD have lobar differences with regard to pulmonary arterial remodeling. We also seek to determine the influence of pulmonary arterial size, pulmonary arterial pressure, regional emphysema severity, and regional pulmonary perfusion upon pulmonary arterial remodeling in COPD.
Methods
Subjects and controls
From 2000 to 2009, 44 subjects with complete preoperative assessments (including an estimate of right ventricular systolic pressure [RVSP] on echocardiography) underwent lung transplantation for severe COPD (not including patients with α-1 antitrypsin deficiency) at our institution. Of these subjects, 42 had adequate stored tissue for analysis. We developed normal control data by interrogating our institution’s autopsy lung specimen database for age-matched, nonsmoking control subjects with no history of chronic cardiorespiratory disease. Of 153 subjects with lung autopsies performed during 2007–2009, only 5 were deemed true control subjects. The study was approved by the institution’s human ethics committee.
Morphologic analysis
At the time of lung transplantation, explanted lungs were placed in formaldehyde solution. The lungs were inflated but not injected before morphologic analysis.14,16 The lungs were sectioned and examined for macroscopic abnormalities before hemotoxylin and eosin staining and standard histologic examination. In addition, for each subject, representative paraffin-embedded sections of approximately 1 cm2 (1 upper lobe and 1 lower lobe) were prepared for histologic evaluation using smooth muscle actin immunohistochemistry with a Verhoeff elastin counterstain as described elsewhere.21
One observer (J.P.W.), blinded to the subjects’ pulmonary arterial pressure and lobar origin of the specimen, prospectively performed morphologic assessment using digital image analysis. Pulmonary arterial remodeling was quantified by percentage wall thickness (%WT) measured as  , as in earlier studies (Fig. 1).15,16
online supplement (29.5KB, pdf)
, as in earlier studies (Fig. 1).15,16
online supplement (29.5KB, pdf)
Figure 1.

The lumen and external elastic lamina circumference were measured from A. This enabled an idealized artery to be drawn (B) on the basis of the radius, which was derived from the circumference measures. The percentage wall thickness (%WT) was then calculated as  .
.
Vessel size
Morphologic analysis was limited to muscular pulmonary arteries 0.10–0.25 mm in diameter on the basis of previous work by Shelton and colleagues that demonstrated increased %WT in muscular pulmonary arteries of this size.15 Muscular pulmonary artery size was categorized, rather than treated as a continuous variable,13,17,22,23 on an a priori basis as small (0.10–0.15 mm), medium (0.15–0.20 mm), and large (0.20–0.25 mm) in diameter.
Lobar emphysema and perfusion indices
Lobar percentage emphysema was determined prospectively via three-dimensional reconstruction of the lung transplant assessment computed tomography (CT) chest images using the “density mask” method.24 See the online supplement for additional details.
During lung transplant assessment, ventilation-perfusion scans were performed using inhaled technegas and intravenous injection of technetium-99m macro-aggregated albumin, respectively. Ventilation and perfusion images were collected with a gamma camera for both phases of the investigation. The distribution and intensity of the radioactivity count from the intravenous technetium-99m allowed imputation of regional perfusion. The lobar perfusion index was calculated as the ratio of lobar-to-total pulmonary perfusion.
Pulmonary arterial pressure
All patients had an echocardiogram during lung transplant assessment. (Our institution does not routinely perform right heart catheterization for the evaluation of severe COPD.) Patients with COPD for whom the RVSP could be determined were stratified into 3 pulmonary arterial pressure groups on the basis of a priori classification of the RVSP obtained on the lung transplant assessment echocardiogram as follows: no PH if RVSP <35 mmHg,25 mild PH if  mmHg, and moderate-to-severe PH if RVSP ≥45 mmHg.
mmHg, and moderate-to-severe PH if RVSP ≥45 mmHg.
Statistical analysis
Statistical analysis was performed using SAS software, version 9.2 (SAS Institute). Repeat measures mixed-effects models for %WT were fitted using lobar origin, pulmonary arterial size, and pulmonary arterial pressure as main effects. Post hoc pairwise comparisons were adjusted by a Bonferroni correction. The effects of lobar percentage emphysema and lobar perfusion index on %WT were assessed after adjusting for lobar origin and pulmonary arterial size. Weighted Pearson correlation compared lobar percentage emphysema and perfusion index. Intra-observer and interobserver reliability were determined using the Bland-Altman method. For all analyses, statistical significance was set at a 2-tailed 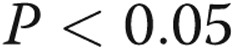 .
.
Results
Five control subjects were identified. They were appropriately age-matched against 42 subjects with COPD (mean age ± standard deviation [SD],  vs.
vs.  years;
years; 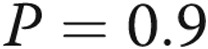 ). Among the subjects with COPD, there were 15 with no PH, 19 with mild PH, and 8 with moderate-to-severe PH. Baseline demographic characteristics are presented in Table 1.
). Among the subjects with COPD, there were 15 with no PH, 19 with mild PH, and 8 with moderate-to-severe PH. Baseline demographic characteristics are presented in Table 1.
Table 1.
Baseline demographic characteristics and physiologic parameters for subjects with chronic obstructive pulmonary disease (COPD)
| Variable | Subjects with COPD (n = 42) |
|---|---|
| Age, years | 58.8 ± 4.9 |
| Ax-Tx time, months | 10.5 ± 10.2 |
| BMI | 23.0 ± 3.7 |
| FEV1, % predicted | 22.7 ± 8.0 |
| FEV1/FVC, % | 30.0 ± 9.6 |
| TLCO, % predicted | 26.5 ± 10.0 |
| RV/TLC, % | 66.0 ± 7.3 |
| PaO2, mmHg | 62.1 ± 15.2 |
| PaCO2, mmHg | 49.4 ± 10.8 |
| Smoking history, pack years | 38.3 ± 23.5 |
| RVEF-GBPS, % | 51.6 ± 11.5 |
| LVEF-GBPS, % | 60.7 ± 9.1 |
| RVSP, mmHg | 40.6 ± 11.7 |
Data are mean values ± standard deviations. Ax-Tx time, duration between lung transplant assessment and lung transplantation; BMI, body mass index, calculated as the weight in kilograms divided by height in meters squared; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GBPS, gated blood pool scan; LVEF, left ventricular ejection fraction; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; RV, residual volume; RVEF, right ventricular ejection fraction; RVSP, right ventricular systolic pressure; TLC, total lung capacity; TLCO, transfer factor of the lung for carbon monoxide.
Qualitative assessment
In the explanted COPD lungs, the parenchyma showed moderate to extensive acinar tissue loss. The vessel changes of PH varied from minor intimal myofibroblastic thickening to significant medial muscular hypertrophy. Plexiform lesions and fibrinoid necrosis were not seen. The large pulmonary arteries showed variable degrees of atherosclerosis. Chronic bronchitis was seen in 3 cases. There was one subject each with extensive fibrosis, pneumonic consolidation, and fibrin thrombi. Scant interstitial granulomata were seen in 6 cases. There was no evidence of neoplasia.
Vessel size and measurement
From 42 subjects with COPD and 5 control subjects, we analyzed 1,513 muscular pulmonary arteries (Table 2). Intra-observer and interobserver reliability for measures of %WT were excellent with an absolute bias (95% limits of agreement) of %WT of only 0.5% (−2.2% to 2.7%) and 1.8% (−4.5% to 8.1%), respectively.
Table 2.
The number of vessels analyzed according to lobar origin and muscular pulmonary arterial size
| Lobe, artery size | Control subjects(n = 5) | COPD(n = 42) |
|---|---|---|
| Upper | ||
| Small | 50 (10.0) | 279 (6.6) |
| Medium | 13 (2.6) | 197 (4.7) |
| Large | 7 (1.4) | 103 (2.5) |
| Lower | ||
| Small | 73 (14.6) | 420 (10.0) |
| Medium | 31 (6.2) | 205 (4.9) |
| Large | 8 (1.6) | 127 (3.0) |
| Total | 182 (36.4) | 1,331 (31.7) |
Values are total number of vessels (average number of vessels per subject). All vessels that met the inclusion criteria were included in the analysis. COPD, chronic obstructive pulmonary disease.
Pulmonary arterial remodeling in COPD
The %WT was substantially increased in the subjects with COPD (irrespective of the presence of PH) compared with control subjects (%WT ± standard error of the mean [SEM]: 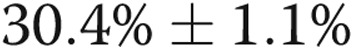 vs.
vs.  ;
; 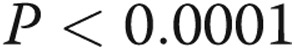 ). Among control subjects, there were no significant differences across pulmonary arterial size or lobar origin. However, in subjects with COPD, %WT was significantly increased in the upper lobes compared with the lower lobes (
). Among control subjects, there were no significant differences across pulmonary arterial size or lobar origin. However, in subjects with COPD, %WT was significantly increased in the upper lobes compared with the lower lobes ( ), and %WT was significantly increased in the small muscular pulmonary arteries compared with the medium and large muscular pulmonary arteries (
), and %WT was significantly increased in the small muscular pulmonary arteries compared with the medium and large muscular pulmonary arteries ( ; Fig. 2).
; Fig. 2).
Figure 2.

Overall, percentage wall thickness (%WT) is greater in the upper lobe compared with the lower lobe (A) and greater in the small muscular pulmonary arteries compared with the medium and large muscular pulmonary arteries (B). The %WT did not vary significantly among the pulmonary arterial pressure group (C). * , †
, †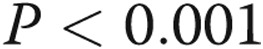 , ‡
, ‡ . ns, not significant; PH, pulmonary hypertension.
. ns, not significant; PH, pulmonary hypertension.
Interaction effects between lobar origin and pulmonary arterial size
For the subjects with COPD, interaction effects were present between lobar origin and muscular pulmonary arterial size, such that %WT was significantly less for the medium ( ) and large (
) and large ( ) muscular pulmonary arteries in the lower lobe compared with the upper lobe, whereas %WT for the small muscular pulmonary arteries was not different across lobar origin (Fig. 3A). The %WT values are presented according to lobar origin and muscular pulmonary arterial size in Table 3.
) muscular pulmonary arteries in the lower lobe compared with the upper lobe, whereas %WT for the small muscular pulmonary arteries was not different across lobar origin (Fig. 3A). The %WT values are presented according to lobar origin and muscular pulmonary arterial size in Table 3.
Figure 3.

Interaction effects of lobar origin, arterial size, and pulmonary hypertension (PH) group on percentage wall thickness (%WT). The %WT for medium and large pulmonary arteries are less in the lower lobes compared with the upper lobes; however, there is no difference in the small pulmonary arteries across lobar origin (A). The %WT for the no PH group is less in the lower lobes compared with the upper lobes; however, there is no difference for mild and moderate-to-severe PH groups across lobar origin (B). There are no interaction effects between pulmonary arterial size and pulmonary arterial pressure group (C). P values are for comparisons between lower and upper lobe. * , †
, †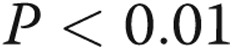 .
.
Table 3.
Mean percentage wall thickness (%WT) according to lobar origin and muscular pulmonary arterial size
| Lobe, artery size | Control subjects (n = 5) | COPD (n = 42) |
|---|---|---|
| Upper | ||
| Small | 13.6 ± 1.3 | 33.0 ± 1.3 |
| Medium | 13.9 ± 1.8 | 32.1 ± 1.3 |
| Large | 10.2 ± 2.2 | 29.1 ± 1.4 |
| Lower | ||
| Small | 13.4 ± 1.2 | 31.9 ± 1.2 |
| Medium | 13.1 ± 1.4 | 27.0 ± 1.3 |
| Large | 12.2 ± 2.1 | 25.2 ± 1.4 |
Values are adjusted mean of %WT ± standard error of the mean. COPD, chronic obstructive pulmonary disease.
Lobar emphysema and perfusion indices
Lobar percentage emphysema and lobar perfusion index measures were only available for 20 and 17 subjects with COPD, respectively. Lobar percentage emphysema was greater in the upper lobes than in the lower lobes (index ± SD, 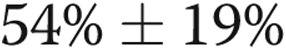 vs.
vs. 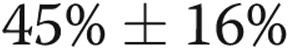 ;
;  ), but was not significantly associated with %WT (
), but was not significantly associated with %WT (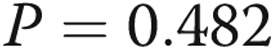 ).
).
The lobar perfusion index was not different between the upper and lower lobes (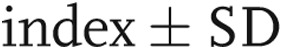 ,
,  vs.
vs.  ;
;  ). Nevertheless, the lobar perfusion index was positively associated with %WT (
). Nevertheless, the lobar perfusion index was positively associated with %WT ( ; Table 4). There were only 14 subjects with COPD with measures for both lobar percentage emphysema and lobar perfusion index and these were not significantly correlated (weighted r = −0.23,
; Table 4). There were only 14 subjects with COPD with measures for both lobar percentage emphysema and lobar perfusion index and these were not significantly correlated (weighted r = −0.23, 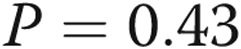 ).
).
Table 4.
Effect of lobar percentage emphysema and lobar perfusion index on the percentage wall thickness (%WT)
| Variable | Parameter estimate | Adjusted P |
|---|---|---|
| Lobar percentage emphysema | 0.36 ± 0.5 | 0.482 |
| Lobar perfusion index | 1.69 ± 0.7 | 0.024 |
Data adjusted for lobe and pulmonary arterial size. Estimates are incremental effects on %WT ± SEM, for every 10% increase in index value.
Pulmonary arterial pressure and pulmonary arterial remodeling
Overall, there were no significant differences in %WT across the pulmonary arterial pressure groups ( ; Fig. 2C). Interaction effects were present between lobar origin and pulmonary arterial pressure group, such that subjects with COPD without PH had less %WT in the lower lobes compared with the upper lobes (
; Fig. 2C). Interaction effects were present between lobar origin and pulmonary arterial pressure group, such that subjects with COPD without PH had less %WT in the lower lobes compared with the upper lobes ( ), but %WT did not change for the other PH categories across lobe origin (Fig. 3B). Despite this interaction, within each lobe there were no significant differences between the PH groups. There were no interaction effects between muscular pulmonary arterial size and pulmonary arterial pressure group (Fig. 3C).
), but %WT did not change for the other PH categories across lobe origin (Fig. 3B). Despite this interaction, within each lobe there were no significant differences between the PH groups. There were no interaction effects between muscular pulmonary arterial size and pulmonary arterial pressure group (Fig. 3C).
Discussion
The key findings of our study are (i) that pulmonary arterial remodeling is consistently present in end-stage COPD; (ii) in COPD, there is increased pulmonary arterial remodeling in the upper lobes compared with the lower lobes; (iii) in COPD, there is greater remodeling in the small muscular pulmonary arteries compared with the medium and larger arteries; and (iv) an increase in regional lung perfusion is associated with increased pulmonary arterial remodeling.
Additionally, no consistent relationship between PH (determined on echocardiography) and pulmonary arterial remodeling was found. Nevertheless, interaction effects are present between lobar origin, pulmonary arterial size, and PH. These findings demonstrate the complexity of the distribution of pulmonary arterial remodeling in COPD.
COPD group versus control group
Our study confirms the presence of pulmonary arterial remodeling universally in end-stage COPD compared with age-matched, nonsmoking controls. Our small number of controls free from chronic cardiac or respiratory disease reflects a small subset of our usual hospital autopsy population. By way of comparison, our measures for pulmonary arterial remodeling among subjects with COPD are slightly less compared with those obtained by Kubo et al.,16 who studied an older cohort of 10 subjects with severe COPD who underwent lung volume reduction surgery (%WT, 30% vs. 36%). Similarly, our control subjects had reduced %WT compared with those reported by Kubo et al.16 (13% vs. 22%), but their control subjects were older nonsmoking patients with lung cancer, which likely accounts for this difference. In addition, slight differences in methodology suggest that our study represents a more conservative estimate of pulmonary arterial remodeling.
Although we have not examined patients with idiopathic pulmonary arterial hypertension (PAH) in this study, Chazova et al.26 examined 19 patients with idiopathic PAH and demonstrated a mean %WT of approximately 60% compared with only 10% in their 7 control subjects. In our institution, we have previously demonstrated increased mean percentage intimal and smooth muscle thickness in muscular pulmonary arteries of patients with pulmonary veno-occlusive disease compared with 6 control subjects (19.3% and 34.1% vs. 9.0% and 25.3%, respectively).21 Unfortunately, each study employs slightly different methodology and assesses different pulmonary artery sizes, so direct comparisons must be made with caution.
Lobe effects
There was increased pulmonary arterial remodeling in the upper lobes compared with the lower lobes in subjects with COPD. Because of the difficulty in finding true control subjects, we were unable to assess for lobar differences in remodeling among control subjects with adequate statistical power. Few previous studies have examined whether differences in pulmonary arterial remodeling exist across different lobes. To our knowledge, the only other study to find increased remodeling in the upper lobes identified increased intimal thickness but not increased medial thickness in the upper lobes of nonsmokers and smokers without significant lung disease.23 However, the authors did not elaborate further on this finding; they did not control their results for pulmonary arterial size, nor did they provide any measure of pulmonary arterial pressure. A subsequent study by the same group22 included subjects with severe COPD but did not report any lobar information regarding pulmonary arterial remodeling.
Other studies reporting lobar-specific measures of pulmonary arterial remodeling have not shown lobar differences.14,27-30 These negative results are consistent with a hypothesis that the entire pulmonary arterial bed is under that same pulmonary arterial pressure and hence should have a similar degree of pulmonary arterial remodeling. However, this presumes that it is the pulmonary arterial pressure that drives the remodeling process independent of pulmonary arterial size or location. In contrast, Botney31 has proposed a 2-hit hypothesis requiring both increased pulmonary blood flow and endothelial injury before the development of neointimal pulmonary arterial remodeling. In reality, pulmonary arterial pressures and remodeling are likely codependent, moderated by arterial resistance, shear forces, and blood flow. Consequently, muscular pulmonary arterial size and location are likely to affect this interaction.
Lobe and pulmonary arterial size
Pulmonary arterial remodeling was increased in the small muscular pulmonary arteries compared with the medium and larger arteries, and this is consistent with some previous studies.15,17,27,29,32 However, other studies have not supported this relationship.13,30 Some caution is needed in inferring a predilection for remodeling of smaller pulmonary arteries, because our measure of remodeling is partially dependent on the size of the vessel.
The distribution of pulmonary arterial remodeling in different-sized pulmonary arteries is also lobe dependant, with relative sparing of lower lobe pulmonary arterial remodeling in the medium and large muscular pulmonary arteries. Of note, the distribution of muscular pulmonary arterial sizes is different between the lower and upper lobes (repeated-measures weighted mean  :
:  mm vs.
mm vs.  mm;
mm;  ), a relationship that was not present in the control group. From our data, we are not able to determine whether this difference in the distribution of pulmonary arterial sizes is associated with greater arterial remodeling in the upper lobes. Nevertheless, the notion that the distribution of vessel size can influence pulmonary hemodynamics is supported by a recent study33 that demonstrated that having fewer small pulmonary vessels was associated with increased pulmonary arterial pressure in a cohort of patients with severe emphysema enrolled in the National Emphysema and Treatment Trial.
), a relationship that was not present in the control group. From our data, we are not able to determine whether this difference in the distribution of pulmonary arterial sizes is associated with greater arterial remodeling in the upper lobes. Nevertheless, the notion that the distribution of vessel size can influence pulmonary hemodynamics is supported by a recent study33 that demonstrated that having fewer small pulmonary vessels was associated with increased pulmonary arterial pressure in a cohort of patients with severe emphysema enrolled in the National Emphysema and Treatment Trial.
Lobar emphysema and perfusion indices
CT quantification of emphysema has been found to be a good marker of the anatomical distribution and severity of emphysema,24,34 but the relationship between histologic emphysema severity and pulmonary arterial remodeling has been inconsistent.14,22,23 Although we demonstrated greater upper lobe emphysema, there was no significant relationship between lobar severity of emphysema and pulmonary arterial remodeling. Furthermore, there was no significant relationship between lobar severity of emphysema and pulmonary artery density, measured as the number of pulmonary arteries identified per lobe per subject.
In contrast, the lobar perfusion index was found to have a significant positive relationship to pulmonary arterial remodeling after adjusting for lobe and muscular pulmonary arterial size. It would be expected that, as a result of destruction of the pulmonary capillary bed, emphysematous areas of the lung receive less perfusion, and this would be further exacerbated by hypoxic pulmonary vasoconstriction. However, if emphysema causes localized pulmonary arterial remodeling, one would expect reduced perfusion to these areas and a negative relationship between pulmonary arterial remodeling and the lobar perfusion index. Surprisingly, we have demonstrated that pulmonary arterial remodeling and lobar perfusion index are positively associated, which suggests that pulmonary arterial remodeling may occur secondary to increased pulmonary perfusion. Hence, our data support the aforementioned 2-hit hypothesis of remodeling.31
Nevertheless, the explanation for increased upper lobe remodeling in this COPD cohort is uncertain. Although not directly associated with increased upper lobe emphysema, the increased upper lobe remodeling may be a result of vascular changes induced by cigarette smoke that, because of regional differences in ventilation, perfusion, inflammation, and clearance of toxins, appear to have a predilection for the upper lobes.35 Furthermore, cigarette smoke exposure has been demonstrated to cause pulmonary vascular changes independent of the development of emphysema in a guinea pig model.33
Pulmonary arterial pressure
Invasive pulmonary hemodynamic testing is not performed as part of routine lung transplant assessment in our center; however, all patients with COPD are assessed by echocardiography. The interaction effects observed between lobe and pulmonary arterial pressure suggest that upper lobe remodeling occurs in the absence of PH. Within the lower lobes, there was a trend to increased pulmonary arterial remodeling among subjects with COPD with moderate-to-severe PH compared with subjects with COPD without PH (unadjusted 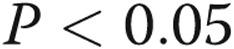 , adjusted
, adjusted 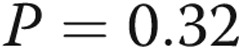 ). It is not apparent whether lower lobe pulmonary arterial remodeling occurs before or after the development of PH.
). It is not apparent whether lower lobe pulmonary arterial remodeling occurs before or after the development of PH.
Limitations
Our study has 2 main limitations. First, lobar percentage emphysema and lobar perfusion index were only performed on a subset of subjects with COPD. Although percentage emphysema scores were calculated prospectively, they were measured using the archived 7.5-mm-thick, contrast-enhanced CT chest scans performed during the lung transplant assessment. Although thinner slice, non–contrast-enhanced CT is generally the preferred mode from which to quantify emphysema scores, the correlation between percentage emphysema and quantitative histological findings is similar across a range of CT section thicknesses ranging from 1 to 10 mm.34
The second main limitation is that pulmonary arterial pressures were estimated from echocardiographic findings rather than the gold standard of right heart catheterization. Clearly, determining COPD to have a high transpulmonary gradient and correlating the vascular remodeling with pulmonary vascular resistance would lead to a more definitive study. Despite the well-documented concerns with using echocardiography to estimate pulmonary arterial pressures, especially in patients with COPD,37,38 echocardiography remains the most accepted noninvasive tool for the assessment of PH in the clinical setting,39 and echocardiographic-determined PH has been associated with increased mortality among patients with COPD.40
Conclusion
This study confirms that severe COPD is associated with a complex pattern of significant pulmonary arterial remodeling. Using histological, radiological, and clinical data, the distribution of remodeling in COPD is lobe dependent and influenced by pulmonary arterial size and pressure. Increased regional blood flow partially contributes to the increased pulmonary arterial remodeling in these subjects. These findings have not been previously demonstrated.
To explain lobar differences in pulmonary arterial remodeling and the interactions between regional blood flow, arterial remodeling, and PH requires additional research. Our study would support future studies using invasive pulmonary hemodynamics, particularly measurement of pulmonary vascular resistance.
Sources of Support: The research was made possible from funding provided to JPW through an Australian National Health and Medical Research Council Postgraduate Scholarship (APP1017853).
Conflict of Interest: None declared.
References
- 1.Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, Kessler R. Prognostic factors in COPD patients receiving long-term oxygen therapy: importance of pulmonary artery pressure. Chest 1995;107:1193–1198. [DOI] [PubMed]
- 2.Doi M, Nakano K, Hiramoto T, Kohno N. Significance of pulmonary artery pressure in emphysema patients with mild-to-moderate hypoxemia. Respir Med 2003;97:915–920. [DOI] [PubMed]
- 3.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:158–164. [DOI] [PubMed]
- 4.Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest 2009;136:412–419. [DOI] [PMC free article] [PubMed]
- 5.Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, Tamm M. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008;32:619–628. [DOI] [PubMed]
- 6.Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, Roca J, Barbera JA. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010;181:270–278. [DOI] [PubMed]
- 7.Sajkov D, McEvoy RD, Cowie RJ, Bradley JA, Antic R, Morris RG, Frith PA. Felodipine improves pulmonary hemodynamics in chronic obstructive pulmonary disease. Chest 1993;103:1354–1361. [DOI] [PubMed]
- 8.Morley TF, Zappasodi SJ, Belli A, Giudice JC. Pulmonary vasodilator therapy for chronic obstructive pulmonary disease and cor pulmonale: treatment with nifedipine, nitroglycerin, and oxygen. Chest 1987;92:71–76. [DOI] [PubMed]
- 9.Rietema H, Holverda S, Bogaard HJ, Marcus JT, Smit HJ, Westerhof N, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Sildenafil treatment in COPD does not affect stroke volume or exercise capacity. Eur Respir J 2008;31:759–764. [DOI] [PubMed]
- 10.Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P, Germann P, Block LH. Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 2003;58:289–293. [DOI] [PMC free article] [PubMed]
- 11.Lederer DJ, Bartels MN, Schluger NW, Brogan F, Jellen P, Thomashow BM, Kawut SM. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD 2012;9:268–275. [DOI] [PMC free article] [PubMed]
- 12.Wrobel JP, Thompson BR, Williams TJ. Mechanisms of pulmonary hypertension in chronic obstructive pulmonary disease: a pathophysiologic review. J Heart Lung Transplant 2012;31:557–564. [DOI] [PubMed]
- 13.Magee F, Wright JL, Wiggs BR, Pare PD, Hogg JC. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax 1988;43:183–189. [DOI] [PMC free article] [PubMed]
- 14.Wilkinson M, Langhorne CA, Heath D, Barer GR, Howard P. A pathophysiological study of 10 cases of hypoxic cor pulmonale. Q J Med 1988;66:65–85. [PubMed]
- 15.Shelton DM, Keal E, Reid L. The pulmonary circulation in chronic bronchitis and emphysema. Chest 1977;71:303–306. [DOI] [PubMed]
- 16.Kubo K, Ge RL, Koizumi T, Fujimoto K, Yamanda T, Haniuda M, Honda T. Pulmonary artery remodeling modifies pulmonary hypertension during exercise in severe emphysema. Respir Physiol 2000;120:71–79. [DOI] [PubMed]
- 17.Wright JL, Petty T, Thurlbeck WM. Analysis of the structure of the muscular pulmonary arteries in patients with pulmonary hypertension and COPD: national institutes of health nocturnal oxygen therapy trial. Lung 1992;170:109–124. [DOI] [PubMed]
- 18.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease: a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958;18:533–547. [DOI] [PubMed]
- 19.Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB. Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest 1977;59:203–216. [DOI] [PMC free article] [PubMed]
- 20.Wedzicha JA. The heterogeneity of chronic obstructive pulmonary disease. Thorax 2000;55:631–632. [DOI] [PMC free article] [PubMed]
- 21.Harch S, Whitford H, McLean C. Failure of medical therapy in pulmonary arterial hypertension: is there an alternative diagnosis? Chest 2009;135:1462–1469. [DOI] [PubMed]
- 22.Hale KA, Ewing SL, Gosnell BA, Niewoehner DE. Lung disease in long-term cigarette smokers with and without chronic air-flow obstruction. Am Rev Respir Dis 1984;130:716–721. [DOI] [PubMed]
- 23.Hale KA, Niewoehner DE, Cosio MG. Morphologic changes in the muscular pulmonary arteries: relationship to cigarette smoking, airway disease, and emphysema. Am Rev Respir Dis 1980;122:273–278. [DOI] [PubMed]
- 24.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest 1988;94:782–787. [DOI] [PubMed]
- 25.Badesch DB, Champion HC, Gomez Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55–S66. [DOI] [PubMed]
- 26.Chazova I, Loyd JE, Zhdanov VS, Newman JH, Belenkov Y, Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 1995;146:389–397. [PMC free article] [PubMed]
- 27.Simons P, Reid L. Muscularity of pulmonary artery branches in the upper and lower lobes of the normal young and aged lung. Br J Dis Chest 1969;63:38–44. [DOI] [PubMed]
- 28.Warnock ML, Kunzmann A. Changes with age in muscular pulmonary arteries. Arch Pathol Lab Med 1977;101:175–179. [PubMed]
- 29.Warnock ML, Kunzmann A. Muscular pulmonary arteries in chronic obstructive lung disease. Arch Pathol Lab Med 1977;101:180–186. [PubMed]
- 30.Fernie JM, Lamb D. Assessment of the effects of age and smoking on the media of muscular pulmonary arteries. J Pathol 1988;155:241–246. [DOI] [PubMed]
- 31.Botney MD. Role of hemodynamics in pulmonary vascular remodeling: implications for primary pulmonary hypertension. Am J Respir Crit Care Med 1999;159:361–364. [DOI] [PubMed]
- 32.Barbera JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, Wiggs BR, Rodriguez-Roisin R. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;149:423–429. [DOI] [PubMed]
- 33.Matsuoka S, Washko GR, Yamashiro T, Estepar RS, Diaz A, Silverman EK, Hoffman E, et al. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med 2010;181:218–225. [DOI] [PMC free article] [PubMed]
- 34.Gierada DS, Bierhals AJ, Choong CK, Bartel ST, Ritter JH, Das NA, Hong C, et al. Effects of CT section thickness and reconstruction kernel on emphysema quantification relationship to the magnitude of the CT emphysema index. Acad Radiol 2010;17:146–156. [DOI] [PMC free article] [PubMed]
- 35.Takahashi M, Fukuoka J, Nitta N, Takazakura R, Nagatani Y, Murakami Y, Otani H, Murata K. Imaging of pulmonary emphysema: a pictorial review. Int J Chron Obstruct Pulmon Dis 2008;3:193–204. [DOI] [PMC free article] [PubMed]
- 36.Wright JL, Churg A. Effect of long-term cigarette smoke exposure on pulmonary vascular structure and function in the guinea pig. Exp Lung Res 1991;17:997–1009. [DOI] [PubMed]
- 37.Arcasoy SM, Christie JD, Ferrari VA, Sutton MSJ, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003;167:735–740. [DOI] [PubMed]
- 38.Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, Fessler AH, Group NR. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J 2007;30:914–921. [DOI] [PubMed]
- 39.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219–1263. [DOI] [PubMed]
- 40.Stone AC, Machan JT, Mazer J, Casserly B, Klinger JR. Echocardiographic evidence of pulmonary hypertension is associated with increased 1-year mortality in patients admitted with chronic obstructive pulmonary disease. Lung 2011;189:207–212. [DOI] [PubMed]


