Abstract Abstract
In patients with chronic obstructive pulmonary disease (COPD), moderate or severe pulmonary hypertension (COPD-PH) is associated with increased rates of morbidity and mortality. Despite this, approaches to treatment and the efficacy of phosphodiesterase type 5 inhibition (PDE-5i) in COPD-PH are unresolved. We present the clinical rationale and study design to assess the effect of oral tadalafil on exercise capacity, cardiopulmonary hemodynamics, and clinical outcome measures in COPD-PH patients. Male and female patients 40–85 years old with GOLD stage 2 COPD or higher and pulmonary hypertension diagnosed on the basis of invasive cardiac hemodynamic assessment (mean pulmonary artery pressure [mPAP] >30 mmHg, pulmonary vascular resistance [PVR] >2.5 Wood units, and pulmonary capillary wedge pressure ≤18 mmHg at rest) will be randomized at a 1∶1 ratio to receive placebo or oral PDE-5i with tadalafil (40 mg daily for 12 months). The primary end point is change from baseline in 6-minute walk distance at 12 months. The secondary end points are change from baseline in PVR and mPAP at 6 months and change from baseline in peak volume of oxygen consumption (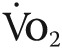 ) during exercise at 12 months. Changes in systemic blood pressure and/or oxyhemoglobin saturation (Sao2) at rest and during exercise will function as safety outcome measures. TADA-PHiLD (TADAlafil for Pulmonary Hypertension assocIated with chronic obstructive Lung Disease) is the first sufficiently powered randomized clinical trial testing the effect of PDE-5i on key clinical and drug safety outcome measures in patients with at least moderate PH due to COPD.
) during exercise at 12 months. Changes in systemic blood pressure and/or oxyhemoglobin saturation (Sao2) at rest and during exercise will function as safety outcome measures. TADA-PHiLD (TADAlafil for Pulmonary Hypertension assocIated with chronic obstructive Lung Disease) is the first sufficiently powered randomized clinical trial testing the effect of PDE-5i on key clinical and drug safety outcome measures in patients with at least moderate PH due to COPD.
Keywords: clinical trial, pulmonary hypertension, tadalafil, phosphodiesterase inhibitor
Introduction
Pulmonary hypertension (PH) is the clinical phenotype that results from adverse remodeling of distal pulmonary arterioles in the setting of genetic or acquired perturbations to cell-signaling pathways responsible for maintaining normal pulmonary vascular function.1 The preponderance of clinical trials in this disease have investigated the effect of pulmonary circulation–specific pharmacotherapy on outcome in patients with World Health Organization (WHO) group 1 PH (pulmonary arterial hypertension [PAH], formerly referred to as “primary pulmonary hypertension”); however, PAH is exceedingly rare in comparison to other clinically important forms of PH.2 For example, chronic obstructive pulmonary disease (COPD), which afflicts ˜46 million Americans and is responsible for a substantial economic burden on the US healthcare system,3,4 is an important promoter of PH. Indeed, moderate or severe PH is increasingly recognized as a pivotal risk factor that is associated with clinical worsening5 and early mortality6 in patients with COPD despite optimal standard of care therapy (i.e., supplemental oxygen). Taken together, these observations underscore the importance of identifying effective pharmacotherapies to improve cardiopulmonary hemodynamics and clinical outcome in patients with PH associated with COPD (COPD-PH).
Rationale
PH due to chronic obstructive lung disease
The pathobiological mechanism(s) underpinning clinical expression of PH in WHO group 3 PH and, specifically, COPD-PH appear to be related to the effects of chronic alveolar hypoxia on the development of pulmonary endothelial dysfunction, pulmonary vasoconstriction, and pulmonary vascular remodeling (concentric medial hypertrophy and muscularization of distal pulmonary arterioles). The destruction of alveolar-capillary units, in turn, results in decreased pulmonary vascular bed cross-sectional area and attendant clinical consequences, including lung hyperinflation, dyspnea, and diminished functional capacity (reviewed in Chaouat et al.7). Longitudinal increases in pulmonary vascular resistance and pressure promotes maladaptive changes to right ventricular (RV) geometry and function and, ultimately, right heart failure (cor pulmonale) that is associated with elevated rates of COPD-PH-associated morbidity and premature death.8
Clinical trials have established that long-term oxygen therapy (LTOT) is an effective strategy by which to improve clinical outcome and decrease mortality in patients with hypoxemia and chronic obstructive lung disease.9,10 However, the absolute risk reduction for adverse clinical events or mortality attributable to LTOT in COPD is moderate and limited only to a subset of the overall population with COPD-PH. Moreover, a paucity of clinical data exist in support of LTOT as an effective therapy by which to attenuate pulmonary vascular complications of COPD, despite the important clinical implications of comorbid PH on quality of life and outcome in the COPD-PH patient population. To the contrary, evidence suggests that the presence of PH in COPD is associated with poor survival and increased hospitalizations despite the initiation of LTOT.11 Collectively, these observations illustrate the importance of assessing the efficacy of pulmonary circulation–specific pharmacotherapies to abrogate pulmonary vascular remodeling and PH in COPD-PH.
Hypoxia and nitric oxide (NO•)–cyclic guanosine monophosphate (cGMP) signaling in pulmonary blood vessels
By inhibiting the degradation of cGMP, phosphodiesterase type 5 inhibitors (PDE-5i) increase bioactive levels of the potent pulmonary vasodilator and antimitogenic molecule NO• (Fig. 1). In experimental animal models of PAH and in humans with this disease, pulmonary vascular dysfunction is linked to increased PDE-5 and decreased NO• levels in pulmonary blood vessels and RV cardiomyocytes.12 We and others have demonstrated previously that levels of the major NO• metabolite, nitrite (NO2−), are decreased in lung tissue harvested from pulmonary hypertensive rats exposed to chronic hypoxia, which, in turn, is associated with pulmonary vascular remodeling and dysfunction.13,14 Numerous potential mechanisms exist by which to account for the observation that pulmonary vascular levels of bioavailable NO• are decreased in the setting of chronic hypoxia, including the adverse effects of increased pulmonary vascular oxidant stress on NO• sensing by soluble guanylyl cyclase (which is the chief biological source of cGMP),15 consumption of NO• by elevated levels of pulmonary vascular reactive oxygen species,16 or diminished endothelin receptor type B–dependent NO• synthesis,13 among others.17 Together, these observations provide biological plausibility to the assertion that restoring cGMP levels in pulmonary vascular tissue through PDE-5i may be an important and as yet underutilized treatment in COPD-PH.
Figure 1.
Adverse effects of hypoxia on normal nitric oxide (NO•)–cyclic guanosine monophosphate (cGMP) signaling in pulmonary artery smooth muscle cells. Upregulation of various pathobiological signaling intermediaries in the pulmonary vasculature by hypoxia may result in increases in pulmonary vascular inflammation, endothelin 1 (ET-1), and oxidant stress levels, which, in turn, promote pulmonary vasoconstriction and adverse remodeling of pulmonary arterioles. This may occur, in part, through the consumption of NO• by oxidant stress and/or oxidation of soluble guanylyl cyclase (sGC) at the prosthetic heme ligand or functional protein cysteinyl thiols (i.e., Cys-122) that are necessary for normal NO•-sGC signaling. By inhibiting phosphodiesterase type 5 (PDE-5) in pulmonary blood vessels (and right ventricular cardiomyocytes), tadalafil attenuates degradation of cGMP, which functions as a key secondary messenger necessary to maintain normal pulmonary vascular tone and prevent vascular remodeling. eNOS: endothelial nitric oxide synthase; PSMC: pulmonary smooth muscle cell; iNOS: inductable nitric oxide synthase; GTP: guanosine triphosphate. Adapted with permission from Francis et al.38
The clinical effects of targeting NO• signaling in COPD-PH patients
The potential therapeutic value of targeting NO• signaling in patients with COPD has been suggested by previous studies.11,18,19 In one prospective, randomized controlled trial, the effect of pulsed inhaled nitric oxide (iNO) therapy for 12 weeks on cardiopulmonary hemodynamics was compared with placebo in COPD patients with mild PH receiving chronic oxygen supplementation. In that study, iNO decreased mean pulmonary artery pressure (mPAP) and indexed pulmonary vascular resistance (PVR) significantly.11 Moreover, several small clinical studies have reported on the therapeutic efficacy of PDE-5i in COPD-PH.20,21 Madden et al.21 demonstrated that sildenafil (50 mg daily for 8 weeks) improved PVR (mean decrease of 3.2 Wood units) in 16 patients with WHO group 3 PH (due to lung disease). However, this and other22,23 published trials investigating PDE-5i in the treatment of COPD-PH were of short duration and lacked adequate statistical power to assess changes in clinically relevant functional outcomes. Hence, the acceptance of PDE-5i into routine clinical practice for COPD-PH has been limited by a lack of sufficiently powered randomized clinical trials. In addition, there are safety concerns in patients with COPD regarding the potential for pulmonary vasodilator therapies to exacerbate ventilation/perfusion (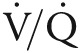 ) mismatch and augment hypoxemia.19 Results from the planned TADA-PHiLD (TADAlafil for Pulmonary Hypertension assocIated with chronic obstructive Lung Disease) trial will address these previous limitations and provide a rigorous examination of the efficacy and safety limitations of PDE-5i in patients with COPD-PH.
) mismatch and augment hypoxemia.19 Results from the planned TADA-PHiLD (TADAlafil for Pulmonary Hypertension assocIated with chronic obstructive Lung Disease) trial will address these previous limitations and provide a rigorous examination of the efficacy and safety limitations of PDE-5i in patients with COPD-PH.
Study design
The TADA-PHiLD study is a multicentered (Providence Veterans Affairs Medical Center, Veterans Affairs Boston Healthcare System, and Veterans Affairs Greater Los Angeles Healthcare System), prospective, randomized, placebo-controlled, double-blind clinical trial comparing oral tadalafil (40 mg daily) to placebo to assess changes in functional capacity, cardiopulmonary hemodynamics, and echocardiographically assessed RV function in patients with at least moderate PH due to COPD (NCT01862536 at http://www.clinicaltrials.gov). The objective of the study is to define the therapeutic efficacy and safety profile of PDE-5i in COPD-PH. Moreover, the overall clinical profile of PH patients to be recruited in this study is akin to the clinical profile of patients encountered in actual practice: this study aims to investigate the effect of tadalafil for COPD-PH under “real-world” conditions in order to inform practitioners regarding the safety profile and potential relevance of this therapy to patients encountered clinically. Results from this trial are anticipated to aid in characterizing the appropriateness of PDE-5i therapy in this disease and may inform future larger randomized clinical trials that promote PDE-5i at alternative doses and/or as part of a combination therapy for COPD-PH.
Patient selection and randomization
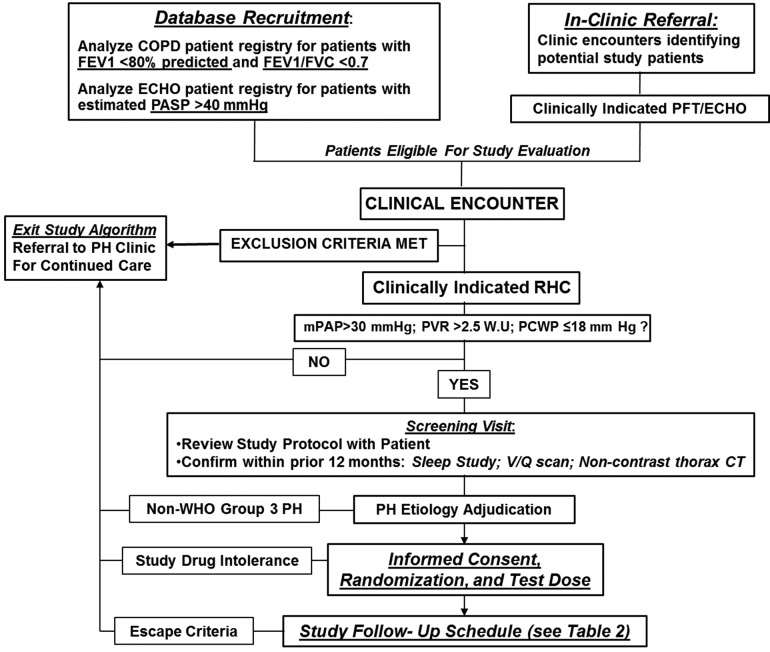
A complete list of inclusion and exclusion criteria is provided in Table 1 and Figure 2. These criteria will be used to identify patients with at least moderate COPD and moderate to severe PH. The rationale for selecting these criteria was based, in part, on reports in the literature and from retrospectively analyzed pilot data from our own clinical experience (unpublished) indicating that mild to moderately elevated pulmonary artery pressure is associated with recurrent hospitalization, decreased quality of life, and shortened life span in COPD.5,24,25 Although COPD is the predominate phenotype anticipated for inclusion in this study, the possibility exists that patients with overlap syndromes involving pulmonary fibrosis and chronic emphysema syndrome (CPFE) or COPD and concomitant obstructive sleep apnea (OSA), referred to as COPD/OSA, may also be included (Table 1). In patients with CPFE and/or COPD/OSA, the contribution of PH to the clinical expression of these disease phenotypes is suggested by the presence of dyspnea, hypoxemia, and decreased diffusion capacity of the lungs for carbon monoxide that are “out of proportion” to the degree of airflow obstruction assessed by standard pulmonary function testing.26,27 Nevertheless, patients with OSA or CPFE as the central mechanism for PH will be identified on the basis of apnea/hypoxia index >15 and the presence of substantial lung fibrosis on thoracic computed tomography, respectively, and excluded from study enrollment.
Table 1.
Patient selection criteria for the TADA-PHiLD (TADAlafil for Pulmonary Hypertension assocIated with chronic obstructive Lung Disease) trial
| Inclusion criteria | Exclusion criteria |
| Male and female patients 40–85 years old | History of WHO group 1, 2, 4, or 5 PH |
| Systemic hypotension (systolic blood pressure <90 mmHg on ≥2 ambulatory measurements) | |
| At least GOLD stage 2 COPD assessed by PFT:a • FEV1 <80% predicted and • FEV1/FVC <0.70 | |
| Child-Pugh B or C hepatic impairment | |
| Severe renal insufficiency (GFR <30 mL/min/1.73 m2) | |
| Severe aortic stenosis (AVA <1.0 cm2) | |
| PH by RHC:b • mPAP >30 mmHg and • PVR >2.5 WU and • PCWP ≤18 mmHg | |
| History of unstable angina, recent myocardial infarction, or stroke (<6 months) | |
| Untreated hypoxemia at rest (Sao2 <92%) | |
| Severe hypoxemia at rest requiring supplemental O2 ≥4 L/min | |
| Diffusion capacity of CO <30% predicted | |
| Known baseline 6-minute walk distance <50 m | |
| Pregnant or breast-feeding women | |
| Moderate or severe obstructive sleep apnea (AHI >15) | |
| Inability to comply with trial tests or a contraindication to tadalafil use, including anatomical deformations of the penis, sickle cell anemia, multiple myeloma, leukemia, bleeding disorders, active peptic ulcer disease, retinitis pigmentosa or other retinal disorders; use or anticipated use of any of the following drugs: nitrate or derivative medication, protease inhibitor, antifungal agent, rifampin, prazosin, and doxazosin |
WHO: World Health Organization; PH: pulmonary hypertension; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second of expiration; FVC: forced vital capacity; RHC: right heart catheterization; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; WU: Wood unit; PCWP: pulmonary capillary wedge pressure; GFR: glomerular filtration rate; AVA: aortic valve area; Sao2: oxyhemoglobin saturation; AHI: apnea-hypoxia index.
Performed within 12 months of randomization.
Performed within 6 month of randomization.
Figure 2.
TADA-PHiLD (TADAlafil for Pulmonary Hypertension assocIated with chronic obstructive Lung Disease) patient enrollment strategy. Potential study candidates are recruited through echocardiogram (ECHO) database analysis or clinical encounter at the Veterans Affairs (VA) Boston Healthcare System (Boston, MA), the Providence VA Medical Center (Providence, RI), or the VA Greater Los Angeles Healthcare System (Los Angeles, CA). For patients meeting study inclusion/exclusion criteria, the etiology of pulmonary hypertension (PH) is adjudicated by a cardiologist and a pulmonologist experienced in the clinical management of PH. Patients may exit the study algorithm voluntarily, in the absence of World Health Organization (WHO) group 3 PH, due to tadalafil intolerance, or by virtue of clinical worsening, which is defined as systemic hypotension, worsening hypoxemia, adverse drug event, or hospitalization requiring intensive care unit level of care. COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second of expiration; FVC: forced vital capacity; PASP: pulmonary artery systolic pressure; PFT: pulmonary function testing; RHC: right heart catheterization; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; WU: Wood unit; PCWP: pulmonary capillary wedge pressure; 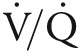 : ventilation/perfusion; CT: computed tomography; WHO: World Health Organization.
: ventilation/perfusion; CT: computed tomography; WHO: World Health Organization.
Only patients meeting the following criteria will be considered for trial enrollment: (i) GOLD stage 2 COPD or higher, defined as forced expiratory volume in the first second of expiration (FEV1)/forced vital capacity <0.70 and FEV1 <80% predicted by pulmonary function testing done within 12 months of recruitment, and (ii) PH documented by invasive hemodynamic assessment (right heart catheterization) to diagnose PH within 6 months of TADA-PHiLD screening according to the following hemodynamic criteria: mPAP >30 mmHg, PVR >2.5 Wood units, and pulmonary capillary wedge pressure ≤18 mmHg at rest. Compared with the currently available WHO hemodynamic definition of PH, our inclusion criteria uses a higher mPAP, which was selected to increase the specificity for identifying patients with at least moderate COPD-PH. Along these lines, we selected a pulmonary capillary wedge pressure threshold of 18 mmHg owing to recently published observations indicating that among patients with PH, a pulmonary capillary wedge pressure of 16–18 mmHg is associated with increased mortality compared with PH patients with pulmonary capillary wedge pressure (PCWP) <15 mmHg, and, thus, this clinical profile may represent a cohort of patients most likely to benefit from the study drug therapy.28
To decrease the probability that patients are enrolled with non–WHO group 3 PH or pulmonary fibrosis as the likely central mediator of dyspnea, a comprehensive clinical review will be performed for each candidate that includes analysis of liver function tests; HIV status; renal function; review of chest imaging, including prior computed tomography (CT) of the thorax and ventilation/perfusion scan; sleep study; and echocardiography. Randomization will occur by computer-generated number assignment.
Tadalafil administration and dose selection
Tadalafil was selected for investigation owing to its pharmacokinetic profile (t1/2 of 17.5 hours; mean oral clearance of 2.48 L/h)29 that favors once-daily dosing and, thus, drug adherence. Tadalafil is not currently approved for the treatment of WHO group 3 PH; however, a Food and Drug Administration off-label exemption was provided for the use of tadalafil in the TADA-PHiLD trial. Following randomization, study participants will then receive a test dose of the study medication (20 mg of tadalafil or placebo) and observed for 4 hours to ensure that blood pressure and oxygen levels are not affected adversely by the medication. Study participants who develop systemic hypotension (defined as a decrease in systolic blood pressure to <90 mmHg or a fall in systolic blood pressure of >20 mmHg accompanied by symptoms such as dizziness or syncope), hypoxemia (defined as a decrease in oxyhemoglobin saturation [Sao2] to <90% or a requirement for increased basal oxygen supplementation levels to >4 L/min), or other evidence of drug intolerance during the study drug test phase will not participate further in the study. Owing to the possibility that PDE-5i may influence adversely patients’ 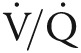 profile, the tadalafil dose will be increased from 20 to 40 mg daily as clinically tolerated over the course of the study duration rather than according to a predetermined schedule per se.
profile, the tadalafil dose will be increased from 20 to 40 mg daily as clinically tolerated over the course of the study duration rather than according to a predetermined schedule per se.
Study visits and procedure schedule
The schedule for patient follow-up, including procedures to be performed during the study period, are outlined in Table 2. These assessments are divided into the following categories related to tadalafil treatment: safety (i.e., patient contact, medical history, physical examination, medication adherence, clinical exacerbation history, adverse event monitoring), exercise response (6-minute walk distance [6MWD], cardiopulmonary exercise test), symptom and quality-of-life response (dyspnea and health-related quality-of-life surveys), and cardiopulmonary and biochemical response (echocardiography, right heart catheterization, plasma N-terminal brain natriuretic peptide concentration). The time interval for each benchmark assessment is based on prudence in the case of safety and predicted time to clinical response to tadalafil for the other measures, which was derived from prior clinical studies involving PDE-5i and PH patients.30-32
Table 2.
Follow-up clinic visits, telephone calls, and schedule of studies
| Month | |||||||
|---|---|---|---|---|---|---|---|
| Initial or preenrollment | Day 3 | 1 | 3 | 6 | 9 | 12 | |
| Contact | Clinic | Tel | Clinic | Clinic | Clinic | Clinic | Clinic |
| Medical history/physical examination | X | X | X | X | X | X | |
| Assess medication compliance | X | X | X | X | X | X | X |
| 6-minute walk test | X | X | X | X | X | X | |
| Echocardiogram | X | X | |||||
| Noninvasive CPET | X | X | |||||
| HRQL dyspnea questionnaires | X | X | X | X | X | X | |
| BNP | X | X | X | ||||
| RHC | X | X | |||||
| Exacerbation historya | X | X | X | X | X | X | |
| Adverse event monitoring | X | X | X | X | X | X | X |
Tel: telephone contact; CPET: cardiopulmonary exercise test; HRQL: health-related quality of life; BNP: N-terminal brain natriuretic peptide concentration; RHC, right heart catheterization.
Exacerbation history is defined as increased dyspnea, increased sputum volume, and increased sputum purulence for at least 3 days requiring treatment with systemic corticosteroids and/or antibiotics and/or emergency department visit or hospitalization.
Sample size determination and clinical end points
The primary clinical end point is the effect of tadalafil on exercise capacity, as measured by change in 6MWD from baseline at 12 months. The secondary clinical end points are change from baseline in PVR and mPAP at 6 months and change from baseline in maximal volume of oxygen extraction (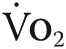 ) during exercise at 12 months. Change in peak
) during exercise at 12 months. Change in peak 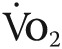 from baseline was selected as an end point owing to recently reported findings identifying this as a clinically relevant measure that correlates positively with improved exercise tolerance and quality of life in patients with PH.33 We selected a time point from randomization to repeat right heart catheterization of 6 months, which we believe is within the time required to demonstrate meaningful cardiopulmonary hemodynamic improvement following study drug therapy, in order to ensure that potential study attrition at longer enrollment intervals does not limit sufficient collection of PVR/mPAP data. These end points are intended to reflect the consequences of drug-induced changes to pulmonary vascular remodeling, cardiopulmonary hemodynamics, aerobic conditioning, and functional capacity and will allow comparisons to results of previous PH trials using this class of drug.31,32
from baseline was selected as an end point owing to recently reported findings identifying this as a clinically relevant measure that correlates positively with improved exercise tolerance and quality of life in patients with PH.33 We selected a time point from randomization to repeat right heart catheterization of 6 months, which we believe is within the time required to demonstrate meaningful cardiopulmonary hemodynamic improvement following study drug therapy, in order to ensure that potential study attrition at longer enrollment intervals does not limit sufficient collection of PVR/mPAP data. These end points are intended to reflect the consequences of drug-induced changes to pulmonary vascular remodeling, cardiopulmonary hemodynamics, aerobic conditioning, and functional capacity and will allow comparisons to results of previous PH trials using this class of drug.31,32
The principal investigator of this study will serve as the sole keeper of all data relevant to the TADA-PHiLD study. We propose to analyze continuous measures compared between two independent groups; thus, power and sample size calculations are derived from a simple two-sample t test. A sample size of 60 subjects in each group (accounting for an estimated 20% dropout rate from a total enrollment of 150 patients) would result in a general effect size of 0.60 standard deviation [SD] units using an α = 0.05 and 90% power. Determination of predicted SD for change in 6MWD was calculated from Lewis et al.,31 who studied the effects of sildenafil on persons with WHO group 2 PH attributable to left ventricular systolic heart failure. On the basis of their report, we predict in our study the SD of change in 6MWD to be 51 m, which is comparable to or greater than clinically important differences reported previously for 6MWD in patients with COPD.34,35 Analyses involving change in 6MWD will be controlled to account for the potentially confounding effect of change in supplemental oxygen level on functional capacity.
A similar statistical strategy was utilized to calculate the number of patients needed to maintain sufficient power with respect to our secondary clinical end points. From Galiè et al.,36 who studied the effects of sildenafil in persons with idiopathic PAH, we obtained the SD of change in PVR (447.1 dyn·s·cm5) and mPAP (8.6 mmHg) that was attributable to treatment at 12 weeks. On the basis of these values, we anticipate detecting a difference of −264 dyn·s·cm5 and −5.2 mmHg for PVR and mPAP, respectively, which are within the range reported previously as clinically meaningful changes in response to PH treatment.
Other outcomes that will be assessed include differences between the two study groups in rates of (i) acute COPD exacerbations (defined as increased dyspnea, increased sputum volume, and increased sputum purulence for at least 3 days and requiring treatment with systemic corticosteroids or antibiotics and emergency department visit or hospitalization) and (ii) pulmonary and all-cause hospitalizations. Additional outcome measures include differences in the following echocardiographically validated indices of RV structure/function: right atrial, left atrial, and RV dimensions; pulmonary artery acceleration time; and presence (or absence) and shape of a systolic “notch” in the RV outflow-tract Doppler profile.37
All cardiac catheterization and cardiopulmonary exercise test data will be interpreted at a central core laboratory. All echocardiography data will be interpreted by an expert echocardiographer with substantial experience in the analysis of PH/RV echocardiographic views.
Drug safety outcome measures
To assess the effect of tadalafil on ventilation/perfusion status, Sao2 levels and change in supplemental oxygen requirements will be assessed at rest and on ambulation during each of the six scheduled 6MWD tests. In addition, to assess other measures of exercise capacity, cardiopulmonary exercise testing will be conducted at baseline and at month 12. Escape criteria (i.e., progressive clinical worsening) meriting temporary withholding of the study drug and/or withdrawal from TADA-PHiLD may be achieved by any of the following events: sustained decrease in Sao2 levels to <90% that is not resolved by (increased) supplemental oxygen therapy, decreased systolic blood pressure to <90 mmHg detected by two consecutive ambulatory blood pressure measurements or a single measurement in the setting of symptomatic hypotension, the development of a major adverse drug reaction, or hospitalization requiring intensive care unit level of care.
Conclusions
PH is increasingly recognized as a key predictor of poor outcomes in patients with COPD, including hospitalization and premature death. However, standard-of-care therapy, including supplemental oxygen, appears to be insufficient to abrogate pulmonary vascular remodeling and PH in this patient population. The TADA-PHiLD trial is a multicenter, prospective, randomized, placebo-controlled, double-blind clinical trial that aims to characterize the efficacy and safety profile of PDE-5i in patients with chronic obstructive lung disease and moderate to severe PH.
Acknowledgments
We thank United Therapeutics and Eli Lilly and Company for their support of this trial and for sponsoring the study drug and placebo. The contents of this scientific article are the work of the listed authors and do not represent the views of the Department of Veterans Affairs or the US government.
Source of Support: The TADA-PHiLD (TADAlafil for Pulmonary Hypertension associated with chronic obstructive Lung Disease) trial is supported by a Veterans Affairs Merit Award from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development Service (to RHG and SIR). This work was supported in part by the US National Institutes of Health (1K08HL111207-01A1 to BAM), the Lerner and Klarman Foundations at Brigham and Women’s Hospital (to BAM), the Pulmonary Hypertension Association and the American Heart Association (11POST6720000 to BAM), and a Veterans Affairs Merit Award from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Service (IBX000711A to GC and 5101BX000811 and P20GM103652 to SIR).
Conflict of Interest: BAM has received grant funding from Gilead Sciences to research pulmonary hypertension.
References
- 1.Shah SJ. Pulmonary hypertension. JAMA 2012;308:1366–1374. [DOI] [PubMed]
- 2.Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186:707–709. [DOI] [PMC free article] [PubMed]
- 3.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5S–9S. [DOI] [PubMed]
- 4.Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev 2003;60:146S–167S. [DOI] [PubMed]
- 5.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367:913–921. [DOI] [PMC free article] [PubMed]
- 6.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013;41:1292–1301. [DOI] [PubMed]
- 7.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008;32:1371–1385. [DOI] [PubMed]
- 8.Terzano C, Conti V, Di Stefano F, et al. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 2010;188:321–329. [DOI] [PubMed]
- 9.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–398. [DOI] [PubMed]
- 10.Bishiop JM, Clark TJ, Dornhorst AC, Cotes JE; Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981;317:681–686. [PubMed]
- 11.Vonbank K, Ziesche R, Higenbottam TW, et al. Controlled prospective randomized trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 2003;58:289–293. [DOI] [PMC free article] [PubMed]
- 12.Nagendran J, Archer SL, Soliman D, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007;116:238–248. [DOI] [PubMed]
- 13.Maron BA, Zhang Y-Y, White K, et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 2012;126:963–974. [DOI] [PMC free article] [PubMed]
- 14.Abe K, Toba M, Alzoubi A, et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 2010;121:2747–2754. [DOI] [PubMed]
- 15.Maron BA, Zhang Y-Y, Handy DE, et al. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem 2009;284:7665–7672. [DOI] [PMC free article] [PubMed]
- 16.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls: the cytotoxic potential of superoxide and nitric oxide. J Biol Chem 1991;266;4244–4250. [PubMed]
- 17.Farber H, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655–1665. [DOI] [PubMed]
- 18.Roger N, Barbera JA, Roca J, et al. Nitric oxide inhalation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997:156:800–806. [DOI] [PubMed]
- 19.Higenbottam T. Pulmonary hypertension and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:12–19. [DOI] [PubMed]
- 20.Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomized, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011;53:81–85. [PubMed]
- 21.Madden BP, Allenby M, Loke TK, Sheth A. A potential role for sildenafil in the management of pulmonary hypertension in patients with parenchymal lung disease. Vasc Pharmacol 2006;44:372–376. [DOI] [PubMed]
- 22.Lederer DJ, Bartels MN, Schluger NW, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD 2012;9:268–275. [DOI] [PMC free article] [PubMed]
- 23.Blanco I, Gimeno E, Munoz PH, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010;181:270–278. [DOI] [PubMed]
- 24.Huynh TN, Weigt SS, Sugar CA, Shapiro S, Kleerup EC. Prognostic factors and outcomes of patients with pulmonary hypertension admitted to the intensive care unit. J Crit Care 2012;27:739.e7–739.e13. [DOI] [PubMed]
- 25.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance—United States, 1980–2002. MMWR Surveill Summ 2005;54:1–28. [PubMed]
- 26.Cottin V, Cordier JF. The syndrome of combined pulmonary fibrosis and emphysema. Chest 2009;136:1–2. [DOI] [PubMed]
- 27.Cottin V, Le Pavec J, Prevot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J 2010;35:105–111. [DOI] [PubMed]
- 28.Frost AE, Farber HW, Barst RJ, et al. Demographics and outcomes of patients diagnosed with pulmonary hypertension with pulmonary capillary wedge pressures 16–18 mmHg: insights from the REVEAL Registry. Chest 2013;143:185–195. [DOI] [PubMed]
- 29.Forgue ST, Patterson BE, Bedding AW, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol 2006;61:280–288. [DOI] [PMC free article] [PubMed]
- 30.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009;119:2894–2903. [DOI] [PubMed]
- 31.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007;116:1555–1562. [DOI] [PubMed]
- 32.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry and clinical status in patients with stable systolic heart failure. Circ Heart Fail 2011;4:8–17. [DOI] [PubMed]
- 33.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 2012;125:324–334. [DOI] [PubMed]
- 34.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. J Am Coll Cardiol 2009;53:1573–1619. [DOI] [PubMed]
- 35.Polkey MI, Spruit MA, Edwards LD, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med 2013;187:382–386. [DOI] [PubMed]
- 36.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Eng J Med 2005;353:2148–2157. [DOI] [PubMed]
- 37.Opotowsky AR, Ojeda J, Rogers F, et al. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging 2012;5:765–775. [DOI] [PMC free article] [PubMed]
- 38.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinase and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharm Rev 2010;62:525–563. [DOI] [PMC free article] [PubMed]