Abstract Abstract
Little is known about the use of lung transplantation in the management of sickle cell disease–associated pulmonary arterial hypertension (SCD-PAH). We present clinical and pathological data and report the first successful outcome of bilateral lung transplantation in a patient with severe SCD-PAH and pulmonary veno-occlusive disease (PVOD). We discuss the complexities of multidisciplinary planning and management of lung transplantation in patients with SCD-associated pulmonary vascular complications. This case reports the first documented successful lung transplant and first case of PVOD in a patient with SCD-PAH.
Keywords: lung transplantation, sickle cell disease, pulmonary arterial hypertension, pulmonary veno-occlusive disease
Sickle cell disease (SCD) is an autosomal, recessive genetic disorder caused by a mutation in the β-globin gene of hemoglobin that produces sickle hemoglobin (HbS), which under certain conditions polymerizes, leading to vaso-occlusive crisis, hemolysis, and end-organ injury and failure.1 Pulmonary arterial hypertension (PAH) is a known complication in patients with SCD, affecting 6%–10% of adult patients with this disorder and elevating mortality risk 10-fold in patients with significant disease.2-5 Medical treatment of SCD-associated pulmonary hypertension (SCD-PAH) can be quite complicated, and to date there is no cure.6 We report the first successful bilateral lung transplant for SCD-PAH and discuss the management and implications of this new surgical treatment option. We further describe the first case of PAH and pulmonary veno-occlusive disease (PVOD) associated with SCD.
Case Description
The patient is a 17-year-old man with a history of SCD (HbSS) complicated by PAH, transfusion-related iron overload, red blood cell (RBC) alloimmunization with anti-C, anti-V, anti-K, and anti-Fya alloantibodies, and a history of moyamoya treated with exchange transfusion since early childhood. He was asymptomatic from a pulmonary perspective until 2006, when he suffered his first acute chest crisis. In January 2007, he suffered an episode of near syncope. In August 2007, he suffered a pulmonary embolism, received a diagnosis of protein S deficiency, and underwent anticoagulation treatment with heparin and warfarin. Subsequently, he presented with a second episode of near syncope and progressive fatigue and dyspnea on exertion. He received a diagnosis of PAH and inoperable chronic thromboembolic pulmonary hypertension (CTEPH), with right ventricular systolic pressure (RVSP) of 65 mmHg and tricuspid regurgitant maximum velocity of 3.9 m/s on echocardiogram (Table 1). Initial right heart catheterization (RHC) demonstrated pulmonary artery (PA) pressures of 60/26 (mean: 38) mmHg, with pulmonary capillary wedge pressure of 4 mmHg (Table 2). Being initially responsive to inhaled nitric oxide (iNO; Table 2), the patient was treated with sildenafil and supplemental oxygen. His SCD was managed by monthly blood transfusions, hydroxyurea, and iron chelation with deferasirox. Despite anticoagulation, he suffered 2 additional uncomplicated pulmonary emboli in 2008; his treatment was transitioned to enoxaparin because of warfarin failure, and he had no further emboli. He continued to receive enoxaparin for protein S deficiency and CTEPH.
Table 1.
Pretransplant echocardiography
| Date | RVSP, mmHg | RVFAC, % | TAPSE, cm | TR maximum velocity, m/s | Myocardial performance index | Diastole EI | Therapy |
|---|---|---|---|---|---|---|---|
| Nov 14, 2007 | 65 | 52 | 2.2 | 3.9 | 0.33 | 1.12 | |
| Jan 15, 2008 | 111 | 59 | 2.3 | 5.3 | 0.29 | 1.36 | |
| Jan 16, 2008a | 43 | 3.3 | Started sildenafil | ||||
| Jan 28, 2010 | 55 | 39 | 3.3 | 3.8 | 0.23 | 1.30 | Sildenafil and bosentan |
| Feb 27, 2010b | 115 | 73 | 2.7 | 4.6 | 0.08 | 1.51 | |
| Feb 27, 2010c | 85 | … | … | … | … | … | Sildenafil, bosentan; started inhaled treprostinil |
| Sep 1, 2010 | 93 | 66 | 2.3 | 4.8 | 0.08 | 1.41 | |
| Oct 8, 2010 | 61 | 65 | 2.5 | 3.9 | 0.07 | 1.46 | Sildenafil, bosentan, and IV treprostinil |
| May 17, 2011 | NA | 62 | 2.9 | NA | 0.07 | 1.28 | Posttransplant |
Early echocardiographic (echo) data (2007–2008) demonstrate evidence of pulmonary arterial hypertension (PAH) with preserved right ventricular (RV) systolic function. Later echo data (2010) demonstrate worsening PAH with increasing eccentricity index (EI) during diastole, suggestive of flattening of the interventricular septum due to RV volume overload. Echo at 3 months after transplant (May 2011) shows resolution of patient’s EI during diastole, consistent with resolution of PAH. RVSP: RV systolic pressure; RVFAC: RV fractional area change; TAPSE: tricuspid annulus peak systolic excursion; TR: tricuspid regurgitant; NA: could not estimate.
Inhaled NO study.
Before inhaled prostacyclin.
After inhaled prostacyclin.
Table 2.
Pretransplant right heart catheterization (RHC) data
| RHC date, test | RAP, mmHg | RVP, mmHg | PAP, mmHg | PCWP, mmHg | TPG, mmHg | PA sat, % | CI | PVRI, WU × m2 | Therapy |
|---|---|---|---|---|---|---|---|---|---|
| Jan 29, 2008 | Sildenafil | ||||||||
| Baseline | 9 | 61/10 | 60/23 (38) | 4 | 34 | 53 | 4.4 | 7.7 | |
| 100% O2 | 9 | 56/0 | 58/22 (36) | 7 | 29 | 80 | 4.5 | 6.5 | |
| 100% O2 + iNO 80 ppm | 9 | 50/1 | 40/14 (25) | 8 | 17 | 83 | 6.32 | 2.9 | |
| Jan 11, 2010 | Sildenafil and bosentan | ||||||||
| Baseline on 2L | 4 | 81/10 | 81/27 (45) | 4 | 41 | 70 | 3.8 | 10 | |
| 100% O2 | 71/26 (40) | 9 | 31 | 80 | 3.9 | 7.9 | |||
| 100% O2 + iNO 40 ppm | 68/19 (36) | 7 | 29 | 79 | 3.7 | 7.9 | |||
| Sep 3, 2010 | |||||||||
| Baseline | 15 | 102/30 (59) | 11 | 48 | 62 | 3.9 | 7.3 | Sildenafil, bosentan, and inhaled treprostinil; transitioned to IV treprostinil |
Early RHC data demonstrate pulmonary arterial hypertension (PAH), initially responsive to oxygen and inhaled nitric oxide (iNO). RHC data after syncopal event (January 11, 2010) demonstrate that the patient was no longer significantly responsive to oxygen or nitric oxide. RHC on September 3, 2010, after a presyncopal event, shows progression of PAH. RAP: right atrial pressure; RVP: right ventricular pressure, systolic/diastolic; PAP: pulmonary arterial pressure, systolic/diastolic (mean); PCWP: pulmonary capillary wedge pressure; TPG: transpulmonary gradient; PA sat: pulmonary artery oxygen saturation; CI: cardiac index (L/min/m2) PVRI: pulmonary vascular resistance index; WU: Wood units; 2L: 2L oxygen by nasal cannula.
In autumn 2009, the patient’s symptoms worsened, and he presented for optimization of PAH therapy. Bosentan was added. Despite dual-agent oral therapy, he suffered a syncopal event in January 2010, and repeat RHC demonstrated worsened PA pressures (81/27 [mean: 45] mmHg), pulmonary vascular resistance index (PVRI; 10 Wood units × m2), and a decline in cardiac index (3.8 L/min/m2). He was less responsive to iNO, no longer technically meeting the definition of having a positive response (decrease in mean PA pressure of <10 mmHg; Table 2). Inhaled treprostinil was added and titrated up to 9 puffs 4 times daily, after which he reported improved stamina. He was also referred for lung transplant evaluation.
In September 2010, the patient was admitted with increasing dyspnea on exertion and presumed chest crisis, accompanied by progressive right ventricular (RV) failure on echocardiogram (RVSP of 88 mmHG, moderately decreased function per report) with trace lower-extremity edema despite augmented PAH therapy. A computed tomography (CT) angiogram showed no evidence of acute or chronic pulmonary embolism. RHC demonstrated PA pressure of 102/30 (mean: 59) mmHg, with right atrial pressure of 15 mmHg. His transpulmonary gradient was 48 mmHg, his cardiac index was 3.67 L/min/m2 by Fick index, and his PVRI was 7.3 Wood units (Table 2). The patient’s treatment was transitioned from inhaled to intravenous treprostinil. Despite a maximal dose of 24 ng/kg/min, he continued to worsen clinically. Furthermore, in addition to his worsening RHC parameters, an echocardiogram also demonstrated an increasing eccentricity index (EI) over time, to a maximum of 1.51, indicative of flattening of the interventricular septum due to RV pressure overload (Table 1). Given his worsening symptoms and history of syncope, the decision was made to list him for lung transplantation.
In preparation for listing, an extremely conservative hematologic goal of less than 10% HbS was set to prevent sickle cell crisis at the time of transplant and postoperatively.7-9 The patient underwent weekly blood transfusions, continuing his deferasirox, hydroxyurea, and enoxaparin anticoagulation. Once he reached the target hemoglobin, he was listed in October 2010 for double lung transplantation. His lung allocation score was 34.6. Given his history of syncope, an appeal was made to the United Network for Organ Sharing committee; however, it was denied because of the perceived high-risk nature of this procedure.
In February 2011, bilateral sequential lung transplantation was performed using alemtuzumab induction via bilateral anteroaxillary thoracotomies. Cold ischemic times were 212 and 346 minutes. Both the recipient and donor were cytomegalovirus (CMV) negative. Preoperative hemoglobin was 9.0 g/dL, and hematocrit was 27.3%. Although the patient’s HbS was 5.5% at the time of transplantation, he received transfusions of 2 units of packed RBCs immediately prior to surgery and 2 units during surgery to minimize the risk of sickle cell chest crisis during the procedure. His postoperative hemoglobin and hematocrit were 10.9 g/dL and 31.4%, respectively. To further minimize his hematologic risk, the procedure was performed entirely off cardiopulmonary bypass (CPB), utilizing iNO, nitroprusside, and milrinone for cardiopulmonary support. The patient was weaned off his preoperative pulmonary vasodilators, and iNO, milrinone, and nitroprusside were discontinued within 48 hours. After extubation on postoperative day 3, he quickly weaned to room air.
On postoperative day 8, he developed hypoxemia, requiring brief mechanical ventilation. Bronchoscopy was consistent with airway ischemia reperfusion injury, and he improved with a short course of antibiotics and diuretics. On postoperative day 14, he was noted to have bilateral loculated pleural effusions, consistent with clotted blood. To avoid potential bleeding and minimize transfusion needs, it was decided to forego washout in the operating room. He underwent placement of pigtail thoracostomy tubes to facilitate tissue plasminogen activator administration with complete resolution. The patient was discharged on room air on postoperative day 19, off all pulmonary vasodilator therapy.
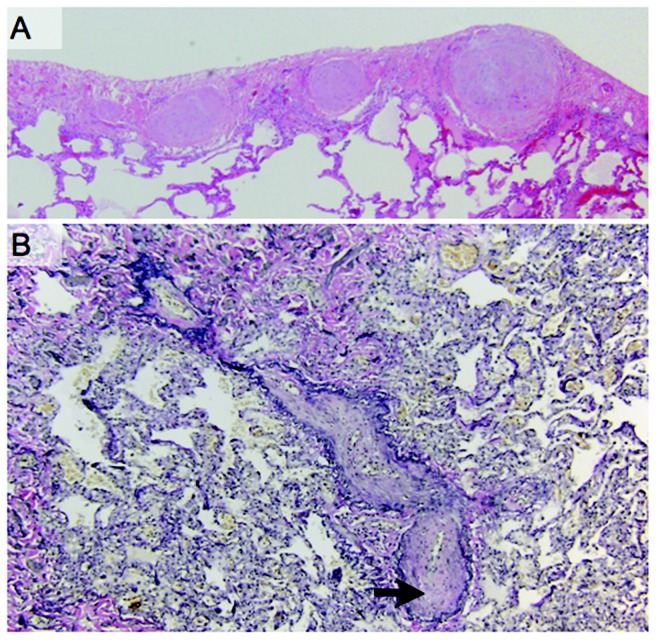
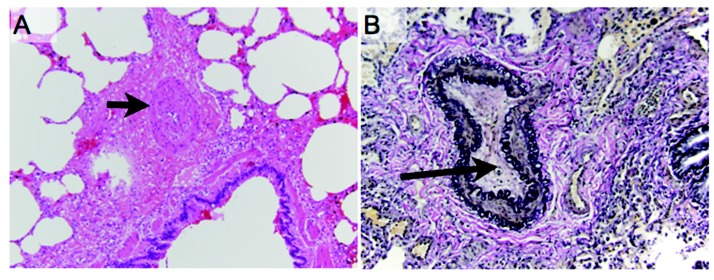
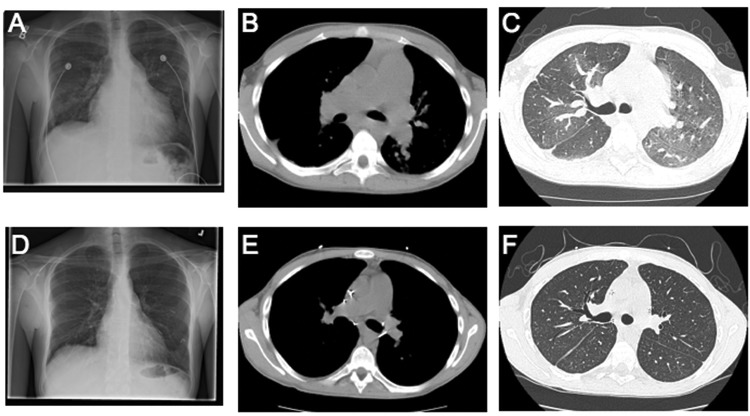
Postoperative pathology of the explanted lungs demonstrated histologic changes consistent with PVOD (Fig. 1), severe PAH (Fig. 2), and CTEPH. The explanted lungs were remarkable for prominent patchy fibrosis with perivascular accentuation. Sections of the lung and pleura showed marked myointimal thickening of pleuropulmonary veins, with “arterialization” of the vessels (Fig. 1A) and complete obliteration of the lumens of veins and venules by myointimal fibroelastosis (Fig. 1B). There was also marked myointimial thickening of the pulmonary artery (Fig. 2A) and nearly complete obliteration of pulmonary arterial lumens (Fig. 2B). Distal pulmonary arteries showed eccentric cellular intimal fibrosis and recanalizing thrombi. There were also broad zones of pleural fibrosis, consistent with old infarcts. Resolution of his disease was also documented on postoperative imaging by chest radiographs and CT scans (Fig. 3). His 3-month postoperative echocardiogram showed normal RV size, thickness, and function, with an inadequate tricuspid regurgitation envelope to estimate pulmonary artery pressures. His EI on diastole decreased to 1.28, suggesting an improvement in RV morphology.
Figure 1.
Pathologic findings revealed pulmonary veno-occlusive disease. A, Sections of the lung and pleura show marked myointimal thickening of pleuropulmonary veins with “arterialization” of the vessels. B, Elastin tissue stain revealed complete obliteration (arrow) of the lumens of veins and venules by myointimal fibroelastosis.
Figure 2.
Pathologic findings consistent with concomitant pulmonary arterial vasculopathy. A, Marked myointimial thickening of the pulmonary artery (arrow). B, Elastic tissue stains highlighting the near complete obliteration of the lumens of pulmonary arteries (arrow).
Figure 3.
Comparison of pre- and posttransplant radiologic changes. Pretransplant chest radiograph (A) and CT scans (B, C) demonstrate an enlarged right ventricle and enlarged pulmonary arteries. In addition, patchy mosaicism is seen in the parenchyma (C). These findings are resolved with transplant, as seen in the virtually normal posttransplant chest radiograph (D) and CT scan (E, F).
Following his discharge, the patient was initially managed with standard 3-drug immunosuppression with prednisone, tacrolimus (target trough levels: 12–15 mg/dL), and mycophenolate mofetil. He was also treated with valganciclovir and voriconazole for CMV and antifungal prophylaxis. Although he suffered 2 episodes of acute cellular rejection, requiring methylprednisolone and thymoglobulin, he responded to augmented immunosuppression therapy, and at 2 years after transplant he was doing well, off oxygen and without any further episodes of syncope. His posttransplant hematologic regimen at that time included blood transfusions every 3 weeks to maintain his HbS percentage under 10%. Interestingly, whereas before transplantation he suffered 6–9 vaso-occlusive pain crises per year while receiving PAH therapy, he did not have any vaso-occlusive pain events on his posttransplant transfusion and immunosuppression regimen. His PA pressures and RV function remain normal.
Discussion
We have reported the first successful bilateral lung transplantation for SCD-PAH. We document the clinical progression of the patient’s disease and the finding that explanted lungs showed extensive pathology, including evidence of PAH, PVOD, and thromboses. To our knowledge, this is the first report of such complicated pathology in a patient with SCD. After definitive surgical treatment, the patient did well 2 years after transplant, without episodes of syncope or sickle cell crisis.
To date there has been no report of lung transplantation for a patient with SCD. However, kidney, combined heart-kidney, liver, and combined liver-kidney transplants have been reported.10 Kidney transplantation has been the most common procedure for patients with SCD-related disease.8,9,11-16
The main challenges of solid-organ transplantation in patients with SCD relate to higher perioperative mortality and risk of graft loss due to vaso-occlusive complications in the transplanted organ. Major surgery in SCD poses a risk of acute vaso-occlusive complications, such as the development of acute chest syndrome, a life-threatening acute lung injury syndrome characterized by bilateral radiographic infiltrates, fever, and hypoxemia.17 In 1995, a clinical trial showed that preoperative simple transfusion to increase the total hemoglobin to 10 mg/dL, regardless of the HbS level, is as effective as a more aggressive transfusion regimen aiming at decreasing the HbS to <30% in reducing operative complications in SCD patients undergoing a number of different surgical procedures.18 Even though a prophylactic simple transfusion has become the standard of care in SCD, questions remain as to the most effective transfusion strategy for selected surgeries and in special situations such as that posed by solid-organ transplantation.19
Red cell vaso-occlusive events in transplant allografts and painful vaso-occlusive crises have been reported in both kidney and liver transplant recipients,9,12,14,16,20,21 and long-term outcomes, such as mortality, are worse in SCD kidney transplant recipients than in non-SCD recipients (reviewed by Scheinman15 in 2009). While these cases are not the majority, such a complication in organ transplantation—and especially lung transplantation—would be expected to increase the risk of primary graft dysfunction and organ failure. While the optimal target HbS is unknown, some authors encourage the use of RBC transfusions to maintain hematocrit at 30% in patients at high risk of postoperative SCD-associated complications.22 In renal transplantation, some have recommended more stringent preoperative transfusion goals to minimize or even eliminate HbS.8,9 Furthermore, there have been associations between HbS percentage and disease severity in other reported complications of SCD.23
The optimal target hematocrit and HbS percentage are debated, however, as frequent blood transfusions are not without risk. In addition to the common transfusion risks experienced by the general population, SCD patients are particularly prone to RBC alloimmunization because of disparate expression of RBC antigens, compared to the mostly Caucasian blood donor pool. This is clinically relevant because it limits availability of matched blood for future transfusions and may be responsible for delayed hemolytic transfusion reactions and the rare but potentially fatal hyperhemolytic syndrome of SCD. The risk of worsening RBC alloimmunization was therefore carefully weighed in this patient with preexisting RBC alloantibodies. To minimize this risk, we consulted the local blood bank and determined, prior to transplantation, that long-term procurement of leukoreduced packed RBCs that had undergone extensive phenotype matching (for C, V, K, Fya, and Jkb) was feasible.24 Moreover, our patient had evidence of transfusional hemosiderosis prior to lung transplantation, requiring upward titration of his deferasirox dose and management of multiple drug interactions with his complex posttransplantation polypharmacotherapy.
Finally, in the setting of solid-organ transplantation, potential transfusion risks include human leukocyte antigen (HLA) alloimmunization, antiallograft antibodies, and humoral rejection. In the renal transplant literature, RBC transfusion has been associated with increased risk of HLA sensitization.25,26 However, to date there has been no association between blood transfusion frequency and development of lung allograft rejection.27 In fact, in the largest temporal study to date, there was an inverse association between frequency of blood transfusions after lung transplantation and acute rejection, leading some to theorize that exposure to HLAs through blood transfusions may have a tolerogenic effect.27,28
The decision to pursue an aggressive transfusion regimen to maintain an HbS of 10% was made for several reasons. Reduction of HbS has been an effective strategy for preventing vaso-occlusive complications in SCD, such as cerebrovascular accidents in children at high risk based on transcranial Doppler results.19,29,30 In addition to prevention of cerebrovascular complications (were CPB deemed necessary intraoperatively), there have been reports in the sickle cell literature describing reduced incidence of acute chest syndrome with aggressive transfusions.19,30 In addition, our patient had a history of moyamoya, placing him at risk for cerebrovascular complications. Finally, reduction of the intracellular HbS concentration has been shown to reduce in vitro and in vivo sickling of RBCs and the incidence of vaso-occlusive episodes in patients homozygous for HbS, due to decreased polymerization of HbS, containing hemoglobin in the presence of increased concentrations of hemoglobin A or hemoglobin F.31,32
To lower the risks of blood transfusion in our patient, surgery was performed off CPB, and the patient tolerated double lung transplant. Whether or not to use CPB while performing double lung transplantation has long been discussed and remains controversial.33 As in other cases of severe PAH, our patient was at risk of complications related to uncontrolled reperfusion off CPB after completion of one lung, such as primary graft dysfunction (acute lung edema) with acute deterioration, emergent CPB institution, and/or major complications such as brain damage, bleeding, and RV failure. However, given the patient’s hematologic complexity, it was believed that the benefits of avoiding CPB as a blood-saving strategy outweighed these theoretical risks.
In the postoperative period and over the subsequent 24 months, the patient has continued to receive regular leukocyte-reduced, cross-matched blood transfusions, and we have been progressively maximizing the dose of hydroxyurea with the hope of increasing his fetal hemoglobin and reducing the frequency of transfusion. We have opted not to place an implantable double-lumen catheter for exchange transfusion, to minimize the risk of catheter-related thrombosis in this patient with protein S deficiency. To date he has not developed significant antiallograft antibodies or further RBC alloantibodies. In keeping with the overall control of his SCD phenotype, his opiate requirements have also decreased drastically, and he no longer receives scheduled long-acting opiates.
We report that bilateral lung transplantation is feasible in patients with SCD and multiple comorbidities. Our case provides a rationale for considering lung transplantation as a treatment for end-stage SCD-PAH on a case-by-case basis. This case underscores the peculiar challenges of lung transplantation in this population, including the intensive medical needs after transplantation. It is paramount that multidisciplinary care include early and ongoing consultation with a blood bank and discussions with the surgical team on the safest technical approach. Although the optimal transfusion strategy in this setting remains to be determined, here we show that an aggressive transfusion approach coupled with hydroxyurea, in addition to routine posttransplant care, is safe and effective in minimizing SCD- and transplant-related complications at 2 years after transplant.
Source of Support: This work was supported by the Parker B. Francis Foundation (MPG).
Conflict of Interest: HCC is a member of the scientific advisory boards of Gilead, Pfizer, Bayer, and Actelion and is a consultant for United Therapeutics. All other authors declare no conflict of interest.
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–2031. [DOI] [PubMed]
- 2.Gladwin MT. Prevalence, risk factors and mortality of pulmonary hypertension defined by right heart catheterization in patients with sickle cell disease. Expert Rev Hematol 2011;4:593–596. [DOI] [PubMed]
- 3.Sachdev V, Kato GJ, Gibbs JSR, Barst RJ, Machado RF, Nouraie M, Hassell KL, et al. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation 2011;124:1452–1460. [DOI] [PMC free article] [PubMed]
- 4.Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 2011;365:44–53. [DOI] [PubMed]
- 5.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J 2012;39:112–118. [DOI] [PubMed]
- 6.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, Gibbs JSR, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 2011;118:855–864. [DOI] [PMC free article] [PubMed]
- 7.Yousafzai SM, Ugurlucan M, Al Radhwan OA, Al Otaibi AL, Canver CC. Open heart surgery in patients with sickle cell hemoglobinopathy. Circulation 2010;121:14–19. [DOI] [PubMed]
- 8.Brennan DC, Lippmann BJ, Shenoy S, Lowell JA, Howard TK, Flye MW. Living unrelated renal transplantation for sickle cell nephropathy. Transplantation 1995;59:794–795. [DOI] [PubMed]
- 9.Nath J, McDaid J, Bentall A, Ball S, Ready AR, Inston NG. Sickle cell and renal transplant: a national survey and literature review. Exp Clin Transplant 2012;10:1–7. [DOI] [PubMed]
- 10.Audard V, Grimbert P, Kirsch M, Habibi MA, Lang P, Rémy P, Abbou C, et al. Successful combined heart and kidney transplantation in a patient with sickle-cell anemia. J Heart Lung Transplant 2006;25:993–996. [DOI] [PubMed]
- 11.O’Rourke EJ, Laing CM, Khan AU, Hussain R, Standish RA, Buscombe JR, Hilson AJW, Harber M. The case: allograft dysfunction in a patient with sickle cell disease. Kidney Int 2008;74:1219–1220. [DOI] [PubMed]
- 12.Chatterjee SN. National study in natural history of renal allografts in sickle cell disease or trait: a second report. Transplant Proc 1987;19:33–35. [PubMed]
- 13.Chatterjee SN. National study on natural history of renal allografts in sickle cell disease or trait. Nephron 1980;25:199–201. [DOI] [PubMed]
- 14.Ojo AO, Govaerts TC, Schmouder RL, Leichtman, AB, Leavey SF, Wolfe RA, Held PJ, Port FK, Agodoa LY. Renal transplantation in end-stage sickle cell nephropathy. Transplantation 1999;67:291–295. [DOI] [PubMed]
- 15.Scheinman JI. Sickle cell disease and the kidney. Nat Rev Nephrol 2009;5:78–88. [DOI] [PubMed]
- 16.Montgomery R, Zibari G, Hill GS, Ratner LE. Renal transplantation in patients with sickle cell nephropathy. Transplantation 1994;58:618–620. [DOI] [PubMed]
- 17.Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med 2000;342:1855–1865. [DOI] [PubMed]
- 18.Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, Pegelow C, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. N Engl J Med 1995;333:206–213. [DOI] [PubMed]
- 19.Hirst C, Williamson L. Preoperative blood transfusions for sickle cell disease. Cochrane Database Syst Rev 2012; (1):CD003149. [DOI] [PubMed]
- 20.Hurtova M, Bachir D, Lee K, Calderaro J, Decaens T, Kluger MD, Zafrani ES, et al. Transplantation for liver failure in patients with sickle cell disease: challenging but feasible. Liver Transplant 2011;17:381–392. [DOI] [PubMed]
- 21.Mekeel KL, Langham MR Jr., Gonzalez-Peralta R, Fujita S, Hemming AW. Liver transplantation in children with sickle cell disease. Liver Transplant 2007;13:505–508. [DOI] [PubMed]
- 22.Firth PG, Head CA. Sickle cell disease and anesthesia. Anesthesiology 2004;101:766–785. [DOI] [PubMed]
- 23.Nagpal KC, Asdourian GK, Goldbaum MH, Raichand M, Goldberg MF. The conjunctival sickling sign, hemoglobin S, and irreversibly sickled erythrocytes. Arch Ophthalmol 1977;95:808–811. [DOI] [PubMed]
- 24.Vichinsky EP, Luban NL, Wright E, Olivieri N, Driscoll C, Pegelow CH, Adams RJ. Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: a multicenter transfusion trial. Transfusion 2001;41:1086–1092. [DOI] [PubMed]
- 25.Magee BA, Martin J, Cole MP, Morris KG, Courtney AE. Effects of HLA-matched blood transfusion for patients awaiting renal transplantation. Transplantation 2012;94:1111–1116. [DOI] [PubMed]
- 26.Obrador GT, Macdougall IC. Effect of red cell transfusions on future kidney transplantation. Clin J Am Soc Nephrol 2013;8:852–860. [DOI] [PubMed]
- 27.Mason DP, Little SG, Nowicki ER, Batizy LH, Murthy SC, McNeill AM, Budev MM, Mehta AC, Pettersson GB, Blackstone EH. Temporal pattern of transfusion and its relation to rejection after lung transplantation. J Heart Lung Transplant 2009;28:558–563. [DOI] [PubMed]
- 28.Jackson A, McSherry C, Butters K, Diko M, Almond PS, Matas AJ, Reinsmoen NL. Pretransplant exposure to donor HLA-DR antigen in random transfusion units and the development of donor antigen-specific hyporeactivity. Hum Immunol 1997;55:148–153. [DOI] [PubMed]
- 29.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 1998;339:5–11. [DOI] [PubMed]
- 30.Miller ST, Wright E, Abboud M, Berman B, Files B, Scher CD, Styles L, Adams RJ. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr 2001;139:785–789. [DOI] [PubMed]
- 31.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med 1995;332:1317–1322. [DOI] [PubMed]
- 32.Voskaridou E, Christoulas D, Bilalis A, Plata E, Varvagiannis K, Stamatopoulos G, Sinopoulou K, Balassopoulou A, Loukopoulos D, Terpos E. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS). Blood 2010;115:2354–2363. [DOI] [PubMed]
- 33.Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med 2011;32:279–293. [DOI] [PubMed]