Abstract
Purpose
To increase childhood influenza vaccination rates using a toolkit and early vaccine delivery in a randomized cluster trial.
Methods
Twenty primary care practices treating children (range for n=536-8,183) were randomly assigned to Intervention and Control arms to test the effectiveness of an evidence-based practice improvement toolkit (4 Pillars Toolkit) and early vaccine supplies for use among disadvantaged children on influenza vaccination rates among children 6 months-18 years. Follow-up staff meetings and surveys were used to assess use and acceptability of the intervention strategies in the Intervention arm. Rates for the 2010-2011 and 2011-2012 influenza seasons were compared. Two-level generalized linear mixed modeling was used to evaluate outcomes.
Results
Overall increases in influenza vaccination rates were significantly greater in the Intervention arm (7.9 percentage points) compared with the Control arm (4.4 percentage points; P<0.034). These rate changes represent 4522 additional doses in the Intervention arm vs. 1,390 additional doses in the Control arm. This effect of the intervention was observed despite the fact that rates increased significantly in both arms - 8/10 Intervention (P<0.001) and 7/10 Control sites (P-values 0.04 to <0.001). Rates in two Intervention sites with pre-intervention vaccination rates >58% did not significantly increase. In regression analyses, a child's likelihood of being vaccinated was significantly higher with: younger age, white race (Odds ratio [OR]=1.29; 95% confidence interval [CI]=1.23-1.34), having commercial insurance (OR=1.30; 95%CI=1.25-1.35), higher pre-intervention practice vaccination rate (OR=1.25; 95%CI=1.16-1.34), and being in the Intervention arm (OR=1.23; 95%CI=1.01-1.50). Early delivery of influenza vaccine was rated by Intervention practices as an effective strategy for raising rates.
Conclusions
Implementation of a multi-strategy toolkit and early vaccine supplies can significantly improve influenza vaccination rates among children in primary care practices but the effect may be less pronounced in practices with moderate to high existing vaccination rates.
Keywords: Influenza vaccine, immunization, children, childhood influenza vaccination
Introduction
Despite the 2008 Advisory Committee on Immunization Practices recommendation that all children over the age of 6 months receive an annual influenza vaccine [1], national vaccination uptake in the United States remains substantially below desired levels of 70% [2], averaging 51.5% An array of [3]. An array of evidence-based interventions to improve childhood influenza vaccine uptake exists [4-7]. While significant gains have been reported, no single intervention has raised rates sufficiently; rather, the evidence suggests the need for a combination of strategies. The Community Preventive Services Task Force (Task Force) [8] recommended using two or more of three strategic approaches in preference to using several techniques within a single strategic approach. They are: 1) enhancing access to vaccination services; 2) increasing demand among patients; and 3) provider- and system-based interventions such as reminders, modified office flow, standing order programs (SOPs) and electronic immunization tracking.
Based on Task Force recommendations [8] and previous research in adult primary care practices [9], we modified an adult immunization toolkit to create the 4 Pillars Toolkit for Increasing Childhood Influenza Immunization (4 Pillars Toolkit) in primary care practices serving children. A practice-based, cluster randomized trial was conducted using the 4 Pillars Toolkit and early delivery of vaccine supplies for Vaccines for Children (VFC)-eligible children. This report describes: 1) the intervention that included the 4 Pillars Toolkit; 2) resultant changes in influenza vaccination rates; 3) the individual and practice level characteristics that affected influenza vaccination from two-level generalized linear mixed modeling; and 4) recommendations for policy and practice.
Methods
This trial took place during the 2011-2012 influenza season and was approved by the University of Pittsburgh Institutional Review Board.
Sample Size and Sites
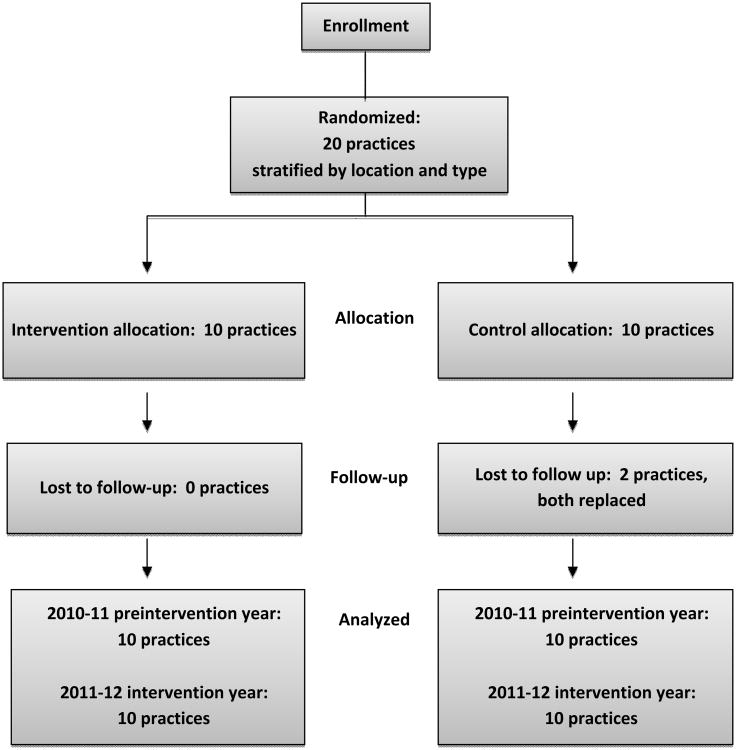
Optimal Design software (University of Michigan, Version 1.77. 2006) was used to calculate sample size, for a randomized trial seeking a 10-15% absolute increase in vaccination rate, and a minimum practice size of 100-200 pediatric patients. A sample size of 20 clusters (10 Intervention and 10 Control practices) was determined necessary to achieve 80% power with an alpha of 0.05. Primary care pediatric and family medicine practices from two practice-based research networks (http://www.pedspittnet.pitt.edu/; http://www.familymedicine.pitt.edu/content.asp?id=2353) and one clinical network in Southwestern Pennsylvania were solicited for participation. When 20 sites agreed to participate, solicitation ceased. All sites were part of the UPMC Health System and used a common electronic medical record (EMR), EpicCare, with the exception of one practice with two offices that used a different EMR system (Allscripts Professional).
Cluster Randomization
Cluster randomization allocates clinical practices rather than individuals to the intervention arms [10]; hence, each practice or office was considered as a cluster. To be eligible, the office must have had a patient population of at least 200 children ages 6 months through 18 years, access to vaccination data via an EMR and willingness to make office changes to increase influenza vaccination rates. Participating practices were stratified by location – inner city (urban practices with primarily disadvantaged children), urban, suburban and rural and by discipline (pediatrics vs. family medicine). The practices were than randomized into the Intervention or Control arms within strata with the two offices of one rural practice assigned one to each arm. Practices randomized to the Control arm were informed that their intervention would take place the following season and were not contacted again until the end of the influenza season.
Interventions
The intervention was designed using Diffusion of Innovations theory [11], and included the 4 Pillars Toolkit, provider education, and vaccine supply interventions which are described in Table 1. One of the investigators (MPN) visited each Intervention site before the beginning of the influenza season, and following a standard procedure, introduced the study and the package of interventions at a staff meeting and worked with staff to develop practice-specific ideas for implementing the toolkit. Each Intervention practice received ≤200 doses of donated vaccine for Vaccines for Children (VFC) eligible children until practices received their VFC supplies allowing sites to vaccinate disadvantaged children as early as commercially insured whose supplies typically become available sooner. The intervention was conducted from September 2011 through March 2012.
Table 1. Intervention strategies used to increase childhood influenza vaccination rates and post intervention effectiveness ratings from Intervention sites.
| Strategy | Number of sites using strategy | Effectiveness score* Range = 0-100 |
Debrief session score** Range = 0-100 |
|
|---|---|---|---|---|
| 4 Pillars Toolkit | ||||
|
| ||||
| Pillar 1 | Convenient vaccination services | |||
|
| ||||
| Convenient influenza vaccination | 10 | 73.8 | 46.2 | |
| Description: Practices were encouraged to reduce access barriers by offering convenient influenza vaccination services such as after-hours vaccine clinics, walk-in vaccination, dedicated vaccination stations, designated vaccination only hours and vaccination offered at any non-febrile illness visit. | ||||
|
| ||||
| Pillar 2 | Notification of parents/patients about the importance of influenza vaccination and the availability of vaccine | |||
|
| ||||
| Office posters | 10 | 76.7 | 100 | |
| Description: The research team downloaded influenza vaccination posters from the CDC website and provided at least enough to post in each practice's exam rooms. Offices were encouraged to create their own posters and fliers to reminder patient, parents and providers. | ||||
|
| ||||
| Patient reminders | 5 | 20.6 | Not rated | |
| Description: Practices were strongly encouraged to notify all parents/patients of the availability of influenza vaccine, date and time of any influenza vaccination clinics, and physician recommendations to be vaccinated. Practices could use any appropriate means, e.g., autodialed phone calls, emails, text messages, “on-hold” messages, fliers, social media, verbal reminders at check-in, etc. | ||||
|
| ||||
| Autodialed phone messages | 9 | 70.0 | 72.6 | |
| Description: The research team worked with the practices to send one or more autodialed message in September, October and December to the entire patient population or a subset (e.g., those still not vaccinated by a certain date) of each practice. | ||||
|
| ||||
| Pillar 3 | Enhanced office systems to facilitate influenza immunization | |||
|
| ||||
| Physician prompts | 10 | 90.7 | 69.3 | |
| Description: The electronic medical record (EMR) was programmed to generate a physician prompt called a best practice alert (BPA) which would appear on the computer screen whenever an unvaccinated child was being seen. | ||||
| Vaccination as part of vital signs(Immunizations given as part of vital signs) | 6 | 31.5 | 39.6 | |
| Description: Practices were to make influenza vaccination routine by having clinical support staff assess immunization status as part of the process of rooming patients and recording vital signs, and by incorporating SOPs into the practice. | ||||
|
| ||||
| Standing Order Protocols (SOPs) | 7 | 58.6 | 29.7 | |
| Description: Staff would inform the parent, obtain consent, give the Vaccine Information Sheet and prepare the vaccine, and when feasible, vaccinate eligible children without the need for a specific physician's order. | ||||
|
| ||||
| Pillar 4 | Motivation through an office immunization champion | |||
|
| ||||
| Immunization champion (IC) | 10 | 67.7 | Not rated | |
| Description: The IC in each practice was an enthusiastic motivator who used her or his time and energy to provide feedback, encourage competition and energize the staff to keep up the efforts throughout the season. The IC shared weekly graphs sent by the research team (see descriptions below under provider and community interventions)depicting the number of vaccines given and missed opportunities to vaccinate and used them as a basis to discuss ways to improve or sustain efforts. | ||||
|
| ||||
| Provider and community interventions | ||||
|
| ||||
| Pre-intervention visits (Staff/Provider education in-service on toolkit) | 10 | 86.6 | 100 | |
| Description: Investigators visited intervention practice to introduce the study and toolkit at a staff meeting and brainstorm ideas for implementing the 4 pillars in practical and meaningful ways for each practice. | ||||
|
| ||||
| Mid-season refresher (Staff/Provider education online) | 9 | 45.0 | Not rated | |
| Description: Staff was offered the opportunity to view a short online slide presentation mid-influenza season, and answer a short survey for which they received participant payment. | ||||
|
| ||||
| Weekly reports (Feedback from investigators) | 10 | 84.2 | 66.0 | |
| Description: Based on the previous year's total number of influenza vaccines given, each practice was given an initial goal of 25% increase over the previous year. This goal was divided into 16 weeks and graphed. Weekly counts of vaccines given were plotted on line graphs comparing actual values with the goal and were emailed to the Immunization Champion (IC) from September through mid-December. | ||||
|
| ||||
| Weekly reports (Comparison of progress with other practices) | 10 | 72.9 | 66.0 | |
| Description: ICs also received a bar graph showing their practices' progress compared with the other intervention sites. | ||||
|
| ||||
| Videos | -- | Not rated | 59.4 | |
| Description: The investigators developed two videos based on focus group findings to encourage teens and younger children to be vaccinated. The teen video was advertised in practices using fliers with a headline to catch teens' attention and a QR code for direct linking to smart phones. A second video was a 30 second public service announcement, produced in collaboration with the county health department and a local television station, and featured a local child celebrity. It played on intervention practices' waiting room electronic message boards and aired 280 times on TV from early September through March 2012. | ||||
| Community outreach | -- | Not rated | Not rated | |
| Description: The research team conducted community outreach, primarily in disadvantaged communities, to reach groups with traditionally low vaccination rates, visiting places of worship, community centers and social service agencies, distributing fliers and talking with people gathered there. | ||||
|
| ||||
| Vaccine supply/policy interventions | ||||
|
| ||||
| Early delivery of Vaccines for Children (VFC) and donated influenza vaccine | 10 | 94.2 | 100 | |
| Description: Selective early delivery of VFC influenza vaccine to Intervention practices and the delivery of 50-750 doses (distributed proportionally to the size of the practice) of donated influenza vaccine for administration to VFC children. Practices were encouraged to extend the vaccination season by vaccinating as soon as supplies arrived until the end of February. | ||||
|
| ||||
| Borrowing of commercial vaccine for VFC patients | 8 | 40.9 | 39.6 | |
| Description: Investigators received permission for practices to borrow commercial supplies of vaccine to administer to VFC patients until VFC supplies arrived. | ||||
Note: NA= not asked.
Effectiveness score: Average rating by Intervention arm sites for effectiveness of strategy for raising influenza vaccination rates, effectiveness range= 1-100 with 0=did not use.
Debrief session score: Overall assessment of Intervention arm sites' staff on techniques. 0=did not use; 1=not effective; 2=moderately effective; 3=very effective. Average score from all practices then multiplied by 33 to adjust to 0=100 range.
Toolkit
The 4 Pillars Toolkit was based on four evidence-based [8, 12] key strategies: Pillar 1 – Convenient vaccination services; Pillar 2 - Notification of patients about the importance of immunization and the availability of vaccines; Pillar 3 - Enhanced office systems to facilitate immunization; Pillar 4 - Motivation through an office immunization champion. Table 1 describes the strategies used in more detail. The 4 Pillars Toolkit includes background on the importance of protecting children against influenza, barriers to increasing influenza vaccination from both provider and parent/patient perspectives and strategies to eliminate those barriers. Practices were expected to implement strategies from each of the 4 pillars.
Data collection
At the end of the influenza season, all Intervention sites were revisited by an investigator who used a discussion guide to get feedback from the staff on which strategies they used and how effective they believed them to be, in order to assess fidelity of the intervention [13]. Notes were summarized and coded into a 4-point scale (0=did not use, 1=not effective, 2= somewhat effective, 3=very effective). In addition, two individuals from each intervention site (head nurse or office manager and lead physician) scored the effectiveness of each study-specific strategy on a scale of 1-100, assigning a zero if their practice did not use the strategy. The scores for each question were averaged across both respondents for each practice. Sites also reported approximate date of receipt of VFC vaccines; months were converted into their corresponding numbers (i.e., September = 9) with the first half of the month (if given) assigned a 0.0 and dates in the second half of the month assigned a 0.5 and dates were averaged for each arm.
De-identified demographic, office visit and influenza vaccination data were derived from EMR data extractions performed by the UPMC Center for Assistance in Research using the eRecord and from a similar data extraction from the EMR by staff of the non-UPMC sites following the 2011-2012 influenza season.
Statistical analyses
Descriptive analyses were performed for patient demographic characteristics (age, sex, race, and health insurance). Chi-square tests were used to examine whether children's characteristics differed between the Intervention and Control arms. Site-specific influenza vaccination rates were calculated for the pre-intervention and intervention years. The denominator was defined as the number of children who had been seen at least once (indicates being an active patient) during 3/1/2010 – 2/28/2011 for the pre-intervention year and 3/1/2011 – 2/29/2012 for the invention year. The numerator was defined as the number of children who had received at least one dose of influenza vaccine during each influenza season (8/1/2010 – 2/28/2011 for the pre-intervention year and 8/1/2011 – 2/29/2012 for the intervention year). Chi-square tests were used to compare vaccination rates in each arm and for each year. Number of doses given was the count of all doses of influenza vaccine given to eligible children between 8/1/2011 and 2/29/2012.
To determine which factors were related to childhood influenza vaccination rates while accounting for the clustered nature of the data, two-level generalized linear modeling was conducted using influenza vaccination status as a binary outcome variable using SAS® 9.3. Patient level variables that were significantly different across arms (age, race, and health insurance) were included in regression analyses. Initially, the practice level independent variables were pre-intervention vaccination rate, intervention arm, number of strategies used to increase vaccination and effectiveness score for individual strategies. Strategies selected for regression analyses were those only available to the Intervention arm (e.g. early delivery of vaccine); Control sites for those strategies were assigned scores of zero. Correlations among all strategy effectiveness scores were tested using correlation coefficients. All independent variables were tested to determine co-linearity removing those with a variance inflation factor (VIF) >10 [14, 15]. A random intercept model with variance components covariance structure was chosen as the final model based on the lowest value of Akaike information criterion. Statistical significance of two-sided tests was set at type I error (alpha) equal to 0.05.
Results
Demographics
Each arm contained two family medicine and 8 pediatric practices, 1 rural and 2 urban practices, but differed in the number of inner city and suburban practices (Table 2). During the pre-intervention year, the Intervention and Control arms did not differ by percent female patients, but Intervention practices overall had a greater proportion of non-white, commercially insured, and younger children than Control practices (P<0.001). The number of eligible children ranged from 536 to 8,183.
Table 2. Demographic characteristics of practices and patients during the pre-intervention season (2010-2011).
| Site | N of children |
Type of practice* |
Location | Race | Insurance | Female (%) |
Age Mean (SD) |
||
|---|---|---|---|---|---|---|---|---|---|
| White (%) |
Non-white (%) |
Public/ Self-pay/ Uninsured/(%) |
Commercial (%) |
||||||
| Intervention sites | |||||||||
|
| |||||||||
| 1 | 536 | FM | Suburban | 86.0 | 14.0 | 22.6 | 77.4 | 51.1 | 11.4 (5.1) |
| 2 | 1,670 | FM | Urban | 14.8 | 85.2 | 68.1 | 31.9 | 52.3 | 8.8 (5.8) |
| 3 | 1,083 | Peds | Inner city | 39.2 | 60.8 | 79.3 | 20.7 | 48.9 | 7.1 (5.1) |
| 4 | 4,317 | Peds | Inner city | 16.4 | 83.6 | 80.5 | 19.5 | 49.3 | 6.0 (4.7) |
| 5 | 6,780 | Peds | Rural | 94.0 | 6.0 | 33.1 | 66.9 | 49.6 | 8.3 (5.3) |
| 6 | 4,424 | Peds | Suburban | 93.1 | 6.9 | 30.6 | 69.4 | 50.1 | 6.4 (4.4) |
| 7 | 4,541 | Peds | Suburban | 88.7 | 11.3 | 31.0 | 69.0 | 48.4 | 7.4 (4.9) |
| 8 | 8,183 | Peds | Suburban | 93.1 | 6.9 | 23.8 | 76.2 | 48.6 | 8.2 (5.3) |
| 9 | 7,040 | Peds | Urban | 71.0 | 29.0 | 22.4 | 77.6 | 49.0 | 7.9 (5.3) |
| 10 | 4,719 | Peds | Suburban | 94.0 | 6.0 | 12.4 | 87.6 | 49.8 | 7.3 (4.8) |
|
| |||||||||
| Control sites | |||||||||
|
| |||||||||
| 11 | 1,276 | FM | Inner city | 38.6 | 61.4 | 87.8 | 12.2 | 55.1 | 9.4 (6.1) |
| 12 | 3,107 | Peds | Suburban | 73.1 | 26.9 | 72.9 | 27.1 | 48.9 | 8.7 (5.3) |
| 13 | 5,810 | Peds | Suburban | 72.7 | 27.3 | 65.7 | 34.3 | 49.3 | 8.8 (5.5) |
| 14 | 549 | FM | Suburban | 94.0 | 6.0 | 27.0 | 73.0 | 50.8 | 11.0 (5.6) |
| 15 | 2,702 | Peds | Rural | 95.5 | 4.5 | 22.2 | 77.8 | 47.0 | 7.9 (5.2) |
| 16 | 5,653 | Peds | Urban | 63.8 | 36.2 | 35.8 | 64.2 | 47.6 | 7.5 (4.9) |
| 17 | 6,264 | Peds | Suburban | 86.6 | 13.4 | 16.2 | 83.8 | 48.9 | 8.3 (5.1) |
| 18 | 4,876 | Peds | Suburban | 93.4 | 6.6 | 12.4 | 87.6 | 49.3 | 8.6 (5.1) |
| 19 | 3,234 | Peds | Suburban | 91.2 | 8.8 | 9.2 | 90.8 | 48.2 | 8.6 (5.0) |
| 20 | 4,835 | Peds | Urban | 68.5 | 31.5 | 29.4 | 70.6 | 48.4 | 7.1 (5.2) |
|
| |||||||||
| Control sites, overall N=38,306 | 77.5 | 22.5 † | 35.6 | 64.4 † | 48.8 | 8.2 (5.2) ‡ | |||
| Intervention sites, overall N=43,293 | 77.2 | 22.8 | 34.0 | 66.0 | 49.3 | 7.6 (5.2) | |||
Type: FM=Family Medicine practice; Peds=Pediatric practice.
SD=Standard Deviation
P<.001 for difference between Intervention and Control arms by Chi square test.
P<.001 for difference between Intervention and Control arms by Wilcoxon test due to non-normal distribution of ages.
Vaccination
Overall pre-intervention influenza vaccination rates were similar in the Intervention (46.0%) and the Control arms (45.7%; P=.373, Table 3). In the intervention season, the rate in the Intervention arm (53.8%) was significantly greater than that for the Control arm (50.1%; P<0.001), with an average pre-intervention to intervention change in vaccination rate of 7.9 percentage points (PP) for the Intervention arm and 4.4 PP for the Control arm (P=0.034). Influenza vaccination rates increased significantly in eight of ten Intervention practices (P<0.001) with absolute differences ranging from 0.6 PP to 21.5 PP, and in seven of ten Control sites (P values=0.04 to <0.001) with differences ranging from -3.2 PP to 9.4 PP. The two Intervention practices that did not significantly increase their vaccination rates were those with pre-intervention rates >58%. Omitting the practices with pre-intervention rates >58% resulted in an average pre-intervention to intervention change in rates of 12.1 PP in the Intervention arm and 4.6 PP in the Control arm (P=.005 for the difference).
Table 3. Influenza Vaccination Rates in Intervention and Control Sites for the Pre-intervention (2010-11) and Intervention (2011-12) Seasons.
| Site | Children (n) | Preintervention season (2010-11) % | Children (n) | Intervention season (2011-12) % | Absolute difference (percentage point) | P value* |
|---|---|---|---|---|---|---|
| Intervention sites | ||||||
|
| ||||||
| 1 | 536 | 14.0 | 712 | 23.0 | 9.0 | <0.001 |
| 2 | 1,670 | 21.0 | 1,661 | 37.5 | 16.5 | <0.001 |
| 3 | 1,083 | 26.1 | 2,123 | 40.6 | 14.5 | <0.001 |
| 4 | 4,317 | 35.5 | 7,925 | 57.0 | 21.5 | <0.001 |
| 5 | 6,780 | 39.0 | 6,743 | 48.0 | 9.0 | <0.001 |
| 6 | 4,424 | 39.4 | 4,821 | 50.8 | 11.4 | <0.001 |
| 7 | 4,541 | 45.4 | 4,748 | 54.7 | 9.3 | <0.001 |
| 8 | 8,183 | 50.3 | 8,376 | 56.2 | 5.8 | <0.001 |
| 9 | 7,040 | 58.2 | 6,942 | 58.8 | 0.6 | 0.49 |
| 10 | 4,719 | 63.6 | 4,988 | 64.5 | 0.9 | 0.37 |
| Overall | 43,293 | 46.0 | 49,039 | 53.9 | 7.9† | <0.001 |
|
| ||||||
| Control sites | ||||||
|
| ||||||
| 11 | 1,276 | 14.7 | 1328 | 24.2 | 9.4 | <0.001 |
| 12 | 3,107 | 31.3 | 2,864 | 40.7 | 9.4 | <0.001 |
| 13 | 5,810 | 31.8 | 5,656 | 36.4 | 4.6 | <0.001 |
| 14 | 549 | 32.2 | 578 | 29.1 | -3.2 | 0.25 |
| 15 | 2,702 | 42.8 | 2,841 | 51.4 | 8.6 | <0.001 |
| 16 | 5,653 | 43.7 | 5,559 | 49.1 | 5.4 | <0.001 |
| 17 | 6,264 | 52.2 | 6,457 | 56.1 | 3.9 | <0.001 |
| 18 | 4,876 | 53.4 | 4,942 | 55.4 | 2.0 | 0.04 |
| 19 | 3,234 | 54.6 | 3,358 | 55.8 | 1.2 | 0.32 |
| 20 | 4,835 | 62.9 | 5,043 | 63.3 | 0.4 | 0.65 |
| Overall | 38,306 | 45.7 | 38,626 | 50.1 | 4.4† | <0.001 |
For difference in vaccination rates between pre-intervention and intervention seasons.
Difference between Intervention and Control arms P<0.034.
Among all Intervention sites 4,522 more doses were given in the intervention year over the previous year for a total of 29,863 doses, whereas among all Control sites in the same season, total doses increased by 1,390 to 22,088. On average, Intervention practices received VFC supplies approximately 1 month earlier (mid-August) than Control practices (mid-September). Some Control sites received VFC influenza vaccine as late as October and November.
Intervention
The average effectiveness scores from the surveys and the debrief sessions for the intervention strategies and the number of Intervention sites using them are shown in Table 1. The strategies rated as most effective by practice leadership were early delivery of influenza vaccines donated by a vaccine manufacturer that could be used for VFC children (94.2); electronic physician prompts (90.7); pre-intervention in-service visits (86.6); weekly feedback on rates from the investigators to the immunization champion (84.2); posters (76.7) and express vaccination services (73.8). These results were generally similar to the ratings given by the staff at the follow-up meetings in which 7 practices reported using physician prompts and express vaccine clinics and 10 practices reported using early delivery of vaccine, provider in-service meetings and posters.
Using effectiveness scores, regression analyses were conducted to examine which of the intervention strategies influenced likelihood of vaccination among children in the Intervention practices. Out of 14 strategies, six had a significant impact on likelihood of vaccination. They were preseason in-service meetings (OR=1.03; 95% CI=1.00-1.05; P=0.038); early delivery of influenza vaccine (OR=1.03; 95%CI=1.00-1.05; P=0. 021); borrowing commercial vaccine for VFC children (OR=1.05; 95%CI=1.02-1.08; P=.002); feedback on immunization rates from the research team to the immunization champion (OR=1.03; 95%CI=1.01-1.06; P=0.010); comparisons of the practices' progress to one another (OR=1.04; 95%CI=1.01-1.06; P=.006); and feedback on immunization rates from the immunization champion to the staff (OR=1.05; 95%CI=1.02-1.07; P<.001). These ORs indicate that for every 10 point increase in a strategy's effectiveness score, the odds of vaccination increased by 3%-5%. Co-linearity among these strategies precluded their inclusion in further regression analyses.
In final regression analyses, (Table 4) younger children, white children (OR=1.29; 95%CI=1.23-1.34) and commercially insured (OR=1.30; 95%CI=1.25-1.35) children were more likely to be vaccinated than their older (OR=0.91; 95%CI=0.90-0.91), non-white and publicly insured counterparts. Furthermore, children in practices with higher pre-intervention vaccination rates (OR=1.25; 95%CI=1.16-1.34) and those in Intervention practices (OR=1.23; 95%CI=1.23-1.50) were significantly more likely to be vaccinated; the latter finding indicates the positive effect of the intervention while controlling for baseline rate.
Table 4. Patient and practice level variables related to vaccination status in two-level generalized linear mixed modeling.
| Variable | Odds Ratio (95% CI) | P value | ||||
|---|---|---|---|---|---|---|
| Patient level variables | ||||||
|
| ||||||
| Age | 0.91 (0.90-0.91) | <0.001 | ||||
| White race (ref. = non-white) | 1.29 (1.23-1.34) | <0.001 | ||||
| Commercial health insurance (ref. = public/self-pay/uninsured) | 1.30 (1.25-1.35) | <0.001 | ||||
|
| ||||||
| Practice level variables | ||||||
|
| ||||||
| Pre-intervention vaccination rate (unit=10% increase) | 1.25 (1.16-1.34) | <0.001 | ||||
| Intervention (ref. = Control) | 1.23 (1.01-1.50) | <0.05 | ||||
Discussion
This study employed provider and patient education, early access to vaccine for low income children and an immunization practice improvement toolkit to raise childhood influenza vaccination rates in pediatric and family medicine practices. These interventions were presented to practices as a package which could be adapted to fit the structure and culture of individual sites. Both Intervention and Control arms significantly increased vaccination rates overall; however the absolute change in rate in the Intervention arm was significantly higher. The observed change in rate in the Control arm may be due to community interventions, secular increases in national rates, or simply because the practices had agreed to participate in the study [16]. The intervention was effective despite the larger practice sizes and the increase in patients in the Intervention sites (Table 3), both of which can inhibit practice change. The final vaccination rate in the Intervention arm (53.8%) is somewhat higher than previous studies among high risk children which reported post intervention rates centering around 30% but reaching as high as 62% [4, 5, 7, 17-23]. Studies of all children 6 months to 18 years of age or healthy infants only, are fewer in number, but reported changes in rates among infants ranged from 20 PP to 34 PP [7, 19, 24], with one intervention study reporting an overall intervention rate of 44% [6].
Practices with pre-intervention vaccination rates above 58% did not significantly improve rates as a result of the intervention, indicating a possible threshold effect. We speculate that practices with a high pre-intervention rate viewed themselves as already doing all that was feasible to vaccinate against influenza. Few studies have reported overall vaccination rates above 50%, with one observational study [25] reporting a maximum of 60% among 118 pediatric and family practices across the country. Thus, it may be difficult to achieve the 70% national goal by relying solely on primary care practices to vaccinate. Perhaps expansion to other venues such as school-based influenza vaccination clinics [26] or for admission to child care [27] are the best means to reach children who are not receiving influenza vaccine from their doctors.
The effect of age on vaccination rates seems to be consistent across studies including the present study, with younger children more likely to be vaccinated than older children [3, 25, 28]. Medicaid-insured children have been reported as more likely to be vaccinated than privately insured or uninsured children at community health centers [29], but were less likely to be vaccinated in the present study, which included some community health centers and may be due to later delivery of VFC vaccines to Control sites. Differences in influenza vaccination rates across racial groups vary, with no differences reported between black and Latino low income children [30], higher rates among Asian and Hispanic children than among white children in community health centers [29], higher rates among white children than black children in inner-city practices [19, 31] and in this study. These differences may be attributed to the demographic differences of the source population and the types of health centers studied.
In this study, vaccination was encouraged as soon as vaccine arrived and continued past December when influenza vaccination typically tapers off. Intervention practices received donated influenza vaccine supplies to be used for non-insured and VFC children, received preferential early delivery of VFC influenza vaccine through arrangements made with the Pennsylvania Department of Health and also were given permission to borrow commercial supplies to vaccinate VFC children if needed for adequate supply. One barrier that may prevent practices from vaccinating as many children as possible is the typical delay in delivery of VFC influenza vaccine supplies relative to commercial supplies [32]. VFC-eligible children who visit the practice before supplies arrive often leave unvaccinated and may not return later in the season to be vaccinated. Although the difference in delivery dates is decreasing, studies have reported that VFC vaccines arrive 2-4 weeks later than commercial supplies, which results in lower two dose compliance rates [32, 33]. Intervention practices rated early delivery of vaccine supplies as the most effective strategy available to them during the intervention. The timing of the distribution of VFC influenza vaccine to providers is determined by individual state immunization programs based on their receipt of vaccine from federal depots and their program priorities. Hence, early distribution of all VFC vaccine is not always possible; however, early distribution of a portion of VFC vaccine early in the season has the potential to increase vaccination rates among VFC-eligible children.
Motivational efforts by the immunization champion were also rated as effective by Intervention practices. With a long vaccination season (up to six months), the efforts of the immunization champion to motivate the office staff are an important element of a successful vaccination program. Recent studies have not reported on a pre-influenza season staff educational session, motivation, or an immunization champion as essential parts of an influenza vaccination improvement package, but they are relatively low cost and easy strategies to implement [12, 34]. Influenza vaccination of children is cost-saving in the US [35], provided that vaccine costs <$20-25 [36]. Furthermore, a variety of quality improvement recommendations are specifying that primary care practices increase immunization rates [37], and in some cases are being financially rewarded for improvements. These benefits should outweigh the potential cost of educational programs and monitoring rates.
These findings suggest that efforts to improve influenza vaccination by practices should include: offering vaccine as early as possible, assigning an immunization champion, educating the staff about vaccination procedures, and providing regular feedback to providers and staff about the practice's vaccination rates and progress towards its goals. The ability to improve childhood influenza vaccination rates may depend upon the demographic distribution of the practice's patient population, its current vaccination rate and its overall efforts to achieve better coverage. If there is a threshold effect for office-based interventions, other types of immunization programs (e.g., school based), may be necessary to reach national vaccination goals. From a policy perspective, contemporaneous early delivery of commercial and VFC influenza vaccines and/or the ability to use supplies on hand, enables practices to serve all children equally, and not require some of them to return to the practice to be vaccinated at a later date.
Strengths and Limitations
To date, this study is the only published randomized cluster trial to examine both patient- and practice level characteristics, including an evidence-based intervention, on childhood influenza vaccination rates. Previous studies have not used the randomized cluster trial and few have focused the intervention on the entire span of childhood. This study was limited by the facts that the rural sites randomly assigned to each arm were two offices of the same practice and that the community educational outreach and/or the knowledge that they were in a study may have led to increases in rates in the Control arm practice, thereby reducing the observed differences between arms. Further, vaccination rates may have been underestimated because vaccines given outside the practice may or may not have been captured from other sources.
Conclusions
A multi-strategy toolkit and provision of early vaccine can significantly improve vaccination rates over secular trends, except in practices with high pre-intervention coverage. Improving access to influenza vaccine by early delivery of vaccine supplies, so that opportunities to vaccinate all children are available early in the season, allows practices to vaccinate more children by extending the timeline of vaccine availability. This toolkit of evidence-based strategies can be implemented by an immunization champion in a variety of primary care practices.
Supplementary Material
Figure 1. Randomization Scheme.
Acknowledgments
The authors thank the University of Pittsburgh Clinical and Translational Science Institute Pediatric PittNet practice based research network and the following site investigators: Tracey Conti, MD, Mark Diamond, MD, Harold Glick, MD, Phillip Iozzi, DO, Kenneth Keppel, MD, John J. Labella, MD, Sanjay Lambore, MD, Sheldon Levine, MD, Thomas G. Lynch, MD, Elaine McGhee, MD, Paul Rowland, MD, Robert Rutowski, MD, Pamela Schoemer, MD, Emeil Shenouda, MD, Aaron Smuckler, MD, Scott Tyson, MD, Donald Vigliotti, MD, David Wolfson, MD, Rana Ziadeh, MD. The authors also thank Sanofi Pasteur for donation of 2,000 doses of influenza vaccine used in the study.
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Pittsburgh. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies.
Funding Source: This investigation was supported by a grant (U01 IP000321) from the Centers for Disease Control and Prevention. The views expressed herein are those of those authors and not those of the Centers for Disease Control and Prevention. The project described was also supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005.
Drs. Zimmerman and Lin have a research grant from Sanofi Pasteur, Inc. Drs. Zimmerman, Nowalk and Lin have a research grant from Merck & Co, Inc. Drs. Lin and Nowalk consult for MedImmune, LLC.
Abbreviations
- 95% CI
Confidence interval (95%)
- Task Force
Community Preventive Services Task Force
- EMR
Electronic medical record
- OR
Odds ratio
- SOPs
Standing order programs
- VFC
Vaccines for Children
- VIF
Variance inflation factor
Appendix
Two-level generalized linear mixed modeling
Patient-level Model
Practice-Level Model
Mixed Model
Footnotes
Conflict of Interest: The other authors have no conflicts of interest to disclose.
Financial disclosures: Richard K. Zimmerman has received research funding from Merck and Co, Inc. and Sanofi Pasteur.
Mary Patricia Nowalk has received research funding from Merck and Co, Inc. and consults for MedImmune, LLC.
Chyongchiou Jeng Lin has received research funding from Merck and Co, Inc. and Sanofi Pasteur and consults for MedImmune, LLC.
Kristin Hannibal has no financial relationships relevant to this article to disclose.
Annamore Matambanadzo has no financial relationships relevant to this article to disclose.
Krissy K. Moehling has no financial relationships relevant to this article to disclose.
Hsin-Hui Huang has received research funding from Merck and Co, Inc. and Sanofi Pasteur.
Judith Troy has no financial relationships relevant to this article to disclose.
Norma J. Allred has no financial relationships relevant to this article to disclose.
Greg Gallik has no financial relationships relevant to this article to disclose.
Evelyn C. Reis has no financial relationships relevant to this article to disclose.
References
- 1.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Morb Mortal Wkly Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Healthy People 2020: Immunization and Infectious Diseases Overview. [Accessed January 18, 2012]. http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=23 .
- 3.Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2011-12 Influenza Season. [Accessed 2012, December 12]. http://www.cdc.gov/flu/fluvaxview/coverage_1112estimates.htm .
- 4.Dombkowski KJ, Harrington LB, Dong S, Clark SJ. Seasonal influenza vaccination reminders for children with high-risk conditions: a registry-based randomized trial. Am J Prev Med. 2012;42:71–5. doi: 10.1016/j.amepre.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Britto MT, Schoettker PJ, Pandzik GM, Weiland J, Mandel KE. Improving influenza immunisation for high-risk children and adolescents. Qual Saf Health Care. 2007;16:363–8. doi: 10.1136/qshc.2006.019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA. 2012;307:1702–8. doi: 10.1001/jama.2012.502. [DOI] [PubMed] [Google Scholar]
- 7.Paul IM, Eleoff SB, Shaffer ML, Bucher RM, Moyer KM, Gusic ME. Improving influenza vaccination rates for children through year-round scheduling. Ambul Pediatr. 2006;6:230–4. doi: 10.1016/j.ambp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Task Force on Community Preventive Services. Guide to Community Preventive Services. [Accessed 2013, January 18]. http://www.thecommunityguide.org/index.html .
- 9.Nowalk MP, Nutini J, Raymund M, Ahmed F, Albert SM, Zimmerman RK. Evaluation of a toolkit to introduce standing orders for influenza and pneumococcal vaccination in adults: a multimodal pilot project. Vaccine. 2012;30:5978–82. doi: 10.1016/j.vaccine.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Campbell MK, Piaggio G, Elbourne DR, A DG. Consort 2010 statement: extension to cluster randomised trials. BMJ. (Clinical research) 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg B, Parcel SG. Diffusion of Innovations. In: Karen Glanz, Rimer BK, Lewis FM., editors. Health Behavior and Health Education. 3rd. San Francisco: John Wiley and Sons, Inc.; 2002. pp. 312–34. [Google Scholar]
- 12.Melinkovich P, Hammer A, Staudenmaier A, Berg M. Improving pediatric immunization rates in a safety-net delivery system. Jt Comm J Qual Patient Saf. 2007;33:205–10. doi: 10.1016/s1553-7250(07)33024-9. [DOI] [PubMed] [Google Scholar]
- 13.Mowbray CT, Holter MC, Teague GB, Bybee D. Fidelity criteria: Development, measurement, and validation. Am J Eval. 2003;24:315–40. [Google Scholar]
- 14.Fox J. Linear statistical models and related methods: With applications to social research. New York: John Wiley; 1984. [Google Scholar]
- 15.Neter J, Wasserman W, Kutner M. Applied linear statistical models. 2nd. Illinois: Richard Irwin, Inc.; 1985. [Google Scholar]
- 16.McCambridge J, Kypri K, Elbourne D. In randomization we trust? There are overlooked problems in experimenting with people in behavioral intervention trials. J Clin Epidemiol. 2014;67:247–53. doi: 10.1016/j.jclinepi.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daley MF, Barrow J, Pearson K, Crane LA, Gao D, Stevenson JM, et al. Identification and recall of children with chronic medical conditions for influenza vaccination. Pediatrics. 2004;113:e26–33. doi: 10.1542/peds.113.1.e26. [DOI] [PubMed] [Google Scholar]
- 18.Gaglani M, Riggs M, Kamenicky C, Glezen WP. A computerized reminder strategy is effective for annual influenza immunization of children with asthma or reactive airway disease. Pediatr Infect Dis J. 2001;20:1155–60. doi: 10.1097/00006454-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman RK, Nowalk MP, Lin CJ, Ko FS, Block B, Anderson G, et al. Interventions over 2 years to increase influenza vaccination of children aged 6-23 months in inner-city family health centers. Vaccine. 2006;24:1523–9. doi: 10.1016/j.vaccine.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Martin E. Improving influenza vaccination rates for pediatric asthmatics by use of an asthma educational tool and a patient electronic care system. Clin Pediatr. 2008;47:588–92. doi: 10.1177/0009922808314902. [DOI] [PubMed] [Google Scholar]
- 21.Fiks AG, Hunter KF, Localio AR, Grundmeier RW, Bryant-Stephens T, Luberti AA, et al. Impact of electronic health record-based alerts on influenza vaccination for children with asthma. Pediatrics. 2009;124:159–69. doi: 10.1542/peds.2008-2823. [DOI] [PubMed] [Google Scholar]
- 22.Esposito S, Pelucchi C, Tel F, Chiarelli G, Sabatini C, Semino M, et al. Factors conditioning effectiveness of a reminder/recall system to improve influenza vaccination in asthmatic children. Vaccine. 2009;27:633–5. doi: 10.1016/j.vaccine.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 23.Kempe A, Daley MF, Barrow J, Allred N, Hester N, Beaty BL, et al. Implementation of universal influenza immunization recommendations for healthy young children: results of a randomized, controlled trial with registry-based recall. Pediatrics. 2005;115:146–54. doi: 10.1542/peds.2004-1804. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman RK, Hoberman A, Nowalk MP, Lin CJ, Greenberg DP, Weinberg ST, et al. Feasibility of influenza immunization for inner-city children aged 6 to 23 months. Am J Prev Med. 2004;27:397–403. doi: 10.1016/j.amepre.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Toback SL, Rothstein E, Bhatt P, Carr W, Ambrose CS. In-Office Influenza Vaccination by US Pediatric Providers Varies Greatly and Is Higher Among Smaller Offices. Clin Pediatr. 2012;51:551–9. doi: 10.1177/0009922812443731. [DOI] [PubMed] [Google Scholar]
- 26.Humiston SG, Schaffer SJ, Szilagyi PG, Long CE, Chappel TR, Blumkin AK, et al. Seasonal Influenza Vaccination at School A Randomized Controlled Trial. Am J Prev Med. 2014;46:1–9. doi: 10.1016/j.amepre.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Hadler JL, Yousey-Hindes K, Kudish K, Kennedy ED, Sacco V, Cartter ML, et al. Impact of requiring influenza vaccination for children in licensed child care or preschool programs - connecticut, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:181–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Poehling KA, Fairbrother G, Zhu YW, Donauer S, Ambrose S, Edwards KM, et al. Practice and Child Characteristics Associated With Influenza Vaccine Uptake in Young Children. Pediatrics. 2010;126:665–73. doi: 10.1542/peds.2009-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor ME, Everhart RM, Berg M, Federico SG, Hambidge SJ. Pediatric influenza immunization in an integrated safety net health care system. Vaccine. 2012;30:2951–5. doi: 10.1016/j.vaccine.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 30.Uwemedimo OT, Findley SE, Andres R, Irigoyen M, Stockwell MS. Determinants of Influenza Vaccination Among Young Children in an Inner-City Community. J Community Health. 2012;37:663–72. doi: 10.1007/s10900-011-9497-9. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman RK, Hoberman A, Nowalk MP, Lin CJ, Greenberg DP, Weinberg ST, et al. Improving influenza vaccination rates of high-risk inner-city children over 2 intervention years. Ann Fam Med. 2006;4:534–40. doi: 10.1370/afm.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrose CS, Toback SL. Improved Timing of Availability and Administration of Influenza Vaccine Through the US Vaccines for Children Program From 2007 to 2011. Clin Pediatr. 2013;52:224–30. doi: 10.1177/0009922812470868. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J. 2011;30:100–6. doi: 10.1097/INF.0b013e3181efff54. [DOI] [PubMed] [Google Scholar]
- 34.Nowalk MP, Nolan BAD, Nutini J, Ahmed F, Albert SM, Susick M, et al. Success of the 4 Pillars Toolkit for Influenza and Pneumococcal Vaccination in Adults. J Healthc Qual. 2013 doi: 10.1111/jhq.12020. [DOI] [PubMed] [Google Scholar]
- 35.White T, Lavoie S, Nettleman MD. Potential cost savings attributable to influenza vaccination of school-aged children. Pediatrics. 1999;103:e73. doi: 10.1542/peds.103.6.e73. [DOI] [PubMed] [Google Scholar]
- 36.Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21:1769–75. doi: 10.1016/s0264-410x(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 37.National Committee for Quality Assurance. Core Set of Children's Health Care Quality Measures for Medicaid and CHIP (Child Core Set): Technical Specifications and Resource Manual for Federal Fiscal Year 2013 Reporting. Washington, D C.: Centers for Medicare & Medicaid Services; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.