Abstract Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a life-threatening condition characterized by single or recurrent pulmonary thromboemboli, which promote pulmonary vascular remodeling. MicroRNA (miRNA), is a small, noncoding RNA that is involved in multiple cell processes and functions and may participate in the pathogenesis of CTEPH. Our aims were to identify the miRNA expression signature in pulmonary artery smooth muscle cells (PASMCs) of CTEPH patients and to study the role of let-7d in CTEPH pathogenesis. The miRNA expression profile was analyzed by microarray in PASMCs of CTEPH and control patients. Differentially expressed miRNAs were selectively validated by stem-loop quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR). The role of let-7d was identified by in silico analysis, and its effect on the proliferation of PASMCs was measured by methyl thiazolyl tetrazolium (MTT). Student’s unpaired t test, the Fisher exact test, and the χ2 test were used for statistical analysis. Eighteen miRNAs were differentially expressed in PASMCs from CTEPH patients, including 12 upregulated miRNAs and 6 downregulated miRNAs; among the latter, let-7d decreased 0.58-fold in CTEPH patients, as validated by qRT-PCR. It was found that let-7d could inhibit the proliferation of PASMCs through upregulation of p21. In conclusion, PASMCs in CTEPH patients have an aberrant miRNA profile and reduced let-7d, which could promote PASMC proliferation and may be involved in the pathogenesis of CTEPH.
Keywords: chronic thromboembolic pulmonary hypertension, let-7d, pulmonary artery smooth muscle cell, proliferation
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is one of the leading causes of severe pulmonary hypertension (PH). CTEPH is characterized by persistent pulmonary embolism, which can in turn increase pulmonary vascular resistance, resulting in PH and consequent right-side heart failure.1 It has been known for many years that CTEPH cannot be explained simply by pulmonary vascular obliteration due to unresolved thromboemboli but that a pulmonary embolism could be the initiating event, while ensuing PH would result from progressive pulmonary vascular remodeling.2,3 The process of pulmonary vascular remodeling involves all layers of the vessel wall and is complicated by cellular heterogeneity within each compartment of the pulmonary arterial wall.4 Until recently, the pathogenesis of pulmonary vascular remodeling in CTEPH was largely unknown, but new genetic and pathophysiological studies have emphasized the involvement of a number of molecular mechanisms, including pathways involving growth factors, cytokines, metabolic signaling, elastases, and proteases.5,6
MicroRNAs (miRNAs) are small, noncoding RNAs (21–23 nucleotides) that participate in diverse aspects of biology, including developmental timing, differentiation, proliferation, cell death, and metabolism.7,8 Recently, differential expression of miRNAs has been implicated in many cardiovascular diseases, including PH. Dysregulated expression of bone morphogenetic protein receptor type II (BMPR2), which is a pathogenetic hallmark of PH, is regulated by miR-17/92.9 MiR-204 plays an important role in the decrease of proliferation, vascular remodeling, and pulmonary artery blood pressure in PH and could be a new therapeutic target for PH.10 MiR-21 also plays a significant role in hypoxia-induced pulmonary vascular smooth muscle cell (VSMC) proliferation and migration.11
Altered expression of miRNAs has now been found in some kinds of PH by microarray analysis, which is an established, high-throughput technique. Researchers who screened lung miRNA profiles in a longitudinal, crossover design during the development of PH caused by chronic hypoxia or monocrotaline in rats found that distinct miRNAs were regulated during the development of PH in rats.12 However, the miRNA expression profile and its possible role in CTEPH patients remain unresolved. Recently, it has been reported that the expression of the fibrinogen alpha gene regulated by miR-759 was associated with susceptibility to CTEPH.13
Let-7 microRNAs form a conserved microRNA family consisting of 12 genes (let-7a to let-7i) encoding different miRNAs.14 Because they are downregulated in various cancers and also can target oncogenes, they are usually known as tumor suppressors, and in lung cancer, low levels of let-7 was associated with shortened postoperative survival.15 It was also reported that let-7 could repress cell proliferation via cell cycle regulation.16 So alternately expressed let-7 may participate in pulmonary vascular remodeling in CTEPH. Of the let-7 family, let-7d could influence VSMC proliferation.17 How its expression changes and how it affects CTEPH are still unknown.
In this study, we performed microarray analysis to determine whether any miRNAs differentially expressed and investigated the role of some alternately expressed miRNAs (e.g., let-7d) in the pathogenesis of CTEPH.
Material and methods
Subjects
The study was approved by the Research Ethics Committee of Beijing Chao-Yang Hospital of Capital Medical University. Written informed consent was obtained from all patients before the procedure. The microarray cohort was composed of 5 CTEPH patients and 3 control subjects.
Isolation and culture of primary pulmonary artery smooth muscle cells (PASMCs)
PASMCs were isolated from tissues of CTEPH patients and control subjects. The PASMCs of CTEPH patients were carefully isolated from the endothelium and the thin layer of media covering the endarterectomy tissues (selected as far as possible from the downstream branches). The isolated cells were all spindle shaped, and almost all of the cells isolated from pulmonary endarterectomy (PEA) tissues were positive for α-smooth muscle actin and major histocompatibility complex, which are both smooth muscle cell–specific markers (data not shown). The selected tissues were cut into small pieces, incubated in fresh Hank’s balanced salt solution (HBSS) containing 2.5 mg/mL collagenase (Worthington Biochemical, Lakewood, NJ) and 1.0 mg/mL bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) for 30 min at 37°C, centrifuged at 300 g for 5 min, resuspended in Medium 231 (Gibco, Grand Island, NE) supplemented with smooth muscle growth supplement, 200 μg/mL of penicillin, and 200 IU/mL streptomycin, seeded in dishes, and incubated in a humidified 5% CO2 atmosphere at 37°C.
MiRNA microarray analysis
A miRCURY LNA Array (ver. 16.0; Exiqon, Vedbaek, Denmark) analysis was performed on RNA extracted from PASMC RNA from 5 CTEPH patients and 3 control subjects to identify the differential miRNA expression profile of CTEPH. Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) and the miRNeasy mini kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. After RNA isolation, the miRCURY Hy3/Hy5 Power labeling kit (Exiqon) was used according to the manufacturer’s guideline for miRNA labeling. The labeled samples were hybridized on the miRCURY LNA Array according to the array manual. The slides were scanned using the Axon GenePix 4000B microarray scanner (Axon Instruments, Foster City, CA), and the scanned images were then imported into GenePix Pro 6.0 software (Axon Instruments) for grid alignment and data extraction.
Stem-loop quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) assays
Real-time PCR was performed using an Applied Biosystems 7500 Sequence Detection system. The 20-μL PCR reaction included 2 μL reverse-transcription product, 9 μL SYBR Green Mix, 2 μL miRNA forward primer, 2 μL miRNA reverse primer, and 5 μL RNase-free H2O. The reactions were heated to 95°C for 20 s, followed by 40 cycles of 95°C for 10 s, 60°C for 20 s, and 70°C for 6 s. The relative amount of miRNAs was normalized against U6 snRNA (small nuclear RNA).
Gene ontology (GO) analysis
GO analysis was applied to analyze the main function of target genes of differentially expressed miRNAs. GO is a key functional classification of the National Center for Biotechnology Information.18 Fisher’s exact test and a χ2 test were used to classify the GO category, and the false discovery rate (FDR) was calculated to correct the P value (the smaller the FDR, the smaller the error in judging the P value). We chose only GOs that had a P value <0.001 and an FDR <0.05.
Pathway analysis
Pathway analysis was used to discover the significant pathways of the target genes of differentially expressed miRNAs according to the Kyoto Encyclopedia of Genes and Genomes (KEGG), Biocarta, and Reatome.19,20 Fisher’s exact test and a χ2 test were used to select the significant pathway, and the threshold of significance was defined by a P value <0.001 and an FDR <0.05.
miRNA-gene network
The relationships between the miRNAs and target genes were calculated on the basis of their differing expression values and according to the interactions of miRNAs and target genes in the Sanger miRNA database to build the miRNA-gene network.21,22 The adjacency matrix of miRNAs and target genes, 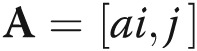 , is made by the attribute relationships among target genes and miRNAs, and [ai, j] represents the relation weight of target gene i and miRNA j. In the miRNA-gene network, circles represent genes and squares represent miRNAs, and their relationship is represented by an edge (see Fig. S1). The center of the network is represented by degree, that is, the contribution of one miRNA to the target genes around it or the contribution of one target gene to the miRNAs around it. The key miRNA and gene in the network always have the highest degrees.
, is made by the attribute relationships among target genes and miRNAs, and [ai, j] represents the relation weight of target gene i and miRNA j. In the miRNA-gene network, circles represent genes and squares represent miRNAs, and their relationship is represented by an edge (see Fig. S1). The center of the network is represented by degree, that is, the contribution of one miRNA to the target genes around it or the contribution of one target gene to the miRNAs around it. The key miRNA and gene in the network always have the highest degrees.
Transfection of miRNA
Transient transfection of let-7d mimics (GenePharma, Shanghai) was carried out with Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s procedure. Briefly, the PASMCs were washed with a serum-free medium and cultured in the serum-free medium without antibiotics. The transfection complex (let-7d and the transfection reagent mixture) were added to the medium in a drop-wise manner and mixed gently by rocking the medium back and forth. After 4–6 hours, the cell culture medium was transferred to a medium containing serum and antibiotics and incubated at 37°C for 48 hours before the proliferation assay, Western blot analysis, or PCR experiments were conducted.
Cell growth assay
We used methyl thiazolyl tetrazolium (MTT; Sigma-Aldrich) to determine the viability of cells. Cells were plated in 96-well plates at 5,000 cells/well and incubated for 48 hours after transfection of let-7d mimics or negative controls. Following this incubation, MTT (5 mg/mL) reagent was added to each well and the cells were incubated for an additional 4 hours, at which time the supernatant was removed and 150 μL/well of dimethyl sulfoxide (DMSO; Sigma-Aldrich) was added to solubilize the formazan salt crystals. Solubilized formazan products were quantified by spectrophotometry at 490 nm using an enzyme-linked immunosorbent assay reader (Bio-Rad, Hercules, CA).
Western blot analysis
PASMC proteins were separated on an SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) gel and subsequently blotted onto a nitrocellulose membrane (Millipore, Billerica, MA). After blocking for 1 hour with 5% nonfat dry milk dissolved in phosphate-buffered saline, the membrane was incubated with anti-p21 primary antibody (1∶1,000; Cell Signaling Technology, Beverly, MA) and anti-β-actin primary antibody (1∶1,000; Sigma-Aldrich) overnight at 4°C. The primary antibody-labeled membranes were then treated with IRDyeTM800 (green) or IRDyeTM700 (red) conjugated, affinity-purified antirabbit immunoglobulin G for 1 hour. The positive protein bands were visualized by LI-COR Odyssey infrared double-fluorescence imaging system (LI-COR, Lincoln, NE). The value of the relative density of the target protein band was normalized to the density of the β-actin band.
Statistical analysis
Data were presented as means ± standard error. Comparison between the groups of data was evaluated with Student’s unpaired t-test. A P value <0.05 was considered statistically significant. Fisher’s exact test and the χ2 test were used in GO and pathway analysis.
Results
Global miRNA profile of PASMCs in CTEPH patients
To analyze the miRNA expression profile, PASMCs were isolated from 5 CTEPH patients’ endarterectomy tissues (Fig. 1) and 3 control patients’ tissues from lobectomy for pneumothorax or lung transplantation in Beijing Chao-Yang Hospital. The clinical characteristics of the CTEPH patients are displayed in Table 1.
Figure 1.
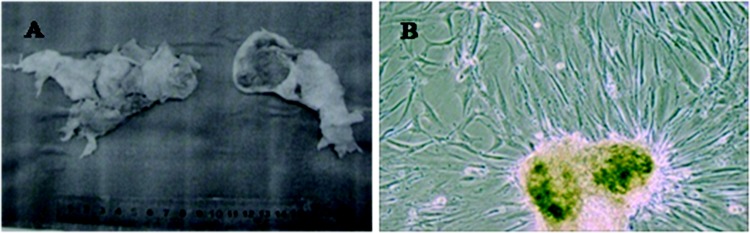
A, Thromboembolic tissue removed from the pulmonary arteries during pulmonary endarterectomy (PEA). The PEA tissues are unresolved thromboemboli covered by a thin layer of media and fibrotic intima. B, Light microscopy image of isolated pulmonary artery smooth muscle cells of chronic thromboembolic pulmonary hypertension patients. The spindle-shaped cells are growing from the adherent tissue pieces at the first passage.
Table 1.
Clinical and hemodynamics data of the 5 CTEPH patients
| CTEPH-1 | CTEPH-2 | CTEPH-3 | CTEPH-4 | CTEPH-5 | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 62 | 37 | 44 | 41 | 49 |
| Sex | Male | Male | Male | Male | Male |
| Weight, kg | 80 | 63 | 68 | 69 | 75 |
| Height, cm | 182 | 173 | 170 | 172 | 174 |
| NYHA functional class | III | IV | III | II | II |
| 6-MWD, m | 340 | 129 | 384 | 324 | 321 |
| Hemodynamics data | |||||
| mPAP, mmHg | 55 | 89 | 49 | 41 | 65 |
| CI, L/min/m2 | 3.1 | 1.5 | 1.8 | 1.44 | 1.3 |
| PVR, dyn/s/cm5 | 645 | 2,463 | 925 | 1,186 | 1,830 |
| mRAP, mmHg | 8 | 15 | 11 | 12 | 3 |
| PWP, mmHg | 6.2 | 12 | 12 | 2 | 8 |
| Risk factors | |||||
| Previous VTE | No | No | No | Yes | Yes |
| Recurrent VTE | No | No | No | Yes | No |
| Malignancy | No | No | No | No | No |
| Ventriculo-atrial shunt | No | No | No | No | No |
| Thyroid hormone replacement | No | No | No | No | No |
| Pacemaker | No | No | No | No | No |
| Infected pacemaker | No | No | No | No | No |
| Splenectomy | No | No | No | No | No |
CTEPH: chronic thromboembolic pulmonary hypertension; NYHA functional class: New York Heart Association functional classification of the World Health Organization; 6-MWD: 6-minute walk distance; mPAP: mean pulmonary artery pressure; CI: cardiac index; PVR: pulmonary vascular resistance; mRAP: mean right atrial pressure; PWP: pulmonary wedge pressure; VTE: venous thromboembolism.
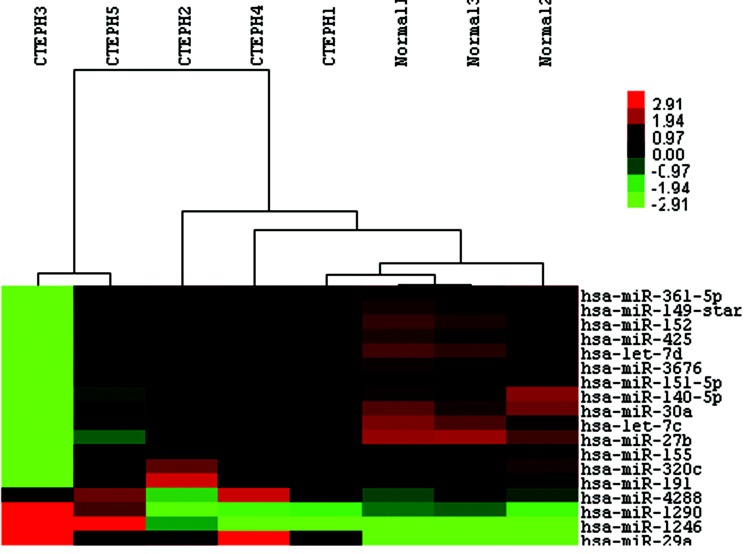
The miRCURY LNA Array (v.16.0) system was then employed to analyze RNA isolated from PASMCs of the 5 CTEPH patients and 3 controls. From the 1,891 capture probes, we found 12 miRNAs that were upregulated and 6 that were downregulated (each with a 2-fold change) in CTEPH patients relative to those in controls, as shown in Table 2. The hierarchical cluster of differentially expressed miRNAs in CTEPH patients versus controls is shown in Figure 2.
Table 2.
Differentially expressed miRNAs identified in cells of CTEPH patients
| miRNA | Accession | Control mean | CTEPH patient mean | Fold change |
|---|---|---|---|---|
| Upregulated miRNAs | ||||
| hsa-miR-320c | MIMAT0000087 | 0.3848122 | 0.7791244 | 2.0246874 |
| hsa-miR-149 | MIMAT0004609 | 0.0388231 | 0.0848726 | 2.1861367 |
| hsa-miR-4288 | MIMAT0016918 | 2.4845110 | 5.4681362 | 2.2008903 |
| hsa-miR-191 | MIMAT0000440 | 0.6079675 | 1.3494137 | 2.2195490 |
| hsa-miR-425 | MIMAT0003393 | 0.0393036 | 0.0880385 | 2.2399576 |
| hsa-miR-151-5p | MIMAT0004697 | 0.1679546 | 0.3951892 | 2.3529527 |
| hsa-miR-29a | MIMAT0000086 | 5.8436868 | 14.850401 | 2.5412725 |
| hsa-miR-361-5p | MIMAT0000703 | 0.1938334 | 0.4987414 | 2.5730411 |
| hsa-miR-3676 | MIMAT0022734 | 0.1243556 | 0.3460382 | 2.7826502 |
| hsa-miR-1246 | MIMAT0005898 | 6.9651019 | 20.463536 | 2.9380096 |
| hsa-miR-155 | MIMAT0000646 | 0.2615062 | 0.8869655 | 3.3917565 |
| hsa-miR-1290 | MIMAT0005880 | 1.1478704 | 5.2392336 | 4.5643078 |
| Downregulated miRNAs | ||||
| hsa-miR-30a | MIMAT0000087 | 0.7497516 | 0.2369417 | 0.3160270 |
| hsa-let-7c | MIMAT0000064 | 0.6185536 | 0.2692877 | 0.4353506 |
| hsa-miR-140-5p | MIMAT0000431 | 0.6613641 | 0.3037604 | 0.4592938 |
| hsa-miR-27b | MIMAT0000419 | 2.0069687 | 0.9541719 | 0.4754294 |
| hsa-let-7d | MIMAT0000065 | 0.2752446 | 0.1325376 | 0.4815267 |
| hsa-miR-152 | MIMAT0000438 | 0.1579967 | 0.0763367 | 0.4831536 |
miRNA: microRNA; CTEPH: chronic thromboembolic pulmonary hypertension.
Figure 2.
Heat maps of the 18 microRNAs (miRNAs) expressed differently between 5 chronic thromboembolic pulmonary hypertension (CTEPH) patients and 3 control patients. Rows represent miRNAs, and columns represent patients. A color change from red to green indicates that expression was downregulated. In contrast, a color change from green to red indicates that expression was upregulated. Color brightness represents the signal values of miRNAs in the microarray chip.
In silico analysis revealed the role of let-7d in CTEPH pathogenesis
In silico analysis was used to analyze and identify differentially expressed miRNAs that could play a role in the pathogenesis of CTEPH. First, a list of messenger RNA (mRNA) targets was identified by screening the 3′ untranslated regions of mRNAs for seed sequences of the 18 differentially expressed miRNAs. Because reliable miRNA target information is still limited, most of the target prediction methods used generate a large number of predicted targets, many of which are presumed to be false. We therefore integrated miRNA targets from both Targetscan and Miranda databases to predict the potential miRNA targets in our study, and we obtained 1,830 target genes. The functions of these target genes were then analyzed according to GO, and their target pathways were determined according to KEGG, Biocarta, and Reatome (data not shown). According to the threshold of significant GOs and pathways (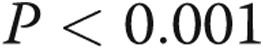 and
and 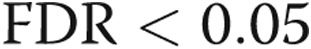 ), 308 target genes were identified.
), 308 target genes were identified.
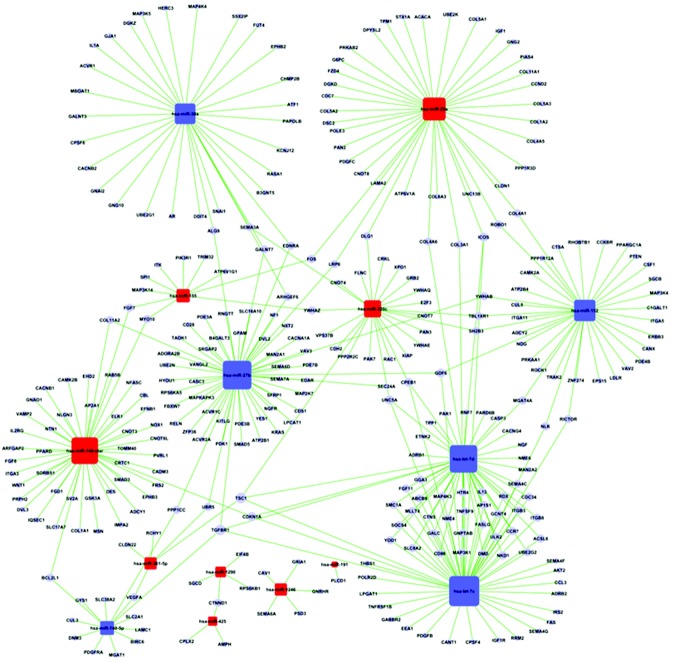
To elaborate the relationship between the 18 differentially expressed miRNAs and the 308 target genes and to find the major differentially expressed miRNAs in CTEPH, the miRNA–gene regulatory network (18 miRNAs and 308 target genes) was established, as shown in Figure S1. As explained in “Material and methods,” the term “degree” describes the contribution of one miRNA to the genes around it or the contribution of one gene to the miRNAs around it, so the key miRNA and gene in the network will have the highest degree. The 18 differentially expressed miRNAs were ranked according to degree, and let-7c, miR-27b, and let-7d were the highest-ranked miRNAs, as shown in Table 3. Meanwhile, the top 10 target genes in the network are shown in Table 4; interestingly, the first three are all genes of the let-7 family.
Table 3.
Degree of differentially expressed microRNAs
| MicroRNA | MicroRNA style | Degree |
|---|---|---|
| hsa-let-7c | Downregulated | 38 |
| hsa-miR-27b | Downregulated | 34 |
| hsa-let-7d | Downregulated | 30 |
| hsa-miR-149* | Upregulated | 29 |
| hsa-miR-152 | Downregulated | 26 |
| hsa-miR-29a | Upregulated | 25 |
| hsa-miR-30a | Downregulated | 16 |
| hsa-miR-320c | Upregulated | 15 |
| hsa-miR-140-5p | Downregulated | 7 |
| hsa-miR-1290 | Upregulated | 4 |
| hsa-miR-155 | Upregulated | 4 |
| hsa-miR-1246 | Upregulated | 3 |
| hsa-miR-361-5p | Upregulated | 3 |
| hsa-miR-425 | Upregulated | 1 |
Table 4.
Top 10 target genes according to degree
| Gene symbol | Description | Degree |
|---|---|---|
| TGFBR1 | Transforming growth factor, beta receptor 1 | 4 |
| TSC1 | Tuberous sclerosis 1 | 4 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) c | 3 |
| CPEB1 | Cytoplasmic polyadenylation element binding protein 1 | 3 |
| GDF6 | Growth differentiation factor 6 | 3 |
| HTR4 | 5-hydroxytryptamine (serotonin) receptor 4 | 2 |
| BCL2L1 | BCL2-like 1 | 2 |
| CTNND1 | Catenin (cadherin-associated protein), delta 1 | 2 |
| CCR7 | Chemokine (C-C motif) receptor 7 | 2 |
Let-7d repressed the proliferation of PASMCs by upregulating p21
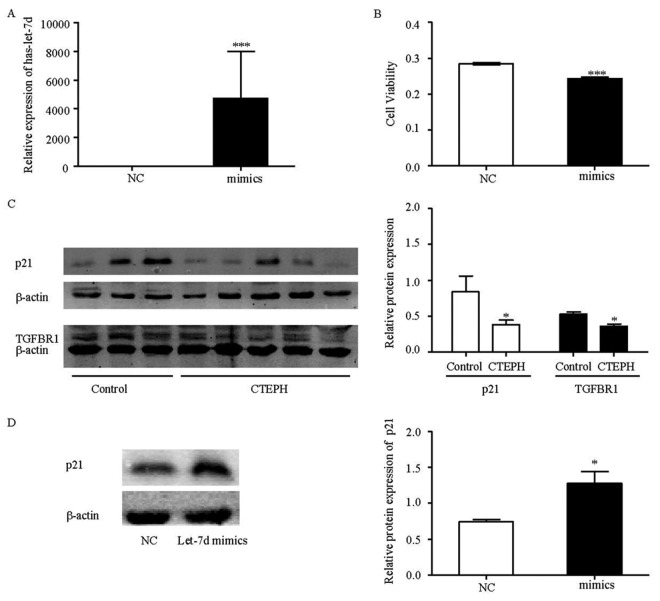
To confirm the findings obtained from the miRNA profile, we validated let-7c and let-7d expression levels in PASMCs from CTEPH patients, using stem-loop qRT-PCR, and found that only let-7d was decreased in our study (Fig. 3). It has been reported that the let-7 family can act as tumor suppressors and key regulators of cell proliferation pathways.23 As a result, it is possible that let-7d, which was downregulated in CTEPH patients, may play an important role in repressing the proliferation of PASMCs. Therefore, the role of let-7d in the proliferation of PASMCs was investigated. As shown in Figure 4, overexpressing let-7d (Fig. 4A) could significantly repress the proliferation of PASMCs (Fig. 4B). To elucidate the mechanisms, the protein expression levels of its target genes, TGFBR1 and p21, were examined (Fig. 4C). It was observed that both of these proteins were downregulated in PASMCs of CTEPH patients.
Figure 3.
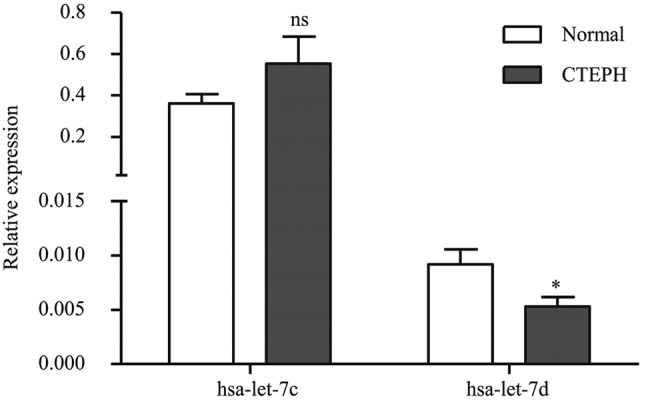
Expression of let-7c and let-7d in pulmonary artery smooth muscle cells of chronic thromboembolic pulmonary hypertension (CTEPH) patients and control (“normal”) patients. The relative let-7c and let-7d expressions were determined by stem-loop quantitative real-time reverse-transcription polymerase chain reaction. Data shown are means + standard error; asterisk indicates 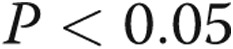 versus controls.
versus controls.
Figure 4.
Let-7d repressed pulmonary artery smooth muscle cell (PASMC) proliferation by upregulating p21. A, Expression of let-7d after transfection of let-7d mimics and negative control (NC). B, Proliferation of PASMCs when treated with let-7d mimics. C, Protein expression levels of p21 (21 kDa), TGFBR1 (52 kDa; see Table 4 for definition), and β-actin (43 kDa) in PASMCs from chronic thromboembolic pulmonary hypertension (CTEPH; 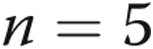 ) and control (
) and control (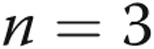 ) patients. D, Protein expression levels of p21 in PASMCs of CTEPH patients after transfection of let-7d mimics. The results are presented as means + standard error; a single asterisk indicates
) patients. D, Protein expression levels of p21 in PASMCs of CTEPH patients after transfection of let-7d mimics. The results are presented as means + standard error; a single asterisk indicates 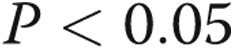 , and three asterisks indicate
, and three asterisks indicate 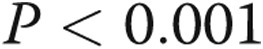 , both versus NCs.
, both versus NCs.
After a detailed literature review, we discovered that let-7 could reduce cell cycle progression, thereby repressing cell proliferation,23 and that p21 is a potent cyclin-dependent kinase inhibitor, which can arrest the cells at the G1 phase of the cell cycle. We therefore investigated the effect of let-7d on p21 expression and observed that let-7d significantly upregulated the expression of p21 (Fig. 4D). Thus, let-7d may act as a tumor suppressor and may repress the proliferation of PASMCs by promoting p21-mediated cell cycle arrest.
Discussion
The role of miRNAs in the progression of several diseases, including PH, is becoming increasingly important. In this study, we profiled the miRNA signature in PASMCs of CTEPH patients and found 18 differentially expressed miRNAs, 12 upregulated and 6 downregulated. One miRNA, let-7d, was decreased 0.58-fold and was found to inhibit the proliferation of PASMCs by upregulating p21.
In CTEPH patients, vascular remodeling in the distal pulmonary vascular bed has been shown to be an important component in the development of PH,24,25 and excessive proliferation of smooth muscle cells contributes to vascular-wall thickening and remodeling in the pulmonary arteries. In addition, it has been reported that many pathways involving growth factors, cytokines, metabolic signaling, elastases, and proteases also participate in vascular remodeling. These include but are not limited to iron channels, BMPR2, 5-hydroxytryptamine (5-HT), angiopoietin-1, and endothelin-1.26 The molecular mechanisms responsible for pulmonary artery remodeling are incredibly complex, and further clarification is required.
MiRNAs are highly conserved, single-stranded, noncoding, small RNAs that control cellular functions. Many miRNAs are highly expressed in the vasculature, and their expression is altered in vascular diseases. Several miRNAs have been found to be critical modulators of vascular pathologies, such as atherosclerosis, lipoprotein metabolism, inflammation, arterial remodeling, angiogenesis, smooth muscle cell regeneration, hypertension, apoptosis, and neointimal hyperplasia.27 The role of miRNAs in PH has previously been investigated, and it has been shown that inappropriate STAT3 (signal transducer and activator of transcription 3) activation in PH is linked to aberrant miR-204 expression. Furthermore, downregulation of miR-204 activates Src kinase and nuclear factor of activated T cells, which sustains PH-PASMC proliferation and resistance to apoptosis.10 Alterations in the surface expression of BMPR2 have also been described in several forms of PH,28,29 and it has been reported that interleukin 6 (IL6)–dependent STAT3 activation leads to the expression of miRNA cluster 17/92, which results in BMPR2 downregulation.9 Hypoxia and oxygen sensing have a strong impact in PH pathogenesis, as several miRNAs have shown to be inducible by hypoxia; miR-34a was increased in whole lung of mice after 2 weeks under hypoxia,30 and miR-210 is also a predominant hypoxia-sensitive miRNA.31 MiR-21 is downregulated in the MCT (monocrotaline) experimental model of PH12 and plays a significant role in hypoxia-induced pulmonary VSMC proliferation and migration by regulating multiple gene targets (PDCD4, SPRY2, and PPARα).11 In our study, we profiled the miRNA signature in PASMCs of CTEPH patients and found that miR-320c, miR-149*, miR-4288, miR-191, miR-425, miR-151-5p, miR-29a, miR-361-5p, miR-3676, miR-1246, miR-155, and miR-1290 were upregulated and miR-30a, let-7c, miR-140-5p, miR-27b, let-7d, and miR-152 were downregulated. Among these 18 differentially expressed miRNAs, we discovered through in silico analysis that let-7d may be involved in the pathogenesis of CTEPH. Caruso et al.12 profiled miRNA signatures in rat hypoxic and MCT models of PH and found that let-7f was downregulated in both hypoxic and MCT models, while let-7a was significantly reduced only in MCT-treated rats.
The let-7 family consists of 11 very closely related genes and is conserved among invertebrates and vertebrates, including humans.32 Let-7 is widely viewed as a tumor suppressor miRNA, and consistent with this idea, the expression of let-7 family members is downregulated in many cancer types when compared to normal tissue and during tumor progression. For some forms of cancer, most or all let-7 family members appear to be downregulated.15,33 In our study, let-7d was downregulated in CTEPH patients, and it repressed the proliferation of PASMCs by upregulating p21, which could arrest the cells at the G1 phase of the cell cycle. Consistent with our results, it has been reported that let-7 acts as a tumor suppressor and can repress cell proliferation by regulating multiple genes involved in cell cycle and cell division.23 Let-7 plays a critical role in cell cycle control, as exogenous addition of pre-let-7 in primary human fibroblasts results in a decrease in cell number and an increased fraction of cells in the G2/M cell cycle phase.16 In addition let-7g can restrict cellular proliferation and induce cell death by triggering a significant shift in cell cycle distribution, with an accumulation of G0/G1- and G2/M-phase cells and a corresponding reduction of S-phase cells.34 Studies have also shown that let-7d reduces cell growth and leads to a greater number of cells in the G1 phase than in the G2/M phases in VSMCs.17 But the role of let-7 in regulation of p21 is not fully clear; one paper showed that overexpression of let-7a caused an enhancement of p21 via let-7a-mediated suppression of NIRF (Np95 ICBP90 ring finger), through which let-7a played a growth-inhibitory effect.35 In papillary thyroid carcinoma (PTC), let-7f was capable of reducing TPC-1 cell (a human PTC cell line) growth by increasing p21 cell cycle inhibitor mRNA, but potential mechanisms were not discussed.36 Therefore, further research is necessary to reveal the possible mechanisms through which let-7d regulates p21.
In conclusion, to the best of our knowledge we are the first to report the global miRNA profile in PASMCs of CTEPH patients and to demonstrate that let-7d could repress the proliferation of PASMCs by upregulating p21. These results provide a potential therapeutic treatment (reestablishing the let-7d level) for CTEPH and suggest that further studies into miRNA expression would prove beneficial in understanding the pathogenesis of CTEPH.
Acknowledgments
We thank doctors at Beijing An-Zhen Hospital and doctors of cardiac surgery at Beijing Chao-Yang Hospital for providing the PEA tissues.
Supplemental figure.
Figure S1.
The miRNA-gene-network. The cycle nodes represent messenger RNA (mRNA), the red box nodes represent upregulated microRNA (miRNA), and the blue box nodes represent downregulated miRNA. Edges describe the inhibitive effect of miRNA on mRNA.
Source of Support: Fund of China 973 program (2009CB522107) and National Natural Science Foundation of China (3081010394, 810700742, 81111130212, 81228001).
Conflict of interest: None declared.
Supplements
Supplemental figurePulmCirc-003-654.s001.pdf (1.4MB, pdf)
References
- 1.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation 2006;113(16):2011–2020. [DOI] [PubMed]
- 2.Moser KM, Braunwald NS. Successful surgical intervention in severe chronic thromboembolic pulmonary hypertension. Chest 1973;64(1):29–35. [DOI] [PubMed]
- 3.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993;103(3):685–692. [DOI] [PubMed]
- 4.Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 2002;45(3):173–202. [DOI] [PubMed]
- 5.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011;8(8):443–455. [DOI] [PMC free article] [PubMed]
- 6.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43(12 Suppl S):13S–24S. [DOI] [PubMed]
- 7.Grosshans H, Filipowicz W. Molecular biology: the expanding world of small RNAs. Nature 2008;451(7177):414–416. [DOI] [PubMed]
- 8.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008;455(7209):58–63. [DOI] [PubMed]
- 9.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 2009;104(10):1184–1191. [DOI] [PubMed]
- 10.Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011;208(3):535–548. [DOI] [PMC free article] [PubMed]
- 11.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol 2010;299(6):L861–L871. [DOI] [PMC free article] [PubMed]
- 12.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 2010;30(4):716–723. [DOI] [PubMed]
- 13.Chen Z, Nakajima T, Tanabe N, Hinohara K, Sakao S, Kasahara Y, Tatsumi K, Inoue Y, Kimura A. Susceptibility to chronic thromboembolic pulmonary hypertension may be conferred by miR-759 via its targeted interaction with polymorphic fibrinogen alpha gene. Hum Genet 2010;128(4):443–452. [DOI] [PubMed]
- 14.Jerome T, Laurie P, Louis B, Pierre C. Enjoy the silence: the story of let-7 microRNA and cancer. Curr Genomics 2007;8(4):229–233. [DOI] [PMC free article] [PubMed]
- 15.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 2004;64(11):3753–3756. [DOI] [PubMed]
- 16.Legesse-Miller A, Elemento O, Pfau SJ, Forman JJ, Tavazoie S, Coller HA. let-7 overexpression leads to an increased fraction of cells in G2/M, direct down-regulation of Cdc34, and stabilization of Wee1 kinase in primary fibroblasts. J Biol Chem 2009;284(11):6605–6609. [DOI] [PMC free article] [PubMed]
- 17.Yu ML, Wang JF, Wang GK, You XH, Zhao XX, Jing Q, Qin YW. Vascular smooth muscle cell proliferation is influenced by let-7d microRNA and its interaction with KRAS. Circ J 2011;75(3):703–709. [DOI] [PubMed]
- 18.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, et al. (the Gene Ontology Consortium). Gene ontology: tool for the unification of biology. Nat Genet 2000;25(1):25–29. [DOI] [PMC free article] [PubMed]
- 19.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res 2004;32(Suppl 1):D277–D280. [DOI] [PMC free article] [PubMed]
- 20.Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res 2007;17(10):1537–1545. [DOI] [PMC free article] [PubMed]
- 21.Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput Biol 2007;3(7):e131. [DOI] [PMC free article] [PubMed]
- 22.Joung JG, Hwang KB, Nam JW, Kim SJ, Zhang BT. Discovery of microRNA-mRNA modules via population-based probabilistic learning. Bioinformatics 2007;23(9):1141–1147. [DOI] [PubMed]
- 23.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res 2007;67(16):7713–7722. [DOI] [PubMed]
- 24.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med 2001;345(20):1465–1472. [DOI] [PubMed]
- 25.Manecke GR Jr., Wilson WC, Auger WR, Jamieson SW. Chronic thromboembolic pulmonary hypertension and pulmonary thromboendarterectomy. Semin Cardiothorac Vasc Anesth 2005;9(3):189–204. [DOI] [PubMed]
- 26.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res 2004;68(2):75–103. [DOI] [PubMed]
- 27.Jamaluddin MS, Weakley SM, Zhang L, Kougias P, Lin PH, Yao Q, Chen C. miRNAs: roles and clinical applications in vascular disease. Expert Rev Mol Diagn 2011;11(1):79–89. [DOI] [PMC free article] [PubMed]
- 28.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118(7):2372–2379. [DOI] [PMC free article] [PubMed]
- 29.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, et al. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2007;27(5):1072–1078. [DOI] [PubMed]
- 30.Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, Voelkel NF, Ishizaki T. p53 gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol 2011;300(5):L753–L761. [DOI] [PubMed]
- 31.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 2009;35(6):856–867. [DOI] [PMC free article] [PubMed]
- 32.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000;408(6808):86–89. [DOI] [PubMed]
- 33.O’Hara AJ, Wang L, Dezube BJ, Harrington WJ Jr., Damania B, Dittmer DP. Tumor suppressor microRNAs are underrepresented in primary effusion lymphoma and Kaposi sarcoma. Blood 2009;113(23):5938–5941. [DOI] [PMC free article] [PubMed]
- 34.Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci USA 2008;105(10):3903–3908. [DOI] [PMC free article] [PubMed]
- 35.He X, Duan C, Chen J, Ou-Yang X, Zhang Z, Li C, Peng H. Let-7a elevates p21WAF1 levels by targeting of NIRF and suppresses the growth of A549 lung cancer cells. FEBS Lett 2009;583(21):3501–3507. [DOI] [PubMed]
- 36.Ricarte-Filho JC, Fuziwara CS, Yamashita AS, Rezende E, da-Silva MJ, Kimura ET. Effects of let-7 microRNA on cell growth and differentiation of papillary thyroid cancer. Transl Oncol 2009;2(4):236–241. [DOI] [PMC free article] [PubMed]