Abstract Abstract
Continuous prostanoid infusions are recommended for patients with advanced pulmonary arterial hypertension. Infusion site pain has discouraged some physicians from considering subcutaneous (SQ) treprostinil therapy even though it has safety and convenience advantages over intravenous epoprostenol. We conducted a 1-year prospective study of patients utilizing SQ treprostinil. We provided counseling on infrequent site changes and a written analgesic protocol including narcotics. After placement of a new site, subjects recorded daily pain scores and analgesic use. Twenty-six of 29 patients consented, including 4 patients who had recently started therapy. They returned 203 diaries, and we captured every site change in a diary. Sixteen subjects returned 8 or fewer diaries during 12 months, and 20% of diaries documented only mild discomfort. The majority of diaries documented brief periods of severe pain, but this had generally abated by day 7. Contrary to published guidelines, infusion site pain was independent of treprostinil dose in a rigorous analysis. There were 3 significant local reactions but no systemic illness. No subject discontinued SQ treprostinil because of site discomfort. Subjects reported satisfaction with their treatment using a validated assessment, and quality-of-life scores were favorable. A strategy emphasizing infrequent site changes and early analgesia can facilitate use of SQ treprostinil. These data may allow physicians to consider treprostinil earlier in the treatment algorithm for this fatal disease.
Introduction
Pulmonary arterial hypertension (PAH) is a severe, progressive disease of the pulmonary circulation that causes exercise intolerance, right ventricular failure, and death. Five-year survival remains a dismal ∼60% even with modern therapies, and incident patients progress faster.1-4
Intravenous (IV) epoprostenol is associated with improvements in symptoms, exercise capacity, pulmonary hemodynamics, and survival.5-9 Although epoprostenol is often recommended for higher-risk patients,10,11 its use is limited by the drug’s short half-life (3–5 minutes) and complex delivery system. Infrequent but serious complications include catheter infections, venous thrombosis, sepsis, and fatal infusion interruptions.12,13 Solutions must be changed at least daily, and some forms of the drug require chilling.
Treprostinil is a stable prostacyclin analogue that offers safety and convenience advantages. It has an elimination half-life of 4 hours, mitigating the consequences of abrupt discontinuation. It is stable at room temperature, and syringes for the subcutaneous (SQ) pump are generally changed every 3 days without mixing.
In a blinded, placebo-controlled trial, SQ treprostinil provided a modest 10-m median benefit in exercise capacity but also improved secondary endpoints including hemodynamics and indexes of dyspnea.14 The most common side effect attributed to treprostinil was infusion site pain (85%), leading to premature study discontinuation in 8% of patients. Infusion site pain was thought to be dose related, and this perception contributed to the low doses achieved (mean, 9 ng/kg/min).
Doses up to 125 ng/kg/min provide linear increases in plasma treprostinil levels.15,16 Transition from IV epoprostenol to SQ treprostinil is a safe and effective treatment option,17,18 and treprostinil seems to be useful as part of a combination strategy with oral PAH therapy.19,20 Treprostinil dose can be titrated to symptoms and side effects, and higher doses are associated with better exercise tolerance.21-24 Lang and colleagues25 recently reported that early SQ treprostinil therapy was associated with good outcomes in a severely ill, treatment-naive population. Nonetheless, many physicians do not consider SQ treprostinil because site pain is perceived as insurmountable.
Other investigators have reported that pain peaks early after initiation of a new site26 and that faster dose escalation was associated with a decreased perception of site pain.23 Recent reviews emphasize a multimodal approach to ameliorating site discomfort,27,28 but data to support such a strategy are lacking. The purpose of our prospective study was to evaluate outcomes with a formal analgesia strategy and to assess the safety of recommending infrequent site changes.
Methods
The University of Rochester (IRB-29553) approved the prospective, diary-based study that we conducted between January 2010 and March 2011. We contacted each of our 29 SQ treprostinil patients by mail before phone follow-up. Three patients (out of the potential 29) declined to participate, and in each case this was consistent with their behavior as patients (frequently missed appointments, difficulty in returning phone calls for delivery of drug and supplies, etc.). All 3 of these patients remain alive and well on treprostinil (2 with idiopathic disease and 1 with HIV-associated PAH). The other 26 patients chose to enroll and provided written informed consent (Fig. 1). Subjects completed the Treatment Satisfaction Questionnaire for Medicines (TSQM)29 and the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR)30 initially and at the end of 12 months of diary collection. We gave subjects a 1-page guide to site management and a diary to record their daily experiences (see the 1-page guide and complete diary collection tool, available online as supplements 1 and 2, respectively). We reminded subjects about indications for placing a new site and counseled them regarding nonnarcotic and narcotic analgesics, which we routinely recommend. In particular, we provide all patients with hydrocodone/acetaminophen 5 mg/500 mg to be used for treprostinil-associated adverse events including infusion site pain. Subjects recorded pain daily (numerical visual-analogue scale) and associated symptoms such as drainage, itching, and redness. They logged analgesic use and amount of relief provided. We instructed subjects to log entries in the diary for 14 days after a site change or until pain level was noted as 0 for 2 consecutive days. Subjects returned diaries by express delivery. The study team made phone or e-mail contact every other week to remind patients to complete diaries, to assure that infusion site changes were not “missed,” and to assess for any unplanned physician visits.
Figure 1.
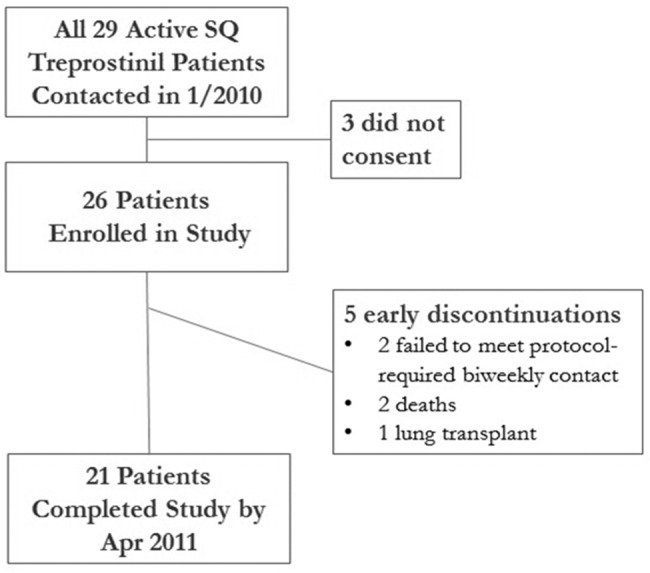
Study population and outcome. All 29 active treprostinil patients at our institution were approached, and most consented. Three did not consent, and all 3 were alive and on subcutaneous (SQ) treprostinil with infrequent site changes at study end. Of the 26 enrolled, 2 were excluded early because they failed to meet protocol-mandated contact with the site every other week; therefore, we could not ascertain appropriate completion of the diaries. They were alive on SQ treprostinil with infrequent site changes at study end. Eight months each of patient diary data were collected for the 2 patients who died and 11 months for the transplanted patient.
Study team members entered demographics and individual diaries into a web-based database. Descriptive statistics were used to summarize data before display using the Tukey box plot method with whiskers covering 90th to 10th percentiles (Graph Pad Prism v5.04). For the correlations in Figure 4, we used Prism to perform linear regression between the dose and pain. For the narcotics utilization data presented in Figure 7, we wanted to assess whether there was a difference in the pain experience for subjects who reported narcotics use as compared to those who did not report using narcotics for infusion site pain. To determine whether there was a difference in the pain experience, we analyzed the data by using generalized estimating equations, which permitted robust estimation and limited concern about the violation of the normal distribution assumption in linear models. These analyses were carried out using SAS 9.3 (SAS Institute, Cary, NC).
Figure 4.

Treprostinil dose does not correlate with infusion site pain. A, We attempted to correlate the treprostinil dose with infusion site pain. We tested the hypothesis that dose might be related either to the total pain experience during a site change (area under the curve, the sum of all pain scores during the 14-day diary) or to the maximum intensity of site pain on any given day. There was clearly no correlation visually, and this was confirmed with a linear regression analysis (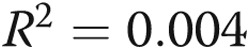 ,
, 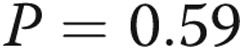 for dose vs. total pain;
for dose vs. total pain; 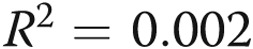 ,
, 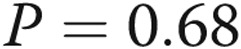 for maximum-intensity pain). B, A similar analysis for the volume of infusion and the pain was also negative. However, in the case of volume, there may have been a very loose relationship, and the statistical analyses left open this possibility (
for maximum-intensity pain). B, A similar analysis for the volume of infusion and the pain was also negative. However, in the case of volume, there may have been a very loose relationship, and the statistical analyses left open this possibility (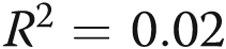 ,
, 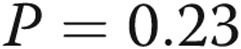 for volume vs. total pain;
for volume vs. total pain; 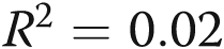 ,
, 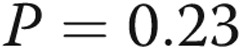 for maximum-intensity pain).
for maximum-intensity pain).
Figure 7.
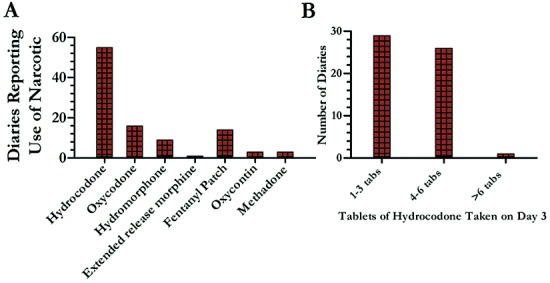
Narcotics use to control infusion site pain was modest. We asked patients to record the number of doses of any narcotic taken specifically for subcutaneous treprostinil infusion site pain; 16 subjects reported narcotics use in 103/203 diaries (conversely, 100 diaries did not document any narcotics use despite having access to a narcotics prescription). In A, the number of patients using any narcotic on day 3 (the day of worst pain as illustrated in Fig. 3) is shown. We routinely prescribe hydrocodone/acetaminophen 5 mg/500 mg, and thus, that is the drug whose use is most commonly documented. We further analyzed the number of hydrocodone tablets taken on day 3 (B). Most patients took 3 tablets or less on the day that was, on average, the most painful. The rigorous analysis described in the text shows that subjects recorded significantly more pain in the diaries for which they also recorded narcotics use.
Results
Of all 29 patients using SQ treprostinil at our institution between January and April, 2010, 26 consented (Fig. 1). Most of these subjects had been on treprostinil for >12 months, but 4 patients began the study within 6 months of starting treprostinil (a time during which patients are at high risk for therapy discontinuation because of site pain).25 Two quickly failed to meet protocol-required biweekly phone contact and were excluded (this obligation, mandated to prevent data loss, was explicit in the consent form); both remain alive and well on SQ treprostinil with infrequent infusion site changes. Two of the remaining 24 enrolled subjects provided 8 months of diary data each before dying, and 1 subject provided 11 months prior to successful lung transplantation. Twenty-one subjects completed 1 year of diary recordings. 1 details patient characteristics typical for PAH. At the time of study enrollment, most of these subjects had already improved clinically on treprostinil; they generally reported class II symptoms with an average 363-m 6-minute walk on the visit immediately prior to study enrollment. Protocol compliance was remarkable; patients completed 192/203 (95%) of diaries correctly, and we captured every site change with a diary.
Table 1.
Subject characteristics at the visit immediately prior to study enrollment
| Characteristic | Value |
|---|---|
| Age, years (range) | 55 (15–78) |
| Female sex | 20 (77) |
| WHO classification/group | |
| Idiopathic/heritable/anorexigen | 14 (54) |
| Connective tissue | 4 (15) |
| Congenital heart disease | 2 (8) |
| Human immunodeficiency or portal hypertension | 2 (8) |
| Chronic thromboembolic (WHO group IV) | 2 (8) |
| Sarcoidosis (WHO group V) | 2 (8) |
| WHO/NYHA functional class | |
| Class II | 22 (85) |
| Class III | 4 (15) |
| 6-minute walk, m | 363 ± 147.2 |
| Right atrial pressure, mmHg | 8 ± 4 |
| Mean pulmonary artery pressure, mmHg | 45 ± 14 |
| Cardiac index, L/min/m2 | 2.6 ± 0.2 |
| Months on SQ treprostinil, months (range) | 29 (1–68a) |
| Other PAH therapy | |
| Endothelin receptor antagonists | 4 (15) |
| Phosphodiesterase-5 inhibitors | 10 (38) |
| Both | 7 (30) |
Data are no. (%) unless otherwise indicated. Rounding of small numbers causes the totals to add to greater than 100% for World Health Organization (WHO) classification. The patients in WHO groups IV and V had extremely severe hemodynamic changes before treprostinil and had a clinically favorable response long prior to study enrollment. Five patients were on treprostinil monotherapy at the time that the study started, with the majority on other pulmonary arterial hypertension therapy as listed. NYHA = New York Heart Association; SQ = subcutaneous.
5 subjects < 12 months.
Utilizing our guidance, patients maintained infusion sites for long periods of time (Fig. 2), changing them only when they had increased pain, itching, erythema, or drainage. Rarely, patients changed sites when they perceived increase in PAH symptoms (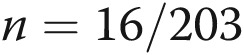 ). About 15% of the infusion sites placed during the study (28/203) were never “good” sites, and subjects thus started new sites within 2 weeks because of local bleeding or persistent pain. However, many (52%) infusion sites lasted >4 weeks, and the majority of subjects (16/24, 66%) returned 8 or fewer diaries during the 12-month study period (Fig. 2).
). About 15% of the infusion sites placed during the study (28/203) were never “good” sites, and subjects thus started new sites within 2 weeks because of local bleeding or persistent pain. However, many (52%) infusion sites lasted >4 weeks, and the majority of subjects (16/24, 66%) returned 8 or fewer diaries during the 12-month study period (Fig. 2).
Figure 2.
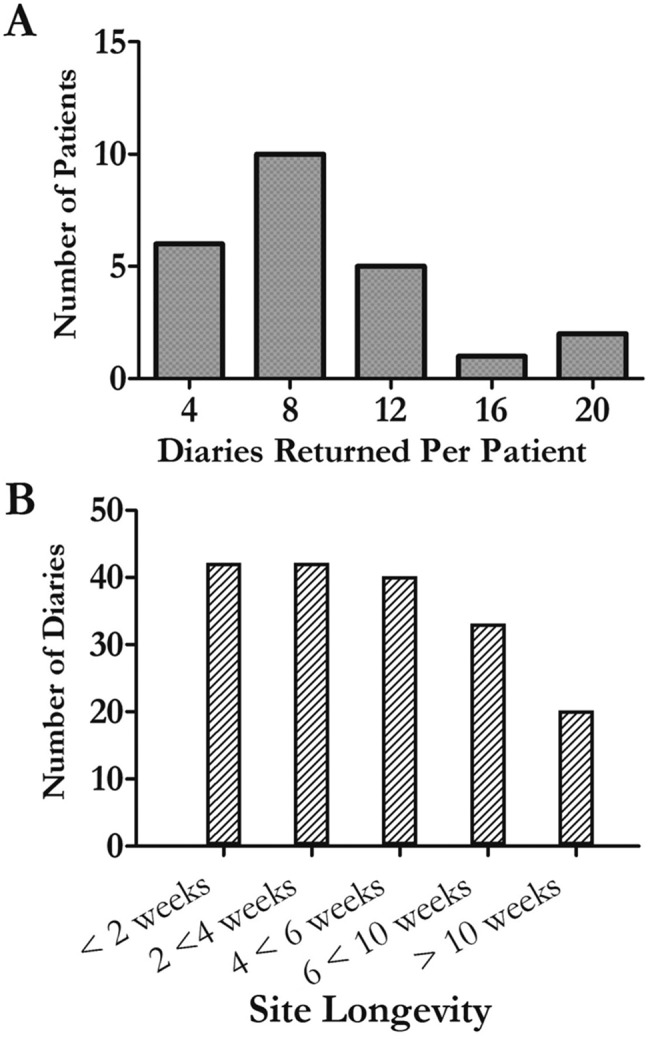
Treprostinil infusion sites can be maintained safely for months. In A, the histogram illustrates the number of diaries returned (no. site changes) per patient. In B, the histogram shows how long infusion sites remained active for the 179 site changes with firm start and end dates (by definition, the last diary returned for each patient would not have an “end date” for that infusion site). Note the large proportion of sites that lasted >4 weeks. A single patient had very little pain when she changed her infusion site, and she accounts for 12 of the diaries that lasted fewer than 14 days. Twelve patients maintained 16 infusion sites for longer than 3 months during the study period; the longest was 225 days. Unfortunately, in 28/203 site changes, the site was changed before 14 days because of bleeding or persistent and severe pain.
Pain
The visual analogue scale listed the word “uncomfortable” above the number 4, and patients verbally confirmed that scores <4 did not reflect severe pain. We therefore dichotomized the diaries for subsequent analysis; diaries that recorded ≥4 on any day (156/203) versus those diaries in which pain scores were always ≤3 (44/203). All subjects returned at least 1 diary during the year documenting severe site pain (≥4 on any day). However, the median pain score for these more severe subject diaries on any given day was never >5 (Fig. 3). Moreover, even in these diaries with severe pain, the median score was 3 by day 7, and 75% of the patients had pain scores of ≤3 by day 10 (Fig. 3). Interestingly, 11 unique subjects recorded only mild pain in 20% of the diaries returned (44/203).
Figure 3.
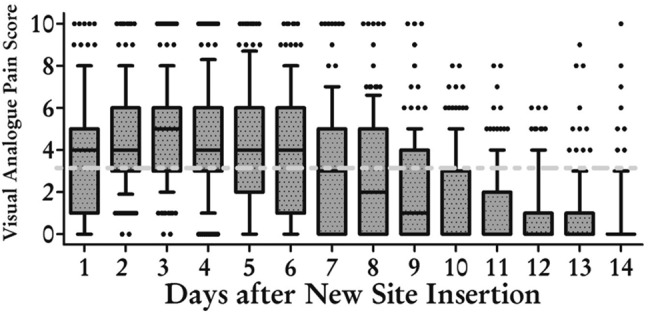
Even severe treprostinil infusion site pain was consistently short-lived. Of 203 diaries returned, 156 documented a pain score (on any day) ≥4. The graph shows the maximal pain rating for each day as a Tukey box-whiskers plot, with the box denoting the middle 50% of the data points and whiskers marking the 10th and 90th percentiles. Outliers are noted by filled circles. Note that most subjects had little if any pain (≤3, indicated at the dashed line) after day 7. For 28 of these 156 diaries, subjects recorded intense pain (sometimes associated with bleeding) and then started a new site within 14 days.
In the initial treprostinil trial, investigators assumed pain to be dose related. We tested this hypothesis rigorously for the first 70 site changes in our study, which occurred over a large range of treprostinil doses (Fig. 4A). There was not a relationship either between the dose of the drug and the worst pain recorded or between the dose and the overall pain experience (sum of pain for all days). Because some patients anecdotally report less pain with smaller infusion volumes, we hypothesized that pain might be related to volumes. While there was not a statistically significant relationship between volume and pain, the analyses left open the possibility of a weak relationship (Fig. 4B).
Safety
During the 12-month time frame, no patient experienced an episode of bacteremia or sepsis or any sign of systemic infection related to the infusion site. Neither death was related to infection or inadequate treprostinil delivery. During the study period, no patient reported an emergency department visit to treat cellulitis or an infusion site abscess. Two patients had 3 office visits to evaluate old sites for signs of infection. In 1 case, the patient had a hematoma that was drained without antibiotics. The other patient reported 2 office visits; once, the provider prescribed a brief course of antibiotics for cellulitis at the old site.
Patients remained clinically stable with long-lasting infusion sites, suggesting that the pharmacodynamic effect was preserved. To complement the clinical perception, we measured plasma treprostinil levels serially in 6 patients who left sites in place 30 days or longer (Fig. 5; Table 2). With stable drug doses between 47 and 170 ng/kg/min, the plasma treprostinil levels were similar during prolonged maintenance of an infusion site. The degree of variation (20%–30%) we observed is similar to that previously reported as diurnal variation.31
Figure 5.
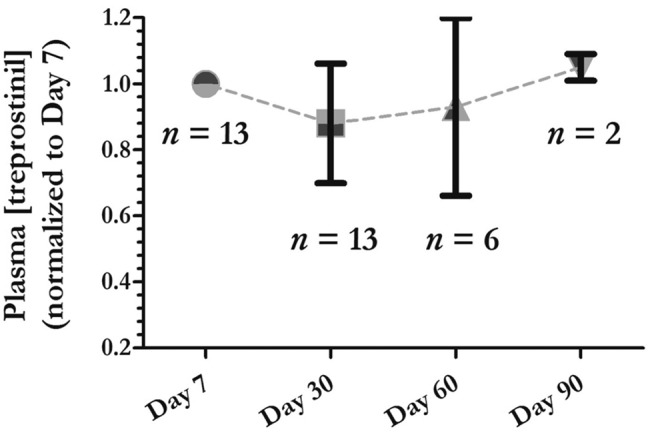
Plasma treprostinil levels were stable with prolonged infusion sites. We measured plasma treprostinil levels by liquid chromatography–tandem mass spectrometry methodology as previously described15 in a cohort of 6 patients proximal to the medical center for whom frequent blood draws were not a burden. For approximately 6 months, these patients had blood drawn at day 7 following the placement of a new site. Six different patients maintained 13 sites for 30 days and had blood drawn on days 7 and 30; 5 patients maintained 7 of those sites for 60 days and had blood redrawn. Two of those patients maintained 1 site each for 90 days, with a final blood draw on day 90. The data are represented as a normalized value compared to the baseline value on day 7; by definition, the plasma level was 1 on day 7, and the subsequent values are normalized to day 7 (mean ± SD, except for day 90, on which we had only 2 samples). In 5 of the 12 sets of data in which the dose was not increased during follow-up, the plasma treprostinil levels were higher at day 30, 60, or 90 than they were at day 7. The plots illustrate mean ± SD; 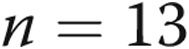 at day 7,
at day 7, 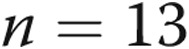 at day 30,
at day 30, 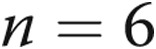 at day 60,
at day 60, 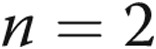 at day 90. The raw data with actual treprostinil levels and doses (range, 47–170 ng/kg/min) are provided in Table 2.
at day 90. The raw data with actual treprostinil levels and doses (range, 47–170 ng/kg/min) are provided in Table 2.
Table 2.
Serial plasma blood draws show stable treprostinil levels with long-lasting infusion sites
| Subject/sample | Day | Plasma concentration (ng/mL) | Normalized concentration (D7) | Treprostinil dose (ng/kg/min) |
|---|---|---|---|---|
| X-01 04-01-11 | 7 | 17.80 | 1.00 | 119 |
| X-01 04-26-11 | 30 | 18.80 | 1.06 | 119 |
| X-01 05-06-11 | 7 | 16.50 | 1.00 | 119 |
| X-01 05-26-11 | 30 | 15.60 | 0.95 | 119 |
| X-01 06-23-11 | 60 | 14.50 | 0.88 | 119 |
| X-01 07-25-11 | 90 | 16.60 | 1.01 | 119 |
| X-01 08-01-11 | 7 | 20.70 | 1.00 | 119 |
| X-01 08-25-11 | 30 | 16.50 | 0.80 | 119 |
| X-01 09-21-11 | 60 | 21.10 | 1.02 | 140 |
| Y-10 04-05-11 | 7 | 13.60 | 1.00 | 73 |
| Y-10 04-25-11 | 30 | 7.08 | 0.52 | 73 |
| Y-10 05-26-11 | 60 | 12.40 | 0.91 | 73 |
| Y-10 08-11-11 | 7 | 14.80 | 1.00 | 73 |
| Y-10 09-06-11 | 30 | 13.00 | 0.88 | 73 |
| Y-10 10-03-11 | 60 | 7.18 | 0.49 | 73 |
| Y-10 03-07-11 | 7 | 13.90 | 1.00 | 73 |
| Y-10 03-28-11 | 30 | 12.20 | 0.88 | 73 |
| Z-07 04-14-11 | 7 | 16.30 | 1.00 | 170 |
| Z-07 05-09-11 | 30 | 14.10 | 0.87 | 170 |
| Z-07 06-13-11 | 7 | 20.30 | 1.00 | 170 |
| Z-07 07-08-11 | 30 | 15.40 | 0.76 | 170 |
| W-06 04-05-11 | 7 | 9.00 | 1.00 | 54 |
| W-06 04-28-11 | 30 | 7.14 | 0.79 | 54 |
| W-06 05-26-11 | 60 | 8.84 | 0.98 | 54 |
| W-06 08-11-11 | 7 | 8.64 | 1.00 | 54 |
| W-06 08-31-11 | 30 | 6.65 | 0.77 | 54 |
| W-06 10-12-11 | 60 | 12.20 | 1.41 | 54 |
| W-06 10-27-11 | 90 | 9.40 | 1.09 | 54 |
| V-24 08-05-11 | 7 | 6.09 | 1.00 | 47 |
| V-24 08-26-11 | 30 | 7.78 | 1.28 | 47 |
| V-24 09-22-11 | 60 | 5.82 | 0.96 | 47 |
| V-24 10-07-11 | 7 | 6.51 | 1.00 | 47 |
| V-24 10-28-11 | 30 | 5.44 | 0.84 | 47 |
| U-21 04-06-11 | 7 | 11.30 | 1.00 | 78 |
| U-21 05-03-11 | 30 | 11.80 | 1.04 | 62 |
| U-21 06-03-11 | 60 | 9.96 | 0.88 | 62 |
For complete details, see legend to Figure 5. Underlining denotes 2 cases in which the treprostinil dose changed during the plasma collection period. In the first case (X-01), the dose increased at day 60, and we excluded that sample from the reported analysis (and the graphs). It is included in this table for completeness. In the second case (U-21), the dose was decreased, but the value at day 30 was still higher than that at day 7 (D7). We therefore included that sample because the variability observed was similar to that for the overall population of samples.
Treatment satisfaction
Because continuous prostacyclin therapy has significant adverse effects, we measured treatment satisfaction and quality of life with validated instruments. The TSQM allows patients to rate a medication across the dimensions of effectiveness, side effects, and convenience; it has demonstrated validity and may predict patient adherence to therapy.29 The mean global rating was 82 (out of 100 potential points), with only 6/26 patients scoring the drug less than 79; the numbers were nearly identical after 1 year of data collection (Fig. 6). Not surprisingly, patients rated the side effects and convenience worse than efficacy. The CAMPHOR instrument scores patients along the dimensions of symptoms, functioning, and quality of life. In this group of patients ill enough to require parenteral prostanoid therapy, the interquartile range (IQR) for all three dimensions was quite similar to that of the large cohort of patients (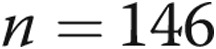 ) in the study, which validated the CAMPHOR instrument for use in the United States.32 In fact, our physical functioning scores appeared to be lower (better). There did not appear to be any changes during the year of study in either quality of life or TSQM for this cohort of treprostinil users.
) in the study, which validated the CAMPHOR instrument for use in the United States.32 In fact, our physical functioning scores appeared to be lower (better). There did not appear to be any changes during the year of study in either quality of life or TSQM for this cohort of treprostinil users.
Figure 6.
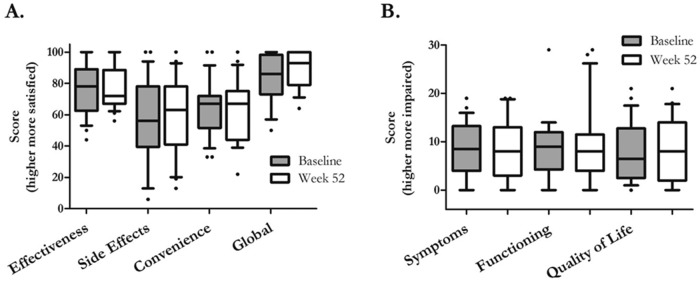
Treatment satisfaction and quality of life. Treatment satisfaction was measured at the beginning and end of the 52-week study using the Treatment Satisfaction Questionnaire for Medicine (TSQM)29 and quality of life by the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR).30 In A, patients scored subcutaneous treprostinil well for efficacy but less so for side effects and convenience. Interestingly, the global rating reflected efficacy more than side effects. In B, patients scored themselves reasonably well with regard to symptoms, physical functioning, and quality of life. There did not appear to be changes in either treatment satisfaction or quality-of-life dimensions during the 12-month period. 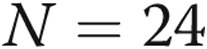 at baseline;
at baseline; 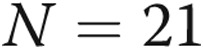 at week 52.
at week 52.
Analgesic use
We asked subjects to record the interventions for pain. In 103/203 diaries (51%), 16 unique subjects recorded narcotics use on at least 1 day to control pain related to the infusion. Figure 3 shows that pain was worst on day 3, and we thus focused further analysis on that day. All patients in our practice have a prescription for hydrocodone/acetaminophen, but we use other narcotics if hydrocodone is insufficient; we sometimes refer to a specialty pain management service. Figure 7 shows the various narcotics subjects used for site pain and the number of diaries that reported use of that narcotic on day 3. Because most subjects used hydrocodone, we further explored the number of hydrocodone tablets used. Figure 7B shows that most patients used ≤3 tablets on day 3. Thus, of all 203 site changes in a group of patients who had easy access to narcotics, only 40% (88) of the diaries reported narcotics usage for site pain on day 3; the dose was relatively modest.
In a separate analysis, we hypothesized that subjects would document greater pain in the diaries for which they also recorded narcotics usage. We used a robust statistical test to incorporate all of the data and make the fewest assumptions about the distributions. The generalized estimating equations made a point estimate of 37 (out of a maximum of 140) for the total pain experience (sum of all pain ratings for the 14 days, or “area under the pain curve”) in the diaries that reported any narcotic usage (95% confidence interval [CI], 28–45.) Total pain ratings in the diaries that did not report narcotics use were much lower, with a point estimate of 21 (95% CI, 10–32; 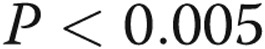 ). An identical analysis for the maximum intensity pain during the 14 days was similar. The worst pain during the recording period was estimated at 6.1 (out of 10) for diaries associated with narcotic usage (95% CI, 5.4–6.9) compared to 4.3 for those without narcotics use (95% CI, 3.1–5.6;
). An identical analysis for the maximum intensity pain during the 14 days was similar. The worst pain during the recording period was estimated at 6.1 (out of 10) for diaries associated with narcotic usage (95% CI, 5.4–6.9) compared to 4.3 for those without narcotics use (95% CI, 3.1–5.6; 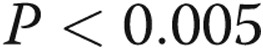 ). We did not attempt to determine the degree of relief subjects obtained when using narcotics; our goal was only to determine whether the use of any narcotics during a specific infusion site change was associated with greater reported pain.
). We did not attempt to determine the degree of relief subjects obtained when using narcotics; our goal was only to determine whether the use of any narcotics during a specific infusion site change was associated with greater reported pain.
Improvements associated with treprostinil therapy
The 26 subjects had stable PAH symptoms, and most were long-term treprostinil users, with an average time of 29 months on therapy before enrollment in the study. Given the limitations inherent in a retrospective review, it appeared that our subjects had experienced a favorable benefit while on therapy. Retrospective chart review showed that the average 6-minute walk test in our group of patients was 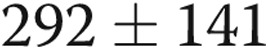 m prior to initiating treprostinil (363 m at study enrollment). In our practice, we invariably repeat right heart catheterizations prior to the initiation of parenteral prostacyclins. On the catheterization prior to starting treprostinil, average right atrial pressure was
m prior to initiating treprostinil (363 m at study enrollment). In our practice, we invariably repeat right heart catheterizations prior to the initiation of parenteral prostacyclins. On the catheterization prior to starting treprostinil, average right atrial pressure was 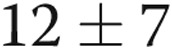 mmHg (which dropped to 8 by study enrollment), average mean pulmonary artery pressure was
mmHg (which dropped to 8 by study enrollment), average mean pulmonary artery pressure was 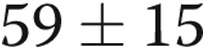 mmHg (which dropped to 45 mmHg), and average cardiac index was
mmHg (which dropped to 45 mmHg), and average cardiac index was 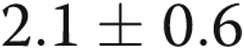 L/min/m2 (which increased to 2.6 L/min/m2).
L/min/m2 (which increased to 2.6 L/min/m2).
Discussion
For chronic outpatient administration, SQ treprostinil has safety and convenience advantages over IV epoprostenol. Nonetheless, infusion site pain has been a major barrier for physicians and patients alike, and no previous prospective study has addressed this systematically. Our report is the only prospective, long-term cohort study to document the occurrence and management of infusion site pain in a relatively unselected group of patients, 4 of whom started treprostinil within 6 months of study enrollment. Our strategy allowed two-thirds of the patients to utilize SQ treprostinil with 8 or fewer infusion site changes over the year, and 75% of diaries recorded only mild pain after day 10 of a new infusion site even when pain had been severe in the first few days. Thus, the majority of our patients reported little or no infusion site pain for much of the 12-month study period. None of the patients in our entire practice discontinued treatment during the study period except for death or transplant (including the 2 that were disqualified early for protocol violations and the 3 who did not consent; see Fig. 1).
It is reassuring that narcotics usage correlated significantly with more intense pain, and ∼50% of the diaries reported no narcotics usage even though all subjects had narcotics available in the home. Our data support the conclusion that patients will not misuse narcotics even when they are provided as part of “routine” management (in anticipation of pain). In our study, patients used narcotics only when they also documented more severe pain; even then, the total narcotics use was modest on the day of most intense pain (Fig. 7B). We did not attempt to address the degree to which narcotics provided relief.
No patient required hospitalization for treatment of bacteremia or sepsis, and we observed only 3 localized reactions requiring provider intervention. This compares favorably with IV epoprostenol. McLaughlin et al.12 provided detailed safety information for 162 patients treated over a 10-year period; 129 local infections (0.26 per patient-year) and 70 episodes of sepsis (0.14 per patient-year) were documented. This study was retrospective, and thus, these authors would have been more likely to miss episodes of infection or sepsis than in our prospective study focused on safety. We had approximately the same number of significant local reactions (3/24 = 0.13 per patient-year) and no episodes of sepsis.
Limitations
There are limitations to our study design. The majority of our cohort was experienced (>12 months) before the start of the study, and this likely improved patients’ experience with the drug and our results. However, nearly all of our eligible patients participated, we observed no discontinuations, and 4/21 study completers had recently (<6 months) initiated treatment with treprostinil and probably experienced more severe pain with a higher potential for discontinuation.25 Since the initiation of the study in January 2010, we have not had a single patient (research or otherwise) discontinue therapy because of site discomfort. Even the 3 eligible patients who did not participate in the study remain on SQ treprostinil with infrequent site changes (in March 2013); none of the original 26 study subjects has discontinued because of site pain (there were an additional 3 deaths since the close of the study, all attributable to disease progression). We have started 98 patients on SQ treprostinil since 2003; our first patient discontinued because of site discomfort, and during the past decade, 4 other patients (total of 5) stopped SQ treprostinil therapy because of infusion site pain or swelling. Thus, our study documents what an experienced treatment team can expect with chronic therapy when following this infusion site management approach. A superior study design would have focused only on new treprostinil starts, but in a rare disease state with multiple competing trials, such a study would take many years, even in a multicenter approach.
We tried to identify or exclude a relationship between treprostinil dose and infusion site pain. We did not identify a significant relationship, but it remains possible that the dose or the volume of treprostinil correlates with infusion site pain. Our study may have had insufficient power to detect a small but consistent effect, but it does seem reasonable to exclude a large relationship between the dose or volume of drug and the intensity of site pain. Our findings complement those of another small study in which more rapid dose escalation actually correlated with less intense site pain.23
This report is also limited because the research probably reinforced patient adherence. We had frequent contact with patients to ensure that we did not miss infusion site changes, physician encounters, or, especially, hospitalizations. It would not have been possible to perform such a rigorous data collection without this sort of frequent contact.
Implications for treatment adherence and quality improvement
On the other hand, it would not be particularly resource intensive to implement our approach broadly. The specialty pharmacies or distribution partners who are in regular contact with patients about medication shipments could reinforce adherence to a physician-prescribed instruction sheet similar to ours. Prescribers could instruct pharmacies to educate patients from a menu of strategies, including infrequent site changes and patient-specific anti-inflammatory and analgesic medications. A simplified version of the diary could reinforce the use of prescribed analgesics and also provide a tool for monitoring adherence to recommended strategies at office visits.
With the TSQM, patients scored SQ treprostinil well globally, and, on average, this did not change over the period of study. The TSQM has not been utilized much in trials for PAH therapeutics, but the patient ratings for efficacy and global satisfaction were similar to those in a recent report of subjects who transitioned from inhaled iloprost to inhaled treprostinil.33 This global satisfaction rating is a bit surprising, as our subjects tended to score SQ treprostinil worse for convenience and side effects as compared to the subject ratings for inhaled treprostinil. Interestingly, in our small study, global satisfaction seemed to track more with efficacy than with side effects.
In summary, our study demonstrates the feasibility of infrequent site changes and an early analgesic approach to facilitate patient comfort and satisfaction with chronic SQ treprostinil. Most subjects reported only mild pain after day 7 of a new site and often left infusion sites in place for months at a time. Measured plasma treprostinil levels in a subset of patients were stable, and this correlated with a stable symptom complex in the entire cohort. Our approach could facilitate earlier adoption of parenteral prostacyclin in PAH patients who are not optimally managed on the currently available oral and inhaled therapies.
Acknowledgments
The authors thank Susan Messing, with support from faculty biostatistician Naiji Lu, PhD, for assistance with the analysis to establish a relationship between narcotic usage and reported pain. We thank the pharmacy staff at the Pittsburgh branch of Accredo for help in identifying patients and in verifying the dosages for the analysis in Figure 4. Data management was provided as a core service of the University of Rochester Department of Biostatistics and Computational Biology under the leadership of Dongwen Wang, PhD. Shirley Zimmerkidd provided layout and formatting assistance for the diary collection tool, and Panos Ginis facilitated the specimen processing for the data in Figure 5.
Source of Support: This study was completed with funds from United Therapeutics.
Conflict of Interest: RJW serves as a paid consultant to United Therapeutics, maker of treprostinil. In each of the past 3 years, he has earned $10,000 in consulting from United Therapeutics, and this has been completely disclosed to and approved by the University of Rochester’s Conflict of Interest Advisory Group. RJW holds no equity in any pharmaceutical or device company. He participates as principal investigator in industry-sponsored studies with Actelion, Gilead, and United Therapeutics; he is also the principal investigator of two other investigator-initiated trials with United Therapeutics. KF has also served as a paid consultant to United Therapeutics, earning less than $10,000 in each of the past 2 years. Her earnings were similarly disclosed to and approved by the University of Rochester. She owns no equity in any pharmaceutical or device company. None of the other authors is conflicted.
Supplements
Supplement 1 (26.9KB, pdf) : A Patient Guide to Remodulin Site Management at the University of Rochester PAH Program
Supplement 2 (292.2KB, pdf) : Symptom Diary for Sub Q Treprostinil
References
- 1.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005;95:199–203. [DOI] [PubMed]
- 2.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164–172. [DOI] [PubMed]
- 3.Humbert M, Sitbon O, Yaici A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010;36:549–555. [DOI] [PubMed]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163. [DOI] [PubMed]
- 5.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol): results of a randomized trial. Ann Int Med 1990;112:485–491. [DOI] [PubMed]
- 6.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension—the Primary Pulmonary Hypertension Study Group. New Engl J Med 1996;334:296–302. [DOI] [PubMed]
- 7.McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. New Engl J Med 1998;338:273–277. [DOI] [PubMed]
- 8.Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease: a randomized, controlled trial. Ann Int Med 2000;132:425–434. [DOI] [PubMed]
- 9.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002;40:780–788. [DOI] [PubMed]
- 10.Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S78–S84. [DOI] [PMC free article] [PubMed]
- 11.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219–1263. [DOI] [PubMed]
- 12.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002;106:1477–1482. [DOI] [PubMed]
- 13.Kuhn KP, Byrne DW, Arbogast PG, Doyle TP, Loyd JE, Robbins IM. Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med 2003;167:580–586. [DOI] [PubMed]
- 14.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002;165:800–804. [DOI] [PubMed]
- 15.McSwain CS, Benza R, Shapiro S, et al. Dose proportionality of treprostinil sodium administered by continuous subcutaneous and intravenous infusion. J Clin Pharmacol 2008;48:19–25. [DOI] [PubMed]
- 16.Laliberte K, Arneson C, Jeffs R, Hunt T, Wade M. Pharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteers. J Cardiovasc Pharmacol 2004;44:209–214. [DOI] [PubMed]
- 17.Rubenfire M, McLaughlin VV, Allen RP, et al. Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. Chest 2007;132:757–763. [DOI] [PubMed]
- 18.Vachiery JL, Hill N, Zwicke D, Barst R, Blackburn S, Naeije R. Transitioning from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension. Chest 2002;121:1561–1565. [DOI] [PubMed]
- 19.Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC. Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest 2008;134:139–145. [DOI] [PubMed]
- 20.Jacobs W, Boonstra A, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Addition of prostanoids in pulmonary hypertension deteriorating on oral therapy. J Heart Lung Transplant 2009;28:280–284. [DOI] [PubMed]
- 21.Lang I, Gomez-Sanchez M, Kneussl M, et al. Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest 2006;129:1636–1643. [DOI] [PubMed]
- 22.Tapson VF, Gomberg-Maitland M, McLaughlin VV, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest 2006;129:683–688. [DOI] [PubMed]
- 23.Skoro-Sajer N, Lang IM, Harja E, Kneussl MP, Sing WG, Gibbs SJ. A clinical comparison of slow- and rapid-escalation treprostinil dosing regimens in patients with pulmonary hypertension. Clin Pharmacokinet 2008;47:611–618. [DOI] [PubMed]
- 24.Hiremath J, Thanikachalam S, Parikh K, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant 2010;29:137–149. [DOI] [PubMed]
- 25.Sadushi-Koliçi R, Skoro-Sajer N, Zimmer D, et al. Long-term treatment, tolerability, and survival with sub-cutaneous treprostinil for severe pulmonary hypertension. J Heart Lung Transplant 2012;31:735–743. [DOI] [PubMed]
- 26.Skoro-Sajer N, Bonderman D, Wiesbauer F, et al. Treprostinil for severe inoperable chronic thromboembolic pulmonary hypertension. J Thromb Haemost 2007;5:483–489. [DOI] [PubMed]
- 27.Skoro-Sajer N, Lang I. Treprostinil for the treatment of pulmonary hypertension. Expert Opin Pharmacother 2008;9:1415–1420. [DOI] [PubMed]
- 28.Mathier MA, McDevitt S, Saggar R. Subcutaneous treprostinil in pulmonary arterial hypertension: practical considerations. J Heart Lung Transplant 2010;29:1210–1217. [DOI] [PubMed]
- 29.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12. [DOI] [PMC free article] [PubMed]
- 30.McKenna SP, Doughty N, Meads DM, Doward LC, Pepke-Zaba J. The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): a measure of health-related quality of life and quality of life for patients with pulmonary hypertension. Qual Life Res 2006;15:103–115. [DOI] [PubMed]
- 31.Wade M, Baker FJ, Roscigno R, et al. Pharmacokinetics of treprostinil sodium administered by 28-day chronic continuous subcutaneous infusion. J Clin Pharmacol 2004;44:503–509. [DOI] [PubMed]
- 32.Gomberg-Maitland M, Thenappan T, Rizvi K, Chandra S, Meads DM, McKenna SP. United States validation of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR). J Heart Lung Transplant 2008;27:124–130. [DOI] [PubMed]
- 33.Bourge RC, Tapson VF, Safdar Z, et al. Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. Cardiovasc Ther 2013;31:38–44. [DOI] [PMC free article] [PubMed]


