Abstract Abstract
There is a paucity of data on infant intravenous prostacyclin use, the gold standard for therapy for severe pulmonary hypertension (PH). This review aimed to evaluate the safety, tolerability, and outcomes of infant prostacyclin use. A retrospective observational study was performed in a large pediatric hospital with a dedicated pediatric PH program. Subject medical records, bedside flow sheets, and progress notes were reviewed to identify use of intravenous epoprostenol or treprostinil within the first year of life. The indication for prostacyclin use was recalcitrant hemodynamic compromise associated with PH, identified as either idiopathic PH, persistent PH of the newborn, PH associated with congenital diaphragmatic hernia, congenital heart disease, bronchiolitis, or chronic lung disease. Prostacyclin-related adverse events included 7 episodes of hypotension, 6 episodes of perceived pain, 2 episodes of cyanosis, and 1 episode of feeding intolerance. Prostacyclin was stopped only for cyanotic episodes associated with use in severe chronic lung disease. Two hemorrhagic events occurred during extracorporeal membrane oxygenation, which were unlikely to be prostacyclin related. Outcomes included 21 deaths unrelated to prostacyclin, 1 lung transplant, 6 PH resolutions, 8 transitions to oral PH medications, and 1 continuation of treprostinil. In conclusion, efficacy could not be evaluated in this study because of the loss of equipoise for neonatal prostacyclin use. Prostacyclin use was well tolerated in neonatal diseases associated with PH, but dose titration was limited by hypotension and hypoxemia.
Keywords: prostacyclin, infant, pulmonary hypertension
Introduction
Prostacyclin (PGI2), an arachadonic acid metabolite that stimulates adenylyl cyclase in vascular smooth muscle cells, causes an increase in intracellular cyclic adenosine monophosphate (AMP) and vasodilation of the systemic and pulmonary circulatory systems. In the United States, PGI2 is commercially available in three formulations. Epoprostenol is available for intravenous administration, while treprostinil, a tricyclic benzidine analogue with a longer half-life and increased stability at room temperature, is available for subcutaneous, intravenous, or inhaled administration. Iloprost is the third product, available only for inhaled administration.
Each PGI2 product has US Food and Drug Administration (FDA) approval for use in pulmonary hypertension (PH) in adults, with extensive studies demonstrating sustained improvement in symptoms and mortality. While these medications have also been utilized in children aged 2–18 years, there is no US FDA approval for this age range. Common adverse reactions seen in adults and older children include flushing, pain, and hypotension.1,2 Additionally, there is a theoretical risk of bleeding due to the antiplatelet effects of PGI2.1,2
There are a number of neonatal and infant disorders that are characterized by increased pulmonary pressures and eventual diagnosis of PH with development of right heart failure symptoms. The classic example is persistent PH of the newborn (PPHN), a disorder resulting from persistence of fetal pulmonary vasoconstriction after birth. Additional neonatal conditions that are associated with increased pulmonary vascular resistance include congenital diaphragmatic hernia (CDH), bronchopulmonary dysplasia (BPD), congenital heart disease (CHD), and severe acute bronchiolitis syndromes. Because of the ability of PGI2 to cause pulmonary vasodilation, reduce pulmonary pressures, and increase pulmonary blood flow, it has been considered as a potentially life-sustaining treatment for neonatal PH. Inhaled nitric oxide (iNO), a potent pulmonary vasodilator, remains the mainstay of treatment for this condition. However, use of iNO has many drawbacks, including the fact that it is not a viable option for long-term use, is effective in only ∼40% of cases, and holds US FDA approval only for PPHN.3-5
While epoprostenol and treprostinil are used in specialized pediatric PH centers, the literature for use of these agents in infants 0–12 months of age is sparse. No studies have evaluated safety or serious adverse events. To date, we have found 18 infants in reports on the use of PGI2 for neonatal PH. In 1997, Eronen et al.6 conducted a prospective study of 8 infants with PPHN who qualified for extracorporeal membrane oxygenation (ECMO). The use of epoprostenol led to improved oxygenation and decreased pulmonary artery pressures. All 8 infants survived without requiring ECMO; 2 infants subsequently developed BPD. Nakwan et al.7 retrospectively evaluated the use of beraprost, a PGI2 product utilized in Japan, in place of iNO in 7 infants with PPHN. The study showed significant improvement, including hospital discharge, for all patients. Deluca et al.8 published a case report of the use of epoprostenol in an infant with CDH, showing a transient but unsustained benefit leading to treatment failure. Golzand et al.9 reported on the use of epoprostenol in an infant with PPHN refractory to iNO with good response, leading to discontinuation of both PH-specific agents. Finally, Kovach et al.10 reported on the use of inhaled epoprostenol in an infant with CHD refractory to iNO that resulted in dramatic improvements in ventilation and oxygenation, leading to discontinuation of both PH-specific agents.
On the basis of the discussed literature, the PH program at Children’s Hospital of Philadelphia (CHOP), a 517-bed tertiary care facility, utilizes epoprostenol and treprostinil intravenously in severely ill infants with this spectrum of disorders. We have lost equipoise for neonatal PGI2 use and therefore cannot evaluate efficacy. The purpose of this study is to report the safety and tolerability of epoprostenol and treprostinil in children <12 months of age.
Subjects and methods
This retrospective observational study included consecutive patients identified through the electronic pharmacy order entry system and the Pulmonary Hypertension Clinical Database from January 1, 1999, through December 12, 2011. This study was conducted in full accordance with all applicable CHOP research policies and procedures and all applicable federal and state laws and regulations. Collection, recording, and reporting of data are accurate and ensured the privacy, health, and welfare of research subjects reviewed in this study. The study was internally funded in full by the Department of Pharmacy at CHOP.
The medical records of patients in whom epoprostenol or treprostinil was initiated prior to 366 days of age were reviewed. All patients were included and reported. The use of epoprostenol and treprostinil at CHOP is restricted to the Section of Pulmonary Hypertension, Division of Cardiology. Indications for use and dose alterations were directed by a clinical protocol and a single cardiologist (BDH).
Collected data from the progress notes, nursing flow sheets, and the Pulmonary Hypertension Clinical Database included age at initiation, indication for use, length of use, and patient outcome. Baseline characteristic data included the presence of mechanical ventilatory/circulatory and/or hemodynamic support (Table 1). A number of additional data points were collected, including those necessary to evaluate dose titration schedules. The time points for data collection included the following: within 1 hour prior to initiation, 24 hours and 1 week after initiation, discharge or drug discontinuation (whichever occurred first), and last follow-up. When applicable, data were collected at the time of any dose reductions and 2 weeks after the maximum dose was achieved.
Table 1.
Baseline characteristics
| Characteristic | All subjects (n = 36) | Survivors (n = 15) | Nonsurvivors (n = 21) |
|---|---|---|---|
| Age at initiation, median (range), days | 44. (1–359) | 162 (1–359) | 27. (1–321) |
| Length of follow-up, median (range), days | NA | 1,219.5 (585–4,283) | NA |
| Ventilator use | 36. (100) | 15 (100) | 21. (100) |
| ECMO use | 11. (30.6) | 4 (36) | 7. (33.3) |
| Medications | |||
| Nitric oxide | 30. (83.3) | 12 (80) | 18. (85.7) |
| Diuretics | 25. (69.4) | 9 (60) | 16. (76.2) |
| Dopamine | 16. (44.4) | 2 (13.3) | 14. (66.7) |
| Sildenafil | 15. (41.7) | 5 (33.3) | 10. (47.6) |
| Milrinone | 6. (16.7) | 2 (13.3) | 4. (19.0) |
| Dobutamine | 1. (2.8) | 0 (0) | 1. (4.8) |
Data are no. (%), unless otherwise indicated. NA: not applicable; ECMO: extracorporeal membrane oxygenation.
The data points utilized to evaluate safety events were selected on the basis of the more common or severe events reported with use of these agents in adults and children, including reports of flushing, hypotension, bleeding events, thrombocytopenia, pain, cyanosis, and feeding intolerance. Blood pressure, platelet counts, hemoglobin values, and pain scores were also collected for further evaluation of these safety events. Hypotension was defined as any value below the lower acceptable range identified for an age group.11 An increase in pain was defined as a consistent increase in pain scores from baseline over a 24-hour period utilizing a consistent, validated pain scale appropriate to the age of the child. Thrombocytopenia was defined as an unexplained decrease of >20% in platelet count after initiation of PGI2.
Because of the small patient population identified for this consecutive convenience sample, the data are reported in a purely descriptive fashion. No statistical analysis was appropriate.
Results
Thirty-six subjects were included in this review, with a median age at initiation of 44 days (range, 1–359 days). All subjects were critically ill and receiving mechanical ventilator support at the time of initiation. Other therapies present at initiation included iNO, sildenafil, diuretics, milrinone, and inotropes (Table 1). The etiology of PH was varied (Table 2). Twenty-eight infants had significant parenchymal lung disease and high supplemental oxygen requirements prior to initiation.
Table 2.
Pulmonary hypertension (PH) etiology
| Diagnosis | n = 36 |
|---|---|
| Congenital heart disease | 6. (16.7) |
| Repaired transposition of the great arteries | 3. (8.3) |
| Atrial septal defect | 1. (2.8) |
| Repaired interrupted aortic arch | 1. (2.8) |
| Postoperative tricuspid and pulmonary stenosis | 1. (2.8) |
| Noncardiac disease | 30. (83.3) |
| Congenital diaphragmatic hernia | 9. (25) |
| Chronic lung disease | 9. (25) |
| Persistent PH of the newborn | 6. (16.7) |
| Acute bronchiolitis | 4. (11.1) |
| Idiopathic PH | 2. (5.6) |
Data are no. (%).
Thirty-four subjects were initiated on epoprostenol, and 2 were initiated on treprostinil. The choice of agent was made on hemodynamic and ventilator stability, with treprostinil chosen only if rapid dose titration was not necessary or expected. Seven subjects were transitioned from epoprostenol to treprostinil using a rapid switch protocol after confirming that no detrimental effects were present. No subject was transitioned back to epoprostenol. At the time of study completion, 1 patient was still receiving treprostinil therapy and was excluded from treatment length analysis. The median overall length of use was 7 days (range, 2 hours to 1,332 days). Eleven subjects received PGI2 for <72 hours, with a median length of use of 23.52 hours (range, 2–72 hours). The remaining 24 subjects received therapy for a median of 29 days (range, 3.6–1,332 days). Table 3 provides length-of-use information in relation to the type of disease process.
Table 3.
Length of prostacyclin use
| All usage | Usage ≤72 hours | Usage >72 hours | ||||
|---|---|---|---|---|---|---|
| No. | Median (range), days | No. | Median (range), hours | No. | Median (range), days | |
| Total | 35 | 7.0 (0.083–1,332) | 11 | 23.5 (2–72) | 24 | 29.0 (3.6–1,322) |
| CHD | 6 | 14.5 (3.6–1,332) | 1 | 48.0 (NA) | 5 | 415.0 (3.6–1,332) |
| CDH | 8 | 3.3 (0.125–7) | 4 | 10.9 (3–67.2) | 4 | 5.2 (3.8–7) |
| CLD | 9 | 78.0 (0.77–489) | 2 | 34.4 (18.48–50.4) | 7 | 91.0 (23–489) |
| PPHN | 6 | 24.0 (0.7–34) | 2 | 44.4 (0.7–72) | 4 | 30.5 (18–34) |
| Bronchiolitis | 4 | 22.5 (1.94–33) | 1 | 46.6 (NA) | 3 | 33.0 (12–33) |
| Idiopathic PH | 2 | 2.5 (0.98–3.96) | 1 | 23.5 (NA) | 1 | 3.96 (NA) |
CHD: congenital heart disease; CDH: congenital diaphragmatic hernia; CLD: chronic lung disease; PPHN: persistent pulmonary hypertension of the newborn; NA: not applicable; PH: pulmonary hypertension.
Safety and tolerability assessment
Eighteen subjects (50%) experienced at least 1 drug-related adverse event (Table 4). Four subjects experienced flushing as documented by nursing staff in the patient chart, none of whom required dose reduction. There were 7 total events of hypotension documented at any point in treatment, 5 requiring temporary dose reduction. In each of these subjects, subsequent increases in dose were tolerated without recurrence of hypotension. Four subjects experienced an increase in pain scores within 24 hours after initiation of the medication. An additional 2 subjects experienced an increase in pain scores 1 week after initiation. There was no documented change in dose associated with documented pain; however, the PH team’s approach calls for a slowed up-titration in situations of documented pain. One subject experienced feeding intolerance within 72 hours clinically attributed to PGI2 initiation, requiring a temporary dose reduction. Two subjects with severe CLD experienced PGI2-associated cyanosis after 2 and 21 hours of treatment, resulting in discontinuation and provision of palliative care. There were 2 major bleeding events that occurred within 48 hours of drug initiation in subjects who were simultaneously receiving heparin for ECMO anticoagulation. One subject with CDH and a 26-day history of ECMO had previous bleeding events prior to PGI2 initiation. The other subject with idiopathic PH and a 7-day history of ECMO had multiple organ failure and a coagulopathic state prior to starting PGI2.
Table 4.
Incidence of safety and tolerability events
| Event | Incidence | Dose reduced | Drug discontinued |
|---|---|---|---|
| Major events | |||
| PGI2-associated cyanosis | 2. (5.6) | … | 2. (100) |
| Significant bleeding event | 2. (5.6) | … | … |
| Minor events | |||
| Hypotension | 7. (19.4) | 5. (71.4) | 1. (14.3) |
| Pain | 6. (16.7) | … | … |
| Flushing | 4. (11.1) | … | … |
| Feeding intolerance | 1. (2.8) | 1. (100) | … |
| Thrombocytopenia | 0. (0) | … | … |
Data are no. (%). PGI2: prostacyclin.
Treatment outcomes
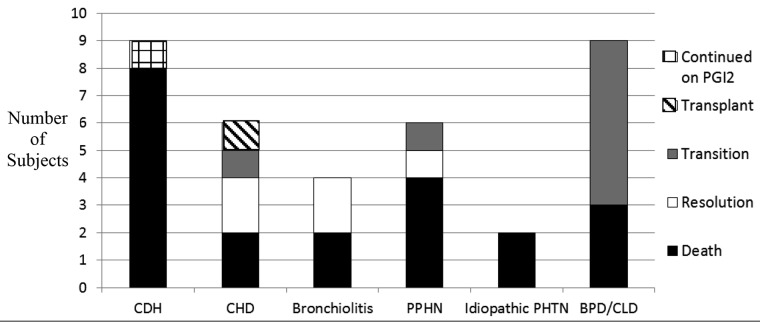
Of the 36 subjects reviewed, 1 (2.9%) was continued on treprostinil at the time of study completion. Mortality occurred in 21 subjects (60%) during the span of the review. Five subjects (14%) experienced complete resolution of symptomatic PH and no longer required treatment, 8 (25%) were transitioned to other PH-specific therapy for symptom control, and 1 (2.9%) underwent lung transplantation with discontinuation of PGI2. Figure 1 shows these outcomes stratified by primary indication for use.
Figure 1.
Outcomes stratified by pulmonary hypertension etiology. PGI2: prostacyclin; CDH: congenital diaphragmatic hernia (1 child not included; see text); CHD: congenital heart disease; PPHN: persistent pulmonary hypertension of the newborn; PHTN: pulmonary hypertension; BPD: bronchopulmonary dysplasia; CLD: chronic lung disease.
Of the 21 mortality events (Table 5), 11 (52.4%) occurred within 72 hours of PGI2 initiation: 5 subjects were initiated on PGI2 to aid in decannulation from ECMO, and 6 were initiated on the medication as a last-line therapy during rapid deterioration. The remaining 10 deaths (47.6%) comprised various lengths of use and causes of death. In every case, mortality was attributed to the underlying pathology and was neither hastened nor caused by a PGI2-related event. In fact, in 9 (90%) of these cases, documented PGI2-associated improvement was observed.
Table 5.
Cause of mortality
| Patient | Etiology | Length of usea | Reason for death | Response to PGI2 |
|---|---|---|---|---|
| PGI2 use ≤72 hours | ||||
| 5 | CLD | 50 | Care withdrawn | LR |
| 8 | CDH | 5 | Care withdrawn | LR |
| 15 | PPHN | 72 | Asystole | LR, SEb |
| 23 | Bronchiolitis | 46.5 | Care withdrawn | LR |
| 28 | CHD | 2 | Disease progression | LR, SEc |
| 34 | CLD | 18.5 | Care withdrawn | LR, SEc |
| Initiated after ECMO decannulation for deterioration | ||||
| 2 | CDH | 3 | Care withdrawn | LR, SEb |
| 4 | CDH | 17 | Care withdrawn | LR |
| 33 | CDH | 67 | Clinical progression | LR |
| Initiated prior to ECMO decannulation | ||||
| 6 | PPHN | 17 | Clinical progression | LR |
| 12 | IPAH | 23.5 | Multiorgan failure | LR, SEb |
| PGI2 use >72 hours | ||||
| 9 | IPAH | 3.96 | Care withdrawn | RS, CP |
| 14 | CHD | 5 | Asystole | RS, CP |
| 18 | CLD | 23 | Care withdrawn | RS, CP |
| 20 | CDH | 5 | Care withdrawn | RS, CP |
| 22 | Bronchiolitis | 12 | Multiorgan failure | RS, CP |
| 24 | CDH | 7 | Care withdrawn | RS, CP |
| 31 | CLD | 90 | Septic shock | RS, CP |
| 32 | PPHN | 31 | Care withdrawn | RS, CP |
| Initiated after ECMO decannulation for deterioration | ||||
| 37 | CDH | 5.46 | Clinical progression | RS, CP |
| Initiated prior to ECMO decannulation | ||||
| 30 | CDH | 3.75 | Clinical progression | LR |
PGI2: prostacyclin; CLD: chronic lung disease; LR: lack of PGI2 response; CDH: congenital diaphragmatic hernia; PPHN: persistent pulmonary hypertension of the newborn; SE: side effect noted; ECMO: extracorporeal membrane oxygenation; IPAH: idiopathic pulmonary hypertension; RS: PGI2 response seen; CP: continued progression of disease.
Data are hours for PGI2 use ≤72 hours and days for PGI2 use >72 hours.
Hypotension requiring dose reduction.
Cyanosis requiring discontinuation.
A total of 5 subjects were discharged on a PGI2, 1 of whom was still receiving treprostinil at the time of study completion. Of the remaining 4 subjects, 1 had lung transplantation after 1,332 total days of medication. The final 3 subjects were successfully transitioned to other PH-specific therapy after discharge, with total lengths of use of 489, 721, and 818 days.
Dosing
Epoprostenol and treprostinil doses were initiated at 1–2 ng/kg/min. In some situations, initiation occurred at an outside hospital, with subsequent titration up or down as needed on transfer. Doses were titrated by 0.5–2 ng/kg/min with only two deviations from this practice, neither of which resulted in dose or titration-related adverse events. Time intervals between titrations were ≥45 minutes for epoprostenol and ≥60 minutes for treprostinil. For those subjects who survived, the mean epoprostenol dose utilized was 6.4 ng/kg/min (standard deviation [SD], 2.25). The mean treprostinil dose utilized in those who survived was 36.44 ng/kg/min (SD, 25.59). For those subjects who died during the span of the review but utilized the medications for >72 hours, the mean epoprostenol dose utilized was 17.6 ng/kg/min (SD, 12.44). Only 1 subject died with treprostinil use of >72 hours, receiving a final dose of 42 ng/kg/min.
Discussion
To our knowledge, this is the largest series of intravenous PGI2 use in children <12 months of age. This retrospective review demonstrates safety and tolerability in 36 critically ill infants; however, since we use PGI2 in all critically ill infants with PH, we cannot appropriately assess efficacy.
Prostacyclins cause vasodilation in the pulmonary vasculature, leading to decreased pulmonary pressures and improved cardiac output.1,2 This mechanism of action has the potential to provide improved oxygen delivery in critically ill infants with PH-associated right heart failure. This is especially true in infants with CDH, BPD, PH, bronchiolitis, and certain presentations of CHD with elevated pulmonary pressures due to acute pulmonary vasoconstriction. Developmental arrest and inflammatory injury to the pulmonary parenchyma and vascular bed causes a fixed, nondilatable reduction in the vascular cross-sectional area. There is no evidence to suggest that PGI2 increases angiogenesis under these clinical circumstances, limiting the acute efficacy of PGI2 to vasodilation and improvement in pulmonary blood flow. We report the survival of 15 infants who were critically ill with right heart failure associated with PH and believe this statistic alone is a reason to strongly consider PGI2 in this age group.
Previously published literature in this population did not focus on the safety and tolerability of PGI2. We report that epoprostenol and treprostinil administration is associated with acceptable safety and tolerability in children <12 months of age. While 50% of subjects experienced at least 1 drug-related adverse event, the majority of these were transient. The incidence of flushing, hypotension, and pain was lower than that reported in adult patients.1,2 All episodes of hypotension or pain were transient, with subsequent increases in dose that did not lead to further complications.
One subject in this study experienced feeding intolerance that was clinically associated with drug initiation. The presentation of feeding intolerance in neonates could be a manifestation of the abdominal pain and diarrhea that has been previously reported in adult patients during clinical trials.1,2 The prevalence estimate for this adverse event might be confounded by the limitations of enteral nutrition in these critically ill infants.
One subject experienced pulmonary hemorrhage and 1 subject experienced intraventricular hemorrhage while receiving PGI2 infusion. In both of these cases, the PGI2 had been in use <48 hours, and both patients had a predisposition to bleed prior to the addition of PGI2. Additionally, both patients were receiving ECMO and continuous therapeutic infusions of heparin. It is unlikely that these hemorrhages were caused by PGI2, since no other infant had an issue with bleeding during the study period. It is unclear whether the infusion was contributory or noncontributory to the bleeds when considering the antiplatelet activity of PGI2.
Finally, 2 of a total of 21 subjects with pulmonary parenchymal disease experienced cyanosis with initiation and titration of PGI2. Cyanosis is a significant theoretical concern in this patient population. Pulmonary vasodilation can lead to a mismatch of ventilation and perfusion that leads to worsened oxygenation status, even when intended to improve pulmonary blood flow and oxygen delivery. In both subjects with documented cyanosis, the medication was rapidly titrated off and discontinued, with subsequent resolution of the hypoxemia but not the right heart failure. The low incidence of cyanosis seen in this review (5.6%) may help to better characterize the true need for concern for cyanosis in this patient population. Additionally, because of the short half-life of epoprostenol, it is an acceptable adverse event when appropriate dosing and monitoring is in place, as resolution occurs quickly after discontinuation of the medication.
A high mortality rate was noted in the outcomes of the reviewed subjects. However, use of PGI2 did not contribute to mortality in any of these cases (Table 5). The use of PGI2 at our institution requires consultation with the Division of Cardiology, Section of Pulmonary Hypertension. While this leads to increased uniformity and safety with administration and monitoring, it may potentially delay treatment as the need for and timing of consultation is determined subjectively by the primary care team. Delays in therapy to periods of rapid deterioration should be avoided, as suggested by the fact that 52.3% of deaths occurred within 72 hours of PGI2 therapy. Furthermore, the risk of mortality in the population of infants with right heart failure is high, and it is currently unknown whether the use of PGI2 modulates this risk. The impact of disease etiology on mortality cannot be overstated, in that survival with PGI2 rescue therapy in infants with CDH (11.1%) and/or receiving ECMO (36.3%) in this study was low.
We have an increased comfort with the use of these medications in this patient population. Thus, the dosing utilized for these subjects mirrors that utilized for all pediatric patients at our hospital. It is important to recognize that dosage increases and rates of titration were not correlated to the occurrence of adverse events in this review. It appears that for the subjects who survived, many were transitioned to treprostinil for longer-term use, explaining the higher doses of treprostinil seen compared with epoprostenol in this group (an average of 6.4 vs. 36.44 ng/kg/min). Final infusion rates also ranged greatly depending on subject response and duration of therapy, with epoprostenol doses ranging from 4 to 50 ng/kg/min and treprostinil doses ranging from 11 to 105 ng/kg/min. The primary concern with the use of high doses is the risk of high-output cardiac failure, which did not occur in any of these subjects. This high dose range can be safely utilized in infants who survive the initial episode of severe cardiovascular dysfunction.
Conclusion
We report that epoprostenol and treprostinil, when used in an experienced pediatric PH center, appear to be tolerated by critically ill infants <12 months of age. Prostacyclins were utilized in a number of disease states that resulted in increased pulmonary pressures and right ventricular failure. It appears that short-term outcomes are dependent on the underlying disease severity at initiation of PGI2. Dosing similar to that utilized in the general pediatric population can be safely implemented in children <12 months of age; however, mild hypotension and worsening hypoxia can be dose-limiting side effects. There is a need for further studies of the use of these medications to assess efficacy in this age range; however, we suspect that many centers have also lost equipoise, as there is currently no other therapeutic intervention available.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Epoprostenol [package insert]. Research Triangle Park, NC: GlaxoSmithKline, 2006.
- 2.Treprostinil [package insert]. Research Triangle Park, NC: United Therapeutics, 2011.
- 3.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 1997;336:597–604. [DOI] [PubMed]
- 4.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med 2000;342:469–474. [DOI] [PubMed]
- 5.Kelly LK, Porta NFM, Goodman DM, Carroll CL, Steinhorn RH. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr 2002;141:830–832. [DOI] [PubMed]
- 6.Eronen M, Pohjavuori M, Andersson S, Pesonen E, Raivio KO. Prostacyclin treatment for persistent pulmonary hypertension of the newborn. Pediatr Cardiol 1997;18:3–7. [DOI] [PubMed]
- 7.Nakwan N, Nakwan N, Wannaro J. Persistent pulmonary hypertension of the newborn successfully treated with beraprost sodium: a retrospective chart review. Neonatology 2011;99:32–37. [DOI] [PubMed]
- 8.DeLuca D, Zecca E, Vento G, DeCarolis MP, Romagnoli C. Transient effect of epoprostenol and sildenafil combined with iNO for pulmonary hypertension in congenital diaphragmatic hernia. Paediatr Anaesth 2006;16:596–602. [DOI] [PubMed]
- 9.Golzand E, Bar-Oz B, Arad I. Intravenous prostacyclin in the treatment of persistent pulmonary hypertension of the newborn refractory to inhaled nitric oxide. Isr Med Assoc J 2005;7:408–409. [PubMed]
- 10.Kovach J, Ibsen L, Womack M, Steusse D, Law YM. Treatment of refractory pulmonary arterial hypertension with inhaled epoprostenol in an infant with congenital heart disease. Congenit Heart Dis 2007;2:194–198. [DOI] [PubMed]
- 11.Kliegman RM, Behrman RE, Jenson HB, Stanton BF. Nelson textbook of pediatrics. 18th ed. Philadelphia: Elsevier, 2011.