Abstract Abstract
Pulmonary hypertension is a debilitating disease with no cure. We have previously shown that peroxisome proliferator–activated receptor (PPAR) β/δ agonists protect the right heart in hypoxia-driven pulmonary hypertension without affecting vascular remodeling. PPARβ/δ is an important receptor in lipid metabolism, athletic performance, and the sensing of prostacyclin. Treatment of right heart hypertrophy and failure in pulmonary hypertension is an emerging target for future therapy. Here we have investigated the potential of GW0742, a PPARβ agonist, to act directly on the right heart in vivo and what transcriptomic signatures are associated with its actions. Right heart hypertrophy and failure was induced in mice using a pulmonary artery banding (PAB) model. GW0742 was administered throughout the study. Cardiovascular parameters were measured using echocardiography and pressure monitoring. Fibrosis and cellular changes were measured using immunohistochemistry. Transcriptomics were measured using the Illumina MouseRef-8v3 BeadChip array and analyzed using GeneSpring GX (ver. 11.0). PAB resulted in right heart hypertrophy and failure and in increased fibrosis. GW0742 reduced or prevented the effects of PAB on all parameters measured. GW0742 altered a number of genes in the transcriptome, with Angptl4 emerging as the top gene altered (increased) in animals with PAB. In conclusion, the PPARβ/δ agonist GW0742 has direct protective effects on the right heart in vivo. These observations identify PPARβ/δ as a viable therapeutic target to treat pulmonary hypertension that may complement current and future vasodilator drugs.
Keywords: pulmonary hypertension, heart failure, peroxisome proliferator–activated receptor (PPAR) β, right ventricular function, angiopoietin-like 4
Introduction
Pulmonary hypertension is a rare but devastating disease in which the normally low pulmonary artery pressure is elevated due to vasoconstriction and remodeling of pulmonary vessels. Increased pulmonary artery pressure results in increased workload on the right side of the heart, causing right heart hypertrophy, fibrosis, and ultimately heart failure. Drugs used to manage pulmonary hypertension are traditionally designed to target the vasculature and enhance vasodilator and antiproliferation pathways, including nitric oxide/cyclic guanosine monophosphate (with phosphodiesterase type 5 inhibitors) and prostacyclin (with prostacyclin and related mimetics), and/or inhibit vasoconstrictor pathways, such as endothelin 1 (with receptor antagonists).1,2 However, it is increasingly recognized that, in addition to the pulmonary vasculature, the right heart is a viable therapeutic target in the treatment of pulmonary hypertension.3,4
We have recently shown, using a hypoxia model of pulmonary hypertension in rats, that GW0742, a ligand for peroxisome proliferator–activated receptor (PPAR) β/δ,5 reduces right heart hypertrophy without influencing pulmonary vascular remodeling.6 This was a somewhat unexpected finding, since we and others found GW0742 to relax pulmonary vessels6 or vascular cells7 and to inhibit lung cell proliferation in vitro.8 Despite this, however, the in vivo data suggested that GW0742 might have a direct protective action on the right heart in this model.6 PPARβ/δ is expressed in the heart, where it regulates metabolism9 and protects against ischemia reperfusion injury.10,11 In addition, a recent study by Liu et al.12 used a transverse aorta constriction model and showed that left ventricular dilation, fibrosis, and mitochondrial abnormalities were reduced in transgenic mice in which constitutively active PPARβ/δ was expressed. This suggests that PPARβ/δ is an attractive protective pathway in the overloaded heart independent of any effects on blood vessels. Drugs that activate PPARβ/δ also enhance physical endurance when given with exercise by a mechanism involving enhanced metabolism in muscle via the adenosine monophosphate (AMP) kinase/PPARγ coactivator 1α pathway.13 Importantly, orally active drugs that activate PPARβ/δ have been developed and tested in humans.
In the current study, we have extended our earlier observations to investigate directly the effect of GW0742 on right heart remodeling and the transcriptome in a model that mimics the increased workload to the right side of the heart seen in pulmonary hypertension.14
Methods
Experiments were performed according to institutional guidelines that comply with national and international regulations. The local authorities for animal research approved the study protocol.
Animals
Male C57BL/6 mice (Charles River, Sulzfeld, Germany) were used. Baseline cardiovascular characteristics were not different between groups (Table 1).
Table 1.
Baseline echocardiographic characteristics of the studied groups
| Sham + placebo | Sham + PPAR 30 mg/kg | PAB + placebo | PAB + PPAR 10 mg/kg | PAB + PPAR 30 mg/kg | |
|---|---|---|---|---|---|
| BW, g | 24.3 ± 0.4 | 23.9 ± 0.2 | 23.0 ± 0.1 | 23.3 ± 0.2 | 23.9 ± 0.1 |
| CI, mL/min/BW | 0.78 ± 0.01 | 0.80 ± 0.02 | 0.61 ± 0.09* | 0.60 ± 0.03* | 0.59 ± 0.02* |
| TAPSE, mm | 1.59 ± 0.02 | 1.60 ± 0.03 | 0.98 ± 0.01* | 1.01 ± 0.02* | 0.99 ± 0.04* |
| RV MPI (Tei) | 0.72 ± 0.05 | 0.73 ± 0.04 | 1.38 ± 0.07* | 1.39 ± 0.04* | 1.41 ± 0.07* |
Data are mean ± SEM. PPAR: peroxisome proliferator–activated receptor; PAB: pulmonary artery banding; BW: body weight; CI: cardiac index; TAPSE: tricuspid annular plane systolic excursion; RV: right ventricle; MPI: myocardial performance index.
P < 0.05 vs. sham-operated mice.
Pulmonary artery banding (PAB)
Briefly, animals were anesthetized with isoflurane (3%), intubated, and mechanically ventilated. Pulmonary arteries were constricted with a small titanium ligating clip (Hemoclip; Edward Weck, Research Triangle Park, NC) with a width of 0.35 mm, after which the chest was closed and the animal was allowed to recover from anaesthesia. Control mice were subjected to sham operations with either drug or placebo (vehicle) treatments.
Experimental protocol
Seven days after surgery, mice were randomized (10 mice per group) to the following groups: group 1, sham-operated mice plus placebo; group 2, sham + GW0742 at 30 mg/kg/d; group 3, PAB plus placebo; group 4, PAB plus GW0742 at 10 mg/kg/d; group 5, PAB plus GW0742 at 30 mg/kg/d. Treatments (oral gavage) were performed for 14 days until the day of the terminal hemodynamic measurements on day 21.
Echocardiography
Echocardiography was performed before and 14 days after treatment using the Vevo770 high-resolution imaging system equipped by 30-MHz transducer (VisualSonics, Toronto). Anesthesia was induced with 3% and maintained with 1.0%–1.5% isoflurane in room air supplemented with 100% O2. Cardiac output (CO), tricuspid annular plane systolic excursion (TAPSE), and myocardial performance index (MPI) were measured as described elsewhere.15 All measurements were performed by an experienced sonographer who was blinded to the results of invasive and morphometric studies.
Hemodynamic and right ventricle (RV) hypertrophy measurements
RV systolic pressure (RVSP) was measured by a catheter inserted into the RV via the right jugular vein; for systemic arterial pressure (SAP), left carotid artery catheterization was performed as described elsewhere.16 The heart was dissected to separate RV from left ventricle plus septum (LV + S), and the ratio RV/(LV + S) was calculated as a measurement for RV hypertrophy.
RV cardiomyocyte size, fibrosis, and vascularization assays
RV cardiomyocyte size, fibrosis, and vascularization were performed as described elsewhere.15,17 Briefly, myocardium was fixed in 4% formalin and stained using picrosirius red (Sirius Red F3B; Niepoetter, Bürstadt, Germany) for collagen fractions. For cardiomyocyte size determination, transverse sections of RV were stained with fluorescein isothiocyanate (FITC)–conjugated wheat germ agglutinin (WGA-FITC; Sigma-Aldrich, St. Louis, MO) mounted with dako mounting medium. Sections without WGA-FITC were used as a negative control. To quantify the capillaries, transverse cryosections of the RV were stained with rat anti–platelet endothelial cell adhesion molecule (Santa Cruz Biotechnology, Heidelberg, Germany) and rabbit anti-dystrophin (Santa Cruz Biotechnology). Alexa Fluor 555–conjugated anti-rat (Cell Signaling Technology, Beverly, MA) and FITC-conjugated anti-rabbit (Invitrogen, Carlsbad, CA) secondary antibodies were used for fluorescent detection. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Invitrogen) and mounted with Dako (Glostrup, Denmark) mounting medium.
Microarray analysis
RNA was extracted from whole right hearts of mice and converted, and samples were hybridized onto probe sets on an Illumina MouseRef-8v3 BeadChip array, as described elsewhere.18 Data were analyzed using the syntax package R (ver. 2.3.1) with Bioconductor and limma.14 Data were calculated using a moderated t test followed by Benjamini-Hochberg false discovery rate correction and Ingenuity Pathway Analysis software, and selected associations are shown. Transcriptomic changes in the heart associated with PAB have been reported elsewhere.14 As shown previously,14 PAB in this study induced significant changes in numerous genes in the heart; transcriptomic analysis of genes in this model have been reported in detail in other studies and are not the direct subject of this report. However, the data obtained have been included for the readers’ information in Tables S5–S8 (1.6MB, pdf) .
Data analysis
Data are mean ± SEM. Differences were considered statistically significant at P < 0.05, determined using analysis of variance and the Student-Newman-Keuls post hoc test for multiple comparisons between studied groups.
Results
Effect of the PPARβ/δ agonist GW0742 on cardiovascular parameters induced by PAB
To investigate the effect of GW0742 on RV hypertrophy and function, we used a mouse model of physical pulmonary artery constriction that leads to increased resistance followed by RV hypertrophy, fibrosis, and RV failure. This model allows elucidation of the effects of treatment on the right heart independently of the pulmonary vasculature. We tested the potential for the PPARβ/δ agonist GW0742 to improve RV function at two doses, 10 and 30 mg/kg. These doses were recommended by the supplier, GlaxoSmithKline, and are consistent with other studies using the compound in vivo.6,19
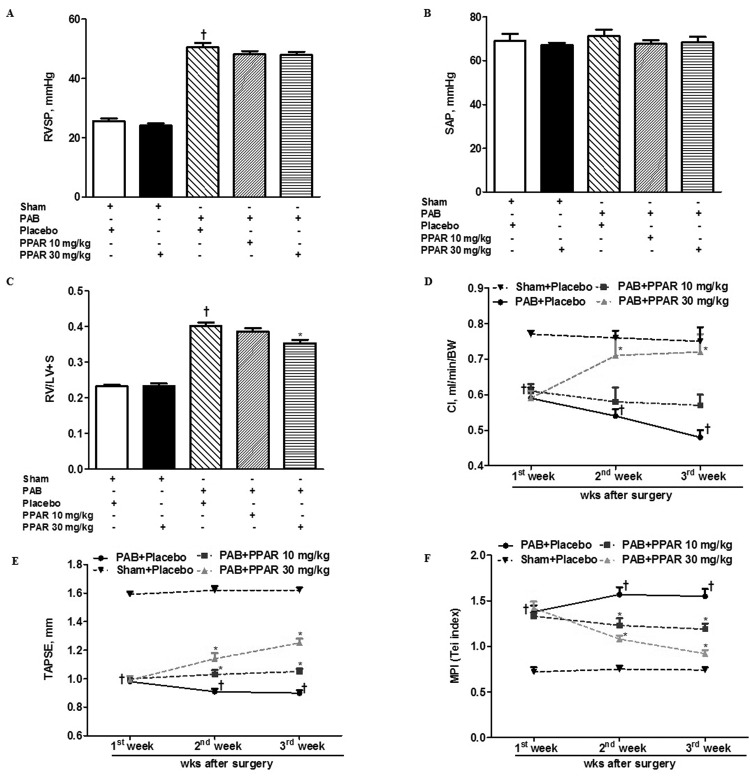
Noninvasive evaluation of RV function demonstrated that 7 days after PAB mice developed RV dysfunction and failure (Table 1), which progressively worsened from day 7 to day 21. Specifically, PAB increased RVSP and RV mass (RV/LV+S), impaired RV MPI, and decreased cardiac index (CI) and TAPSE. PAB had no effect on SAP (Fig. 1B).
Figure 1.
Effects of GW0742 on right heart mass and performance. A, Right ventricular systolic pressure (RVSP). B, Systemic arterial pressure (SAP). C, Ratio of right ventricle (RV) mass to left ventricle and septum (RV/LV+S). D, Cardiac index (CI). E, Tricuspid annular plane systolic excursion (TAPSE). F, RV myocardial performance (or Tei) index. Data are mean ± SEM. Asterisks indicate P < 0.05 versus pulmonary artery banding (PAB) + placebo; daggers indicate P < 0.05 for PAB + placebo versus sham + placebo. BW: body weight.
Treatment with GW0742 at a dose of 30 mg/kg had no effect on any parameter measured in sham-operated animals. GW0742 (10 or 30 mg/kg) similarly had no effect on increased RVSP after PAB (Fig. 1A). By contrast, GW0742 (30 mg/kg) significantly decreased RV hypertrophy and improved CI, TAPSE, and RV MPI in PAB mice compared with placebo-treated animals (Fig. 1D–1F). GW0742 (10 mg/kg) significantly prevented further worsening of RV function in PAB mice compared with PAB mice treated with placebo (Fig. 1D–1F). The improvement in MPI in response to GW0742 treatment was associated with reduction in the isovolumic relaxation time with a slight increment in ET interval. Additionally, we found that treatment with GW0742 increased Sm peak velocity, indicating improvement in systolic function of the RV (data not shown).
Effect of GW0742 on fibrosis and vascularization in the RV induced by PAB
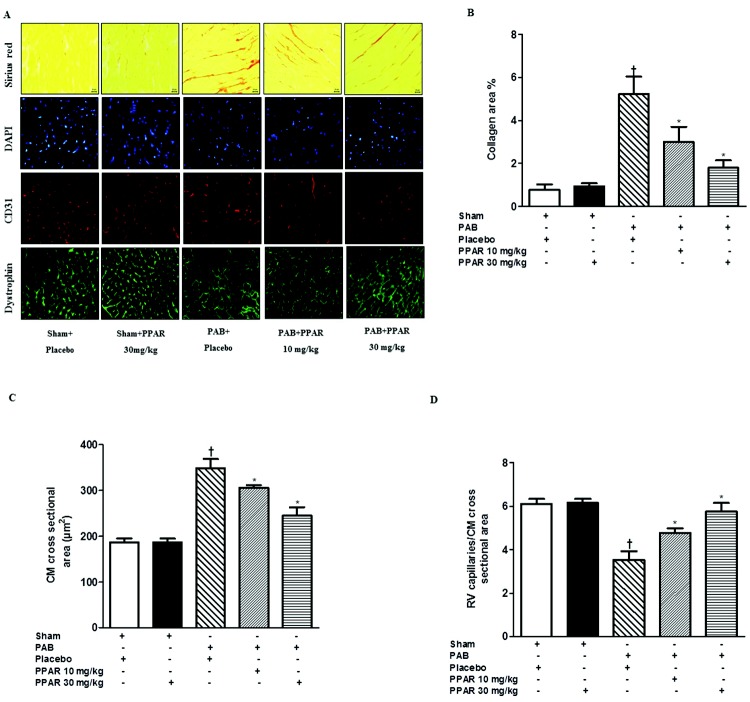
Right heart hypertrophy and dysfunction induced by PAB is accompanied by fibrosis and associated with increased levels of collagen, increase in cardiomyocyte size, and reduction in vascularization (number of capillaries; Fig. 2). GW0742 (30 mg/kg) had no effect on collagen, cardiomyocyte size, or capillary density in the RV of sham-operated animals (Fig. 2). However, the protective effects of GW0742 seen on ventricular mass and function were recapitulated at the cellular level in the heart. Increased collagen deposition and cardiomyocyte size were dose-dependently reduced by GW0742 at 10 and 30 mg/kg (Fig. 2A–2C). Vascularization was also significantly increased by GW0742 (10 and 30 mg/kg) in a dose-dependent manner (Fig. 2D).
Figure 2.
Effects of GW0742 on right ventricle (RV) fibrosis and vascularization. A, Representative images of right ventricular staining. B, Right ventricular collagen contents (collagen area). C, Right ventricular cardiomyocyte (CM) cross-sectional area. D, Right ventricular vascularization of the different groups. Data are mean ± SEM. Asterisks indicate P < 0.05 versus pulmonary artery banding (PAB) + placebo; daggers indicate P < 0.05 for PAB + placebo versus sham + placebo.
Effect of GW0742 on the transcriptome in sham-operated animals and in animals with right heart failure induced by PAB
PPARβ/δ agonists such as GW0742 are known to cause many or most of their actions through modulating induction of genes.20 In sham-operated animals, treatment with GW0742 induced significant changes (increase or decrease) in 59 genes, with 36 being increased and 23 being decreased. Using a cutoff of genes altered ≥1.5-fold resulted in a list of 20 genes being significantly changed in right heart tissue of sham-operated mice treated with GW0742; 14 genes were increased, and 6 were decreased. Importantly, however, the PPARβ/δ target gene, Angptl4, was increased by ≥3-fold in the heart tissue of sham-operated mice treated with GW0742 (Table 2). Pathway analysis of the 59 genes altered by GW0742 in sham-operated mice revealed associations with the following network functions: cell cycle and development/death, amino acid metabolism, and developmental/metabolic disorders (Table 3). These network functions were linked with the following biofunctions: cancer, cell cycle, and connective tissue development and function (Table S1 (1.6MB, pdf) ). In line with this, canonical pathways identified in the hearts of sham-operated mice that received GW0742 included the cell cycle, ubiquinone biosynthesis, inositol metabolism, mitochondrial dysfunction, and oxidative phosphorylation (Table S2 (1.6MB, pdf) ). In PAB mice, GW0742 treatment resulted in 70 genes being significantly altered, compared with PAB mice treated with placebo. Again taking a cutoff of ≥1.5-fold, GW0742 altered 7 genes in PAB mice (Table 4). Importantly, again, as in sham-operated animals, the target gene Angptl4 was increased by GW0742 in the right heart tissue of the PAB animals. In fact, Angptl4 was ranked number one in the list of altered genes with a >3-fold increase in GW0742- versus vehicle-treated animals. Using the full list of 70 altered genes in PAB mice treated with GW0742, pathway analysis revealed (as was seen in the sham-operated group) associations with cellular development and cell death network functions (Table 5). Biofunctions associated with GW0742 treatment in this group included cardiovascular disease (Table S3 (1.6MB, pdf) ). Four of the canonical pathways identified as being linked to gene alteration by GW0742 treatment in sham-operated animals were also linked in animals treated with GW0742 in the PAB model: inositol metabolism, ubiquinone biosynthesis, mitochondrial dysfunction, and oxidative phosphorylation (Table S4 (1.6MB, pdf) ).
Table 2.
Transcripts altered ≥1.5-fold in sham-operated C57BL/6 mice that were treated with GW0742
| Symbol | Probe ID | Accession no. | Definition | FC | P |
|---|---|---|---|---|---|
| Egr2 | ILMN_2623983 | NM_010118.1 | Early growth response 2 | 4.18 | 1.52 × 10−5 |
| Errfi1 | ILMN_2714031 | NM_133753.1 | ERBB receptor feedback inhibitor 1 | 3.38 | 5.29 × 10−5 |
| Angptl4 | ILMN_2759365 | NM_020581.1 | Angiopoietin-like 4 | 3.22 | 6.75 × 10−4 |
| Erg1 | ILMN_2662926 | NM_007913.5 | Early growth response 1 | 2.71 | 1.03 × 10−6 |
| Slc41a3 | ILMN_3154553 | NM_001037493.1 | Solute carrier family 41, member 3 (Slc41a3), transcript variant 2 | 2.42 | 2.06 × 10−2 |
| Fos | ILMN_2750515 | NM_010234.2 | FBJ osteosarcoma oncogene | 2.23 | 1.49 × 10−3 |
| Junb | ILMN_1220034 | NM_008416.1 | Jun-B oncogene (Junb) | 2.20 | 9.81 × 10−5 |
| Cyr61 | ILMN_2794645 | NM_010516.1 | Cysteine-rich protein 61 | 2.19 | 6.99 × 10−4 |
| Cyr61 | ILMN_2710253 | NM_010516.1 | Cysteine-rich protein 61 | 2.18 | 2.30 × 10−4 |
| Fosb | ILMN_2778279 | NM_008036.2 | FBJ osteosarcoma oncogene B | 1.87 | 1.03 × 10−6 |
| Nfkbiz | ILMN_2755008 | NM_030612.2 | Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, zeta | 1.87 | 1.89 × 10−3 |
| Clock | ILMN_2610973 | NM_007715.5 | Circadian locomoter output cycles kaput | 1.57 | 1.84 × 10−4 |
| Slc41a3 | ILMN_3075513 | NM_001037493.1 | Solute carrier family 41, member 3, transcript variant 2 | 1.56 | 2.07 × 10−2 |
| Zfp36 | ILMN_3162407 | NM_011756.4 | Zinc finger protein 36 (Zfp36) | 1.54 | 2.84 × 10−2 |
| Podn | ILMN_1229370 | NM_172874.2 | Podocan | 0.66 | 1.89 × 10−3 |
| Prei4 | ILMN_2601884 | NM_001042671.1 | Mus musculus preimplantation protein 4, transcript variant 3 | 0.57 | 8.94 × 10−3 |
| EG241041 | ILMN_2911009 | NR_002858.1 | Predicted gene, EG241041 on chromosome 1 | 0.50 | 1.52 × 10−3 |
| Prei4 | ILMN_1251682 | NM_001042672.1 | Preimplantation protein 4 (Prei4), transcript variant 4 | 0.48 | 1.52 × 10−5 |
| Coq10b | ILMN_3031099 | NM_001039710.1 | Coenzyme Q10 homolog B (S. cerevisiae) (Coq10b), transcript variant 1 | 0.47 | 1.89 × 10−3 |
| 2210407C18Rik | ILMN_1226767 | NM_144544.1 | RIKEN cDNA 2210407C18 gene | 0.38 | 5.76 × 10−5 |
Animals were sham operated and treated with placebo (vehicle) or 30 mg/kg/d GW0742. After 14 days, the right ventricle was excised, and RNA was extracted from each sample. Gene expression was measured using the Illumina MouseRef-8v3 BeadChip array, and data were analyzed using the syntax package R (ver. 2.3.1) with Bioconductor and limma. Genes that were significantly altered were calculated using a moderated t test followed by Benjamini-Hochberg false-discovery rate correction. FC: fold change.
Table 3.
Top five networks in sham-operated C57BL/6 mice that were treated with GW0742
| Associated network functions | Molecules in network | Focus molecules | Score |
|---|---|---|---|
| Cell cycle, cellular development, cellular growth and proliferation | BMP4, CCNA1, CCNE1, CCNE2, CDC25A, CDK2, CDKN1A, CDX2, CHEK1, CSF1 (includes EG:12977), CUL1, cyclin A, cyclin E, ELAVL1, FOXM1, HDAC3, HDAC4, histone H1, KLF4, LGALS1, MAP2K1, NCOR1, NCOR2, NPM1, OSM, PCNA, POLR2A, PRKCE, PYHIN1, RBL2 (includes EG:100331892), SFN, SKP2 (includes EG:27401), SP3, TOPBP1, TSPYL2 | 2 | 3 |
| Amino acid metabolism, small-molecule biochemistry, connective tissue disorders | PPAT, SREBF1 | 1 | 3 |
| Amino acid metabolism, auditory and vestibular system development and function, cellular development | DIO2, MARCH6 | 1 | 3 |
| Cell death, protein synthesis, cellular development | DGAT2, ERN1 | 1 | 3 |
| Developmental disorder, genetic disorder, metabolic disease | CTSD, HEXB, MREG | 1 | 3 |
Animals underwent a sham operation and after several days were orally dosed with vehicle or GW0742 (30 mg/kg). After 14 days, the right ventricle was excised, and RNA was extracted from each sample. Gene expression was measured using the Illumina MouseRef-8v3 BeadChip array, and data were analyzed using the syntax package R (ver. 2.3.1) with Bioconductor and limma. Genes that were significantly modified, as calculated using a moderated t test followed by Benjamini-Hochberg false-discovery rate correction, were imported into Ingenuity Pathway Analysis software. The table shows the top five networks identified from genes differentially expressed in C57BL/6 mice treated with GW0742 compared with sham-operated controls.
Table 4.
Transcripts altered ≥1.5-fold in C57BL/6 mice undergoing pulmonary artery banding that were treated with placebo or GW0742
| Symbol | Probe ID | Accession no. | Definition | FC | P |
|---|---|---|---|---|---|
| Angptl4 | ILMN_2759365 | NM_020581.1 | Angiopoietin-like 4 | 3.50 | 6.38 × 10−4 |
| Serpina3g | ILMN_2725927 | NM_009251.1 | Serine (or cysteine) peptidase inhibitor, clade A, member 3G | 2.30 | 4.79 × 10−2 |
| Fos | ILMN_2750515 | NM_010234.2 | FBJ osteosarcoma oncogene | 1.77 | 4.22 × 10−2 |
| Junb | ILMN_1220034 | NM_008416.1 | Jun-B oncogene | 1.5 | 4.22 × 10−2 |
| Mertn | ILMN_2739843 | NM_113719 | Meteorin, glial cell differentiation regulator | 0.65 | 1.52 × 10−2 |
| Aqp1 | ILMN_2980661 | NM_007472.1 | Aquaporin 1 | 0.63 | 4.08 × 10−3 |
| Aqp1 | ILMN_2980663 | NM_007472.1 | Aquaporin 1 | 0.62 | 4.08 × 10−3 |
Animals were subjected to pulmonary artery banding and treated with placebo (vehicle) or 30 mg/kg/d GW0742. After 14 days, the right ventricle was excised, and RNA was extracted from each sample. Gene expression was measured using the Illumina MouseRef-8v3 BeadChip array, and data were analyzed using the syntax package R (ver. 2.3.1) with Bioconductor and limma. Genes that were significantly altered were calculated using a moderated t test followed by Benjamini-Hochberg false discovery rate correction. FC: fold change.
Table 5.
Top five networks in C57BL/6 mice undergoing pulmonary artery banding (PAB) that were treated with 30 mg/kg GW0742
| Associated network functions | Molecules in network | Focus molecules | Score |
|---|---|---|---|
| Cellular development, embryonic development, cell death | ACADS, ADAM12, ALDOA, ANKRD40, APP, ARHGEF2, CFL2, CKAP2, CRTC1, CSRP3, DYRK1A, ENSA, EPN2, HGF, HSPH1, IGF1R, IL5, KIF5C, LBR (includes EG:368360), LIF, LMO7, MAP4, MAPK1, NDUFV3, NUMBL, PACSIN3, PLOD1, PLXDC1, PSMB5, RAE1, RGS12, SH3BP4, TLR3, TP53 (includes EG:22059), YWHAG | 13 | 27 |
| Cell death, cellular movement, cell morphology | ADAM10, APOA1, AQP1, AQP7, CACNA2D1, calcineurin protein(s), calpain, DNAI1, DNAJB6, ERK, FCER1G, FHOD1, FOS, GATA6, HBB, HOXA10, HTT, JUP, KLF1, LCN2, LPAR1, MAP7D1, MDH2 (includes EG:17448), PAK1, PCYT1A, PDGFB, PECAM1, PRKCB, REM1, RGS5, SIRPA, SUZ12, TGFA, TGFB1 (includes EG:21803), VASP | 12 | 25 |
Animals’ pulmonary arteries were occluded, and after several days animals were orally dosed with vehicle or 30 mg/kg GW0742. After 14 days, the right ventricle was excised, and RNA was extracted from each sample. Gene expression was measured using the Illumina MouseRef-8v3 BeadChip array, and data were analyzed using the syntax package R (ver. 2.3.1) with Bioconductor and limma. Genes that were significantly modified, as calculated using a moderated t test followed by Benjamini-Hochberg false discovery rate correction, were imported into Ingenuity Pathway Analysis software. The table shows the top five networks identified from genes significantly expressed in PAB C57BL/6 mice treated with GW0742 compared with vehicle controls.
Discussion
Pulmonary hypertension is a rare but debilitating disease with no known cure. As the disease progresses, the additional workload on the right heart causes hypertrophy, fibrosis, and failure. As such, the right heart has now become a valid therapeutic target in the treatment of pulmonary hypertension. We have previously shown that the PPARβ/δ agonist GW0742 inhibits right heart hypertrophy in a hypoxia-driven model of pulmonary hypertension and speculated that this may be due to actions directly on the heart.6 In the current study, we have addressed this possibility directly and used a PAB model to induce changes in the right heart consistent with those seen in pulmonary hypertension. Using this model, we have demonstrated that the PPARβ/δ agonist GW0742 reduces hypertrophy and fibrosis. Analysis of the entire mouse transcriptome in the right heart tissue of animals with PAB treated with GW0742 revealed the PPARβ/δ target, Angptl4, as the top gene induced. These data support the idea that PPARβ/δ is a therapeutic target in the treatment of pulmonary hypertension and indicate that GW0742 and similar drugs are the first therapies to directly target right heart failure in pulmonary hypertension.
GW0742 is a highly selective PPARβ/δ agonist5 shown to be active in a range of cell types, including lung fibroblasts,8 platelets,21 and—relevant to this study—cardiomyocytes.22 In vivo, GW0742 has been shown to protect against atherosclerosis,23 colon cancer,24 ischemia reperfusion injury in the heart,11,25 acute lung injury,26,27 and septic shock.27 In addition, there is mounting evidence that PPARβ/δ activated by other means plays an important role in the heart. Of direct relevance to this study, others have shown that PPARβ/δ activation in adult hearts facilitates mitochondrial function and improves cardiac performance under pressure-overload conditions.12 In our study, we found that GW0742 reduced right heart hypertrophy and fibrosis. These observations are in line with others showing that activation of PPARβ/δ with the selective agonist L-165041 inhibits hypertrophy in neonatal rat cardiomyocytes28 and proliferation of cardiac fibroblasts through inhibition of nuclear factor κβ signaling.29 In our study, the effects of GW0742 on fibrosis in the right heart were associated with reduced collagen expression. Of direct relevance to our work, others have shown that activation of PPARβ/δ using GW501516 inhibits collagen synthesis in cardiac fibroblasts.30 Thus, reduced fibrosis may in part help to explain the reduction in right heart remodeling and subsequent improvements in CO and performance observed in our model. However, it should be noted that a reduction of pressure overload–induced RV hypertrophy by pharmacological intervention might be either beneficial or deleterious for heart function. The overall effect may depend on many factors, including fibrosis, vascularization, and intrinsic metabolic demand. Indeed, since the mechanisms of transition from compensatory RV hypertrophy to maladaptive RV remodeling are not completely understood, it is difficult to make firm conclusions regarding the overall clinical benefit of PPARβ/δ agonists in the complex setting of clinical pulmonary hypertension. Nevertheless, in our study we have clearly demonstrated that PPARβ/δ activation reduced RV hypertrophy and improved vascularization and heart function. This observation, together with our previous work showing that GW0742 improved RVSP and reduced RV hypertrophy in a hypoxic model of pulmonary hypertension,6 suggests that this pathway warrants further study. In line with this and of direct relevance to patients with pulmonary hypertension, where exercise capacity is reduced, it should also be noted that PPARβ/δ activation in association with exercise or activators of the AMP kinase pathway improves athletic performance at the level of muscle metabolism.13 Small-molecule activators of AMP kinase have been developed,31 and, while it is beyond the scope of our current study to investigate this pathway, it is tempting to speculate that these drugs may act in synergy with PPARβ/δ agonists in the treatment of pulmonary hypertension. Indeed, the AMP kinase activator AICAR inhibits cardiac fibroblast proliferation32,33 and protects against myocardial injury after ischemia reperfusion.33
PPARβ/δ mediates its effects via genomic34 and nongenomic20,35 pathways. In the current study, we analyzed genomic influences by monitoring global gene induction in the right hearts of animals treated with GW0742. PAB itself has profound effects on gene induction in both the right and the left heart, which has been described in a previous study.14 In other systems, the PPAR target gene, Angptl4, is consistently induced by PPARβ/δ agonists,34 including GW0742.36 In line with this, we found that Angptl4 was the top gene induced from the entire transcriptome in right heart tissue from PAB mice treated with GW0742. We also found that Angptl4 was among the top three genes induced by GW0742 in the right heart of sham-operated animals. As we saw previously,6 GW0742 had no effect on cardiovascular or cardiac parameters in sham-operated animals. These observations suggest that the changes in the transcripome are protective and, therefore, are only apparent functionally when there is a pathological change. Angptl4 is a member of the angiopoietin-like family and regulates angiogenesis and lipid metabolism.37 While no data currently exist relating Angptl4 to idiopathic pulmonary hypertension, it has recently been associated with high-altitude adaptation in Tibet.38 Angptl4 has also been associated with left heart failure; a recent study found Angptl4 to protect against myocardial infarction and no reflow through preservation of vascular integrity.39 It was demonstrated that vascular permeability, hemorrhage, edema, inflammation, and infarct severity were increased in Angptl4-deficient mice, whereas injection of recombinant human Angptl4 reduced myocardial infarct size and the extent of no reflow in mice and rabbits. These observations support the idea that Angptl4 is a viable mechanism by which PPARβ/δ activation leads to cardioprotection.
PPARβ/δ agonists induce Angptl4 gene expression, protect the right heart at the level of reduced fibrosis, and improve CO and failure in a PAB model. These findings identify the right heart as a viable therapeutic target in the treatment of pulmonary hypertension and add further weight to the hypothesis that PPARβ/δ agonists may be of benefit to patients with the disease. It should, however, be noted that drugs like GW0742 may interact with other therapies used to treat pulmonary hypertension, and more preclinical studies should be carried out6 using combinations of drugs before clinical trials begin.
Source of Support: This work was supported by the Wellcome Trust and the British Heart Foundation. GW0742 was a gift from Dr. Tim Wilson at GlaxoSmithKline.
Conflict of Interest: None declared.
References
- 1.Dewachter L, Dewachter C, Naeije R. New therapies for pulmonary arterial hypertension: an update on current bench to bedside translation. Expert Opin Investig Drugs 2010;19:469–488. [DOI] [PubMed]
- 2.Stamm JA, Risbano MG, Mathier MA. Overview of current therapeutic approaches for pulmonary hypertension. Pulm Circ 2011;1:138–159. [DOI] [PMC free article] [PubMed]
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011;8:443–455. [DOI] [PMC free article] [PubMed]
- 4.Handoko ML, de Man FS, Allaart CP, Paulus WJ, Westerhof N, Vonk-Noordegraaf A. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: lessons from the left heart. Eur Respir Rev 2010;19:72–82. [DOI] [PMC free article] [PubMed]
- 5.Sznaidman ML, Haffner CD, Maloney PR, et al. Novel selective small molecule agonists for peroxisome proliferator–activated receptor δ (PPARδ)––synthesis and biological activity. Bioorg Med Chem Lett 2003;13:1517–1521. [DOI] [PubMed]
- 6.Harrington LS, Moreno L, Reed A, et al. The PPARβ/δ agonist GW0742 relaxes pulmonary vessels and limits right heart hypertrophy in rats with hypoxia-induced pulmonary hypertension. PLoS ONE 2010;5:e9526. [DOI] [PMC free article] [PubMed]
- 7.Li Y, Connolly M, Nagaraj C, et al. Peroxisome proliferator–activated receptor–β/δ, the acute signaling factor in prostacyclin-induced pulmonary vasodilation. Am J Respir Cell Mol Biol 2012;46:372–379. [DOI] [PubMed]
- 8.Ali FY, Egan K, FitzGerald GA, et al. Role of prostacyclin versus peroxisome proliferator–activated receptor β receptors in prostacyclin sensing by lung fibroblasts. Am J Respir Cell Mol Biol 2006;34:242–246. [DOI] [PubMed]
- 9.Cheng L, Ding G, Qin Q, et al. Cardiomyocyte-restricted peroxisome proliferator–activated receptor–δ deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 2004;10:1245–1250. [DOI] [PubMed]
- 10.Burkart EM, Sambandam N, Han X, et al. Nuclear receptors PPARβ/δ and PPARα direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 2007;117:3930–3939. [DOI] [PMC free article] [PubMed]
- 11.Kapoor A, Collino M, Castiglia S, Fantozzi R, Thiemermann C. Activation of peroxisome proliferator–activated receptor–β/δ attenuates myocardial ischemia/reperfusion injury in the rat. Shock 2010;34:117–124. [DOI] [PubMed]
- 12.Liu J, Wang P, Luo J, et al. Peroxisome proliferator–activated receptor β/δ activation in adult hearts facilitates mitochondrial function and cardiac performance under pressure-overload condition. Hypertension 2011;57:223–230. [DOI] [PMC free article] [PubMed]
- 13.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARδ agonists are exercise mimetics. Cell 2008;134:405–415. [DOI] [PMC free article] [PubMed]
- 14.Kreymborg K, Uchida S, Gellert P, et al. Identification of right heart–enriched genes in a murine model of chronic outflow tract obstruction. J Mol Cell Cardiol 2010;49:598–605. [DOI] [PubMed]
- 15.Kojonazarov B, Sydykov A, Pullamsetti SS, et al. Effects of multikinase inhibitors on pressure overload–induced right ventricular remodeling. Int J Cardiol 2013;167:2630–2637. [DOI] [PubMed]
- 16.Dahal BK, Heuchel R, Pullamsetti SS, et al. Hypoxic pulmonary hypertension in mice with constitutively active platelet-derived growth factor receptor–β. Pulm Circ 2011;1:259–268. [DOI] [PMC free article] [PubMed]
- 17.Lang M, Kojonazarov B, Tian X, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS ONE 2012;7:e43433. [DOI] [PMC free article] [PubMed]
- 18.Wright WR, Parzych K, Crawford D, Mein C, Mitchell JA, Paul-Clark MJ. Inflammatory transcriptome profiling of human monocytes exposed acutely to cigarette smoke. PLoS ONE 2012;7:e30120. [DOI] [PMC free article] [PubMed]
- 19.Haskova Z, Hoang B, Luo G, et al. Modulation of LPS-induced pulmonary neutrophil infiltration and cytokine production by the selective PPARβ/δ ligand GW0742. Inflamm Res 2008;57:314–321. [DOI] [PubMed]
- 20.Belvisi MG, Mitchell JA. Targeting PPAR receptors in the airway for the treatment of inflammatory lung disease. Br J Pharmacol 2009;158:994–1003. [DOI] [PMC free article] [PubMed]
- 21.Ali FY, Davidson SJ, Moraes LA, et al. Role of nuclear receptor signaling in platelets: antithrombotic effects of PPARβ. FASEB J 2006;20:326–328. [DOI] [PubMed]
- 22.Cheng L, Ding G, Qin Q, et al. Peroxisome proliferator–activated receptor δ activates fatty acid oxidation in cultured neonatal and adult cardiomyocytes. Biochem Biophys Res Commun 2004;313:277–286. [DOI] [PubMed]
- 23.Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARδ agonist GW0742X reduces atherosclerosis in LDLR−/− mice. Atherosclerosis 2005;181:29–37. [DOI] [PubMed]
- 24.Marin HE, Peraza MA, Billin AN, et al. Ligand activation of peroxisome proliferator–activated receptor β inhibits colon carcinogenesis. Cancer Res 2006;66:4394–4401. [DOI] [PubMed]
- 25.Yue TL, Nerurkar SS, Bao W, et al. In vivo activation of peroxisome proliferator–activated receptor–δ protects the heart from ischemia/reperfusion injury in Zucker fatty rats. J Pharmacol Exp Ther 2008;325:466–474. [DOI] [PubMed]
- 26.Di Paola R, Crisafulli C, Mazzon E, et al. GW0742, a high-affinity PPAR-β/δ agonist, inhibits acute lung injury in mice. Shock 2010;33:426–435. [DOI] [PubMed]
- 27.Kapoor A, Shintani Y, Collino M, et al. Protective role of peroxisome proliferator–activated receptor–β/δ in septic shock. Am J Respir Crit Care Med 2010;182:1506–1515. [DOI] [PubMed]
- 28.Planavila A, Rodriguez-Calvo R, Jove M, et al. Peroxisome proliferator–activated receptor β/δ activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc Res 2005;65:832–841. [DOI] [PubMed]
- 29.Teunissen BE, Smeets PJ, Willemsen PH, De Windt LJ, Van der Vusse GJ, Van Bilsen M. Activation of PPARδ inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc Res 2007;75:519–529. [DOI] [PubMed]
- 30.Zhang H, Pi R, Li R, et al. PPARβ/δ activation inhibits angiotensin II–induced collagen type I expression in rat cardiac fibroblasts. Arch Biochem Biophys 2007;460:25–32. [DOI] [PubMed]
- 31.Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 1994;353:33–36. [DOI] [PubMed]
- 32.Du J, Guan T, Zhang H, Xia Y, Liu F, Zhang Y. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem Biophys Res Commun 2008;368:402–407. [DOI] [PubMed]
- 33.Paiva MA, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther 2010;24:25–32. [DOI] [PMC free article] [PubMed]
- 34.Grimaldi PA. Metabolic and nonmetabolic regulatory functions of peroxisome proliferator–activated receptor β. Curr Opin Lipidol 2010;21:186–191. [DOI] [PubMed]
- 35.Ali FY, Hall MG, Desvergne B, Warner TD, Mitchell JA. PPARβ/δ agonists modulate platelet function via a mechanism involving PPAR receptors and specific association/repression of PKCα—brief report. Arterioscler Thromb Vasc Biol 2009;29:1871–1873. [DOI] [PubMed]
- 36.Ge H, Cha JY, Gopal H, et al. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J Lipid Res 2005;46:1484–1490. [DOI] [PubMed]
- 37.Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med 2005;11:473–479. [DOI] [PubMed]
- 38.Simonson TS, Yang Y, Huff CD, et al. Genetic evidence for high-altitude adaptation in Tibet. Science 2010;329:72–75. [DOI] [PubMed]
- 39.Galaup A, Gomez E, Souktani R, et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 2012;125:140–149. [DOI] [PubMed]