Abstract Abstract
This review highlights our current knowledge regarding expression of transient receptor potential (TRP) cation channels in lung endothelium and evidence for their involvement in regulation of lung endothelial permeability. Six mammalian TRP families have been identified and organized on the basis of sequence homology: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), and TRPA (ankyrin). To date, only TRPC1/4, TRPC6, TRPV4, and TRPM2 have been extensively studied in lung endothelium. Calcium influx through each of these channels has been documented to increase lung endothelial permeability, although their channel-gating mechanisms, downstream signaling mechanisms, and impact on endothelial structure and barrier integrity differ. While other members of the TRPC, TRPV, and TRPM families may be expressed in lung endothelium, we have little or no evidence linking these to regulation of lung endothelial permeability. Further, neither the expression nor functional role(s) of any TRPML, TRPP, and TRPA family members has been studied in lung endothelium. In addition to this assessment organized by TRP channel family, we also discuss TRP channels and lung endothelial permeability from the perspective of lung endothelial heterogeneity, using outcomes of studies focused on TRPC1/4 and TRPV4 channels. The diversity within the TRP channel family and the relative paucity of information regarding roles of a number of these channels in lung endothelium make this field ripe for continued investigation.
Keywords: TRP channels, calcium, lung endothelial permeability, lung endothelial heterogeneity
Introduction
The transient receptor potential (TRP) channel superfamily is perhaps the largest known cation channel family contributing to calcium entry in lung endothelium. Since calcium influx through plasmalemmal calcium channels can impair the integrity of the lung endothelial barrier, and thus its permeability to fluid and protein, TRP channels have the potential to be both critical participants in and therapeutic targets for acute lung injury. This review focuses on our current knowledge regarding TRP channel expression in the lung and evidence supporting a role for TRP channels in regulation of lung endothelial barrier integrity and permeability. Changes in lung endothelial permeability due to calcium influx can certainly be modulated by the availability of cyclic nucleotide pools and mediated by myriad downstream signaling pathways. However, these issues have been reviewed at length.1-6 Thus, we focus on TRP channel–mediated calcium entry and loss of endothelial barrier integrity. Given the growing recognition of structural and functional heterogeneity of lung endothelium,7-10 we also discuss the evidence for TRP channels’ involvement in regulation of lung endothelial permeability from that perspective as well.
Currently, 27 human TRP isoforms have been identified (28 in mouse), grouped into 6 families based on amino acid homology: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), and TRPA (ankyrin). A seventh family—TRPN (NO-mechano potential C)—has not been identified in any vertebrate species other than zebra fish.11 TRP channel proteins contain 6 transmembrane spanning domains, with a pore loop region between transmembrane domains 5 and 6. Domains within the intracellular N- and C-termini contribute to channel assembly and regulation. Individual TRP proteins assemble into homo- or heterotetramers to form functioning cation channels. While Nilius and Owsianik11 recently suggested that most TRP channels exist as homotetramers, heteromultimeric assembly of several TRP proteins from within or between subfamilies into functional cation channels has been described.12-15 Nonetheless, aside from a recent report using Förster resonance energy transfer (FRET) to document assembly of TRPC1 and TRPC4 into heteromeric channels in pulmonary artery endothelial cells and endothelial caveolar fractions isolated from intact lung,16 there is very little direct evidence for TRP channel composition and stoichiometry in lung endothelium. To further complicate this issue, protein expression, channel assembly, and trafficking to the plasma membrane may potentially be dictated by developmental stage and/or disease in vivo,17,18 passage of endothelial cells in culture,19 or the degree of confluency of endothelial monolayers in culture.20
TRP channels, calcium entry, and lung endothelial permeability
TRPC (canonical)
TRPC family members 1–7 share an invariant amino acid sequence (EWKFAR) in the C-terminus called the TRP box, as well as 3–4 NH2-terminal ankyrin repeat domains. Generally, TRPCs form nonselective cation channels, with calcium-to-sodium selectivity ratios ranging from 1.1 to 9.21 Protein expression for TRPC1, 3, 4, 6, and 7 has been variously identified in pulmonary vascular endothelium in the intact lung by Western blot or immunohistochemical approaches.18,22-24 In addition, expression of TRPC1, 2, 4, and 6 messenger RNA (mRNA) and protein has been identified in human, mouse, and rat pulmonary artery endothelial cells.7,23,25-30
In terms of activation mechanisms, TRPC channels can be distinguished as those activated by depletion of endoplasmic reticulum (ER) calcium stores (store-operated channels)—TRPC1/4—and those activated by diacylglycerol (DAG)—TRPC3/6/7. While the latter are often termed receptor-operated channels, this is somewhat of a misnomer, as synthesis of both inositol-1, 4, 5-trisphosphate (IP3, an endogenous initiator of store depletion) and DAG can result from receptor-ligand interactions in lung endothelial cells. However, this functional distinction may not clearly delineated between TRPC subfamily groups because of potential heterologous channel assembly, i.e., interaction between TRPCs typically associated with store-operated channels (TRPC1 and 4) and those associated with receptor-operated channels (TRPC3, 6, and 7). Resolution of this issue is complicated by the relative lack of information regarding stoichiometry for TRPC channel assembly in lung endothelium. The only available information has been provided by Cioffi and colleagues,16 who used a FRET-based reporter system to identify the composition of the channel responsible for the calcium-selective store-operated current (ISOC) in cultured pulmonary artery endothelium and in caveolar fractions harvested from intact lung endothelium. They found that the ISOC channel consists of at least one TRPC1 and 2 TRPC4 proteins and that interaction of TRPC4 with Orai1 is responsible for the channel’s calcium selectivity. While this study resolves stoichiometry for one store-operated channel, lung endothelium also expresses nonselective cation channels that are activated by store depletion.22
Agents that evoke depletion of ER calcium stores, resulting in activation of store-operated channels, include IP3 and ER calcium ATPase (SERCA) inhibitors such as the plant alkyloid thapsigargin and cyclopiazonic acid, as well as the calcium chelators 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and N,N, N′,N′-tetrakis(2-pyridylmethyl)ethylene diamine (TPEN). The mechanisms by which these agents act vary: IP3 binds IP3 receptors, leading to efflux of calcium from ER stores; SERCA inhibitors prevent calcium reuptake into ER stores and thus cause depletion; and finally, BAPTA and TPEN chelate either cytosolic or ER calcium, thus preventing refilling or store depletion, respectively. Endogenous agonists, such as thrombin and angiotensin II, act through plasma membrane receptors to evoke store depletion in lung endothelium.29,31 Store depletion, rather than the subsequent rise in cytosolic calcium, is responsible for activation of store-operated channels per se.32-34
Regardless of the tools used to elicit store depletion, it is clear that calcium entry into lung endothelium following store depletion increases lung endothelial permeability, both in vitro and in vivo.18,29,35-37 The mechanisms linking store depletion and activation of store-operated channels are actively debated.38-40 However, the increased permeability response to ISOC activation is due to interendothelial gap formation, classically attributed to myosin light-chain kinase–dependent retraction of endothelial cell borders.5,6,41,42 For example, thapsigargin induces gap formation in cultured pulmonary artery endothelial cells (visualized via light microscopy) and in extra-alveolar vessels from isolated rat lung (visualized via scanning electron microscopy), structural changes that correlate with increased transendothelial diffusion of macromolecules and increases in the filtration coefficient (Kf), respectively.24,35,37,42 At the ultrastructural level, interendothelial gap formation was observed in extra-alveolar vessels at least 100 μm in diameter, signifying that the increased permeability effect induced by store-operated calcium entry activation is due to weakening of endothelial barrier integrity in the extra-alveolar compartment (Fig. 1).35 Notably, despite a 3-fold or more increase in Kf, thapsigargin-induced store depletion does not evoke alveolar flooding.7 Tiruppathi et al.29 found that the calcium entry response to thrombin-induced PAR-1 activation in lung endothelium, which initiates store depletion, was significantly reduced in endothelial cells isolated from TRPC4−/− mice. Further, in TRPC4−/− lung endothelial cells, the decrement in thrombin-induced calcium entry correlated with loss of actin stress-fiber formation and less cell retraction. In parallel studies, they found that the thrombin-induced increase in Kf was attenuated in lungs from TRPC4−/− mice. In agreement with these observations, the lung endothelial permeability response to thapsigargin-induced store depletion is ablated in animals with congestive heart failure,18,31,36 coincident with downregulated expression of TRPC1, 3, and 4 in extra-alveolar vessels.18
Figure 1.
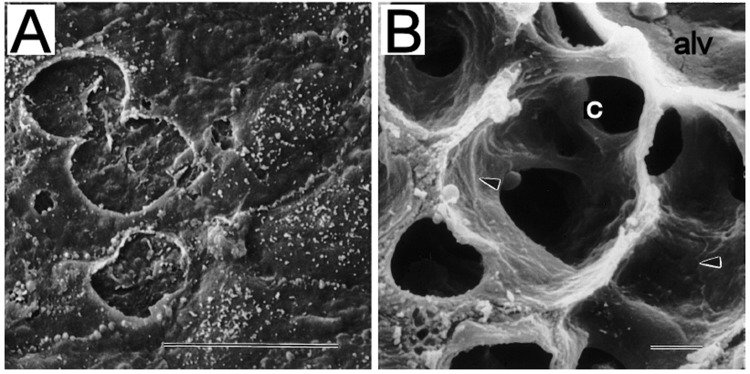
Scanning electron microscopy in thapsigargin-treated rat lung. Thapsigargin-mediated activation of store-operated calcium entry promoted interendothelial gap formation in pulmonary arteries (A) but not in alveolar capillary endothelium (B). These findings are consistent with the notion that extra-alveolar and septal capillary endothelium have distinctive phenotypes, such that store-operated calcium entry via transient receptor potential channels increases lung endothelial permeability only in the extra-alveolar compartment. alv: alveolar space; c: alveolar capillary; arrowheads indicate interendothelial junctions. Scale bars: 10 μm. Adapted from Chetham et al.35
Unlike store-operated calcium entry, activation of TRPC3, 6, and 7 is triggered by DAG and occurs independent of store depletion.43 Alvarez et al.18 found that Kf did not increase in isolated rat lungs treated with the DAG mimic 1-oleoyl-2-acetyl-sn-glycerol (OAG), despite confirmed protein expression for TRPC 3 and 6/7 in extra-alveolar vessels. They concluded that calcium entry through a putative TRPC 3-, 6-, and/or 7-containing channel does not play a role in mediating increases in lung permeability. However, Singh et al.28 reported that activation of calcium entry by OAG in human pulmonary artery endothelial cells led to protein kinase C-α (PKCα)-dependent activation of RhoA, which in turn induced interendothelial cell gap formation. In their study, both OAG- and thrombin-induced calcium entry was significantly attenuated in cells treated with small interfering RNA (siRNA) against TRPC6. In concert with decreased calcium entry, PKCα activity, RhoA activity, myosin light-chain phosphorylation, actin stress-fiber formation, and monolayer permeability were all decreased. In an extension of this study, Kini et al.26 found that phosphatase and tensin homolog (PTEN) interacts with TRPC6 but not with TRPC1, 3, or 4 in human pulmonary artery endothelial cells. The PTEN interaction with TRPC6 was required for OAG-induced calcium entry and subsequent increases in monolayer permeability. Consistent with the latter findings, ischemia and OAG-induced rises in intracellular calcium levels and subsequent increases in monolayer permeability were attenuated in endothelial cells isolated from TRPC6−/− mouse lungs.26 Similarly, TRPC6−/− mouse lungs are protected from ischemia/reperfusion-induced increases in lung permeability and edema.26 Collectively, these results support the notion that calcium entry via TRPC6 can mediate increases in lung permeability. Nonetheless, the disparate findings with respect to the permeability response to OAG-induced calcium entry in the intact rat lung versus those in mouse lung and human pulmonary artery endothelial monolayers have yet to be resolved.
TRPV (vanilloid)
The TRPV family members (1–6) are broadly classified into 4 groups based on their structure and function: TRPV1/2, TRPV3, TRPV4, and TRPV5/6. The TRPV proteins contain versions of the TRP box, the TRP domain, and 3–6 N-terminal ankyrin repeat domains. TRPV1 has been described as both nonselective and calcium selective.21,44 TRPV2–4 form nonselective channels, with calcium-to-sodium selectivity ratios ranging from ∼1 to 10. In contrast, TRPV5 and 6 have been identified as selective calcium channels, with calcium-to-sodium ratios greater than 100.21 TRPV proteins tend to form homomeric channels,11-13 although interactions between TRPV1 and 2 and between TRPV5 and 6 have been observed by FRET, co-immunoprecipation, and colocalization studies in heterologous expression systems.13
Expression of TRPV1 has been identified in human pulmonary artery and human lung microvascular endothelial cells by reverse-transcription polymerase chain reaction (RT-PCR).23,45 TRPV1 is activated by the vanilloid capsaicin, capsaicin analogs (e.g., nonivamide, resiniferatoxin, and olvanil), moderate heat (≥43°C), and low pH (≤5.9).21,44,46 TRPV1 can also be activated by endogenous lipid compounds referred to as “endovanilloids,” such as anandamide, 2-arachidonoyl glycerol, and N-arachidonoyl amino acids.47-51 There is little evidence supporting a role for TRPV1 in regulation of lung endothelial permeability. Neither pharmacological silencing nor genetic deletion of TRPV1 affected the edema response to lipopolysaccharide, or LPS.45 In support of these findings, treatment of isolated rat lungs with the TRPV1 agonist 4α-phorbol-12,13-didecanoate-20 homovanillate (4αPDDHV) had no impact on Kf in rat lung.7 Finally, in a model of lung ischemia/reperfusion injury in rabbits,52 activation of TRPV1 by capsaicin actually attenuated the increased lung wet-to-dry weight ratio and protein levels in the bronchoalveolar lavage fluid. Although TRPV1 mRNA is highly expressed in the alveolar region of the mouse lung, expression is minimal in human lung microvascular endothelial cells.45 Thus, any role of TRPV1 in regulation of lung endothelial permeability in the intact lung may be indirect.
TRPV4 protein is expressed in cultured rat pulmonary arterial and microvascular endothelial cells and in endothelial cells from rat lung.7,25 Further, immunohistochemistry reveals prominent expression of TRPV4 in the alveolar septal wall of human, rat, and mouse lung, while expression in extra-alveolar vessels is more variable.7 TRPV4 can be activated by heat,53 cell swelling,54,55 mechanical and shear stress,53,55,56 epoxyeicosatrienoic acids (EETs),57,58 and synthetic phorbol esters such as 4α-phorbol-12,13-didecanoate (4αPDD).59 The impact of TRPV4 activation on Kf was clearly dependent on calcium entry, because the response was mitigated by either perfusion with a low calcium buffer or pretreatment of lungs with the TRPV antagonist ruthenium red.7 Further, the 4αPDD-induced increase in Kf in isolated mouse lungs, but not that evoked by thapsigargin, was absent in lungs from TRPV4−/− mice (Fig. 2). Despite similar increases in Kf due to activation of store-operated channels or TRPV4, only the latter is associated with alveolar flooding (Fig. 3). Transmission electron microscopy revealed that TRPV4 activation, but not activation of store-operated channels, elicits disruption of the alveolar septal endothelial barrier leading to alveolar flooding in isolated rat and mouse lungs.7 Notably, this disruption does not appear to involve interendothelial cell gap formation but rather a complex array of perturbations in cell morphology (Fig. 4). More recently, the advent of specific small-molecule agonists and antagonists for TRPV4 has provided new tools. Intravenous administration of the TRPV4 agonist GSK1016790A in rats significantly increases lung wet-to-dry weight ratio, resulting in perivascular edema as well as multifocal alveolar flooding.60 Similarly, we have found increases in lung wet-to-dry weight ratio and albumin levels in the bronchoalveolar lavage fluid after GKS1016790A administration to perfused mouse lungs (P. C. Villalta and M. I. Townsley, unpublished observations), effects mitigated in lungs from TRPV4−/− mice.
Figure 2.
Activation of either TRPV4 or store-operated channels increases endothelial permeability in isolated, perfused mouse lung. Challenge of lungs with either 4α-phorbol-12,13-didecanoate-20 (4αPDD) or thapsigargin (TG) to activate TRPV4 or store-operated transient receptor potential (TRP) channels, respectively, similarly increased endothelial permeability, as measured by the filtration coefficient Kf. Only the response to 4αPDD was abrogated in lungs from TRPV4−/− mice. Asterisk indicates 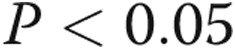 versus baseline. DMSO: dimethyl sulfoxide. From Alvarez et al.7
versus baseline. DMSO: dimethyl sulfoxide. From Alvarez et al.7
Figure 3.
Activation of calcium entry via TRPV4, but not that via store-operated channels, elicits lung endothelial barrier disruption via distinct targeting of the alveolar septal barrier. Activation of TRPV4 by 4α-phorbol-12,13-didecanoate-20 (4αPDD) significantly increased the frequency of blebs and breaks in the alveolar septal wall in both rat and mouse lung (not shown), which led to significant increases in the alveolar fluid volume fraction ( ) in mouse lung. A similar trend was observed in rat lung. Despite 2–3-fold elevation in Kf, thapsigargin had no impact on the integrity of the alveolar septal barrier or alveolar fluid accumulation in either group. Asterisk indicates
) in mouse lung. A similar trend was observed in rat lung. Despite 2–3-fold elevation in Kf, thapsigargin had no impact on the integrity of the alveolar septal barrier or alveolar fluid accumulation in either group. Asterisk indicates  versus control; pound sign indicates
versus control; pound sign indicates  versus thapsigargin. Data from Alvarez et al.7
versus thapsigargin. Data from Alvarez et al.7
Figure 4.
TRPV4 activation preferentially targets the alveolar septal network. Isolated rat and mouse lungs treated with 4α-phorbol-12,13-didecanoate-20 to activate TRPV4 were visualized by transmission electron microscopy. The increase in  noted in Figure 3 was not associated with development of interendothelial gaps (redarrowheads). Rather, TRPV4 activation resulted in endothelial cell detachment from the basement membrane (A, arrow) and microparticle formation (B, arrow), as well as endothelial cell vacuolization (C) and swelling (D).
noted in Figure 3 was not associated with development of interendothelial gaps (redarrowheads). Rather, TRPV4 activation resulted in endothelial cell detachment from the basement membrane (A, arrow) and microparticle formation (B, arrow), as well as endothelial cell vacuolization (C) and swelling (D).
TRPV4 activation by mechanical stress induces lung injury as well. Jian et al.61 found that high venous pressure–induced increases in lung permeability were mediated by cytochrome P450 expoxygenase–dependent activation of TRPV4 in the mouse lung. P450 epoxygenases are enzymes that synthesize EETs from arachidonic acid.62 Thus, these data are consistent with EET-induced activation of TRPV4 noted in vitro.57,58,63 In a model of ventilator-induced lung injury, calcium-dependent increases in lung permeability were exacerbated by heat in TRPV4+/+ mice but were absent in TRPV4−/− mice. This mechanical stress–induced lung injury was also attenuated by use of cytochrome P450 epoxygenase inhibitors.64 However, the permeability response to ventilator-induced injury in mouse lung appears to specifically require TRPV4 expression in alveolar macrophages, because repletion of wild-type macrophages to lungs of TRPV4−/− mice restored the injury response.65 Collectively, these studies support a role for EETs in initiating TRPV4 activation and the permeability response to mechanical stress in the intact lung, but they also suggest differences in the cellular repertoire recruited to elicit lung injury. The role of TRPV4 in the permeability response to high airway and vascular pressure in mouse lung has been well documented by us and others.61,64-66 In both experimental paradigms, the permeability response is significantly attenuated by pretreatment with the nonspecific TRPV antagonist ruthenium red and by use of TRPV4−/− mice. However, until recently, extension of these observations to rats and larger mammals has been hampered by the lack of pharmacologic and genetic tools to specifically target TRPV4 in these species. New studies using small-molecule antagonists—GSK2193874 and GSK2263095—that are selective for TRPV4 have confirmed a TRPV4-mediated permeability response to high vascular pressure in rat and canine lung.67 Further, use of these specific TRPV4 antagonists has provided support for involvement of TRPV4 in the development of pulmonary edema in acute pulmonary venous hypertension induced by aortic banding in rats, as well as the development and resolution of pulmonary edema in a murine model of chronic myocardial infarction.67
The downstream targets of TRPV4-mediated calcium entry that mediate permeability increases in the lung are unknown. Our observation that interendothelial junctions of capillary endothelial cells in mouse or rat lung appear to be intact after TRPV4 activation (Fig. 4)7 challenges the established paradigm that increases in lung endothelial permeability are invariably due to endothelial cell retraction and subsequent gap formation. In cultured human umbilical vein endothelial cells, TRPV4 activation leads to dose-dependent cell detachment,60 leading us to consider activation of proteases to mediate basement membrane degradation, loss of cell-matrix tethering, and/or initiation of cell death processes as potential mechanisms for injury. In preliminary work, we have identified increased active matrix metalloproteinase (MMP) 2 and 9 expression in mouse lungs in concert with TRPV4-induced lung injury,68 although whether these MMPs actually cause the TRPV4-induced increase in lung permeability remains to be determined. Increases in intracellular calcium, if sufficient, can orchestrate cell injury and death,69,70 and TRPV4 activation is capable of initiating cell death in several tissues.71-73 However, we do not observe activated caspase-3 or positive TUNEL (terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling) staining in mouse lungs where TRPV4-mediated lung injury occurred (P. C. Villalta and M. I. Townsley, unpublished observations). As an alternative, the cell swelling, blebbing, and vacuolation induced by TRPV4 activation resemble structural defects induced by another form of programmed cell death, oncosis.74 Oncosis, implicated as a mode of cell death in a few models of acute lung injury,75-77 can be initiated by calcium-dependent activation of calpains, a family of calcium-dependent cysteine proteases.78 Although there are no reports linking calpains and oncosis in TRPV4-induced lung injury, calpain inhibition does attenuate lung interstitial and air space edema during inflammation,79 suggesting a potential role for deformation of the septal barrier. In vitro, separate reports have demonstrated that cyclic stretch both activates TRPV480 and induces calpain-mediated alveolar epithelial barrier disruption.81 Mechanisms by which TRPV4 activation leads to endothelial barrier disruption in the lung should be further resolved.
Involvement of other TRPV channels in regulation of lung endothelium permeability is uncertain. Although TRPV2 mRNA and protein are indeed expressed in rat lung endothelium,23,25 there is no evidence to date linking this TRP channel to regulation of endothelial permeability. In human pulmonary artery endothelium, mRNA for TRPV3, 5, and 6 was not identified with RT-PCR.23 No studies to date have assessed expression of these TRPV channels in lung microvascular endothelium.
TRPM (melastatin)
The TRPM (1–8) family members, divided into the TRPM1/3, 2/8, 4/5, and 6/7 groups, lack N-terminal ankyrin repeat domains.21 TRPM channels vary greatly in calcium selectivity ratio, ranging from those that are not calcium permeable (TRPM4/5) to those with selectivity ratios greater than 3.
Expression of all TRPMs, with the exception of TRPM5, has been verified by RT-PCR in human pulmonary artery endothelial cells.23,82 In addition, protein expression of the alternate splice form TRPM2-s and the full-length protein TRPM2-l, as well as TRPM4, has been confirmed by Western blot in human pulmonary artery endothelial cells.23,82 TRPM2 can be activated in the setting of oxidant stress, although this effect is now recognized to be indirect. Direct activation of TRPM2 is elicited by intracellular adenosine diphosphate ribose (ADPR),83,84 cyclic ADPR, H2O2,85 nicotinic acid adenine dinucleotide phosphate,86 and arachidonic acid.87 Nonetheless, the role of TRPMs in lung endothelial permeability is unclear. Hecquet et al.82 provided evidence for involvement of TRPM2 in oxidative stress–induced damage to human pulmonary artery endothelium. They determined that treatment of human pulmonary artery endothelial monolayers with sublytic concentrations of H2O2 led to calcium influx and decreased monolayer transendothelial resistance in a dose-dependent fashion. Inhibition of calcium entry by blockade of intracellular ADPR generation or siRNA-mediated suppression of TRPM2-l expression diminished the decrease in monolayer barrier integrity. The authors also confirmed a regulatory mechanism—noted in other cell lines88,89—by which TRPM2-s isoform overexpression modulates the activity of the TRPM2 channel and the increased monolayer permeability response to H2O2. The involvement of TRPM2 in the intact lung is more complex. In a model of endotoxin-induced lung injury, where oxidative stress is prevalent, Di and colleagues90 found that lung edema increased and survival decreased in TRPM2−/− mice bred into a C57BL/6 background, compared to wild-type controls. However, Hardaker et al.91 reported that TRPM2 deficiency in a BALB/c mouse strain had no effect on bronchoalveolar-lavage albumin levels following LPS challenge, compared to that in wild-type controls. These discrepancies between the permeability effects of TRPM2 activation in human pulmonary artery endothelial cells and those in mouse lung could be due a number of factors. However, the varied sensitivity to lung injury among different mouse strains may be an important consideration. Notably, BALB/c mice display a lower sensitivity to lung injury induced by LPS or ischemia/reperfusion than do C57BL/6 mice.92,93 Thus, no definitive conclusions can be reached yet regarding the role of TRPM2 in mediating increases in lung endothelial permeability.
TRPML (mucolipin)
The mammalian members of the TRPML family, TRPML1–3, have been identified largely as endolysosomal nonselective cation channels that mediate intraendosomal calcium release in the endocytic pathway.94-96 There is no evidence of TRPML expression or activity in the plasma membrane, and only recently have technological advances allowed for assessment of the channel in lysosomal membranes.97 TRPML1 mRNA is ubiquitously expressed in all major tissues, and TRPML3 mRNA has been detected in mouse lung.98 Neither the expression nor the functional role of TRPML channels in lung endothelium is known. Studies are needed to determine whether these channels influence endothelial barrier properties.
TRPP (polycystin)
The TRPP family members TRPP2, 3, and 5 are more homologous to the TRPML family than to any other TRP family.99 These calcium-regulated, nonselective channels have been extensively studied in renal epithelium, where their role in mechanosensation and involvement in polycystic kidney disease have been reported.99-102 Berrout et al.103 implicated TRPP2 in a model of traumatic brain injury, where mechanical stretch of brain microvascular endothelial cells promoted calcium influx and actin stress-fiber formation independent of store depletion. These investigators reported protein expression of TRPP2, along with TRPC1, TRPV4, and other TRPs, using immunocytochemistry and Western blot in mouse brain microvascular endothelial cells. Knockdown of TRPP2, TRPC1, or both by siRNA, as well as nonselective inhibition of TRPPs and TRPCs by amiloride and LOE908, respectively, resulted in a decreased calcium response and stress-fiber formation, suggesting that at least TRPP2 and TRPC1 may mediate calcium-dependent rearrangement of the cytoskeleton that could promote increases in endothelial permeability. Indeed, inhibition of calcium-permeable channels in the plasma membrane of brain microvascular endothelial cells, which prevents rises in cytosolic calcium, results in protection against increases in blood-brain barrier permeability.104,105
The channels that may influence the barrier properties of the microvascular component of the blood-brain barrier are highlighted here because there are many parallels between brain and lung microvasculature. In both brain and lung, the microvascular barrier segments are very tight, compared to their arterial counterparts.106-108 Further, cytosolic calcium influx via TRPC 1/4 and TRPV4 in the lung5,7,22,24,35,37,109 and via TRPC and TRPV in brain110-113 leads to a compromise of barrier integrity and increases in endothelial permeability. This may be particularly relevant because TRPP2 has been described as part of a functional, mechanosensitive complex with TRPC1 and TRPV4.114-116 Thus, studies are needed to determine whether TRPP2 is expressed in lung endothelium, whether it interacts with other members of the TRPC and TRPV families known to mediate increases in lung endothelial permeability, and whether its activation per se compromises lung endothelial barrier integrity.
TRPA (ankyrin)
The lone member of the TRPA family, TRPA1, is unique in that it possesses 14–19 ankyrin repeats on its amino terminus.117 In the lung, TRPA1 mRNA and protein expression has been verified in human A549 cells and human lung fibroblasts by RT-PCR and Western blot, respectively,118 but an expression profile in lung endothelium is lacking. In cerebral arteries, TRPA1 activation evokes dynamic cytosolic calcium transients and vasodilation.129,120 While TRPA1 appears to play a role in mediating cough and airway inflammation,121-125 no studies to date have explored a functional role for TRPA1 in lung endothelium.
Impact of lung endothelialheterogeneity
Our understanding of the molecular players and mechanisms that mediate lung endothelial permeability responses to TRP channel activation is emerging with a key message: not all endothelial cells, endothelial calcium pools, and functional endothelial responses to calcium entry are cut from the same cloth.
First, the notion that calcium-mediated loss of barrier integrity is solely a consequence of endothelial cell retraction is now being challenged. As discussed above, we have documented increases in lung endothelial permeability in the alveolar septal compartment without the formation of interendothelial gaps,7 suggesting that other mechanisms are responsible for the loss in barrier integrity.
Second, we have determined that TRP channels and other calcium channels may be coexpressed in the same lung endothelial compartment but still elicit unique downstream signals. Specifically, calcium entry via TRPV4 and that via the voltage-gated T-type channel, both expressed in lung septal capillary endothelium, have divergent effects. Only calcium transients resulting from TRPV4 activation increase lung endothelial permeability, and only those resulting from activation of the T-type channel lead to surface expression of P-selectin.10
Third, work from our group and others suggests that calcium entry–dependent alterations in lung endothelial barrier integrity can have distinct outcomes for overall lung function based on the targeted endothelial compartment (Fig. 5). Specifically, activation of store-operated TRP channels in extra-alveolar vessels leads to perivascular/peribronchial cuffing and decreased lung compliance.126 In contrast, activation of TRPV4 leads to alveolar flooding and depressed gas exchange.7,126
Figure 5.
Activation of store-operated channels and TRPV4 leads to distinctive leak sites in the extra-alveolar and alveolar septal networks, respectively. Corrosion casting revealed no leak sites in the normal rat lung (A) but extra-alveolar leak sites in rat lungs treated with thapsigargin to activate store-operated calcium entry (B). In contrast, in rat lungs treated with 14,15-epoxyeicosatrienoic acid (14,15-EET) to activate TRPV4, microvascular leak resulted in alveolar flooding evident just subjacent to the pleural surface (C) and blebbing across the alveolar surface (D). From Townsley et al.127
Finally, heterogeneity should be interpreted with recognition of potential signaling- or remodeling-induced endothelial plasticity. This concept can be evidenced acutely with manipulation of signaling pathways and/or chronically with modulation of TRP channel expression. For example, Wu et al.22 found that acute rolipram pretreatment of rat pulmonary artery and microvascular endothelial cells abolished or unmasked thapsigargin-induced ISOC activation in these cells, respectively. In the intact rat lung, rolipram pretreatment shifted the thapsigargin-induced leak site from the extra-alveolar endothelial compartment to the alveolar septal compartment. Close association of the ER and the plasma membrane has been proposed as a requirement for activation of store-operated TRP channels,40 and the distance between the ER and the plasma membrane is more than 2-fold greater in pulmonary microvascular endothelial cells than in pulmonary artery endothelium.9 Interestingly, Wu et al.109 also found that rolipram treatment leads to translocation of the ER closer to the plasma membrane in pulmonary microvascular endothelial cells. They further demonstrated that dynein, a microtubule motor protein that directs organelle movement away from the plasma membrane, predominates in microvascular endothelial cells, while kinesin, a motor protein that directs organelle movement toward the plasma membrane, predominates in pulmonary artery endothelial cells. Disruption of dynein expression in pulmonary microvascular endothelial cells unmasked thapsigargin-induced ISOC activation, while disruption of kinesin in pulmonary artery endothelial cells ablated thapsigargin-induced ISOC activation. The authors concluded that the ER distribution, influenced by rolipram-exposed cyclic adenosine monophospate (cAMP) pools and microtubule motor function, plays a crucial and functional role in ISOC entry–mediated increases in lung endothelial permeability. In addition to such acute mediator-induced remodeling, chronic remodeling in lung with disease can evoke different alterations. For example, we found that in a model of heart failure, downregulated expression of TRPC1, 3, and 4 in extra-alveolar vessels led to loss of thapsigargin-mediated permeability responses unrelated to alteration in the ER-plasma membrane distance, yet also led to retention of TRPV4-mediated increases in permeability.18
Collectively, we interpret these observations as reflecting endothelial heterogeneity, not only between extra-alveolar and microvascular compartments in lung but also between unique calcium microdomains in any one lung endothelial compartment. Calcium channels expressed in lung endothelium may be coupled to distinct downstream signaling pathways and thus linked to distinct functional outcomes. As a corollary, some TRP channels expressed in lung endothelium are likely not coupled to regulation of lung endothelial permeability.
Summary and future directions
From the large library of mammalian TRP channels now identified—28 members to be exact—evidence links a number of TRP channels to regulation of lung endothelial barrier integrity. We are just beginning to appreciate the complex nature by which specific endothelial responses to calcium entry via TRP channels are orchestrated. Thus far, the TRPC1/4 (ISOC) and TRPV4 channels provide models for overall concepts as we begin to unravel the role of other TRP channels in lung endothelial barrier function. One very important concept is that calcium entry through TRP channels can mediate divergent responses that hinge on lung endothelial cell phenotype (Fig. 6). That is, phenotypic heterogeneity in lung endothelium dictates segmental expression of TRP channels. For example, activation of the ISOC channel (TRPC1/4) can increase lung endothelial permeability by compromising the barrier properties of the extra-alveolar vasculature, thus promoting perivascular cuff formation. Conversely, TRPV4 activation increases lung endothelial permeability by affecting the alveolar septal compartment, thus promoting alveolar flooding. These differences in regional changes in permeability are due in part to innate phenotypic differences between the pulmonary artery and microvascular endothelium. Another important concept is that permeability increases are not always reflected simply by interendothelial gap formation. TRPV4 activation leads to barrier disruption with no apparent alteration in interendothelial junctional integrity. Thus, different signaling pathways must mediate the impact of TRP channel activation in different vascular segments to orchestrate changes in endothelial barrier properties.
Figure 6.
Heterogeneity of injury model. The sequential-filling model of pulmonary edema accumulation initially described by Staub and others,128-131 shown at left, is applicable when lungs are challenged with nonspecific agents to increase endothelial permeability. However, given newer evidence on endothelial heterogeneity, we should appreciate that extra-alveolar and alveolar septal endothelium can be differentially targeted. We, and others, have provided evidence for such a differential distribution of effects following activation of store-operated channels and TRPV4.7,35,127If lung endothelial phenotypic heterogeneity extends to differential, compartment-specific expression of other transient receptor potential (TRP) channels, then their activation may similarly elicit direct edema formation in the extra-alveolar or septal compartments of the lung, thus bypassing the sequential filling model. A: artery; B: bronchiole. Adapted from Townsley et al.127
Increasing evidence for TRP channel involvement in lung endothelial permeability makes this an exciting opportunity for discovery, and much research is still needed. The set of TRP channels that mediate increases in lung endothelial permeability must be completely identified. Once identified, the physiological and signaling mechanisms involved in mediating the permeability increases should be resolved. In addition, we have yet to fully understand whether permeability responses are modulated by the influx of other ions, such as magnesium and sodium, through activated but nonselective TRP channels. In the context of therapeutic value, a global understanding of the way these channels and their downstream effectors influence alterations in endothelial barrier properties could open up a new array of targets for drug development.
Source of Support: This work was supported by grants from the National Institutes of Health (HL066299 and HL081851). PCV was supported in part by National Heart, Lung, and Blood Institute training grant T32 HL076125.
Conflict of Interest: None declared.
References
- 1.Garcia J. Molecular mechanisms of thrombin-induced human and bovine endothelial cell activation. J Lab Clin Med 1992;120:513–519. [PubMed]
- 2.Garcia JGN, Verin AD, Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost 1996;22:309–315. [DOI] [PubMed]
- 3.Malik AB, Lo SK. Vascular endothelial adhesion molecules and tissue inflammation. Pharmacol Rev 1996;48:213–229. [PubMed]
- 4.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006;86:279–367. [DOI] [PubMed]
- 5.Moore TM, Chetham PM, Kelly JJ, Stevens T. Signal transduction and regulation of lung endothelial cell permeability: interaction between calcium and cAMP. Am J Physiol Lung Cell Mol Physiol 1998;275:L203–L222. [DOI] [PubMed]
- 6.Stevens T, Garcia JGN, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol 2000;279:L419–L422. [DOI] [PubMed]
- 7.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 2006;99:988–995. [DOI] [PMC free article] [PubMed]
- 8.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res 2004;68:1–12. [DOI] [PubMed]
- 9.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 2004;67:139–151. [DOI] [PubMed]
- 10.Wu S, Jian M-Y, Xu Y-C, Zhou C, Al-Mehdi A-B, Liedtke W, Shin H-S, Townsley MI. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 2009;297:L650–L657. [DOI] [PMC free article] [PubMed]
- 11.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol 2011;12:218. [DOI] [PMC free article] [PubMed]
- 12.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 2002;99:7461–7466. [DOI] [PMC free article] [PubMed]
- 13.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 2005;118:917–928. [DOI] [PubMed]
- 14.Schilling WP, Goel M. Mammalian TRPC channel subunit assembly. In: Mammalian TRP channels as molecular targets. Novartis Foundation Symposium 258. Chichester: Wiley, 2004:18–43. [PubMed]
- 15.Liedtke WB, Heller S, eds. TRP ion channel function in sensory transduction and cellular signaling cascades. Boca Raton, FL: CRC, 2007. [PubMed]
- 16.Cioffi DL, Wu S, Chen H, Alexeyev M, St. Croix CM, Pitt BR, Uhlig S, Stevens T. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ Res 2012;110:1435–1444. [DOI] [PMC free article] [PubMed]
- 17.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 2003;278:39014–39019. [DOI] [PubMed]
- 18.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med 2005;172:1153–1160. [DOI] [PMC free article] [PubMed]
- 19.Köhler R, Brakemeier S, Kühn M, Degenhardt C, Buhr H, Pries A, Hoyer J. Expression of ryanodine receptor type 3 and TRP channels in endothelial cells: comparison of in situ and cultured human endothelial cells. Cardiovasc Res 2001;51:160–168. [DOI] [PubMed]
- 20.Groschner K. Polymodal TRPC signaling: emerging role in phenotype switching and tissue remodeling. Commun Integr Biol 2010;3:393–395. [DOI] [PMC free article] [PubMed]
- 21.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium 2005;38:233–252. [DOI] [PubMed]
- 22.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, et al. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res 2005;96:856–863. [DOI] [PubMed]
- 23.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX-J. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 2003;285:L1233–L1245. [DOI] [PubMed]
- 24.Moore TM, Brough GH, Babal P, Kelly JJ, Li M, Stevens T. Store-operated calcium entry promotes shape change in pulmonary endothelial cells expressing Trp1. Am J Physiol Lung Cell Mol Physiol 1998;275:L574–L582. [DOI] [PubMed]
- 25.Kerem A, Yin J, Kaestle SM, Hoffmann J, Schoene AM, Singh B, Kuppe H, Borst MM, Kuebler WM. Lung endothelial dysfunction in congestive heart failure: role of impaired Ca2+ signaling and cytoskeletal reorganization. Circ Res 2010;106:1103–1116. [DOI] [PubMed]
- 26.Kini V, Chavez A, Mehta D. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem 2010;285:33082–33091. [DOI] [PMC free article] [PubMed]
- 27.Samapati R, Yang Y, Yin J, Stoerger C, Arenz C, Dietrich A, Gudermann T, et al. Lung endothelial Ca2+ and permeability response to platelet-activating factor is mediated by acid sphingomyelinase and transient receptor potential classical 6. Am J Respir Crit Care Med 2012;185:160–170. [DOI] [PubMed]
- 28.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Gαq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem 2007;282:7833–7843. [DOI] [PubMed]
- 29.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ Res 2002;91:70–76. [DOI] [PubMed]
- 30.Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, Brandes RP, et al. Activation of TRPC6 channels is essential for lung ischaemia–reperfusion induced oedema in mice. Nat Commun 2012;3:649, doi:10.1038/ncomms1660. [DOI] [PMC free article] [PubMed]
- 31.Roy BJ, Pitts VH, Townsley MI. Pulmonary vascular response to angiotensin II in canine pacing-induced heart failure. Am J Physiol Heart Circ Physiol 1996;271:H222–H227. [DOI] [PubMed]
- 32.Hofer AM, Fasolato C, Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+]. J Cell Biol 1998;140:325–334. [DOI] [PMC free article] [PubMed]
- 33.Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA 1990;87:2466–2470. [DOI] [PMC free article] [PubMed]
- 34.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci 1998;19:131–135. [DOI] [PubMed]
- 35.Chetham PM, Babal P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 1999;276:L41–L50. [DOI] [PubMed]
- 36.Ivey CL, Roy BJ, Townsley MI. Ablation of lung endothelial injury after pacing-induced heart failure is related to alterations in Ca2+ signaling. Am J Physiol Heart Circ Physiol 1998;275:H844–H851. [DOI] [PubMed]
- 37.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol Lung Cell Mol Physiol 1998;274:L810–L819. [DOI] [PubMed]
- 38.Putney JW, Jr. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 2007;42:103–110. [DOI] [PMC free article] [PubMed]
- 39.Putney JW. Origins of the concept of store-operated calcium entry. Frontiers Biosci 2011;3:980–984. [DOI] [PMC free article] [PubMed]
- 40.Parekh A. On the activation mechanism of store-operated calcium channels. Pfluegers Archiv Eur J Physiol 2006;453:303–311. [DOI] [PubMed]
- 41.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vasc Pharmacol 2002;39:173–185. [DOI] [PubMed]
- 42.Moore TM, Norwood NR, Creighton JR, Babal P, Brough GH, Shasby DM, Stevens T. Receptor-dependent activation of store-operated calcium entry increases endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 2000;279:L691–L698. [DOI] [PubMed]
- 43.Large WA. Receptor-operated Ca2+-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol 2002;13:493–501. [DOI] [PubMed]
- 44.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 2001;24:487–517. [DOI] [PubMed]
- 45.Thomas KC, Roberts JK, Deering-Rice CE, Romero EG, Dull RO, Lee J, Yost GS, Reilly CA. Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am J Physiol Lung Cell Mol Physiol 2012;302:L111–L119. [DOI] [PMC free article] [PubMed]
- 46.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell 2002;108:595–598. [DOI] [PubMed]
- 47.van der Stelt M, Di Marzo V. Endovanilloids: putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem 2004;271:1827–1834. [DOI] [PubMed]
- 48.Ang S-FB, Moochhala SMP, Bhatia MP. Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med 2010;38:619–628. [DOI] [PubMed]
- 49.Hwang SW, Cho H, Kwak J, Lee S-Y, Kang C-J, Jung J, Cho S, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 2000;97:6155–6160. [DOI] [PMC free article] [PubMed]
- 50.Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry 2006;45:9007–9015. [DOI] [PubMed]
- 51.De Petrocellis L, Di Marzo V. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci 2005;77:1651–1666. [DOI] [PubMed]
- 52.Wang M, Ji P, Wang R, Zhao L, Xia Z. TRPV1 agonist capsaicin attenuates lung ischemia-reperfusion injury in rabbits. J Surg Res 2012;173:153–160. [DOI] [PubMed]
- 53.Gao X, Wu L, O’Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 2003;278:27129–27137. [DOI] [PubMed]
- 54.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Šali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000;103:525–535. [DOI] [PMC free article] [PubMed]
- 55.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA 2003;100:14531–14536. [DOI] [PMC free article] [PubMed]
- 56.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 2009;284:21257–21264. [DOI] [PMC free article] [PubMed]
- 57.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, et al. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 2005;97:908–915. [DOI] [PubMed]
- 58.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 2004;101:396–401. [DOI] [PMC free article] [PubMed]
- 59.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 2002;277:13569–13577. [DOI] [PubMed]
- 60.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: part 2. J Pharmacol Exp Ther 2008;326:443–452. [DOI] [PubMed]
- 61.Jian M-Y, King JA, Al-Mehdi A-B, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 2008;38:386–392. [DOI] [PMC free article] [PubMed]
- 62.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 2004;43:55–90. [DOI] [PubMed]
- 63.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003;424:434–438. [DOI] [PubMed]
- 64.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 2007;293:L923–L932. [DOI] [PubMed]
- 65.Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, Eyal FG, Clapp MM, Parker JC. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2010;299:L353–L362. [DOI] [PMC free article] [PubMed]
- 66.Yin J, Hoffmann J, Kaestle SM, Neye N, Wang L, Baeurle J, Liedtke W, et al. Negative-feedback loop attenuates hydrostatic lung edema via a cGMP-dependent regulation of transient receptor potential vanilloid 4. Circ Res 2008;102:966–974. [DOI] [PubMed]
- 67.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 2012;4:159ra48, doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed]
- 68.Villalta PC, Rocic P, Townsley MI. Role of matrix metalloproteinases (MMP) 2 and 9 in TRPV4-induced lung injury. FASEB J 2012;26:696.12.
- 69.Trump BF, Berezesky IK. The role of altered [Ca2+]i regulation in apoptosis, oncosis, and necrosis. Biochim Biophys Acta 1996;1313:173–178. [DOI] [PubMed]
- 70.Verkhratsky A. Calcium and cell death. In: Carafoli E, Brini M, eds. Calcium signalling and disease: molecular pathology of calcium. New York: Springer, 2008:465–480.
- 71.Casas S, Novials A, Reimann F, Gomis R, Gribble F. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 2008;51:2252–2262. [DOI] [PMC free article] [PubMed]
- 72.Bai JZ, Lipski J. Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 2010;31:204–214. [DOI] [PubMed]
- 73.Klein CJ, Shi Y, Fecto F, Donaghy M, Nicholson G, McEntagart ME, Crosby AH, et al. TRPV4 mutations and cytotoxic hypercalcemia in axonal Charcot-Marie-Tooth neuropathies. Neurology 2011;76:887–894. [DOI] [PMC free article] [PubMed]
- 74.Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol 1997;25:82–88. [DOI] [PubMed]
- 75.Jernigan TW, Croce MA, Fabian TC. Apoptosis and necrosis in the development of acute lung injury after hemorrhagic shock. Am Surg 2004;70:1094–1098. [PubMed]
- 76.Sakashita A, Nishimura Y, Nishiuma T, Takenaka K, Kobayashi K, Kotani Y, Yokoyama M. Neutrophil elastase inhibitor (sivelestat) attenuates subsequent ventilator-induced lung injury in mice. Eur J Pharmacol 2007;571:62–71. [DOI] [PubMed]
- 77.Franek WR, Morrow DMP, Zhu H, Vancurova I, Miskolci V, Darley-Usmar K, Simms HH, Mantell LL. NF-κB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic Biol Med 2004;37:1670–1679. [DOI] [PubMed]
- 78.Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol 2004;44:349–370. [DOI] [PubMed]
- 79.Cuzzocrea S, McDonald MC, Mazzon E, Siriwardena D, Serraino I, Dugo L, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Calpain inhibitor I reduces the development of acute and chronic inflammation. Am J Pathol 2000;157:2065–2079. [DOI] [PMC free article] [PubMed]
- 80.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 2009;104:1123–1130. [DOI] [PMC free article] [PubMed]
- 81.Wang Y, Minshall RD, Schwartz DE, Hu G. Cyclic stretch induces alveolar epithelial barrier dysfunction via calpain-mediated degradation of p120-catenin. Am J Physiol Lung Cell Mol Physiol 2011;301:L197–L206. [DOI] [PMC free article] [PubMed]
- 82.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 2008;102:347–355. [DOI] [PubMed]
- 83.Perraud A-L, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001;411:595–599. [DOI] [PubMed]
- 84.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furiuchi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001;293:1327–1330. [DOI] [PubMed]
- 85.Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 2005;18:61–69. [DOI] [PubMed]
- 86.Beck A, Kolisek M, Bagley LA, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J 2006;20:962–964. [DOI] [PubMed]
- 87.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 2002;9:163–173. [DOI] [PubMed]
- 88.Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 2003;278:16222–16229. [DOI] [PubMed]
- 89.Zhang W, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun S-C, Penn L, Barber DL, et al. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol 2006;290:C1146–C1159. [DOI] [PubMed]
- 90.Di A, Gao X-P, Qian F, Kawamura T, Han J, Hecquet C, Ye RD, Vogel SM, Malik AB. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol 2012;13:29–34. [DOI] [PMC free article] [PubMed]
- 91.Hardaker L, Bahra P, de Billy B, Freeman M, Kupfer N, Wyss D, Trifilieff A. The ion channel transient receptor potential melastatin-2 does not play a role in inflammatory mouse models of chronic obstructive pulmonary diseases. Respir Res 2012;13:30. [DOI] [PMC free article] [PubMed]
- 92.Corteling R, Wyss D, Trifilieff A. In vivo models of lung neutrophil activation: comparison of mice and hamsters. BMC Pharmacol 2002;2:1, doi:10.1186/1471-2210-2-1. [DOI] [PMC free article] [PubMed]
- 93.Dodd-o JM, Hristopoulos ML, Welsh-Servinsky LE, Tankersley CG, Pearse DB. Strain-specific differences in sensitivity to ischemia-reperfusion lung injury in mice. J Appl Physiol 2006;100:1590–1595. [DOI] [PubMed]
- 94.Song Y, Dayalu R, Matthews SA, Scharenberg AM. TRPML cation channels regulate the specialized lysosomal compartment of vertebrate B-lymphocytes. Eur J Cell Biol 2006;85:1253–1264. [DOI] [PubMed]
- 95.Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem 2006;281:17517–17527. [DOI] [PMC free article] [PubMed]
- 96.Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A. TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem 2005;280:43218–43223. [DOI] [PubMed]
- 97.Cheng X, Shen D, Samie M, Xu H. Mucolipins: intracellular TRPML1–3 channels. FEBS Lett 2010;584:2013–2021. [DOI] [PMC free article] [PubMed]
- 98.Cuajungco M, Samie M. The varitint–waddler mouse phenotypes and the TRPML3 ion channel mutation: cause and consequence. Pfluegers Archiv Eur J Physiol 2008;457:463–473. [DOI] [PubMed]
- 99.Qian F, Noben-Trauth K. Cellular and molecular function of mucolipins (TRPML) and polycystin 2 (TRPP2). Pfluegers Archiv Eur J Physiol 2005;451:277–285. [DOI] [PubMed]
- 100.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 2002;4:191–197. [DOI] [PubMed]
- 101.Chen X-Z, Vassilev PM, Basora N, Peng J-B, Nomura H, Segal Y, Brown EM, Reeders ST, Hediger MA, Jing Zhou J. Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature 1999;401:383–386. [DOI] [PubMed]
- 102.Köttgen M. TRPP2 and autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2007;1772:836–850. [DOI] [PubMed]
- 103.Berrout J, Jin M, O’Neil RG. Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood–brain barrier endothelial cells. Brain Res 2012;1436:1–12. [DOI] [PubMed]
- 104.De Bock M, Culot M, Wang N, Bol M, Decrock E, De Vuyst E, da Costa A, et al. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J Cereb Blood Flow Metabol 2011;31:1942–1957. [DOI] [PMC free article] [PubMed]
- 105.De Bock M, Wang N, Decrock E, Bol M, Gadicherla AK, Culot M, Cecchelli R, Bultynck G, Leybaert L. Endothelial calcium dynamics, connexin channels and blood-brain barrier function. Prog Neurobiol 2013;108:1–20. [DOI] [PubMed]
- 106.Parker JC. Hydraulic conductance of lung endothelial phenotypes and Starling safety factors against edema. Am J Physiol Lung Cell Mol Physiol 2007;292:L378–L380. [DOI] [PubMed]
- 107.Parker JC, Stevens T, Randall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 2006;291:L30–L37. [DOI] [PubMed]
- 108.Parker JC, Yoshikawa S. Vascular segmental permeabilities at high peak inflation pressure in isolated rat lungs. Am J Physiol Lung Cell Mol Physiol 2002;283:L1203–L1209. [DOI] [PubMed]
- 109.Wu S, Chen H, Alexeyev MF, King JAC, Moore TM, Stevens T, Balczon RD. Microtubule motors regulate ISOC activation necessary to increase endothelial cell permeability. J Biol Chem 2007;282:34801–34808. [DOI] [PubMed]
- 110.Brown RC, Wu L, Hicks K, O’Neil RG. Regulation of blood-brain barrier permeability by transient receptor potential type C and type V calcium-permeable channels. Microcirculation 2008;15:359–371. [DOI] [PMC free article] [PubMed]
- 111.Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther 1999;289:668–675. [PubMed]
- 112.Nag S. Protective effect of flunarizine on blood-brain barrier permeability alterations in acutely hypertensive rats. Stroke 1991;22:1265–1269. [DOI] [PubMed]
- 113.Hicks K, O’Neil RG, Dubinsky WS, Brown RC. TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am J Physiol Cell Physiol 2010;298:C1583–C1593. [DOI] [PMC free article] [PubMed]
- 114.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 2008;9:472–479. [DOI] [PMC free article] [PubMed]
- 115.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 2008;182:437–447. [DOI] [PMC free article] [PubMed]
- 116.Zhang P, Luo Y, Chasan B, González-Perrett S, Montalbetti N, Timpanaro GA, de Rocío Cantero M, et al. The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum Mol Genet 2009;18:1238–1251. [DOI] [PMC free article] [PubMed]
- 117.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003;112:819–829. [DOI] [PubMed]
- 118.Mukhopadhyay I, Gomes P, Aranake S, Shetty M, Karnik P, Damle M, Kuruganti S, Thorat S, Khairatkar-Joshi N. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct Res 2011;31:350–358. [DOI] [PubMed]
- 119.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res 2009;104:987–994. [DOI] [PMC free article] [PubMed]
- 120.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation 2013;20:138–148. [DOI] [PMC free article] [PubMed]
- 121.Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, et al. Cigarette smoke–induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 2008;118:2574–2582. [DOI] [PMC free article] [PubMed]
- 122.Andrè E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, P Geppetti. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol 2009;158:1621–1628. [DOI] [PMC free article] [PubMed]
- 123.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med 2009;180:1042–1047. [DOI] [PMC free article] [PubMed]
- 124.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, Witek JS, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 2009;106:9099–9104. [DOI] [PMC free article] [PubMed]
- 125.Geppetti P, Patacchini R, Nassini R, Materazzi S. Cough: the emerging role of the TRPA1 channel. Lung 2010;188:63–68. [DOI] [PubMed]
- 126.Lowe K, Alvarez D, King J, Stevens T. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance. J Surg Res 2007;143:70–77. [DOI] [PMC free article] [PubMed]
- 127.Townsley MI, King JA, Alvarez DF. Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation 2006;13:725–739. [DOI] [PubMed]
- 128.Guyton AC, Lindsey AW, Howell JO, Williams JW, Franklin MA. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res 1959;7:649–657. [DOI] [PubMed]
- 129.Staub NC. Pulmonary edema. Physiol Rev 1974;54:678–811. [DOI] [PubMed]
- 130.Staub NC, Nagano H, Pearce ML. Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J Appl Physiol 1967;22:227–240. [DOI] [PubMed]
- 131.Taylor AE, Drake RE. Fluid and protein movement across the pulmonary microcirculation. In: Staub NC, ed. Lung water and solute exchange. New York: Dekker, 1978:129–166.







