Abstract Abstract
Cardiac catheterization is important for the management of patients with pulmonary arterial hypertension (PAH). It is used for diagnosis, assessment, and monitoring of PAH patients, as well as to perform interventions such as balloon atrial septostomy and coil embolization of collateral vessels. Although reports on the risks of catheterization in PAH patients are scarce, many centers hesitate to perform these procedures in such fragile patients. We performed a retrospective chart review of all cardiac catheterizations performed in PAH patients over 10 years at our pulmonary hypertension center. Demographic, hemodynamic, and outcome data were collected. Complication rates were determined, and multivariate proportional hazards modeling was performed to identify predictors of catheterization-related complications. There were 1,637 catheterizations performed in 607 patients over 10 years. Pediatric patients accounted for 50% of these cases, 48% were performed in patients with idiopathic PAH, and 49% were performed under general anesthesia. While the overall complication rate was 5.7%, the rate of major complications was only 1.2% (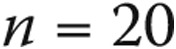 ). Although there were 8 deaths during the admission following catheterization, only 4 of these were related to the procedure, yielding a catheterization-related mortality of 0.2%. In conclusion, when performed at a pulmonary hypertension center with expertise in the care of PAH patients, cardiac catheterization is associated with low complication rates and mortality, and it should remain an important tool in the management of these patients.
). Although there were 8 deaths during the admission following catheterization, only 4 of these were related to the procedure, yielding a catheterization-related mortality of 0.2%. In conclusion, when performed at a pulmonary hypertension center with expertise in the care of PAH patients, cardiac catheterization is associated with low complication rates and mortality, and it should remain an important tool in the management of these patients.
Keywords: pulmonary arterial hypertension, cardiac catheterization, safety, pediatrics, adults
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by vascular proliferation and remodeling in the pulmonary vasculature, leading to increased pulmonary vascular resistance (PVR) and eventually right heart failure. If untreated, PAH is a fatal disease with a median survival of 2.8 years in adults and 10 months in pediatric patients.1,2 Advances in evaluation, management, and treatment over the last 2 decades have led to dramatic improvements in prognosis. Recent data provided by the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) demonstrate a 5-year survival of 68% in newly or previously diagnosed PAH patients between 2006 and 2009, including a 5-year survival of 76% in pediatric patients.3,4 Cardiac catheterization, including acute vasodilator testing, is extremely important in the management of PAH; it is used for diagnosis, prognosis, and therapy guidance as well as to perform interventions such as balloon atrial septostomy and coil embolization of aortopulmonary collateral vessels.1,5-8 Many centers are reluctant to perform invasive procedures in such a fragile patient population because of the potential for serious complications. However, there are scant data regarding the risk of catheterization in PAH patients. The Congenital Cardiac Catheterization Project on Outcomes (C3PO) registry has reported on outcomes and complications in pediatric and adult congenital cardiac catheterizations;9,10 however, there are no reports by this group specific to PAH patients. Reports on complications specific to PAH are limited to a few multicenter collaborative efforts and smaller single-center experiences.11-14 We sought to review our single-center experience performing cardiac catheterization at a pulmonary hypertension center that specializes in the care of both adults and children with PAH, reporting on the safety of the procedure in this complicated patient population when performed in this setting.
Methods
The cardiac catheterization database at Columbia University Medical Center–Morgan Stanley Children’s Hospital of New York (CUMC-MSCHONY) was queried to identify all patients with either idiopathic PAH (IPAH) or associated PAH (APAH) who were referred by the pulmonary hypertension center at CUMC for cardiac catheterization by a specialized team over a 10-year period from July 2002 through June 2012. Clinical records of all pediatric and adult PAH patients who underwent catheterization were included and reviewed. This study was approved by the Institutional Review Board of Columbia University.
We retrospectively collected demographic, hemodynamic, and outcome data on all included cases. Demographics included age, gender, height, weight, body surface area, and diagnosis (IPAH vs. APAH due to repaired or unrepaired congenital heart disease [CHD], connective tissue disease, repaired congenital diaphragmatic hernia [CDH], chronic lung disease of prematurity, and other). Presence of an identified genetic syndrome was recorded, as was use of general anesthesia (GA) and type of airway maintenance during these cases (endotracheal tube with positive pressure vs. laryngeal mask airway vs. nasal cannula oxygen).
Catheterization technique depended on cardiac anatomy but involved either femoral or internal jugular venous access and in some cases femoral arterial access. In all cases a prograde right heart catheterization was performed. Pulmonary arterial blood samples were obtained to determine mixed venous saturation; in cases of intracardiac shunting, saturations were also obtained from the superior vena cava to determine degree of shunting. Catheters were advanced into the right atrium and then into the pulmonary artery to obtain right atrial, pulmonary artery, and pulmonary capillary wedge pressures. When possible, oxygen consumption was measured directly using either the Waters MRM2 oxygen consumption monitor (Waters Instruments, Rochester, MN) or the Oxycon Mobile ergospirometry testing device (ver. 01.00; Carefusion, Hoechberg, Germany). In the remaining cases, including patients receiving positive pressure ventilation, oxygen consumption was assumed from previously published norms.15 Cardiac output was determined by the Fick principle and was then divided by body surface area to determine cardiac index. PVR was determined using Ohm’s law and was indexed to body surface area (PVRI) by using cardiac index in the calculation. Although saturation and pressure measurements were obtained in several rest states, including with the addition of inhaled nitric oxide with or without 100% inspired oxygen, data collection for this study was limited to the initial rest state under baseline conditions. The addition of a left heart catheterization was noted, as was the performance of any catheter-based intervention, which included but was not limited to balloon atrial septostomy, coil embolization of aortopulmonary collateral vessels, and device closure of a patent ductus arteriosus (PDA).
Catheterization-related adverse events were defined as any anticipated or unanticipated event for which avoidable injury occurred or could have occurred as a consequence of the catheterization procedure. Complications included in this analysis were those related directly to the catheterization as well as those related to the use of GA for the procedure. The seriousness of adverse events was designated according to the following definitions published by Bergersen et al.16 in 2008:
-
•
Severity level 1: no harm, no change in condition, may have required monitoring to assess for potential change in condition with no intervention indicated.
-
•
Severity level 2: transient change in condition, not life threatening, condition returns to baseline, required monitoring, required minor intervention such as holding a medication or obtaining a laboratory test.
-
•
Severity level 3: transient change in condition may be life threatening if not treated, condition returns to baseline, required monitoring, required intervention such as reversal agent, additional medication, transfer to the intensive care unit for monitoring, or moderate transcatheter intervention to correct condition.
-
•
Severity level 4: change in condition, life threatening if not treated, change in condition may be permanent, may have required an intensive care unit admission or emergent readmission to the hospital, may have required invasive monitoring, required interventions such as electrical cardioversion or unanticipated intubation, or required major invasive procedures or transcatheter interventions to correct condition.
-
•
Severity level 5: any death and emergent surgery or extracorporeal membrane oxygenation to prevent death with failure to wean from bypass support.
Events described by severity levels 1 and 2 were considered to be minor complications, while severity levels 3–5 were considered to be major complications. Deaths related to catheterization, deaths occurring within 24 hours of catheterization, and deaths occurring during the same hospitalization as the catheterization were also recorded.
Complication rates were determined for the entire population as well as for groups based on age, diagnosis, and GA use. Continuous data were compared using means and standard deviations. Univariate and multivariate proportional hazards modeling was performed to identify predictors of catheterization-related complications. In building the multivariate model, variables that had significance levels of 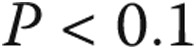 in the univariate model were included. For the purposes of these analyses, age was converted into a categorical variable (<2, 2–9, 10–17, 18–40, and >40 years). Patients <18 years old were considered pediatric patients, and for the purposes of this study patients <2 years old were considered the infant/toddler group.
in the univariate model were included. For the purposes of these analyses, age was converted into a categorical variable (<2, 2–9, 10–17, 18–40, and >40 years). Patients <18 years old were considered pediatric patients, and for the purposes of this study patients <2 years old were considered the infant/toddler group.
Results
Demographics
Between July 2002 and June 2012, 1,637 cardiac catheterizations were performed in 607 patients with PAH at CUMC-MSCHONY. Demographic and hemodynamic data from these cases can be found in Table 1. Approximately two-thirds of the catheterizations were performed in females (68%), and about half of the cases were adults (50%). About half of the patients had IPAH (48%); of the APAH patients, the majority had repaired or unrepaired CHD (62%). About half of the cases were performed under GA (49%), and the majority of these were managed without endotracheal intubation (74%). Overall, 13% of the cases were performed under GA and with the patient intubated for airway management. The vast majority of the 802 GA cases (93%) and 210 GA/intubated cases (95%) were performed in pediatric patients. Left heart catheterization was performed in 26% of cases, and there were 114 interventions performed during 112 catheterizations (7% of cases), mostly including balloon atrial septostomy, coil embolization of aortopulmonary collateral vessels, or device closure of a PDA. A complete list of catheter-based interventions is found in Table 2. Hemodynamic measures included mean right atrial and pulmonary artery pressures of 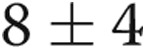 and
and 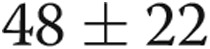 mmHg, respectively. PVRI of the population was
mmHg, respectively. PVRI of the population was 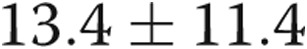 Wood units × m2.
Wood units × m2.
Table 1.
Demographic and hemodynamic characteristics of pulmonary arterial hypertension (PAH) patients who underwent cardiac catheterization at Columbia University Medical Center–Morgan Stanley Children’s Hospital of New York from July 2002 through June 2012
| Characteristic | Value |
|---|---|
| Total no. of catheterization (total no. of patients) | 1,637 (607) |
| Gender | |
| Female | 1,119 (68) |
| Male | 518 (32) |
| Age | |
| <2 years | 121 (7) |
| 2–9 years | 322 (20) |
| 10–17 years | 369 (23) |
| 18–40 years | 442 (27) |
| >40 years | 383 (23) |
| Height, mean ± SD, cm | 142.9 ± 31.8 |
| Weight, mean ± SD, kg | 49.2 ± 27.8 |
| Body surface area, mean ± SD, m2 | 1.4 ± 0.5 |
| Diagnosis | |
| Idiopathic PAH | 786 (48) |
| Associated PAH: congenital heart disease | 525 (32) |
| Associated PAH: other | 326 (20) |
| Syndrome | |
| None | 1,517 (93) |
| Trisomy 21 | 99 (6) |
| Other | 21 (1) |
| Catheterization type | |
| Right heart catheterization | 1,219 (74) |
| Right/left heart catheterization | 418 (26) |
| Anesthesia | |
| None | 835 (51) |
| General: intubation/tracheostomy | 210 (13) |
| General: noninvasive ventilation | 592 (36) |
| Intervention | |
| None | 1,525 (93) |
| Yes | 112 (7) |
| Hemodynamics | |
| Mean right atrial pressure, mean ± SD, mmHg | 8 ± 4 |
| Mean pulmonary artery pressure, mean ± SD, mmHg | 48 ± 22 |
| Cardiac index, mean ± SD, L/min/m2 | 3.6 ± 1.8 |
| Pulmonary vascular resistance indexed to body surface area, mean ± SD, WU × m2 | 13.4 ± 11.4 |
Data are no. (%), unless otherwise indicated. SD: standard deviation; WU: Wood unit.
Table 2.
List of 114 catheter interventions performed in 112 pulmonary arterial hypertension patients at Columbia University Medical Center–Morgan Stanley Children’s Hospital of New York from July 2002 through June 2012
| Intervention | No. |
|---|---|
| Balloon atrial septostomy | 45 |
| Collateral vessel coil embolization | 24 |
| Patent ductus arteriosus device closure | 21 |
| Atrial septal defect device closure | 6 |
| Balloon angioplasty (right ventricle–pulmonary artery conduit [×2], left lower pulmonary vein, right pulmonary artery stent, aortic stent) | 5 |
| Ventricular septal defect device closure | 4 |
| Device retrieval (broken Broviac [×2], migrated patent ductus arteriosus device) | 3 |
| Stent placement (right pulmonary artery, collateral vessels, aortic coarctation) | 3 |
| Transcatheter pulmonary valve implantation | 2 |
| Transthoracic left ventricular puncture (performed for diagnostic purposes in a patient with mechanical mitral and aortic valves) | 1 |
One patient had closure of patent ductus arteriosus and balloon atrial septostomy in 1 procedure, and 1 patient had device closure of patent ductus arteriosus and collateral vessel coiling in 1 procedure.
Morbidity and mortality
There were 93 catheterization-related complications out of 1,637 cases, for an overall complication rate of 5.7%. Of the complications, 73 were minor (4.5%) and 20 were major (1.2%). A closer look at these complications is found in Table 3.
Table 3.
Breakdown of 93 catheterization-related complications (73 minor, 20 major)
| n | Total complications | Minor complications | Major complications | |
|---|---|---|---|---|
| Age group | ||||
| Adults | 825 | 22 (2.7) | 17 (2.1) | 5 (0.6) |
| Pediatrics (<18 years) | 812 | 71 (8.7) | 56 (6.9) | 15 (1.8) |
| Infants/toddlers (<2 years) | 121 | 31 (25.6) | 23 (19.0) | 8 (6.6) |
| GA status | ||||
| No GA | 835 | 16 (1.9) | 13 (1.5) | 3 (0.4) |
| GA | 802 | 77 (9.6) | 60 (7.5) | 17 (2.1) |
| GA with intubation | 210 | 45 (21.4) | 33 (15.7) | 12 (5.7) |
| PAH type | ||||
| IPAH | 786 | 35 (4.4) | 25 (3.2) | 10 (1.3) |
| APAH | 851 | 58 (6.8) | 48 (5.6) | 10 (1.2) |
| Intervention status | ||||
| No intervention | 1,525 | 75 (4.9) | 62 (4.1) | 13 (0.8) |
| Associated intervention | 112 | 18 (16.1) | 11 (9.8) | 7 (6.3) |
Data are no. (%). GA: general anesthesia; PAH: pulmonary arterial hypertension; IPAH: idiopathic PAH; APAH: associated PAH.
Among the 20 major complications (listed in detail in Table 4), there were 6 respiratory arrests, 4 cardiac arrests, 3 arrhythmias requiring defibrillation, and 3 device-related complications. All 6 of the respiratory arrests occurred in pediatric patients following the procedure. Four of these were due to either bronchospasm or poor respiratory effort in infant patients, while a fifth occurred due to poor respiratory effort in a child with complex CHD. The sixth patient was a teenager with hereditary hemorrhagic telangiectasia who had severe epistaxis postprocedure and required reintubation. Of the 4 patients who suffered cardiac arrest, 3 were pediatric patients and 2 were infants. One infant with severe persistent pulmonary hypertension of the newborn suffered cardiac arrest during the procedure and required extracorporeal membrane oxygenation, which served as a bridge to a full recovery. The second infant had APAH associated with CDH, arrested the day following the procedure, and was found to have sepsis. This infant made a full recovery from this episode. The third pediatric patient who suffered cardiac arrest was an adolescent with severe IPAH who arrested following GA induction, and he recovered fully after cardiopulmonary resuscitation. One adult patient with APAH related to a PDA and chronic mitral regurgitation underwent catheterization and PDA device closure, suffered a cardiac arrest after an anaphylactic reaction to protamine given at the conclusion of the case, and fully recovered. Three patients (1 infant, 1 teenager, and 1 adult) required defibrillation for ventricular fibrillation during the procedure. The 3 device-related complications included PDA device migration in 2 patients (1 infant and 1 young adult), and an aortic tear requiring covered stent placement that occurred following stenting of an aortic coarctation in an adolescent with APAH related to CHD. Two patients (1 teenager and 1 young adult) suffered transient ischemia with chest pain and ST changes, 1 adult had pulmonary hemorrhage during pulmonary capillary wedge pressure acquisition, and 1 child had anesthesia-related malignant hyperthermia.
Table 4.
Major catheterization-related complications (n = 20) among 1,637 catheterizations performed at Columbia University Medical Center–Morgan Stanley Children’s Hospital of New York from July 2002 through June 2012
| Complication | No. |
| Respiratory arrest | 6 (all pediatric) |
| Cardiac arrest | 4 (3 pediatric, 1 adult) |
| Arrhythmias requiring defibrillation | 3 (2 pediatric, 1 adult) |
| Device-related complications | 3 (2 pediatric, 1 adult) |
| Chest pain, transient ischemia including ST changes | 2 (1 pediatric, 1 adult) |
| Pulmonary hemorrhage during pulmonary capillary wedge pressure acquisition | 1 (adult) |
| Malignant hyperthermia | 1 (pediatric) |
Of the 73 minor complications, the majority were related to airway issues ( ) and transient rhythm disturbances (
) and transient rhythm disturbances (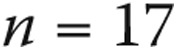 ). All of the airway issues included transient desaturation and/or respiratory acidosis requiring increased monitoring. Eighteen of the 21 occurred in pediatric patients, all of whom underwent GA for catheterization and 7 of whom were in the infant/toddler group. Transient rhythm disturbances included sinus bradycardia in 5 patients (4 pediatric and 1 adult), supraventricular arrhythmias in 4 patients (1 pediatric and 3 adult), junctional bradycardia in 3 patients (all adult), complete heart block in 3 patients (all pediatric), right bundle branch block in 1 infant, and bigeminy in 1 adult. In addition, there were 12 patients (10 pediatric, 9 of whom underwent GA and 2 of whom were in the infant/toddler group) who had hypotension requiring fluid resuscitation during the case. There were 9 patients with vascular access issues, including transient loss of pulse in 6 patients (5 infant/toddler and 1 adult), excessive bleeding in 1 pediatric patient, groin hematoma in 1 pediatric patient, and accidental carotid access without complication in 1 adult patient.
). All of the airway issues included transient desaturation and/or respiratory acidosis requiring increased monitoring. Eighteen of the 21 occurred in pediatric patients, all of whom underwent GA for catheterization and 7 of whom were in the infant/toddler group. Transient rhythm disturbances included sinus bradycardia in 5 patients (4 pediatric and 1 adult), supraventricular arrhythmias in 4 patients (1 pediatric and 3 adult), junctional bradycardia in 3 patients (all adult), complete heart block in 3 patients (all pediatric), right bundle branch block in 1 infant, and bigeminy in 1 adult. In addition, there were 12 patients (10 pediatric, 9 of whom underwent GA and 2 of whom were in the infant/toddler group) who had hypotension requiring fluid resuscitation during the case. There were 9 patients with vascular access issues, including transient loss of pulse in 6 patients (5 infant/toddler and 1 adult), excessive bleeding in 1 pediatric patient, groin hematoma in 1 pediatric patient, and accidental carotid access without complication in 1 adult patient.
Overall, there were 4 procedure-related deaths among 1,637 cases (0.2%), described in Table 5. There were 2 fatalities within 24 hours of catheterization, although only 1 was considered to be related to the procedure, and this occurred in the adult patient who suffered a pulmonary hemorrhage during wedge pressure acquisition. Six other patients suffered fatalities during the admission following catheterization at a range of 5–178 days following the procedure; 3 were attributable to the catheterization (Table 5).
Table 5.
All deaths (n = 8) that occurred during the same hospitalization as catheterization
| Category | Description |
|---|---|
| Catheterization-related deaths | |
| Patient 1 | 75-year-old woman with IPAH, no GA, with pulmonary hemorrhage during wedge pressure acquisition, died within 24 hours of procedure |
| Patient 2 | 10-month-old girl (former 26-week premature baby) with APAH and chronic lung disease of prematurity, GA with intubation, with respiratory arrest from bronchospasm postprocedure (included PDA closure), died 178 days later from disease progression |
| Patient 3 | 10-month-old girl with APAH and repaired total anomalous pulmonary venous return, GA with intubation, with respiratory arrest from increased work of breathing postprocedure, died 59 days later from disease progression |
| Patient 4 | 18-year-old woman with IPAH, GA with nasal cannula oxygen, with progressive hypoxia postprocedure (included balloon atrial septostomy), died 25 days later from disease progressiona |
| Deaths unrelated to catheterization | |
| Patient 5 | 2-year-old girl with IPAH, GA with nasal cannula oxygen, malignant hyperthermia postprocedure, died the day following the procedure from pulmonary edema after initiation of intravenous epoprostenol |
| Patient 6 | 4-month-old girl (former 27-week premature baby) with APAH and chronic lung disease of prematurity, GA with intubation, unremarkable procedure, died 60 days later from disease progression |
| Patient 7 | 4-month-old girl with APAH related to a history of CDH, GA with intubation, suffered a sepsis-related cardiac arrest the day following the procedure and fully recovered, died 94 days later from disease progression |
| Patient 8 | 13-month-old boy with APAH related to complex CHD, GA with intubation, unremarkable procedure, died 5 days later from sepsis unrelated to the catheterization |
IPAH: idiopathic pulmonary arterial hypertension; GA: general anesthesia; APAH: associated pulmonary arterial hypertension; PDA: patent ductus arteriosus; CDH: congenital diaphragmatic hernia; CHD: congenital heart disease.
Since hypoxia was partially a result of septostomy and was part of this patient’s disease progression, this was considered to be a catheterization-related death; however, this was not considered a catheterization complication as it was an acknowledged risk of the procedure.
Risk analysis
Risk analyses performed to identify predictors of catheterization-related complications are detailed in Table 6. With univariate analyses, infants/toddlers were at higher risk for catheterization-related complications, as were patients with smaller body surface area, those with APAH, those who underwent GA, those who had left heart catheterization, and those who underwent a catheter-based intervention. In addition, higher mean right atrial and pulmonary capillary wedge pressures were associated with increased risk of complications, while higher mean pulmonary artery pressure and PVR as well as lower cardiac index were not. In a multivariate model controlling for diagnosis, infants/toddlers were more likely to have complications, as were patients who underwent GA and those who underwent a catheter-based intervention. Higher mean right atrial pressure was also associated with increased risk of complications.
Table 6.
Univariate and multivariate proportional hazards analyses to identify predictors of catheterization-related complications in the pulmonary arterial hypertension (PAH) population
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age <2 years | 8.08 (4.99–13.07) | <0.001 | 5.26 (3.03–9.12) | <0.001 |
| General anesthesia | 5.44 (3.14–9.40) | <0.001 | 3.52 (1.93–6.45) | <0.001 |
| Intervention | 3.70 (2.13–6.45) | <0.001 | 1.94 (1.03–3.67) | 0.04 |
| Mean right atrial pressure | 1.07 (1.02–1.12) | 0.005 | 1.09 (1.03–1.14) | 0.002 |
| Diagnosis (APAH vs. IPAH) | 1.57 (1.02–2.42) | 0.04 | 1.19 (0.74–1.91) | 0.48 |
| Pulmonary capillary wedge pressure | 1.08 (1.03–1.14) | 0.002 | ||
| Body surface area | 0.19 (0.12–0.29) | <0.001 | ||
| Catheterization type (right/left vs. right only) | 3.55 (2.32–5.42) | <0.001 | ||
| Mean pulmonary artery pressure | 1.00 (0.99–1.01) | 0.35 | ||
| Cardiac index | 1.00 (0.88–1.14) | 0.95 | ||
| Pulmonary vascular resistance | 1.00 (0.98–1.02) | 0.95 | ||
HR: hazard ratio; CI: confidence interval; IPAH: idiopathic pulmonary arterial hypertension; APAH: associated pulmonary arterial hypertension.
Discussion
Cardiac catheterization is an important tool in the process of evaluating and managing patients with PAH. As it is the only way to directly measure pulmonary arterial and venous pressures as well as cardiac output, it is a requirement for hemodynamic diagnosis and is often required for entry into major clinical trials in pulmonary hypertension.6 It is also a useful prognosticator that is used to help monitor treatment response and guide management decisions.1,5,7,8 However, in a population as fragile as PAH patients, there is often hesitance to perform invasive procedures such as catheterization, especially when it includes the use of GA. Much of this hesitation comes from anecdotal reporting and higher rates of mortality and serious complications from older series in the 1980s,17,18 as there is very little recent literature regarding the risks and complication rates of catheterization in PAH patients. Hofmann et al.11 reported their 10-year single-center experience performing pulmonary angiography in 202 mostly adult patients with PAH in 2004 and found an overall complication rate of 2.0%, although all complications were major and 3 were fatal. In 2007, Taylor et al.12 reported their 5-year single-center experience performing catheterization under GA in 70 pediatric patients, finding an overall complication rate of 8.6%, a major complication rate of 5.7%, and 1 fatal outcome (1.4%). The largest series to report on catheterization outcomes in pulmonary hypertension patients was a multicenter study from 2006, which had both a retrospective and a prospective arm and reported on >7,000 catheterizations performed at experienced centers in adult patients with pulmonary hypertension.14 Findings included a very low risk of serious adverse events (1.1%) and mortality (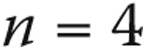 ; 0.06%). Results of the Mid-Atlantic Group of Interventional Cardiology (MAGIC) registry, a multicenter collaboration of pediatric interventional cardiologists from 2010, reported a complication rate of 4.0% among 177 catheterizations in pediatric pulmonary hypertension patients, with no procedural deaths.13
; 0.06%). Results of the Mid-Atlantic Group of Interventional Cardiology (MAGIC) registry, a multicenter collaboration of pediatric interventional cardiologists from 2010, reported a complication rate of 4.0% among 177 catheterizations in pediatric pulmonary hypertension patients, with no procedural deaths.13
We present our 10-year experience performing catheterizations at a single center that specializes in the care of both adult and pediatric patients with pulmonary hypertension, which includes the largest cohort of pediatric PAH patients analyzed for catheterization-related complications. While the overall complication rate in our cohort was 5.7%, the majority of complications were minor (78%) and the rate of major complications was only 1.2%, with procedure-related deaths occurring in only 0.2% of cases.
There was an observable difference in complication rates between pediatric and adult patients. The overall complication rate in adults is quite low at 2.7% and is very similar to that reported by Hofmann and colleagues (2.0%), although our rates of major complications (0.6% vs. 2.0%) and fatalities (0.1% vs. 1.5%) are favorable in comparison.11 One would assume Hofmann’s cohort to be composed mostly of adult patients, since the mean age was around 53 years; however, it is not an exclusively adult patient population, as one of the reported fatalities did occur in a 17-month-old girl. In addition, Hofmann’s data report on the risk of catheterization with pulmonary angiography, which confers a higher risk of complications compared solely with right heart catheterization, and in our population only a very small percentage of patients underwent angiography. Our complication and mortality data also compare similarly with those of the relatively large review of Hoeper et al.,14 who reported major complications in 1.1% and procedure-related mortality in 0.06% of adult PAH catheterizations performed at centers experienced in the care of pulmonary hypertension patients.
Our catheterization-related complication rate in pediatric patients was 8.7%. This finding is very similar to that in the pediatric cohort of Taylor et al.12 (8.6%); however, it is somewhat higher than that reported by the MAGIC registry, in which there was a 4.0% complication rate and no procedural deaths.13 The majority of complications in our pediatric cohort were minor in nature (79%), and the rate of major pediatric complications was relatively low (1.8%) and compares quite favorably with the rate demonstrated by Taylor et al.12 (5.7%). A closer look at our pediatric cohort shows that 31 of the 71 complications, including 8 of 15 major complications, occurred in patients <2 years old (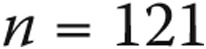 ), yielding overall and major complication rates of 25.6% and 6.6%, respectively, in this age group. The remainder of the pediatric population (
), yielding overall and major complication rates of 25.6% and 6.6%, respectively, in this age group. The remainder of the pediatric population (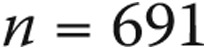 ) suffered 40 complications (5.8%), only 7 of which were major complications (1.0%), a rate very similar to that among their adult counterparts (0.6%).
) suffered 40 complications (5.8%), only 7 of which were major complications (1.0%), a rate very similar to that among their adult counterparts (0.6%).
There appears to be a difference in complication rate when dividing the cohort into nonanesthesia and GA cases (overall complication rate, 1.9% vs. 9.6%; major complication rate, 0.4% vs. 2.1%). A closer look at the GA population shows that 45 of the 77 complications, including 12 of the 17 major complications, occurred in patients who received positive pressure ventilation invasively via either endotracheal tube or tracheostomy (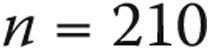 ), yielding overall and major complication rates of 21.4% and 5.7%, respectively, in these patients. There is definite overlap between the increased complication rates in pediatric and GA patients, and this may in part explain the difference in complication rate between our pediatric cohort and that reported by the MAGIC registry. All of our infant/toddler cases received GA, and GA was utilized in 97.0% of patients under the age of 5 years, whereas the MAGIC registry reports GA use in 74% of infants <1 year old and in 83% of patients between 1 and 5 years old.13 It was especially for this reason that we performed multivariate proportional hazards modeling to identify predictors of catheterization-related complications.
), yielding overall and major complication rates of 21.4% and 5.7%, respectively, in these patients. There is definite overlap between the increased complication rates in pediatric and GA patients, and this may in part explain the difference in complication rate between our pediatric cohort and that reported by the MAGIC registry. All of our infant/toddler cases received GA, and GA was utilized in 97.0% of patients under the age of 5 years, whereas the MAGIC registry reports GA use in 74% of infants <1 year old and in 83% of patients between 1 and 5 years old.13 It was especially for this reason that we performed multivariate proportional hazards modeling to identify predictors of catheterization-related complications.
In constructing a multivariate proportional hazards model that controlled for diagnosis (APAH vs. IPAH), we found that complication risk was significantly increased in infants/toddlers, cases involving GA, and cases involving a catheter-based intervention and was associated with increases in mean right atrial pressure. Although there was significant overlap between pediatric cases and the use of GA, each was identified as an independent predictor of complication risk. Similar overlap existed between the performance of a catheter-based intervention and the use of GA (72% of cases); however, the performance of an intervention also proved to be an independent risk factor for increased risk of complication. While the addition of left heart catheterization was found to be predictive of increased complication risk as a univariate variable, it was not an independent predictor of complication risk in the multivariate analysis. This is likely because left heart catheterization was often employed in patients undergoing intervention or as a means of blood pressure and blood gas monitoring in higher-risk cases, and it likely did not independently contribute increased risk in these cases.
The association between hemodynamic parameters and increased complication risk has not been previously evaluated in PAH patients. In general, mean right atrial and pulmonary artery pressures as well as cardiac output have been shown to be prognostic of outcome in patients with PAH.1,7,8 In the case of evaluating our cohort for increased risk of catheterization-related complications, only mean right atrial pressure was associated with increased complication rate, whereas other hemodynamic measures were unrelated.
Limitations of the study are mostly related to its retrospective nature. Although all catheterization reports at CUMC-MSCHONY have been maintained in one electronic system since 2002, anesthesia reports are maintained separately and not electronically, and they were not reviewed separately for the purposes of this study. Each patient’s complete medical record was thoroughly reviewed; thus, it is unlikely that procedure-related complications were missed, even those related solely to GA. Patients were captured on the basis of diagnoses used for billing purposes. Patients incorrectly coded as having either IPAH or APAH were removed from inclusion in the study during chart review. However, patients incorrectly coded as not having IPAH or APAH would have escaped chart review and therefore would have been inappropriately excluded. Patients included in this data analysis were limited to those referred to a specialized team at CUMC-MSCHONY for catheterization by the pulmonary hypertension center at CUMC. Patients with PAH cared for by physicians at CUMC outside of the pulmonary hypertension center may have been referred for a catheterization that would not have been identifiable within the queried database of CUMC-MSCHONY. A final limitation is that this study reports only a single pulmonary hypertension center’s experience. While the observed complication and mortality rates in this cohort stress the importance of performing such procedures in centers of excellence, it should be realized that these results may not be applicable to centers without such expertise.
Conclusion
In summary, cardiac catheterization is an important clinical tool for the diagnosis, assessment, and monitoring of PAH patients. In our pulmonary hypertension center that provides multidisciplinary expert care to adult and pediatric patients with pulmonary hypertension, there is relatively low morbidity and mortality associated with the procedure, although increased complication rates are noted in patients <2 years old, patients undergoing GA, and patients undergoing associated catheter-based interventions. In this setting, we continue to advocate for the use cardiac catheterization as an important tool. However, we do encourage careful consideration before recommending the procedure in the pediatric population, especially in infants and toddlers. We also advocate that cardiac catheterization be performed in a pulmonary hypertension center of excellence in which cardiologists, anesthesiologists, nursing, and ancillary staff are well acquainted with caring for this at-risk patient population.
Acknowledgments
We acknowledge Dr. Marc E. Richmond for his assistance with the statistical analyses conducted during this project.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115:343–349. [DOI] [PubMed]
- 2.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation 1999;99:1197–1208. [DOI] [PubMed]
- 3.Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012;125:113–122. [DOI] [PubMed]
- 4.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 2012;142:448–456. [DOI] [PubMed]
- 5.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S97–S107. [DOI] [PubMed]
- 6.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55–S66. [DOI] [PubMed]
- 7.Clabby ML, Canter CE, Moller JH, Bridges ND. Hemodynamic data and survival in children with pulmonary hypertension. J Am Coll Cardiol 1997;30:554–560. [DOI] [PubMed]
- 8.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005;95:199–203. [DOI] [PubMed]
- 9.Bergersen L, Marshall A, Gauvreau K, et al. Adverse event rates in congenital cardiac catheterization—a multi-center experience. Catheter Cardiovasc Interv 2010;75:389–400. [DOI] [PubMed]
- 10.Learn CP, Holzer RJ, Daniels CJ, et al. Adverse events rates and risk factors in adults undergoing cardiac catheterization at pediatric hospitals-results from the C3PO. Catheter Cardiovasc Interv 2013;81:997–1005. [DOI] [PubMed]
- 11.Hofmann LV, Lee DS, Gupta A, et al. Safety and hemodynamic effects of pulmonary angiography in patients with pulmonary hypertension: 10-year single-center experience. AJR Am J Roentgenol 2004;183:779–786. [DOI] [PubMed]
- 12.Taylor CJ, Derrick G, McEwan A, Haworth SG, Sury MR. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth 2007;98:657–661. [DOI] [PubMed]
- 13.Hill KD, Lim DS, Everett AD, Ivy DD, Moore JD. Assessment of pulmonary hypertension in the pediatric catheterization laboratory: current insights from the Magic registry. Catheter Cardiovasc Interv 2010;76:865–873. [DOI] [PMC free article] [PubMed]
- 14.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 2006;48:2546–2552. [DOI] [PubMed]
- 15.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res 1970;4:23–30. [DOI] [PubMed]
- 16.Bergersen L, Gauvreau K, Jenkins KJ, Lock JE. Adverse event rates in congenital cardiac catheterization: a new understanding of risks. Congenit Heart Dis 2008;3:90–105. [DOI] [PubMed]
- 17.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 1984;70:580–587. [DOI] [PubMed]
- 18.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med 1987;107:216–223. [DOI] [PubMed]


