Abstract Abstract
Hematopoiesis and vascular homeostasis are closely linked to each other via subsets of circulating bone marrow–derived cells with potent activity to repair endothelial injury and promote angiogenesis. As a consequence, abnormalities in hematopoiesis will eventually affect vascular health. Pulmonary arterial hypertension (PAH) is a vascular disease characterized by severe remodeling of the pulmonary artery wall. Over the past decade, circulating hematopoietic cells have been assigned an increasing role in the remodeling, such that these cells have been used in new therapeutic strategies. More recently, research has been extended to the bone marrow where these cells originate to identify abnormalities in hematopoiesis that may underlie PAH. Here, we review the current literature and identify gaps in knowledge of the myeloid effects on PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by severe remodeling of the pulmonary artery and, as a consequence, increased pulmonary artery pressure and ultimately right ventricular failure. Bone marrow (BM)–derived cells are essential to vascular homeostasis, and circulating hematopoietic cells and the marrow-resident mesenchymal stromal cells equally affect vascular health. Among these cells, the fibrocytes and mesenchymal stem cells (MSCs) are well characterized using a combination of cell surface markers and/or cell culture techniques, with fibrocytes being a specific subset of collagen-I-producing myeloid-derived cells1,2 and MSCs as a subset of BM stromal cells with the capacity to differentiate into adipocytes, chondrocytes, and osteoblasts.3 The population of BM-derived “endothelial progenitor cells,” a subset of BM cells with potent angiogenesis activity, is less well defined. For the past several years, it was believed that a subset of these cells exhibited the capacity to differentiate into endothelial cells. Endothelial progenitor cells define a heterogeneous group of cells that are classified into 3 groups in humans on the basis of the method of isolation, including circulating angiogenic cells (CACs), colony-forming unit Hill (CFU-Hill; sometimes also referred to as CFU-EC), and endothelial colony-forming cells (ECFCs).4 The latter are true endothelial cells, as evidenced by their in vivo capacity to form blood vessels, and are defined as endothelial cell colonies appearing after 7–21 days of culture of blood mononuclear cells (MNCs) in endothelial conditions.5 CAC do not form colonies but appear earlier during cell culture (starting on day 4) as spindle-shaped or cobblestone-morphology cells and typically bind Ulex europeas lectin and uptake acetylated low-density lipoprotein (acLDL).6-8 Peripheral blood CAC originate from proangiogenic myeloid cells.5 Murine BM-derived endothelial progenitor cells are isolated using a similar method. CFU-Hill colonies contain a mixture of proangiogenic myeloid progenitors and angiogenic T cells.9-12 In addition, circulating or BM cells expressing hematopoietic stem cell markers such as CD34, CD133, or c-Kit in humans or SCA-1 and c-Kit in murine in combination with VEFGR-2 have also been referred to as endothelial progenitor cells.4 Thus, it is evident now that, except for ECFC, the methods used to identify endothelial progenitor cells in the peripheral blood circulation and BM in fact detect or enrich for proangiogenic myeloid hematopoietic cells.13,14 Plating of mononuclear cell on fibronectin or gelatin substrates, used to isolate endothelial progenitor cells, selects monocytic cells.15 Two frequently used fluorescent labels, Dil-conjugated acLDL and fluorescently labeled plant lectin U. europaeus agglutin 1 (UEA-1), are not specific for endothelial cells and will also stain myeloid cells.10,13,16,17 In vitro tube formation assays used to access angiogenic cord formation by endothelial progenitors are not useful to identify these cells, because many other types are able to form these structures.13 Moreover, although several in vivo studies initially reported that endothelial progenitor cells contribute to endothelial repair and regeneration by differentiating into endothelial cells and integrating into the endothelium,18-25 subsequent in-depth studies using genetically tagged BM or endothelial-cell-specific reporter genes26-29 showed that the endothelial progenitor cells localize adjacent to blood vessels or only temporarily incorporate into the endothelium. Current paradigm in vascular biology is that these BM-derived myeloid cells acquire endothelial-like cell mimicry. Nevertheless, these cells are essential paracrine actors as proangiogenic hematopoietic cells critical for new blood vessel formation and endothelial repair30-35 that are composed of a heterogeneous group of myeloid hematopoietic progenitor and mature cells, including mast cells. Their diverse phenotype and angiogenic activities and the controversy surrounding endothelial progenitor cells have been reviewed in detail elsewhere.13,36 Smooth muscle cells of hematopoietic origin,37-39 derived from BM stromal cells,40 or of unidentified BM origin41,42 have also been reported. Here, we provide a review of the roles of the BM-derived cells in PAH, including proangiogenic hematopoietic cells, mast cells, fibrocytes, and MSCs. We provide review of studies involving animals and patients and discuss the clinical implications of the findings and future perspectives.
Proangiogenic hematopoietic cells in PAH
Proangiogenic hematopoietic cells are subsets of myeloid hematopoietic progenitors and monocytic cells. The majority of the studies involving BM in PAH have investigated these cells in animal models as well as in patients as endothelial progenitor cells. Tables 1 and 2 provide a detailed overview of the characterization and terminology used for these cells in the individual publications.
Table 1.
Phenotype of bone marrow (BM)–derived proangiogenic cells in animal models of pulmonary arterial hypertension (PAH)
| Study | Model | Cells studied | Phenotype | Terminology |
|---|---|---|---|---|
| Luan et al.52 | Canine MCT | BM MNC | CD34 | BMC MNC |
| Zhao et al.55 | Canine MCT | Cultured blood MNC | CD31, CD34, vWF | Endothelial progenitor cells |
| Yip et al.57 | Rat MCT | Cultured BM MNC | Spindle shaped, cobblestone morphology, CD29, CD31, CD90, VEGFR2, vWF | Endothelial progenitor cells |
| Sun et al.53 | Rat MCT | Cultured BM MNC | Spindle shaped, cobblestone morphology, acLDL, tube formation, CD31, VEGFR2, CD133, CD34, c-Kit | Endothelial progenitor cells |
| Zhao et al.55 | Rat MCT | Cultured BM MNC | vWF, Flk-1, Tie-2, acLDL, UEA-1, eNOS | Endothelial progenitor cells |
| Sun et al.58 | Rat MCT | Cultured BM MNC | Spindle shaped, cobblestone morphology, acLDL, CD31, CD34, VEGF, vWF | Endothelial progenitor cells |
| Raoul et al.44 | Mouse MCT or hypoxia | Unfractionated eGFP BM | CD45+Lin−, CD45−Lin− | BM-derived cells |
| Mirsky et al.56 | Nude rats MCT xenografted with human CAC | Cultured human peripheral blood MNC | CD34/VEGFR2, CD133/VEGFR2, acLDL, CD45−VEGFR-2+ c-Kit+ | Circulating angiogenic cells |
| Marsboom et al.46 | Mouse hypoxia | Cultured spleen MNC; circulating cells | VEGFR2, Sca-1, c-Kit, CXCR4, acLDL, CD45−VEGFR-2+, c-Kit+ | Endothelial progenitor cells |
| Hayashida et al.47 | Mouse hypoxia | Unfractionated eGFP BM | GFP α-SMA+ | BM-derived cells |
| Satoh et al.45 | Mouse hypoxia | Tie-2-GFP BM MNC | Tie-2-GFP CD133+VEGFR2+, acLDL, VEGFR2 | Endothelial progenitor cells |
| Davie et al.43 | Calf hypoxia | BM | c-Kit, vWF, eNOS, acLDL, capillary tube | BM progenitor cells, endothelial progenitor cells |
| Launay et al.49 | Mouse hypoxia or MCT | BM | c-Kit | BM-derived stem cells, endothelial precursor cells, smooth muscle precursor cells |
| Gambaryan et al.48 | Mouse hypoxia | BM | c-Kit+, SCA-1+, CXCR4, αSMA | c-Kit+ hematopoietic progenitors |
acLDL = acetylated low-density lipoprotein; CAC = circulating angiogenic cells; eGFP = enhanced green fluorescent protein; eNOS = nitric oxide synthase; MCT = monocrotaline; MNC = mononuclear cells; UEA-1 = Ulex europaeus agglutin 1; vWF = von Willebrand factor; α-SMA = α smooth muscle actin.
Table 2.
Phenotype of bone marrow (BM)–derived proangiogenic cells in patients
| Study | Cells studied | Phenotype | Terminology |
|---|---|---|---|
| Diller et al.64 | Blood MNC | CD34+AC133+, VEGFR2 subsets, acLDL, UEA-1, incorporation into tube-like structure | Endothelial progenitor cells |
| Wang et al.67 | CAC | VE-Cadherin, KDR, CD34, AC133, acLDL, UEA-1 | Endothelial progenitor cells |
| Zhu et al.68 | CAC | KDR, CD34, AC133, acLDL, UEA-1 | Endothelial progenitor cells |
| Junhui et al.65 | CAC | CD133+VEGFR2+, UEA-1, acLDL | Endothelial progenitor cells |
| Asosingh et al.59 | Blood MNC | CD34+CD133+, CFU-EC | BM-derived angiogenic precursors |
| Farha et al.63 | Blood and BM MNC | CD34+CD133+ | Progenitor cells |
| Toshner et al.61 | Blood MNC | CD34+ CD133+VEGFR2+ | Endothelial progenitor cells |
| Smadja et al.60 | Blood MNC | CD34+ CD133+ | Circulating progenitor cells |
| Smadja et al.66 | Blood MNC | CD34+ CD133+, CFU-EC, Matrigel tube formation | Circulating progenitor cells |
| Asoingh et al.71 | Human BM MNC xenografted into NODSCID mice | CD133+ | Progenitors |
| Montani et al.62 | Blood MNC | CD34+ CD133+CXCD4+Lin− | Mast cells and hematopoietic progenitors |
acLDL = acetylated low-density lipoprotein; CAC = circulating angiogenic cells; CFU-EC = colony-forming unit endothelial cell; MNC = mononuclear cells; NODSCID = nonobese diabetic/severe combined immunodeficiency; UEA-1 = Ulex europaeus aglutin 1.
Proangiogenic hematopoietic cells in animal models of PAH
Proangiogenic progenitors in experimental PAH have been studied in small and large animal models, as contributors to the remodeling and as cellular vehicles for gene therapy. Animals exposed to hypoxia or monocrotaline (MCT) are used as models of PAH. In the hypoxia-induced PAH model, animals were placed in a hypobaric chamber, using 10% oxygen (O2) for an extended period of time.43-49 The levels of circulating progenitors expressing hematopoietic cell surface markers were consistently increased in these models, which suggests a role in pathogenesis.43-46 In green fluorescent protein BM chimeric mice, mobilization and recruitment of BM cells into pulmonary arteries and adventitia were observed. A fraction of he BM-derived cells detected in the angiogenic remodeled areas expressed α-smooth muscle actin (α-SMA), suggesting that these cells differentiated into myofibroblasts or smooth muscle cells.43,47 It is possible that these α-SMA cells are derived from BM stromal cells or fibrocytes. Stromal-derived factor 1α (SDF-1α) is a primary factor in the recruitment of BM progenitors into tissues. Animals exposed to hypoxia showed increased expression of SDF-1α in the lung tissue as well as increased presence of SDF-1α-receptor-positive (CXCR4 or CXCR7) c-Kit+ progenitors in the remodeled areas.48 Pharmacological blockade of SDF-1α receptors before the exposure to hypoxia, but not in animals with established PAH, showed parallel reduction in c-Kit+ cell recruitment and decreased right ventricular systolic pressure.48 In 2 other studies, the development of PAH was related to proangiogenic hematopoietic cells with impaired angiogenesis capacity.45,46 Serotonin binding its receptor 2B receptor (5-HT2BR) induces vasoconstriction of the pulmonary arteries by stimulating smooth muscle cell contraction.50 5-HT2BR is overexpressed in patients with PAH, and 5-HT2BR-deficient (5-HT2B−/−) mice fail to develop PAH under hypoxia.49 Early evidence that the BM contributes to the origin of PAH came from a landmark BM transplantation study demonstrating that wild-type BM led to development of PAH in 5-HT2B−/− mice.49 In contrast, engraftment of 5-HT2B−/− BM into wild-type mice prevented PAH in animals under hypoxia or treated with MCT.49 Similar mechanisms may operate in large animals. For example, increased numbers of c-Kit+ cells were found in the expanded vaso vasorum in remodeled areas of the pulmonary artery wall in calves exposed to chronic hypoxia.43 Overall, these reports suggest that proangiogenic hematopoietic cells contribute to PAH in hypoxic animal models.
MCT is a potent pyrrolizine alkaloid endothelial toxin that causes pulmonary vascular disease in rodents.51,52 Therapeutic effects of ex vivo expanded mature hematopoietic proangiogenic cells have mostly been studied in this model.5,13-18,52-55 In all but one of these studies,56 administration of the proangiogenic cells before pulmonary hypertension onset decreased right ventricular systolic pressure and/or reduced remodeling.44,52,54,55,57 One mechanism of benefit seems to be via increased expression of endothelial nitric oxide synthase (eNOS).44,57 Additional improvement was observed when the hematopoietic cells were given in conjunction with vasodilator therapy.53,58 Animals with established pulmonary hypertension showed further reduction of right ventricular systolic pressure and survival when proangiogenic cells were transduced with eNOS.55 Thus, ex vivo expanded differentiated proangiogenic hematopoietic cells have beneficial effects in MCT models of pulmonary hypertension.
Proangiogenic hematopoietic cells in human PAH
Although animal studies of pulmonary hypertension have high levels of circulating progenitors, findings are controversial in human PAH. Some groups reported elevated numbers in the peripheral circulation,59-63 whereas others found a reduction64,65 or no difference,66 compared with healthy control subjects. There are several possible explanations for these discrepancies. First, all groups analyzed different CD34+, CD133+, or c-Kit+ progenitor cell subsets with or without a combination of lineage markers. Second, studies vary in the stage of patients evaluated. Patients with end stage of PAH who are on a waiting list for lung transplantation have significantly reduced numbers of circulating progenitors (KA, personal observation). Another confounder is insufficient number of events acquired and/or nonstandard gating strategy. The latter is critical for detection of circulating progenitors, which are rare events. Although there is no single correct way to analyze for these cells, a uniform set of specific guidelines should be followed (Fig. 1).
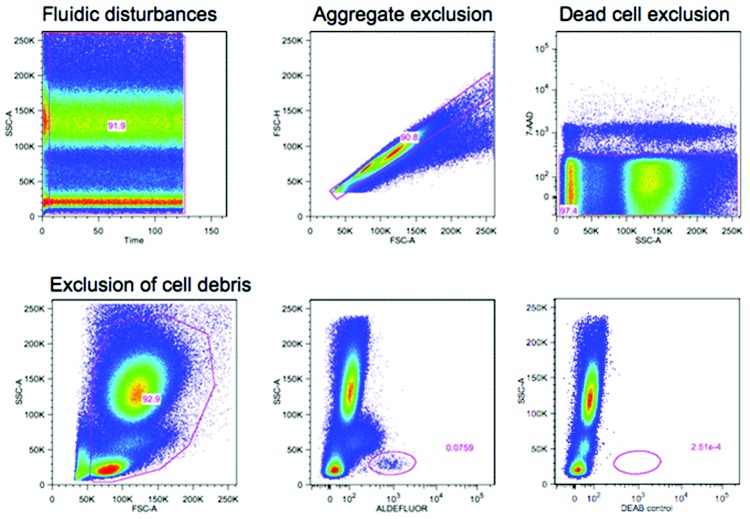
Figure 1.
Guidelines for flow cytometric quantification of circulating proangiogenic progenitors. Proangiogenic progenitors are infrequent in the peripheral blood, and therefore all guidelines for rare event analysis apply.91 The number of events acquired is critical and should be based on the desired coefficient of variance (CV). Poisson distribution best describes the random and independent occurrence of rare events. In this statistic, 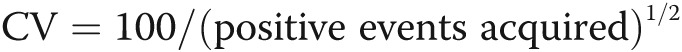 ; for example, acquisition of 100 positive events will give a CV of 10%. If the frequency of positive events is 0.02%, 500,000 events need to be acquired. After acquisition, gating-out sources of artifacts should be part of the gating strategy and applied to each sample. Fluidic disturbances in the flow cell will increase variability in the measured parameter. Time-gating should be performed to control for this; any irregularities and burst of events should be excluded. Aggregate correction should be applied to exclude cell aggregates. Dead and dying cells can lead to nonspecific binding and mask true frequency; therefore, a dead cell exclusion dye should be used. Some dead cells will fragment into cell debris during the staining process and should be excluded by gating out events with light scatters too low to be intact cells. Knowing the spatial distribution of the positive events is helpful to gate them, even when the number of events acquired is low. Backgating of the positive events is always recommended to check which events have been excluded during the logical gating process. In this illustration, red blood cells in whole blood were lysed using fixative free ammonium chloride followed by staining for aldehyde dehydrogenase activity (aldefluor), a recently described enzymatic probe for circulating proangiogenic progenitor cells.92-95 Blocking of the aldehyde dehydrogenase activity using diethylamino benzaldehyde (DEAB) in parallel staining was used as control. 7-AAD was used as a dead cell exclusion dye. The progenitors are identified as aldefluor positive cells with low side scatter (SSC) properties. Identical principles apply for other markers of circulating progenitors.
; for example, acquisition of 100 positive events will give a CV of 10%. If the frequency of positive events is 0.02%, 500,000 events need to be acquired. After acquisition, gating-out sources of artifacts should be part of the gating strategy and applied to each sample. Fluidic disturbances in the flow cell will increase variability in the measured parameter. Time-gating should be performed to control for this; any irregularities and burst of events should be excluded. Aggregate correction should be applied to exclude cell aggregates. Dead and dying cells can lead to nonspecific binding and mask true frequency; therefore, a dead cell exclusion dye should be used. Some dead cells will fragment into cell debris during the staining process and should be excluded by gating out events with light scatters too low to be intact cells. Knowing the spatial distribution of the positive events is helpful to gate them, even when the number of events acquired is low. Backgating of the positive events is always recommended to check which events have been excluded during the logical gating process. In this illustration, red blood cells in whole blood were lysed using fixative free ammonium chloride followed by staining for aldehyde dehydrogenase activity (aldefluor), a recently described enzymatic probe for circulating proangiogenic progenitor cells.92-95 Blocking of the aldehyde dehydrogenase activity using diethylamino benzaldehyde (DEAB) in parallel staining was used as control. 7-AAD was used as a dead cell exclusion dye. The progenitors are identified as aldefluor positive cells with low side scatter (SSC) properties. Identical principles apply for other markers of circulating progenitors.
In preliminary studies, ex vivo expanded circulating proangiogenic cells have been used as cell therapy in idiopathic PAH. These patients showed clinical improvement as indicated by increased 6-minute walk distance, decreased mean pulmonary artery pressure, improved pulmonary vascular resistance, and increased cardiac output, compared with individuals who received conventional therapy alone.67,68 It is important to note that the angiogenic cells in these studies67,68 were CAC, which are low-proliferative and monocyte-derived cells, as shown by others.4
Although there is controversy regarding the levels of circulating progenitors, reports consistently show that these cells are present in high numbers in remodeled areas of the pulmonary artery wall.61-63,69 In paraffin-embedded lung tissues obtained from explanted lungs or lungs rejected for transplantation, CD133+ cells were found at consistently higher levels within the lung parenchyma and intima in idiopathic PAH compared with control lungs.69 In another study, CD133+ cells were found at high levels in concentric lesions and, in higher number, in plexiform lesions, in contrast to healthy lungs.61 Employing flow cytometry, we found that levels of CD34+CD133+ cells in the pulmonary artery wall were tenfold higher than levels in controls.63 Using another progenitor cell marker, c-Kit, elevated numbers of c-Kit+ cells were observed in PAH pulmonary arteries with the majority of the cells located in the perivascular area and vasa vasorum.62 In all these studies,61-63 increased expression of progenitor cell chemoattractant SDF-1α by pulmonary artery endothelial cells in the vascular lesions was found, suggesting upregulated SDF-1α as one of the mechanism involved in the recruitment of progenitors into the pulmonary artery wall.
Key questions remain as to whether these progenitors contribute to the disease or attenuate the pathological remodeling. To address this, studies were extended to the BM where these cells originate. Reticulin, an extracellular matrix fiber, is present in low amounts in the normal BM around the blood vessels as a component of the basement membrane. Increased reticulin deposition, which is an indicator for abnormal hematopoiesis,70 was observed in the marrow of patients with PAH.63 Higher numbers of CD34+CD133+ and CD34+CD133− subsets of progenitors were found in the BM aspirates of patients with PAH compared with the BM from healthy control donors.63 Additional analysis using ex vivo hematopoietic colony-forming assays and hematopoietic transcription factor gene array analysis revealed that the hematopoietic stem cell in PAH is skewed in the myeloid lineage toward erythroid differentiation.71 Surprisingly, in the familial form of the disease, unaffected family members had similar BM abnormalities and elevated mobilization in the peripheral circulation, suggesting that increased hematopoietic stem cell proliferation may start before the onset of PAH.63 Engraftment of human BM into nonobese diabetic/severe combined immunodeficiency mice is a standard assay to study hematopoietic stem cells in vivo. Transplantation of PAH BM CD133+ hematopoietic stem cells into these mice confirmed the increased myeloerythroid proliferation and, strikingly, induced pulmonary vascular disease, including endothelial cell injury associated with increased oxidative stress, in situ thrombi, and right ventricular hypertrophy. These findings advance the idea of a causal link between BM and PAH.71 Intriguingly, independent from these findings, gene expression profiling of peripheral blood mononuclear cells showed increased levels of erythropoiesis-related genes, correlated to disease severity in patients with idiopathic PAH.72 Collectively, reports point toward increased myeloerthyroid proliferation and mobilization of hematopoietic progenitor cells in human PAH. These cells home to the PAH pulmonary arterial wall, preferentially in the remodeled areas, and contribute to pulmonary vascular disease.
Mast cells in PAH
Mast cells originate from the myeloid lineage. In contrast to other hematopoietic cells, mast cells do not egress the BM fully formed but, rather, as a progenitor, which shares many of the proangiogenic hematopoietic progenitor cell markers and matures in the peripheral tissues. Mast cells release various angiogenic factors, and their angiogenic potential has been well documented, in particular in the field of cancer biology.73 There is evidence from animal models and samples obtained from explanted PAH lungs that mast cells are involved in the disease. Increased numbers of mast cells in perivascular remodeled areas of the pulmonary artery were detected in MCT-injected rats. Inhibition of mast cell degranulation by inhibiting c-Kit or by mast cell stabilizer cromolyn reduced right ventricular systolic pressure, right ventricular hypertrophy, and vascular muscularization in a preventive (before onset of PAH), but not in a therapeutic approach.74 In another study, preventive treatment of rats in the MCT model had a similar favorable outcome.75 Several groups have reported that mast cells are abundantly present in and around vascular lesions in tissue sections of explanted PAH lungs.62,76,77 Mast cell product tryptase was also found to be increased in the serum of patients with PAH and the levels decreased after treatment with mast cell inhibitors cromolyn and fexofenadine.77 However, no clinical improvement was observed in this small cohort of patients. These studies provide additional support for the myeloid origins of PAH and point to possible cell mechanism (e.g., mast cells and their proangiogenic and vasoconstrictive products).
Fibrocytes in PAH
Fibrocytes are CD45+ collagen-I+ circulating cells in the myeloid lineage. They co-express a specific combination of markers, such as CD45RO and macrophage antigens 25F9 and S100A8/A9, that distinguish them from monocytes, tissue macrophages, and fibroblasts.2 Two detailed animal studies reported progressive homing of these cells into the pulmonary artery wall, where they obtained an α-SMA-positive myofibroblast phenotype and contributed to thickening of the vessel wall in hypoxia-induced PAH models.78,79 Depletion of the fibrocytes by treatment of the animals with clodronate or gadolinium chloride78 resulted in reduced myofibroblast accumulation and decreased thickening of the pulmonary artery wall, indicating that the vascular remodeling can be attributed to the recruitment of nonresident blood-borne subsets of mononuclear cells.78 These findings that a subset of monocytes transdifferentiate into fibrocytes and contribute to the disease may open new therapeutic strategies but need confirmation in human studies.
MSCs in PAH
MSCs have immunomodulatory benefits in several pathological conditions. In animal models of MCT- or hypoxia-induced PAH, administration of MSCs prevented or attenuated development of disease,80-87 most probably via paracrine mechanisms.88 Two studies performed autologous MSC transplantation in rats with established MCT-induced PAH.43,44 Both studies found reduced right ventricular hypertrophy and right ventricular systolic pressure and attenuated thickening of the pulmonary artery wall.44,45 In yet another report, MSC therapy did not have beneficial effects in hypoxia-induced PAH.88 In some studies, MSCs were used as vehicles for gene therapy.85-87 Treatment of animals with MSCs overexpressing eNOS86 or prostacyclin,85 a vasodilating and platelet-inhibiting lipid molecule, in the rat MCT model or anti-inflammatory and anti-oxidant enzyme heme oxygenase-1 in the mouse hypoxia model87 was more effective in reducing right ventricular hypertrophy and pressure and was associated with prolonged survival compared with animals treated with naive MSC. Overall, these findings suggest that use of MSCs as vehicles for gene therapy may be a promising approach, but additional studies are needed.
Applications of BM stem cell therapies
In contrast to the available therapies targeting mainly the vasoconstrictive component of PAH, focus has shifted to develop drugs that would halt and reverse the pulmonary vascular remodeling. Therapies modulating the BM progenitors are being looked into on the basis of the established link between the BM abnormalities and the pulmonary vasculopathy. Imatinib, a tyrosine kinase inhibitor that is used for the treatment of chronic myeloid leukemia and has been shown to decrease circulating progenitors in PAH (unpublished data) has been described in case reports to be beneficial for the treatment of PAH. In a phase II study, imatinib therapy was well tolerated by patients with PAH and led to a significant reduction in pulmonary vascular resistance in association with an increase in cardiac output.89 Results from the phase III study showed clinical benefits in patients treated with add-on therapy with imatinib for 24 weeks compared with placebo, with improved exercise capacity and hemodynamics, but serious adverse events were common.90 Other drugs targeting the BM progenitors are being considered for the treatment of PAH. Further delineating the interaction between the BM abnormalities and the pulmonary vasculopathy would help develop more selective and targeted therapies.
Conclusion and future directions
The current paradigm is that abnormalities in hematopoiesis are innate to PAH (Fig. 2). The findings point toward an imbalanced differentiation in the hematopoietic system in which the subsets that promote vascular health are predominated by increased numbers of pathologic progenitors. Additional studies with larger cohorts are necessary to establish a potential therapeutic benefit of modified proangiogenic hematopoietic cells. Because hematopoiesis is a continuous hierarchic process of proliferation and differentiation, the distinction between detrimental and beneficial lineages will be critical to the development of novel therapeutic approaches. Understanding the molecular mechanisms underlying the hematopoietic anomalies and the pathways via which these cells contribute or benefit PAH are still required; likewise, fibrocytes may have a role in pulmonary hypertension, but findings need to be confirmed in patients. MSCs are promising as immunodulatory cells for a wide variety of human diseases, and early findings in pulmonary hypertension models suggest a potential beneficial role in PAH.
Figure 2.
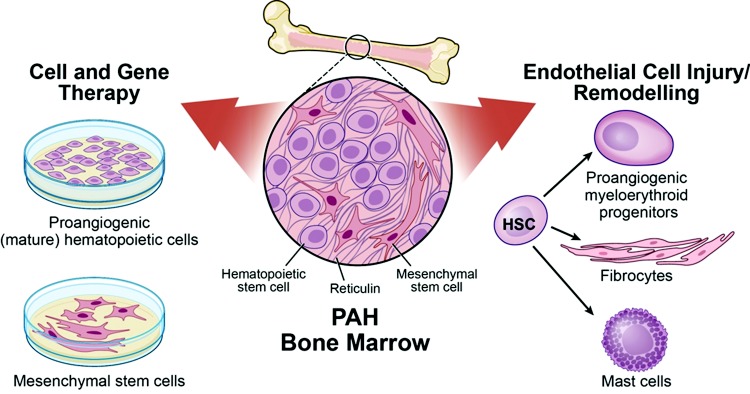
Bone marrow in pulmonary arterial hypertension (PAH). Both the hematopoietic and mesenchymal compartments have been implicated in PAH. Ex vivo expanded monocytic proangiogenic cells, isolated from the peripheral circulation, and mesenchymal stem cells, directly derived from the bone marrow, are used as cell therapy to attenuate the vascular remodeling and/or dampen down the inflammation. These cells have also been used as vehicle for gene delivery. Conversely, increased mobilization of myeloid-erythroid hematopoietic progenitors contributes to the disease by inducing endothelial cell injury and coagulopathy. A subset of the circulating myeloid cells differentiate into fibrocytes that home into the media and adventitia and add to the remodeling by collagen I deposition and by differentiation into myofibroblastic cells. Mast cells also accumulate in the remodeled areas and have been associated with disease progression. HSC: hematopoietic stem cell. Illustration by David Schumick, BS, CMI. Reprinted with the permission of the Cleveland Clinic Center for Medical Art and Photography.
Source of Support: This work is supported by grants HL60917, P01 HL103453, P01HL076491, and M01 RR018390 from the National Institutes of Health, American Thoracic Society/Pulmonary Association Research grant (PH-07-003), and the Hematopoietic Stem Cell Core Facility of the Case Comprehensive Cancer Center (P30 CA43703). KA is a scholar of the International Society for Advancement of Cytometry.
Conflict of Interest: None declared.
References
- 1.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 2011;11:427–435. [DOI] [PMC free article] [PubMed]
- 2.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 2009;4:e7475. [DOI] [PMC free article] [PubMed]
- 3.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736. [DOI] [PubMed]
- 4.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia 2007;21:1141–1149. [DOI] [PubMed]
- 5.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004;104:2752–2760. [DOI] [PubMed]
- 6.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 2001;108:391–397. [DOI] [PMC free article] [PubMed]
- 7.Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res 2000;87:434–439. [DOI] [PubMed]
- 8.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000;97:3422–3427. [DOI] [PMC free article] [PubMed]
- 9.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:1801–1809. [DOI] [PMC free article] [PubMed]
- 10.Rohde E, Malischnik C, Thaler D, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells 2006;24:357–367. [DOI] [PubMed]
- 11.Rohde E, Bartmann C, Schallmoser K, et al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells 2007;25:1746–1752. [DOI] [PubMed]
- 12.Hur J, Yang HM, Yoon CH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation 2007;116:1671–1682. [DOI] [PubMed]
- 13.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008;28:1584–1595. [DOI] [PMC free article] [PubMed]
- 14.Yoder MC, Ingram DA. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim Biophys Acta 2009;1796:50–54. [DOI] [PMC free article] [PubMed]
- 15.Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods 1986;95:273–276. [DOI] [PubMed]
- 16.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 1984;99:2034–2040. [DOI] [PMC free article] [PubMed]
- 17.Holthofer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest 1982;47:60–66. [PubMed]
- 18.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed]
- 19.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 2006;6:835–845. [DOI] [PubMed]
- 20.Dome B, Timar J, Ladanyi A, et al. Circulating endothelial cells, bone marrow-derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: from biology to therapy. Crit Rev Oncol Hematol 2009;69:108–124. [DOI] [PubMed]
- 21.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999;5:434–438. [DOI] [PubMed]
- 22.Ciarrocchi A, Jankovic V, Shaked Y, et al. Id1 restrains p21 expression to control endothelial progenitor cell formation. PLoS ONE 2007;2:e1338. [DOI] [PMC free article] [PubMed]
- 23.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol 2008;45:530–544. [DOI] [PMC free article] [PubMed]
- 24.Seandel M, Butler J, Lyden D, Rafii S. A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization. Cancer Cell 2008;13:181–183. [DOI] [PMC free article] [PubMed]
- 25.Pompilio G, Capogrossi MC, Pesce M, et al. Endothelial progenitor cells and cardiovascular homeostasis: clinical implications. Int J Cardiol 2009;131:156–167. [DOI] [PubMed]
- 26.De Palma M, Mazzieri R, Politi LS, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 2008;14:299–311. [DOI] [PubMed]
- 27.Gothert JR, Gustin SE, van Eekelen JA, et al. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood 2004;104:1769–1777. [DOI] [PubMed]
- 28.Purhonen S, Palm J, Rossi D, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 2008;105:6620–6625. [DOI] [PMC free article] [PubMed]
- 29.Ohle SJ, Anandaiah A, Fabian AJ, Fine A, Kotton DN. Maintenance and repair of the lung endothelium does not involve contributions from marrow-derived endothelial precursor cells. Am J Respir Cell Mol Biol 2012;47:11–19. [DOI] [PMC free article] [PubMed]
- 30.Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost 2009;7:1401–1408. [DOI] [PubMed]
- 31.Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol 2002;22:1567–1572. [DOI] [PubMed]
- 32.Iwakura A, Luedemann C, Shastry S, et al. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 2003;108:3115–3121. [DOI] [PubMed]
- 33.O’Neill TJ IV, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res 2005;97:1027–1035. [DOI] [PubMed]
- 34.Thyberg J. Re-endothelialization via bone marrow-derived progenitor cells: still another target of statins in vascular disease. Arterioscler Thromb Vasc Biol 2002;22:1509–1511. [DOI] [PubMed]
- 35.Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation 2007;115:509–517. [DOI] [PubMed]
- 36.Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl) 2013;91:285–295. [DOI] [PMC free article] [PubMed]
- 37.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 2002;8:403–409. [DOI] [PubMed]
- 38.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation 2002;106:1199–1204. [DOI] [PubMed]
- 39.Imamura H, Ohta T, Tsunetoshi K, et al. Transdifferentiation of bone marrow-derived endothelial progenitor cells into the smooth muscle cell lineage mediated by transforming growth factor-β1. Atherosclerosis 2010;211:114–121. [DOI] [PubMed]
- 40.Kashiwakura Y, Katoh Y, Tamayose K, et al. Isolation of bone marrow stromal cell-derived smooth muscle cells by a human SM22-α promoter: in vitro differentiation of putative smooth muscle progenitor cells of bone marrow. Circulation 2003;107:2078–2081. [DOI] [PubMed]
- 41.Religa P, Bojakowski K, Maksymowicz M, et al. Smooth-muscle progenitor cells of bone marrow origin contribute to the development of neointimal thickenings in rat aortic allografts and injured rat carotid arteries. Transplantation 2002;74:1310–1315. [DOI] [PubMed]
- 42.Zernecke A, Schober A, Bot I, et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res 2005;96:784–791. [DOI] [PubMed]
- 43.Davie NJ, Crossno JT Jr, Frid MG, et al. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L668–L678. [DOI] [PubMed]
- 44.Raoul W, Wagner-Ballon O, Saber G, et al. Effects of bone marrow-derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice. Respir Res 2007;8:8. [DOI] [PMC free article] [PubMed]
- 45.Satoh K, Kagaya Y, Nakano M, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation 2006;113:1442–1450. [DOI] [PubMed]
- 46.Marsboom G, Pokreisz P, Gheysens O, et al. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells 2008;26:1017–1026. [DOI] [PubMed]
- 47.Hayashida K, Fujita J, Miyake Y, et al. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest 2005;127:1793–1798. [DOI] [PubMed]
- 48.Gambaryan N, Perros F, Montani D, et al. Targeting of c-kit+ haematopoietic progenitor cells prevents hypoxic pulmonary hypertension. Eur Respir J 2011;37:1392–1399. [DOI] [PubMed]
- 49.Launay JM, Herve P, Callebert J, et al. Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood 2012;119:1772–1780. [DOI] [PubMed]
- 50.Launay JM, Herve P, Peoc’h K, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 2002;8:1129–1135. [DOI] [PubMed]
- 51.Huxtable R, Ciaramitaro D, Eisenstein D. The effect of a pyrrolizidine alkaloid, monocrotaline, and a pyrrole, dehydroretronecine, on the biochemical functions of the pulmonary endothelium. Mol Pharmacol 1978;14:1189–1203. [PubMed]
- 52.Luan Y, Zhang ZH, Wei DE, Lu Y, Wang YB. Effects of autologous bone marrow mononuclear cells implantation in canine model of pulmonary hypertension. Circ J 2012;76:977–985. [DOI] [PubMed]
- 53.Sun CK, Lin YC, Yuen CM, et al. Enhanced protection against pulmonary hypertension with sildenafil and endothelial progenitor cell in rats. Int J Cardiol 2011;162:45–48. [DOI] [PubMed]
- 54.Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng 2004;10:771–779. [DOI] [PubMed]
- 55.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 2005;96:442–450. [DOI] [PubMed]
- 56.Mirsky R, Jahn S, Koskenvuo JW, et al. Treatment of pulmonary arterial hypertension with circulating angiogenic cells. Am J Physiol Lung Cell Mol Physiol 2011;301:L12–L19. [DOI] [PubMed]
- 57.Yip HK, Chang LT, Sun CK, et al. Autologous transplantation of bone marrow-derived endothelial progenitor cells attenuates monocrotaline-induced pulmonary arterial hypertension in rats. Crit Care Med 2008;36:873–880. [DOI] [PubMed]
- 58.Sun CK, Lee FY, Sheu JJ, et al. Early combined treatment with cilostazol and bone marrow-derived endothelial progenitor cells markedly attenuates pulmonary arterial hypertension in rats. J Pharmacol Exp Ther 2009;330:718–726. [DOI] [PubMed]
- 59.Asosingh K, Aldred MA, Vasanji A, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 2008;172:615–627. [DOI] [PMC free article] [PubMed]
- 60.Smadja DM, Gaussem P, Mauge L, et al. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation 2009;119:374–381. [DOI] [PubMed]
- 61.Toshner M, Voswinckel R, Southwood M, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 2009;180:780–787. [DOI] [PMC free article] [PubMed]
- 62.Montani D, Perros F, Gambaryan N, et al. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2011;184:116–123. [DOI] [PubMed]
- 63.Farha S, Asosingh K, Xu W, et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities. Blood 2011;117:3485–3493. [DOI] [PMC free article] [PubMed]
- 64.Diller GP, van Eijl S, Okonko DO, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 2008;117:3020–3030. [DOI] [PubMed]
- 65.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med 2008;102:1073–1079. [DOI] [PubMed]
- 66.Smadja DM, Mauge L, Sanchez O, et al. Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J 2010;36:1284–1293. [DOI] [PubMed]
- 67.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 2007;49:1566–1571. [DOI] [PubMed]
- 68.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant 2008;12:650–655. [DOI] [PubMed]
- 69.Majka SM, Skokan M, Wheeler L, et al. Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2008;295:L1028–L1039. [DOI] [PMC free article] [PubMed]
- 70.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Brit J Haematol 2007;139:351–362. [DOI] [PubMed]
- 71.Asosingh K, Farha S, Lichtin A, et al. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension. Blood 2012;120:1218–1227. [DOI] [PMC free article] [PubMed]
- 72.Cheadle C, Berger AE, Mathai SC, et al. Erythroid-specific transcriptional changes in PBMCs from pulmonary hypertension patients. PLoS ONE 2012;7:e34951. [DOI] [PMC free article] [PubMed]
- 73.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol 2011;31:475–529. [DOI] [PMC free article] [PubMed]
- 74.Dahal BK, Kosanovic D, Kaulen C, et al. Involvement of mast cells in monocrotaline-induced pulmonary hypertension in rats. Respir Res 2011;12:60. [DOI] [PMC free article] [PubMed]
- 75.Bartelds B, van Loon RL, Mohaupt S, et al. Mast cell inhibition improves pulmonary vascular remodeling in pulmonary hypertension. Chest 2012;141:651–660. [DOI] [PubMed]
- 76.Savai R, Pullamsetti SS, Kolbe J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186:897–908. [DOI] [PubMed]
- 77.Farha S, Sharp J, Asosingh K, et al. Mast cell number, phenotype, and function in human pulmonary arterial hypertension. Pulm Circ 2012;2:220–228. [DOI] [PMC free article] [PubMed]
- 78.Frid MG, Brunetti JA, Burke DL, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 2006;168:659–669. [DOI] [PMC free article] [PubMed]
- 79.Nikam VS, Schermuly RT, Dumitrascu R, et al. Treprostinil inhibits the recruitment of bone marrow-derived circulating fibrocytes in chronic hypoxic pulmonary hypertension. Eur Respir J 2010;36:1302–1314. [DOI] [PubMed]
- 80.Baber SR, Deng W, Master RG, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol 2007;292:H1120–H1128. [DOI] [PubMed]
- 81.Patel KM, Crisostomo P, Lahm T, et al. Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction by a paracrine mechanism. J Surg Res 2007;143:281–285. [DOI] [PubMed]
- 82.Umar S, de Visser YP, Steendijk P, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009;297:H1606–H1616. [DOI] [PubMed]
- 83.Luan Y, Zhang ZH, Wei DE, et al. Implantation of mesenchymal stem cells improves right ventricular impairments caused by experimental pulmonary hypertension. Am J Med Sci 2012;343:402–406. [DOI] [PubMed]
- 84.Jiang L, Song XH, Liu P, et al. Platelet-mediated mesenchymal stem cells homing to the lung reduces monocrotaline-induced rat pulmonary hypertension. Cell Transplant 2012;21:1463–1475. [DOI] [PubMed]
- 85.Takemiya K, Kai H, Yasukawa H, Tahara N, Kato S, Imaizumi T. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol 2010;105:409–417. [DOI] [PubMed]
- 86.Kanki-Horimoto S, Horimoto H, Mieno S, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation 2006;114:I181–I185. [DOI] [PubMed]
- 87.Liang OD, Mitsialis SA, Chang MS, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 2011;29:99–107. [DOI] [PMC free article] [PubMed]
- 88.Rochefort GY, Vaudin P, Bonnet N, et al. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies? Respir Res 2005;6:125. [DOI] [PMC free article] [PubMed]
- 89.Ghofrani HA, Morrell NW, Hoeper MM, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 2010;182:1171–1177. [DOI] [PMC free article] [PubMed]
- 90.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 2013;127:1128–1138. [DOI] [PubMed]
- 91.Zimmerlin L, Donnenberg VS, Donnenberg AD. Rare event detection and analysis in flow cytometry: bone marrow mesenchymal stem cells, breast cancer stem/progenitor cells in malignant effusions, and pericytes in disaggregated adipose tissue. Methods Mol Biol 2011;699:251–273. [DOI] [PubMed]
- 92.Balber AE. Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells 2011;29:570–575. [DOI] [PubMed]
- 93.Capoccia BJ, Robson DL, Levac KD, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood 2009;113:5340–5351. [DOI] [PMC free article] [PubMed]
- 94.Putman DM, Liu KY, Broughton HC, Bell GI, Hess DA. Umbilical cord blood-derived aldehyde dehydrogenase-expressing progenitor cells promote recovery from acute ischemic injury. Stem Cells 2012;30:2248–2260. [DOI] [PubMed]
- 95.Povsic TJ, Zavodni KL, Kelly FL, et al. Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol 2007;50:2243–2248. [DOI] [PubMed]