Abstract
Ant protection of extrafloral nectar-secreting plants (EFN plants) is a common form of mutualism found in most habitats around the world. However, very few studies have considered these mutualisms from the ant, rather than the plant, perspective. In particular, a whole-colony perspective that takes into account the spatial structure and nest arrangement of the ant colonies that visit these plants has been lacking, obscuring when and how colony-level foraging decisions might affect tending rates on individual plants. Here, we experimentally demonstrate that recruitment of Crematogaster opuntiae (Buren) ant workers to the extrafloral nectar-secreting cactus Ferocactus wislizeni (Englem) is not independent between plants up to 5m apart. Colony territories of C. opuntiae are large, covering areas of up to 5000m2, and workers visit between five and thirty-four extrafloral nectar-secreting barrel cacti within the territories. These ants are highly polydomous, with up to twenty nest entrances dispersed throughout the territory and interconnected by trail networks. Our study demonstrates that worker recruitment is not independent within large polydomous ant colonies, highlighting the importance of considering colonies rather than individual workers as the relevant study unit within ant/plant protection mutualisms
Keywords: extrafloral nectar, mutualism, polydomy, social insects, foraging
Introduction
Ant protection of extrafloral nectar (EFN)-secreting plants is a common mutualism found in over 100 plant families (Keeler 2008) in most habitats around the world. In these interactions, ants visit the plants for food and act aggressively towards other plant consumers, often resulting in reduced levels of herbivory. These interactions are accessible, easy to manipulate, and range from obligate to facultative, making them useful model systems for the study of mutualism (Bronstein 1998; Heil and McKey 2003; Rico-Gray and Oliveira 2007). As for nearly all mutualisms, however, research has been biased towards the perspective of one of the two partner species (Bronstein 1994). In studies of facultative ant-plant protection mutualisms, a plant-centered approach has been nearly ubiquitous. For instance, recent studies have focused on the benefits of ant visitation to plants in terms of herbivore deterrence (e.g. Dutra et al. 2006; Ness et al. 2006; Oliveira et al. 1999) and fitness (e.g. Cuautle and Rico-Gray 2003; Styrsky and Eubanks 2010); and these benefits have been the subject of three recent meta-analyses (Chamberlain and Holland 2009b; Rosumek et al. 2009; Trager et al. 2010). Previous studies have also examined the costs of producing rewards (e.g. Holland et al. 2009; Rudgers and Gardener 2004), and the relative quality of different ant partner species from the perspective of the plant (Miller 2007; Ness et al. 2006). The structure of protective ant assemblages and the network properties of ant/plant associations are also of increasing interest (Chamberlain and Holland 2009a; Diaz-Castelazo et al. 2010; Guimaraes et al. 2006).
In contrast, our understanding of these interactions from the ant perspective is much less developed. Previous work has shown that EFN is an important dietary resource for many ants (Bluthgen and Fiedler 2004a; Bluthgen and Fiedler 2004b; Bluthgen et al. 2004; Davidson 1997), contributing to colony growth (Byk and Del-Claro 2011; Wilder et al. 2011) and comprising up to 90% of the total food collected b y some species (Tillberg and Breed 2004). The availability of EFN as an easily accessible, plentiful source of carbohydrates can also change the behavior of ants, making them more aggressive towards insect herbivores (Ness et al. 2009). Beyond these few studies, however, we know very little about the consequences for ants of participating in facultative protection mutualisms. How does the availability of extrafloral nectar in the environment influence colony traits such as nesting and foraging strategy? Here, we take an ant-colony perspective on a facultative protection mutualism involving a common extraflora-nectar secreting cactus in the southwestern United States that has been extensively studied from the plant, but not the ant point of view.
For an ant colony that feeds upon EFN, the spatial and temporal arrangement of this resource poses unique challenges. Many other foods collected by ants including insect prey, carrion, and seeds vary in spatial distribution over time. Colonies often collect these ephemeral resources using rapid pheromone recruitment or foraging trunk trails leading from a central nest entrance (Hölldobler and Wilson 1990). In contrast, some types of plants can secrete EFN for months or years. Because these resource patches are stationary, ant colonies that collect EFN may need to position foragers near many plants simultaneously over long periods of time. One way colonies might deal with patchily distributed, temporally persistent resources is through the spatial arrangement of their nests (Holway and Case 2000). Many ant species are polydomous, a condition in which the colony is distributed among multiple, spatially segregated nests that regularly exchange workers (Debout et al. 2007). One hypothesized advantage of polydomy is that it enables colonies to position foragers closer to patches and reduce forager travel distance (Davidson 1997; Holway and Case 2000; McIver 1991; Pfeiffer and Linsenmair 1998). We therefore predicted that t he ants in our study would exhibit this dispersed nest distribution due to their reliance on extra floral nectar, a resource that is both patchy and temporally persistent.
Nearly all studies of facultative ant plant protection mutualisms have only considered the subset of worker ants that visit the plants, rather than the colony as a whole (but see Byk and Del-Claro 2011). Part of the reason for this may be the difficulty of locating and studying whole colonies. Ant species that participate in facultative EFN mutualisms tend to be ground-nesting, and the foraging range and location of nests is often difficult to determine. However, in an ecological sense, it is useful to think of the entire colony as an individual organism. Ant colonies are often large in spatial extent and number of workers; and like individual organisms they are usually sessile, long-lived, and reproduce at the colony level through production of alates (Hölldobler and Wilson 1990). The wide variety of behaviors, cues and signals used by ants enable information sharing and coordination of collective actions at the colony level, including decisions about where to forage. Thus, although only a few individual workers may be observed interacting with a nectar-secreting plant, it is inappropriate to treat them as independent individuals as one would solitary insects. Even the behavior of workers visiting separate plants can be influenced by the same, potentially cryptic factors operating at the colony level. As a result, it may therefore also be inappropriate to treat the fates of neighboring, reward-producing plants as independent from each other in the likely case that they are tended by ants from the same colony. Despite the potential problem that this poses for researchers using plants as replicates in experimental studies of ant-plant mutualisms, we know of no direct examination of this phenomenon.
In this study, we test the hypothesis that a ground-nesting ant species tending EFN-secreting plants will exhibit dispersed polydomous nest organization, and that this organization will in turn affect the plant. We investigated the distribution of territories, trails, and nest entrances for individual colonies of the ant Crematogaster opuntiae foraging among a landscape of extra-floral nectar-secreting barrel cacti (Ferocactus wislizeni) in the Sonoran Desert of southeastern Arizona, USA. This ant species is one of the most common visitors to nectar-secreting plants in this habitat (Ness 2006; Ness et al. 2006), and extrafloral nectar is an important component of its diet (Ness et al. 2009). We then used the data we collected on colony spatial arrangement to investigate how proximity of individual extra-floral nectar-secreting barrel cacti to colony features such as nest entrances and territory boundaries affected worker visitation and recruitment to the plants. Specifically, we tested whether (1) the landscape of extrafloral nectar-bearing F. wislizeni influenced the spatial organization of C. opuntiae colonies, and (2) whether the frequency of C. opuntiae visitation to the plants varied based on plant proximity to territory boundaries and nest entrances. We then used this knowledge of colony structure and location to (3) test whether ant recruitment to neighboring plants within a colony territory is independent at distances from 1m to 10m.
Methods
Study species and location
The fishhook barrel cactus Ferocactus wislizeni (Cactaceae) is common in deserts and grasslands that experience summer rainfall, from southern Arizona and southeastern California to northern Sonora, Mexico (Benson 1981). It secretes EFN from approximately 5–200 nectaries (glands derived from spines on the aureoles) at the crown of the plant. Although it co-occurs in the Sonoran Desert with other EFN-secreting plants, including saguaro (Carnegiea gigantea), cholla and prickly pear (Opuntia spp.), senna (Senna covesii), and white-thorn acacia (Acacia constricta), F. wislizeni is unusual for secreting sufficient amounts of extrafloral nectar to attract ants year-round (Ness et al. 2009). Censuses and experimental studies of this ant-plant association have been conducted since 2003 in Sonoran Desert scrub habitat at the Desert Laboratory in Tucson, Arizona, USA (32°13’11”N; 111°00’14”W) (Morris et al. 2005, Ness 2006, Ness et al. 2006, 2009). At least twenty-five species of ants are associated with F. wislizeni across southern Arizona (M. C. Lanan, unpublished data). However, at the Desert Laboratory the cacti are commonly visited by four ant species; Solenopsis xyloni, Solenopsis aurea, Forelius pruinosus, and Crematogaster opuntiae (Ness et al. 2006). In this study we focused our attention on C. opuntiae, the most common ant species that interacted with the cacti at the site. It was possible to conduct this study on the behavior of a single ant species, due to the spatial and temporal segregation exhibited by the ants in this system. The four common species avoid one another and rarely interact on the plants, such that single plants are almost always tended by a single ant species at any given time.
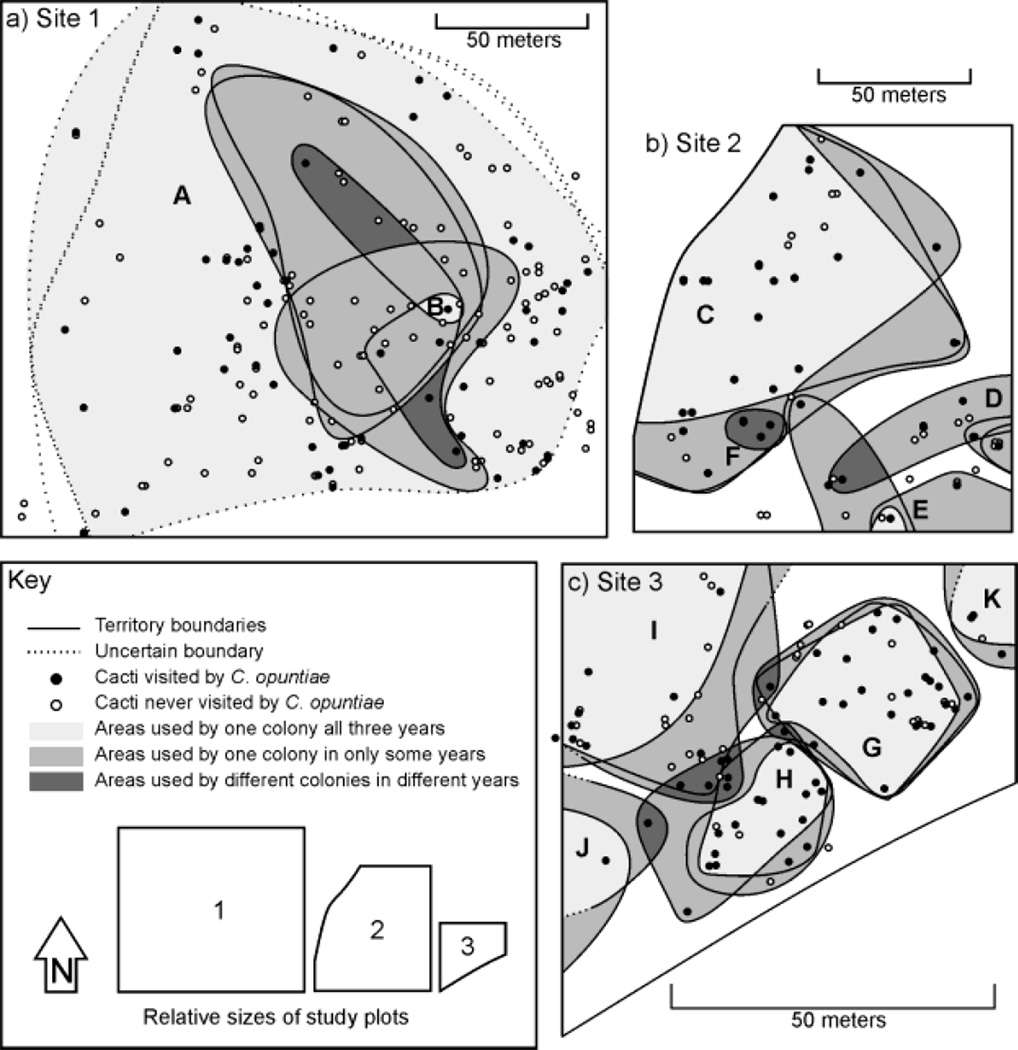
This study was conducted at three sites at the Desert Laboratory where previous research on these interactions has been conducted (Morris et al. 2005; Ness 2006; Ness et al. 2006; Ness et al. 2009). Site 1, a 4-hectare plot, is located on relatively flat alluvial soil at 739 m elevation and contains 186 F. wislizeni. Sites 2 and 3 are located on rocky, sloped terrain at 850–860m elevation. Site 2 is 1.5 hectares in area and contains 58 F. wislizeni, while Site 3 is one hectare in area and contains 107 plants. Site 2 is bounded on the western edge by a cliff face and Site 3 is bounded on the southern edge by a road. All other site boundaries were arbitrary, with cacti growing both inside and outside the borders. Because of the importance of accurate spatial data for portions of this study, we created detailed maps of the locations of all F. wislizeni on these three sites using a combination of GPS and surveying techniques. In addition, a 5 m grid was marked out on Site 3 using steel stakes in order to make more accurate measurements. Sites 1–3 are shown in Fig. 1.
Fig. 1.
Maps showing the 2007, 2008, and 2009 territory boundaries that we deduced using the behavioral assay method for C. opuntiae colonies on a) Site 1, b) Site 2, and c) Site 3. Areas used by a single colony during all three years are shown in light gray, while areas that were sometimes used by one colony are highlighted in medium gray. Areas that changed colony ownership over the course of the study are shown in dark gray. C. opuntiae were never observed on cacti in white areas.
Does the spatial distribution of EFN-bearing plants influence the spatial organization of the ant colonies?
In order to test the hypothesis that C. opuntiae will exhibit dispersed polydomous nest organization influenced by the spatial arrangement of EFN-bearing F. wislizeni, we used several methods to determine the territory boundaries, nest entrances, and trail locations of the ant colonies
Ant colony territories
Previous observations of both laboratory and field colonies suggested that C. opuntiae is highly territorial, and that workers quickly respond to foreign con specific workers (i.e., from a different colony) by attacking them. In highly territorial ants, pair wise confrontation tests are effective methods to determine colony membership and to deduce territory boundaries (Dejean et al. 2010). We conducted confrontation tests by placing pairs of worker ants inside clean, dry plastic vials and observing their behavior for 5 min. Behaviors were classified as either nonthreatening (grooming, trophyllaxis, spending time in close proximity to the other ant) or threatening (agitated running, biting, exuding liquid on the sting, dismemberment of the opponent). As a control, we conducted these confrontation tests on 100 pairs of ants collected from the same nest entrance, as well as 82 pairs of ants from two nest entrances >100 m apart, known from our preliminary work to be entrances to nests of different colonies. We observed consistently threatening behaviors in 0% of the known within-colony confrontations, but within 90% of the known between-colony matchups. These data suggest that confrontation tests are, in the absence of genetic data, a reliable indicator of colony identity for this species. Based on this evidence, in the experiments described below, we rejected the hypothesis that ants belonged to the same colony when they engaged in constantly threatening behaviors for 5 min. This is a conservative test of colony boundaries: that is, we may have erroneously concluded that some pairs belonged to the same colony if they were slightly less aggressive to each other
To determine the size and location of C. opuntiae colony territories at each study site, we used the same confrontation test protocol as above. Because foraging C. opuntiae workers in this habitat are found almost exclusively on barrel cacti and workers are rarely observed on the surface of the ground or around nest entrances (M. C. Lanan, unpublished data), we assessed the spatial extent of territories based on which barrel cacti individual colonies occupied. For the confrontation tests, we collected 3–10 ants from each F. wislizeni using an aspirator, and brought them to the laboratory in plastic vials. All confrontation tests were conducted within 3 hours of worker collection. The pairwise tests yielded clear results in 97.5% of trials. In the remaining 2.5%, the first confrontation test was not conclusive (for example, we observed biting followed by grooming rather than dismemberment); in these few cases, we repeated the test with a new pair of ants. We did not test every possible pairing of ants between the cacti; rather we selected pairings that would enable us to deduce boundaries, such that all aggressive and non-aggressive pairs were supported by at least two trials. The results of the experiments were overlaid on site maps to deduce ant colony territories and territorial boundaries. The experiment was conducted three times at each study site, in November 2007, January 2008, and January 2009.
Nest and trail locations
One C. opuntiae colony (Colony G, Fig. 1) at Site 2 was used for a further investigation of the spatial arrangement of nest entrances and trails. We selected Colony G because its territory boundary was completely contained within the site, and because the relatively flat terrain made it possible to locate its cryptic nest entrances. Although several more colonies also occurred at Site 2, the difficulty in finding nest entrances and the mainly nocturnal foraging behavior of this species required us to focus on only one colony. To locate nest entrances, at the onset of the summer rainy season in July 2007 and July 2008 we made observations of winged alates as they emerged before the mating flight. We located the remainder of the nest entrances in July–August 2007, 2008, and 2009 by placing bait (ground-up Pecan Sandies cookies, Kellogg Company) mixed with UV-reflective, fluorescent yellow or red powder (Dayglo Color Corporation) at the crown of each barrel cactus within the territory. After dark, using a hand-held UV light, we followed workers returning with these baits to their nests. Because alates do not travel outside the nest before their mating flight and must therefore be reared in situ as brood, we assumed that all entrances where alates were observed led to true nests containing both workers and brood. Entrances that were found using the baiting method were checked for the presence of brood by carefully lifting and replacing rocks. Outstations (nest-like structures containing only workers, not brood Anderson and McShea 2001; Lanan 2010) were noted but not included in this study.
We also used the baiting method to locate trails that workers in Colony G followed between barrel cacti and nest entrances, by marking the routes along which ants carried the bait and checking for workers walking these trails on subsequent days. We mapped the location of nests and trails for this colony in July and August of 2007, 2008, and 2009.
Does plant proximity to colony features affect the frequency of ant visitation to the plants?
In order to test the hypothesis that the distance between the plant and the nearest nest entrance would affect the frequency of ant visitation, we used census data collected in 2006–2009 on ant visitation to the plants. Approximately once each month in 2006 and four times per year in subsequent years, the ant species present at the nectaries of each of 351 permanently tagged F. wislizeni at sites 1, 2 and 3 were recorded. At each census we categorized each plant as a) C. opuntiae present, b) a different ant species present, or c) no ants present. Using the territory boundaries delineated during 2007–2009, we classified each barrel cactus as growing in one of three areas: habitat that for all three years was within the boundary of one C. opuntiae territory (interior), habitat that sometimes was within one territory and at other times within no territory (edge), and habitat that was included within the boundary of different territories in different years (contested). In order to relate nest location to EFN availability, we also counted the number of nectaries on all plants within the boundaries of Colony G, Site 2.
Is ant behavior between plants independent?
To test whether increased C. opuntiae recruitment to one F. wislizeni individual would influence ant recruitment to neighboring con specific plants, we supplemented food on a focal cactus by placing a cotton ball soaked with 25 ml 1:10 diluted honey solution at the crown of the plant. We then observed subsequent ant activity on that individual as well as on con specifics 1 m, 5 m, and >10 m distant from it. Ant numbers were recorded on each of the four cacti in each experimental replicate every 10 min during a 60 min control period, in order to determine whether ant numbers were stable over time. If factors such as changes in weather and temperature or the presence of other ant species appeared to cause the number of workers to increase or decrease dramatically during the control period, we aborted the trial and repeated it on a subsequent day. The supplemental food was only added once stable numbers were observed during the control period. We then continued to record the number of ants present on all four cacti at 10 min intervals for a further 60 min period. We repeated this experiment five times during 2009–2010. In one experimental trial, the C. opuntiae workers on the 5 m cactus were displaced by a different ant species partway through the experiment. We include this trial without data from that individual cactus. All trials were conducted using cacti that we had previously determined to be included within the boundaries of individual colonies.
Results
Does the spatial distribution of EFN-bearing plants influence the spatial organization of the ant colonies?
Ant colony territories
Using the behavioral assay method, we were able to determine clear ant territory boundaries at each of the three sites (Fig. 1). These territories overlapped from year to year, although the shape and position of the boundaries varied due to gains or losses of area (Fig. 2). Classifying area as interior (within the boundary of one C. opuntiae territory for all years), edge (sometimes within one territory and sometimes within no territory), and contested (within the boundary of different territories during different years), we found that colonies maintained ownership of the majority of their territory area over the three-year study (Fig. 1). 60% of the area (including 71% of cacti) was classified as interior, while 36% (18% of cacti) was classified as edge and 4% (11% of cacti) was contested. In no cases did colonies share a plant.
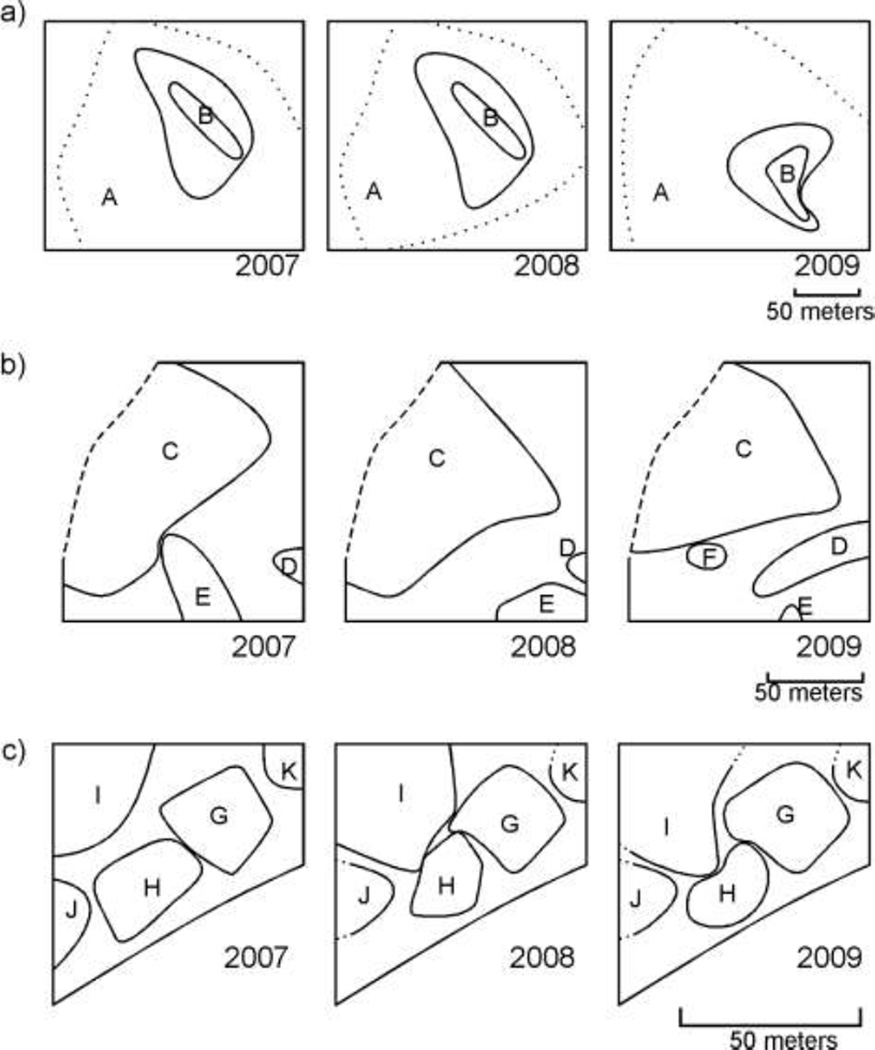
Fig. 2.
Maps showing how C. opuntiae territory boundaries changed over the three year period for 2007, 2008, and 2009 on a) Site 1, b) Site 2, and c) Site 3.
During the period studied two colony territories occurred at Site 1, four occurred at Site 2, and five were found on Site 3. Detailed information about these colonies including estimated sizes are listed in Table 1. All territories except one (Colony F, Site 2) were found during all three years, suggesting that colonies may be long-lived. Colony F appeared in 2009 in an area previously occupied by Colony C, indicating either the establishment of a new colony or colonization of the area by a colony from outside the plot. Interestingly, Colony B (Site 1) appeared to be completely surrounded by Colony A for all years (Fig. 2).
Table 1.
The average territory size, surveys in which the colony was present, proportion of cacti used by each colony, and density of cacti within the territories for each colony.
| Colony | Average territory size | Surveys in which colony was present |
Proportion of cacti used within territory |
Density of cacti within territory |
|---|---|---|---|---|
| A, Site 1 | 18100 m2 May have extended beyond plot in some directions | November 2007, January 2008, January 2009 | 26/114 Cacti 23% | 0.0063/ m2 |
| B, Site 1 | 1100 m2 Contained within plot | November 2007, January 2008, January 2009 | 3/8 Cacti 37% | 0.0073/ m2 |
| C, Site 2 | 7400 m2 Contained within plot, bordered by cliff | November 2007, January 2008, January 2009 | 17/28 Cacti 61% | 0.0038/ m2 |
| D, Site 2 | Majority likely not within pot | November 2007, January 2008, January 2009 | Undetermined | Undetermined |
| E, Site 2 | Majority likely not within plot | November 2007, January 2008, January 2009 | Undetermined | Undetermined |
| F, Site 2 | 200 m2 Contained within plot | January 2009 only | 3/3 Cacti 100% | 0.015/ m2 |
| G, Site 3 | 700 m2 Contained within plot, bordered by road | November 2007, January 2008, January 2009 | 13/31 Cacti 42% | 0.044/ m2 |
| H, Site 3 | 625 m2 Contained within plot, bordered by road | November 2007, January 2008, January 2009 | 13/21 Cacti 62% | 0.034/ m2 |
| I, Site 3 | >750 m2 May have extended beyond plot in some directions | November 2007, January 2008, January 2009 | 9/25 Cacti 36% | 0.033/ m2 |
| J, Site 3 | Majority likely not within plot | November 2007, January 2008, January 2009 | Undetermined | Undetermined |
| K, Site 3 | Majority likely not within plot | November 2007, January 2008, January 2009 | Undetermined | Undetermined |
Nest and trail locations
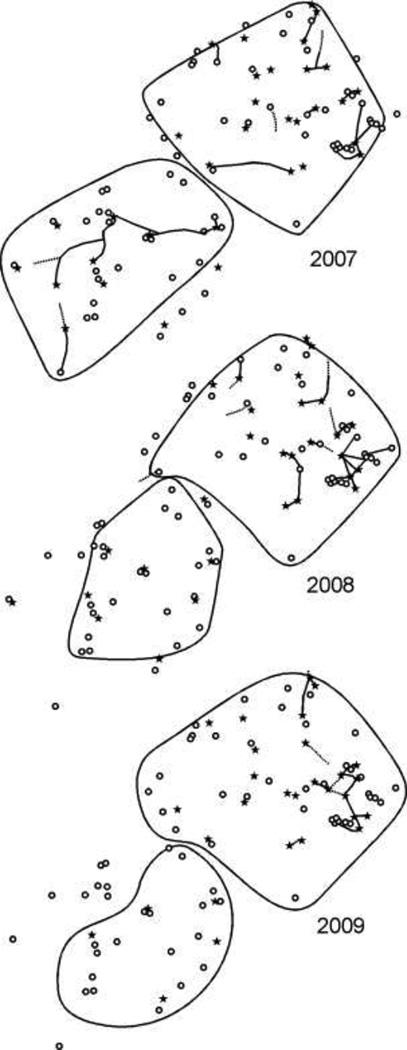
We mapped the location of nest entrances and trails within the territory of Colony G on Site 3 in August of 2007, 2008, and 2009 (Fig. 3). Colony G had 22 nest entrances in 2007, 20 in 2008, and 24 in 2009. The nest entrances typically occurred in soil as small cryptic holes 2 mm in diameter near rocks. Seventeen of the nests occurred in the same location all three years. Due to the difficult terrain and dense vegetation, we were only able to locate portions of the trails used by Colony G. However, the portion of the trails that we did identify were persistent over time. All trails we observed appeared with the onset of the monsoon season in mid-July, and remained in the same location until worker activity on the ground declined in October or November. Only nests that we could confirm as containing brood are shown in Fig. 3. Because multiple, spatially segregated nest entrances were interconnected by trails, we can conclude that the colony occupying this area was polydomous. However, due to the difficult terrain we were unable to determine whether all nests within the territory of Colony G were interconnected by trails.
Fig. 3.
Maps showing the trails and nests found for Colony G at Site 3 in August 2007, August 2008, and August 2009. Cacti are indicated as open circles, and nest entrances are represented by black stars. Trails with heavy traffic (10–30 ants per minute) are shown as solid lines, while trails with lighter traffic (<10 ants per minute) are shown as dotted lines. Some trails appear to dead-end on this map where they entered areas of vegetation that were impassible to researchers.
Over the course of this study, we also noted that C. opuntiae created outstations (small, nest-like chambers that house only workers, not brood) and nectary shelters (small coverings over the cactus nectaries created from soil and debris) within the territories of Colonies A, B, C, G, H, and I. The outstations were distributed throughout the territories and were frequently located at the base of cacti, in vegetation next to the cacti, or in areas of the cactus tissue that had previously been damaged by cactus beetles (Moneilima gigas).
Does plant proximity to colony features affect the frequency of ant visitation to the plants?
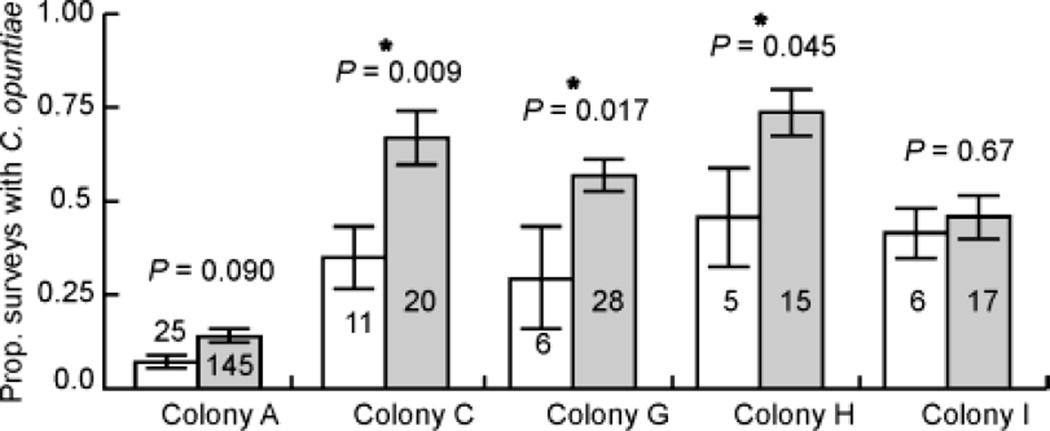
Using the survey data on ant visitation to the cacti from 2006–2009, we compared the extent of C. opuntiae visitation received by plants growing in the edge and interior areas of territories (Fig. 1, medium and light gray areas respectively) for the five colonies that had at least 5 cacti in each area. For colonies C, G, and H, the interiors of territories were visited by C. opuntiae in a significantly greater proportion of the surveys than were plants growing on territory edges (Fig. 4, Colony C: t-test, F1,29 = 7.903, P = 0.0088, Colony G: t-test, F1,32 = 6.3296, P = 0.0171, Colony H: t-test, F1,18 = 4.619, P = 0.0455). For colonies A and I, there was no significant difference in C. opuntiae visitation between the edge and interior areas (Fig. 4, Colony A: t-test, F1,168 = 2.9013, P = 0.0904, Colony I: t-test, F1,21 = 0.1817, P = 0.6742).
Fig. 4.
The proportion of the total surveys in which each cactus was occupied by C. opuntiae in edge (white boxes) and interior (gray boxes) areas for colonies A, C, G, H, and I. Error bars indicate standard error, and the number of cacti (N) is given for each sample. Stars indicate a significant difference between samples at P=0.05.
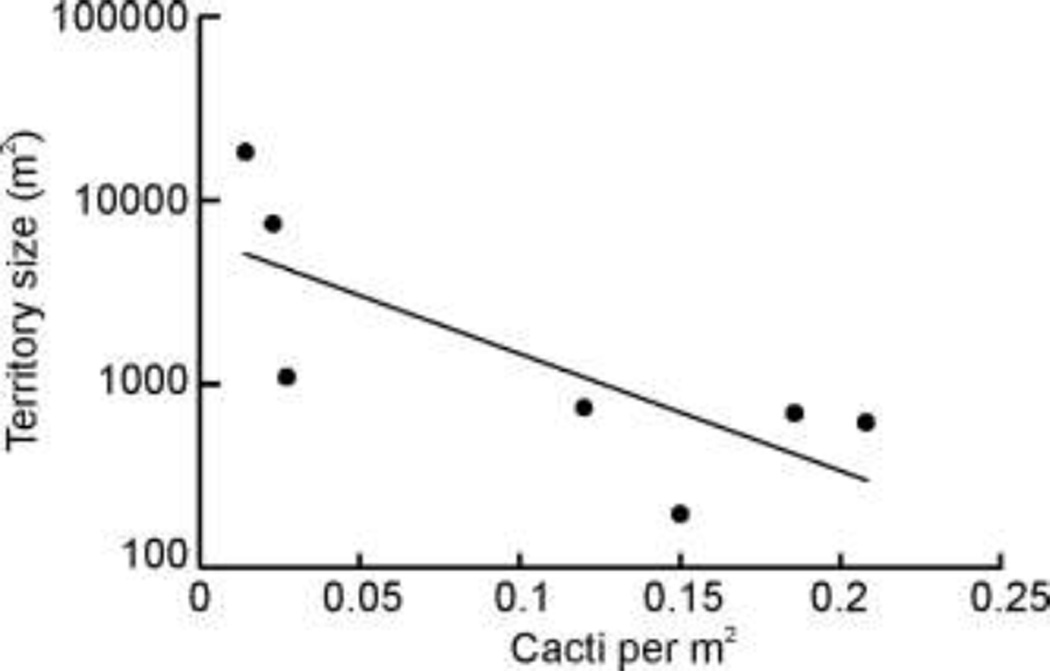
The sizes of the colony territories were related to the density of C. opuntiae-tended cacti, such that the smallest territories occurred in the areas with the greatest density of cacti. Combining data from all three sites, we found that territory size increased as the density of tended cacti decreased (Linear Regression, F1,6 = 6.879, P = 0.0469, r2 =0.579 Fig. 5). A similar but non-significant pattern persisted when we included cacti occupied by other ant species in the analysis (Linear Regression, F1,6 = 2.270, P = 0.192, r2 =0.312).
Fig. 5.
Relationship between territory size and cactus density for the six colonies for which territory sizes could be determined (Linear Regression, F1,6 = 6.879, P = 0.0469, r2 =0.579).
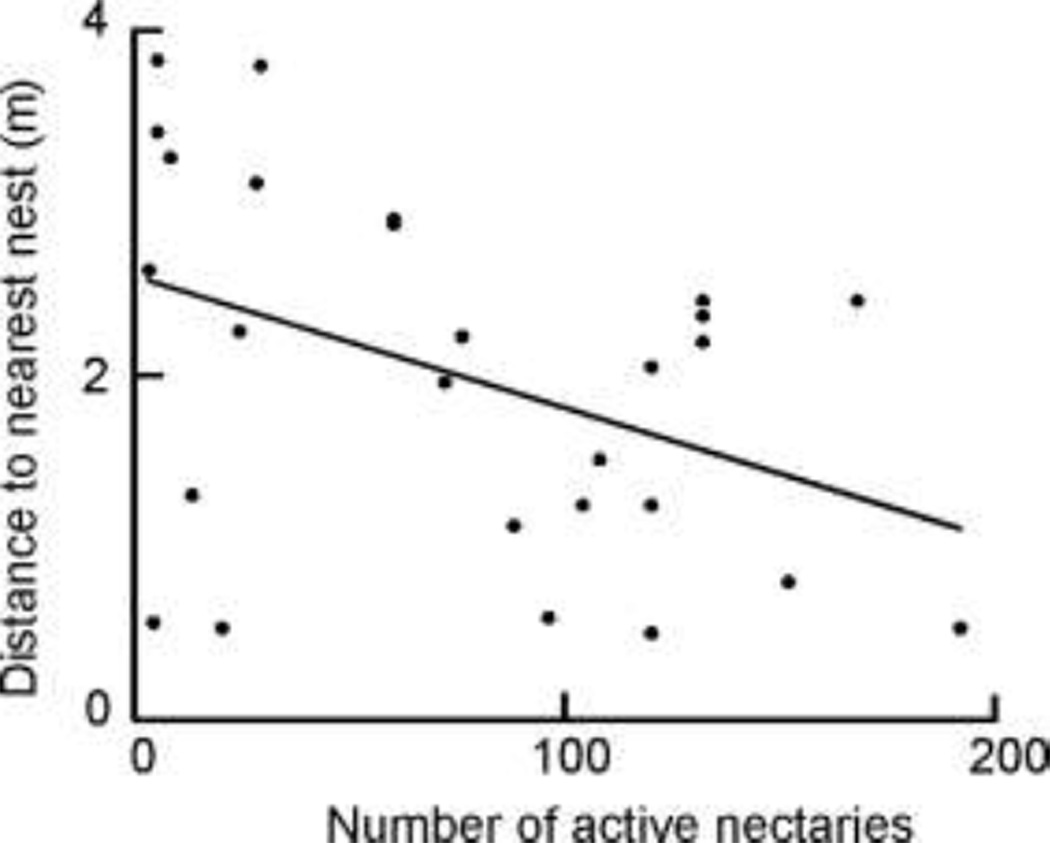
The information we collected on the spatial structure of Colony G enabled us to investigate the relationship between nest placement and ant visitation in greater detail. Distance to the nearest nest had a significant effect on how frequently C. opuntiae workers visited individual barrel cacti within the boundaries of Colony G. The proportion of surveys in which each plant within Colony G was occupied by C. opuntiae decreased significantly as the distance to the nearest nest entrance increased (Logistic Regression, χ2 =4.513, P = 0.034). Plants with more nectaries were more likely to be located near nests; as the number of nectaries per plant increased, the distance to the nearest nest decreased (Linear Regression, F1,25 = 5.2197, P = .0311, r2 =0.173, Fig. 6).
Fig. 6.
In Colony G, distance to the nearest nest de creased as the number of active nectaries on the plants increased (Linear Regression, F1,25 = 5.2197, P = .0311, r2 =0.173).
Is ant behavior between plants independent?
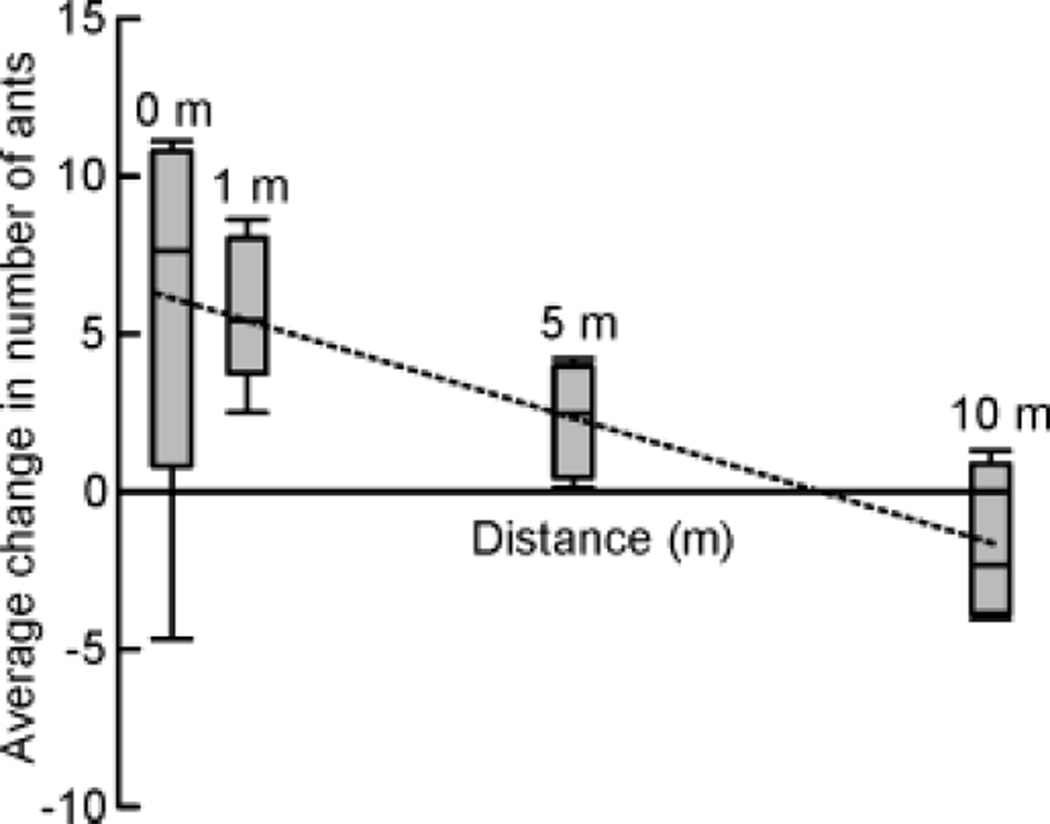
Ant behavior on neighboring cacti was not independent. When we induced ant recruitment to a focal cactus through addition of nectar, we observed an increase in ant recruitment to nearby cacti. The change in the average number of ants was positive on the 1 m and 5 m plants (average increase of 5.5 ants and 2.3 ants, respectively), but recruitment decreased on the 10 m plant (average decrease of 1.7 ants, Fig. 7). There was a negative relationship between distance from the focal plant and the change in worker number we observed (Linear Regression, F1,17 = 15.242, P =0.0011, r2 =0.383). During this experiment we observed that when food was added to the focal cactus, workers would begin running up and down the spines on the top of the plant with their gasters held up above their body, sometimes with drops of liquid visible on the sting. In many cases, workers on the 1 m plant would also exhibit this behavior after food was added to the focal plant. The behavior was never observed on the 5 m or 10 m plants, nor was it observed prior to food addition. We hypothesize that these ants may use a volatile recruitment pheromone that can be detected by workers on neighboring plants.
Fig. 7.
The change in the number of C. opuntiae workers visiting each cactus before and after nectar addition. The average number of ants increased on the focal (0m), 1m, and 5m plants, and decreased on the 10m plants (Linear Regression, F1,17 = 15.242, P =0.0011, r2 =0.383).
Discussion
Viewing this mutualism from the ant rather than the plant perspective reveals that individual Crematogaster opuntiae ant colonies are large, temporally stable features of the environment in which the nectar-secreting cactus Ferocactus wislizeni grows. The placement of colony features such as nests and territory boundaries influenced the likelihood that plants would interact with this ant. Reciprocally, plant features such as the number of nectaries and plant density affected the spatial arrangement of nests and boundaries in the ant colonies. Our most striking result, that the behavior of ant workers within a colony was non-independent between plants, indicates that that the colony level is the most appropriate scale at which to study ant participation in facultative mutualisms such as this.
Implications for mutualism
Our study highlights the importance of the local neighborhood in determining the interaction outcome for both the plant and the ant in EFN protection mutualisms. Because plants and most ant colonies are sessile, spatial distribution of partners determines the types of interactions that can occur. The way in which ant colony structure responds to the local distribution of plants will therefore influence when and where ant-plant interactions occur. In our system, we found that the nest distribution and foraging strategy of C. opuntiae was correlated with the patchy, temporally persistent distribution of the F. wislizeni EFN resource. The numerous polydomous nests were distributed near cacti, temporally persistent over three years, and located nearest to plants that had the greatest number of extrafloral nectaries (and presumably the greatest amount of nectar). It is likely that these correlations are due to ant colony response to cactus distribution, although colony features could also influence cactus distribution if variation in ant protection influences plant recruitment or survival. The size of the colony territories also appeared to be influenced by plant neighborhood effects: territory area was inversely correlated with plant density. A recent study by Wagner and Nicklen (2010) similarly found that the polydomous ant Dorymrmex sp. (smithi complex) preferentially nests under nectar-secreting shrubs and establishes new polydomous nests under shrubs with supplemented nectar.
The spatial arrangement of the ants had a reciprocal effect on the F. wislizeni plants. Plants that grew near nests were more frequently tended by C. opuntiae than were distant plants. The quality of protection a particular plant receives is dependent upon both ant abundance and recruitment rate (Ness et al. 2006), and nest distance can affect both of these factors, with more workers visiting closer plants (Inouye and Taylor 1979) and recruitment occurring faster over shorter distances (Morales 2000). Plants that are tended by colonies with a dispersed, polydomous nest arrangement such as the one we describe in C. opuntiae may therefore be better protected compared to plants tended by colonies with one central nest. The quality of protection a particular plant receives may also be strongly influenced by its location in relation to ant colony features such as nests and territory boundaries, factors rarely considered in studies of facultative ant protection mutualisms (but see Cogni et al. 2003; Dreisig 2000; Lenoir 2003).
The effect of the local neighborhood has been considered in studies of other types of mutualism such as fruit-frugivore interactions (Prasad and Sukumar 2010; Saracco et al. 2005), ant-membracid interactions (Cushman and Whitham 1991) and pollination (Somanathan et al. 2004). Here, we demonstrate that neighborhood effects are also important to consider in facultative ant-plant protection mutualisms. Despite the near ubiquity of ants in this habitat, it would be erroneous to assume that all plants in a habitat have an equal likelihood of being visited by all potential ant partners. Our results show just the opposite- that the particular location in which a plant grows can strongly affect the ant partners it interacts with
Implications for ant colony structure
The polydomous nest structure we observed in C. opuntiae is similar to the nesting strategy that has been reported for several other extra-floral nectar-collecting ants including Camponotus gigas (Pfeiffer and Linsenmair 1998), Crematogaster pygmaea (Quinet et al. 2009), Dorymyrmex sp. (Wagner and Nicklen 2010), and the arboreal Oecophylla longinoda (Holldobler and Lumsden 1980). Due to the limited number of studies that report the nesting behavior of EFN-collecting ants compared to the large number of species that participate in these mutualisms, it is difficult to make broad statements about how widespread polydomous nesting habits are in these ants, compared to monodomy. However, one hypothesized advantage of polydomy is that it enables colonies to position for agers closer to resource patches, thereby reducing forager travel distance (Holway and Case 2000) and increasing the ability of the colony to defend resources (Debout et al. 2007). This dispersed central place foraging (McIver 1991; Pfeiffer and Linsenmair 1998) would be most effective when resources are both patchy and temporally persistent, such as in the case of EFN-secreting plants. We therefore expect that future studies of facultative ant-plant mutualisms will reveal several common features among ant foraging strategies on EFN. Specifically we expect polydomy (either seasonal or permanent), long-lived and numerically large colonies, distinct territories and aggression toward con specifics (with the exception of super colonial species), and well-established trail networks with both between-nest and foraging trails. Future studies are needed to determine how these colony features correlate with resource type, as well as how monodomous species respond to patchy and persistent resources.
Implications for the study of ant protection mutualisms
This study holds broader implications for how ant protection mutualisms are studied in the field. Experimental studies of ant defense mutualisms often manipulate some subset of neighboring patches through exclusion of ants or augmentation of nectar, comparing subsequent levels of herbivore attack or measures of plant fitness between manipulated and unmanipulated patches. As our study demonstrates, however, single ant colonies can visit many patches simultaneously. Foraging decisions are made collectively at the colony level; thus, a short-term change in resource availability at one patch can cause changes in foraging effort that will affect other patches too. We could reasonably expect that augmenting or eliminating resources at one patch could cause either an increase (as in this study) or a decrease in ant attention to neighboring patches whose resources remain unchanged. Hence, it is likely that in ecological experiments, m anipulated and unmanipulated plants or patches placed too close to each other will not respond independently to the experimental treatment.
How close is too close? In this study, nonindependence of patches was seen at a scale of 5 m. To determine the scale at which experiments are commonly conducted, we examined 26 published studies from 1980 to 2011 in which ants were excluded from certain plants and subsequent comparisons were made between manipulated vs. unmanipulated plants. The average distance between control and treatment plants was not reported in 46% of studies; in those in which it was, it ranged from 0.2 to 5 meters, i.e., shorter or equal to the distance between non-independent patches in the experiment reported here. Furthermore, 15% of these studies compared shoots or leaves on the same plant, in which case the visiting ants are very likely to belong to the same colony. In the two studies that used randomly distributed experimental and control plants (Mathews et al. 2011; Oliveira et al. 1999), distances between treatments were sometimes greater, ranging from 0.5 m to 20 m and from approx. 3 m to 15 m. Depending on the characteristics of the ants being investigated, some of these studies may have used distances sufficient to avoid non-independent behavior. However, in no case did the researchers attempt to determine the number or location of the ant colonies they were working with.
Many of the 26 studies we examined cited the advantage of close placement of treatments and controls because other variables, e.g., microhabitat, were held as constant as possible. Control of these variables is a significant advantage in many studies, when variation occurs between microhabitat or individual plants. However, our study demonstrates a possible disadvantage of this experimental design: proximity comes at a potentially significant cost in interpreting the experimental results when the non-independence of ant behavior is considered. We suggest that this potential cost should be weighed against the advantages of close placement of treatments and controls in the design of these experiments.
The results of the present study suggest that ideally, in experimental studies of ant protection mutualisms, each control and treatment patch should be associated with a different ant colony. In the many cases where genetic investigations of colony identity are impractical, behavioral assays such as the ant-transfer experiment reported here can be informative as a quick method for determining ant colony identity in many species that retain a sense of colony identity (but see Steiner et al. 2007). However, given the spatial extent of many colonies, including the largest we describe in this study, using separate colonies for all treatments could require impractically large study areas or result in insufficient sample sizes. Tests to determine the distances at which non-independence occur could therefore be useful; for instance our study indicated that there is a “sphere of influence” within C. opuntiae colonies beyond which the effects of nectar augmentation will have no effect on recruitment. Clearly, the specific traits of each ant species and the spatial distributions of EFN plants will affect non-independence of ant behavior differently among study systems. Our conclusion, however, is that given the likely non-independence of close patches, it is essential that replicates and treatments either be very widely dispersed, or that a pilot experiment of the type reported here be conducted in advance of selecting the appropriate spatial scale of the experiment.
We would also caution that, although we only observed non-independence of recruitment at short distances within colonies, longer-term experiments could have wider-ranging effects on worker behavior among plants. Furthermore, although we only tested the effect of augmenting food on recruitment to neighboring plants, we expect that other common experimental treatments such as excluding ants from certain plants may also cause non-independence of behavior. Ant species with polydomous nesting habits frequently transfer both food and workers between nests (Debout et al. 2007; Pfeiffer a nd Linsenmair 1998). The diet of all members of the colony may therefore be fairly homogeneous, with food collected throughout the colony territory shared among workers. Colony-level nutritional needs can change both the distribution of foragers among resources (Portha et al. 2002) and the aggressiveness of workers (Ness et al. 2009). Thus, experiments that supplement or reduce food availability over long periods of time could eventually cause significant changes in worker behavior, or even nest distribution throughout entire colonies. For long-term manipulative studies of facultative ant-plant mutualisms, the only certain way to avoid these potential problems is to limit the unit of replication to the colony level.
Acknowledgements
We would like to thank Joshua Ness and Bill Morris for their input throughout the design and conduct of these experiments. We also wish to thank David Holway, and all the members of the Bronstein lab group for their advice and comments on this manuscript. Carolyn Camp, Rebecca Ruppel, and Andrew Waser provided assistance in the field. This work was funded by an International Arid Lands Consortium (No. 03R-25) grant to Judith Bronstein, Ido Izhaki, and Ran Nathan, a University of Arizona Center for Insect Science grant to Michele Lanan, and a NIH Postdoctoral Excellence in Research and Teaching (PERT) fellowship to Michele Lanan
Footnotes
Author Contributions: ML and JB conceived and designed the experiments. ML performed the experiments and analyzed the data. ML wrote the manuscript with editorial advice from JB.
All experiments described herein comply with the laws of the country in which they were performed. The authors declare that they have no conflict of interest.
References
- Anderson C, McShea DW. Intermediate-level parts in insect societies: adaptive structures that ants build away from the nest. Insectes Sociaux. 2001;48:291–301. [Google Scholar]
- Bluthgen N, Fiedler K. Competition for composition: Lessons from nectar-feeding ant communities. Ecology. 2004a;85:1479–1485. [Google Scholar]
- Bluthgen N, Fiedler K. Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community. Journal of Animal Ecology. 2004b;73:155–166. [Google Scholar]
- Bluthgen N, Gottsberger G, Fiedler K. Sugar and amino acid composition of ant-attended nectar and honeydew sources from an Australian rainforest. Austral Ecology. 2004;29:418–429. [Google Scholar]
- Bronstein JL. Our Current Understanding of Mutualism. Quarterly Review of Biology. 1994;69:31–51. [Google Scholar]
- Bronstein JL. The contribution of ant plant protection studies to our understanding of mutualism. Biotropica. 1998;30:150–161. [Google Scholar]
- Byk J, Del-Claro F. Ant-plant interaction in the Neotropical savanna: direct beneficial effects of extrafloral nectar on ant colony fitness. Population Ecology. 2011;53:327–332. [Google Scholar]
- Chamberlain SA, Holland JN. Body size predicts degree in ant-plant mutualistic networks. Functional Ecology. 2009a;23:196–202. [Google Scholar]
- Chamberlain SA, Holland JN. Quantitative synthesis of context dependency in ant-plant protection mutualisms. Ecology. 2009b;90:2384–2392. doi: 10.1890/08-1490.1. [DOI] [PubMed] [Google Scholar]
- Cogni R, Freitas AVL, Oliveira PS. Interhabitat differences in ant activity on plant foliage: ants at extrafloral nectaries of Hibiscus pernambucensis in sandy and mangrove forests. Entomologia Experimentalis Et Applicata. 2003;107:125–131. [Google Scholar]
- Cuautle M, Rico-Gray V. The effect of wasps and ants on the reproductive success of the extrafloral nectaried plant Turnera ulmifolia (Turneraceae) Functional Ecology. 2003;17:417–423. [Google Scholar]
- Cushman JH, Whitham TG. Competition mediating the outcome of a mutualism: protective services of ants as a limiting resource for membracids. American Naturalist. 1991;138 [Google Scholar]
- Davidson DW. The role of resource imbalances in t he evolutionary ecology of tropical arboreal ants. Biological Journal of the Linnean Society. 1997;61:153–181. [Google Scholar]
- Debout G, Schatz B, Elias M, McKey D. Polydomy in ants: what we know, what we think we know, and what remains to be done. Biological Journal of the Linnean Society. 2007;90:319–348. [Google Scholar]
- Dejean A, et al. Spatial Distribution of Dominant Arboreal Ants in a Malagasy Coastal Rainforest: Gaps and Presence of an Invasive Species. Plos One. 2010;5 doi: 10.1371/journal.pone.0009319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Castelazo C, Guimaraes PR, Jordano P, Thompson JN, Marquis RJ, Rico-Gray V. Changes of a mutualistic network over time: reanalysis over a 10-year period. Ecology. 2010;91:793–801. doi: 10.1890/08-1883.1. [DOI] [PubMed] [Google Scholar]
- Dreisig H. Defense by exploitation in the Florida carpenter ant, Camponotus floridanus at an extrafloral nectar resource. Behavioral Ecology and Sociobiology. 2000;47:274–279. [Google Scholar]
- Dutra HP, Freitas AVL, Oliveira PS. Dual ant attraction in the neotropical shrub Urera baccifera (Urticaceae): the role of ant visitation to pearl bodies and fruits in herbivore deterrence and leaf longevity. Functional Ecology. 2006;20:252–260. [Google Scholar]
- Guimaraes PR, Rico-Gray V, dos Reis SF, Thompson JN. Asymmetries in specialization in ant-plant mutualistic networks. Proceedings of the Royal Society B-Biological Sciences. 2006;273:2041–2047. doi: 10.1098/rspb.2006.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, McKey D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annual Review of Ecology Evolution and Systematics. 2003;34:425–453. [Google Scholar]
- Holland JN, Chamberlain SA, Horn KC. Optimal defence theory predicts investment in extrafloral nectar resources in an ant-plant mutualism. Journal of Ecology. 2009;97:89–96. [Google Scholar]
- Holldobler B, Lumsden CJ. Territorial strategies in ants. Science. 1980;210:732–739. doi: 10.1126/science.210.4471.732. [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Massachusetts: Belknap Press of Harvard University Press Cambridge; 1990. [Google Scholar]
- Holway DA, Case TJ. Mechanisms of dispersed central-place foraging in polydomous colonies of the Argentine ant. Animal Behaviour. 2000;59:433–441. doi: 10.1006/anbe.1999.1329. [DOI] [PubMed] [Google Scholar]
- Inouye DW, Taylor OR., Jr A temperate region plant-ant-seed predator system: consequences of extra floral nectar secretion by Helianthella quinquenervis. Ecology. 1979;60:1–7. [Google Scholar]
- Keeler KH. World list of angiosperms with extrafloral nectaries. University of Nebraska Lincoln; 2008. [Google Scholar]
- Lanan MC. PhD dissertation. Tucson, Arizona, USA: University of Arizona; 2010. Collective decision-making and foraging in a community of desert ants. [Google Scholar]
- Lenoir L. Response of the foraging behaviour of red wood ants (Formica rufa group) to exclusion from trees. Agricultural and Forest Entomology. 2003;5:183–189. [Google Scholar]
- Mathews CR, Bottrell DG, Brown JH. Interactions between extrafloral nectaries, ants (Hymenoptera: Formicidae), and other natural enemies affect biological control of Grapholita molesta (Lepidoptera: Tortricidae) on Peach (Rosales: Rosaceae) Environmental Entomology. 2011;40:42–51. doi: 10.1603/EN10161. [DOI] [PubMed] [Google Scholar]
- McIver JD. Dispersed central place foraging in Australian meat ants. Insectes Sociaux. 1991;38:129–137. [Google Scholar]
- Miller TEX. Does having multiple partners weaken the benefits of facultative mutualism? A test with cacti and cactus-tending ants. Oikos. 2007;116:500–512. [Google Scholar]
- Morales MA. Survivorship of an ant-tended membracid as a function of ant recruitment. Oikos. 2000;90:469–476. [Google Scholar]
- Morris WF, Wilson WG, Bronstein JL, Ness JH. Environmental forcing and the competitive dynamics of a guild of cactus-tending ant mutualists. Ecology. 2005;86:3190–3199. [Google Scholar]
- Ness JH. A mutualism's indirect costs: the most aggressive plant bodyguards also deter pollinators. Oikos. 2006;113:506–514. [Google Scholar]
- Ness JH, Morris WF, Bronstein JL. Integrating quality and quantity of mutualistic service to contrast ant species protecting Ferocactus wislizeni. Ecology. 2006;87:912–921. doi: 10.1890/0012-9658(2006)87[912:iqaqom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ness JH, Morris WF, Bronstein JL. For ant-protected plants, the best defense is a hungry offense. Ecology. 2009;90:2823–2831. doi: 10.1890/08-1580.1. [DOI] [PubMed] [Google Scholar]
- Oliveira PS, Rico-Gray V, Diaz-Castelazo C, Castillo-Guevara C. Interaction between ants, extrafloral nectaries and insect herbivores in Neotropical coastal sand dunes: herbivore deterrence by visiting ants increases fruit set in Opuntia stricta (Cactaceae) Functional Ecology. 1999;13:623–631. [Google Scholar]
- Pfeiffer M, Linsenmair KE. Polydomy and the organization of foraging in a colony of the Malaysian giant ant Camponotus gigas (Hym./Form.) Oecologia. 1998;117:579–590. doi: 10.1007/s004420050695. [DOI] [PubMed] [Google Scholar]
- Portha S, Deneubourg JL, Detrain C. Self-organized asymmetries in ant foraging: a functional response to food type and colony needs. Behavioral Ecology. 2002;13:776–781. [Google Scholar]
- Prasad S, Sukumar R. Context-dependency of a complex fruit-frugivore mutualism: temporal variation in crop size and neighborhood effects. Oikos. 2010;119:514–523. [Google Scholar]
- Quinet Y, Hamidi R, Ruiz-Gonzalez MX, de Biseau J-C, Longino JT. Crematogaster pygmaea (Hymenoptera: Formicidae: Myrmicinae), a highly polygynous and polydomous Crematogaster from northeastern Brazil. Zootaxa. 2009:45–54. [Google Scholar]
- Rico-Gray V, Oliveira AT. The Ecology and Evolution of Ant-Plant Interactions. Chicago: The University of Chicago Press; 2007. [Google Scholar]
- Rosumek FB, et al. Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia. 2009;160:537–549. doi: 10.1007/s00442-009-1309-x. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Gardener MC. Extrafloral nectar as a resource mediating multispecies interactions. Ecology. 2004;85:1495–1502. [Google Scholar]
- Saracco JF, Collazo JA, Groom MJ, Carlo TA. Crop size and fruit neighborhood effects on bird visitation to fruiting Schefflera morototoni trees in Puerto Rico. Biotropica. 2005;37:81–87. [Google Scholar]
- Somanathan H, Borges RM, Chakravarthy VS. Does neighborhood floral display matter? Fruit set in carpenter bee-pollinated Heterophragma quadriloculare and beetle-pollinated Lasiosiphon eriocephalus. Biotropica. 2004;36:139–147. [Google Scholar]
- Steiner FM, et al. Abandoning aggression but maintaining self-nonself discrimination as a first stage in ant supercolony formation. Current Biology. 2007;17:1903–1907. doi: 10.1016/j.cub.2007.09.061. [DOI] [PubMed] [Google Scholar]
- Styrsky JD, Eubanks MD. A facultative mutualism between aphids and an invasive ant increases plant reproduction. Ecological Entomology. 2010;35:190–199. [Google Scholar]
- Tillberg CV, Breed MD. Placing an omnivore in a complex food web: Dietary contributions to adult biomass of an ant. Biotropica. 2004;36:266–272. [Google Scholar]
- Trager MD, et al. Benefits for plants in ant-plant protective mutualisms: A meta-analysis. Plos One. 2010;5 doi: 10.1371/journal.pone.0014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Nicklen EF. Ant nest location, soil nutrients and nutrient uptake by ant-associated plants: does extrafloral nectar attract ant nests and thereby enhance plant nutrition? Journal of Ecology. 2010;98:614–624. [Google Scholar]
- Wilder SM, Holway D, Suarez AV, Eubanks MD. Macronutrient content of plant-based food affects growth of a carnivorous arthropod. Ecology. 2011;92:325–332. doi: 10.1890/10-0623.1. [DOI] [PubMed] [Google Scholar]