Abstract
Countries with high lactase nonpersistence (LNP) or low lactase persistence (LP) populations have lower rates of some “western” diseases, mimicking the effects of sunshine and latitude. Inflammatory bowel disease (IBD), ie, Crohn’s disease and ulcerative colitis, is putatively also influenced by sunshine. Recent availability of worldwide IBD rates and lactase distributions allows more extensive comparisons. The aim of this study was to evaluate the extent to which modern day lactase distributions interact with latitude, sunshine exposure, and IBD rates. National IBD rates, national distributions of LP/LNP, and population-weighted average national annual ultraviolet B exposure were obtained, estimated, or calculated from the literature. Negative binomial analysis was used to assess the relationship between the three parameters and IBD rates. Analyses for 55 countries were grouped in three geographic domains, ie, global, Europe, and non-Europe. In Europe, both latitude and ultraviolet B exposure correlate well with LP/LNP and IBD. In non-Europe, latitude and ultraviolet B exposure correlate weakly with LP/LNP, but the latter retains a more robust correlation with IBD. In univariate analysis, latitude, ultraviolet B exposure, and LP/LNP all had significant relationships with IBD. Multivariate analysis showed that lactase distributions provided the best model of fit for IBD. The model of IBD reveals the evolutionary effects of the human lactase divide, and suggests that latitude, ultraviolet B exposure, and LP/LNP mimic each other because LP/LNP follows latitudinal directions toward the equator. However, on a large scale, lactase patterns also follow lateral polarity. The effects of LP/LNP in disease are likely to involve complex interactions.
Keywords: lactase, latitude, ultraviolet B exposure, evolution, inflammatory bowel disease
Introduction
The ability of adults to digest lactose in milk divides humanity into two phenotypes. Those able to digest this disaccharide in adulthood are considered lactase-persistent (LP), while those who lose this ability are called lactase-nonpersistent (LNP). The enzyme lactase phlorizin hydrolase, residing on the brush border of the proximal intestine, is genetically determined.1 Several polymorphisms have been identified which control lactase transcription in a cis position distally on chromosome 2.1–5 There are distinct geographic patterns of the LP/LNP phenotype, with global population distributions of roughly one third LP and two thirds LNP. Modern day geographic distributions were determined by evolution and migrations 7,000–10,000 years ago (approximate time of appearance of LP phenotype). Independent emergence of the LP phenotype in Africa and the Middle East and further migrations after the discovery of the New World contributed to modern day LP/LNP patterns.6–8 The hypotheses regarding emergence of LP status concern latitude, sunshine, and ultraviolet B exposure (the calcium assimilation hypothesis) and simultaneous evolution of genes related to pastoralism and animal husbandry (the gene-culture coevolution hypothesis). In Africa and the Middle East, pastoralism is dominant, while both are possible in Europe.1,9,10
Within the last six decades, studies have compared national lactase distributions with certain national disease rates.11–13 In a previous publication, five “western” cancers and one “eastern” cancer were compared according to distribution of LP/LNP, and it was reported that cancers of the colon, ovary, prostate, breast, and lung were less common in populations with increasing LNP frequency, but stomach cancer was proportionately increased. These conclusions were based on the limited available data.13 Crohn’s disease (CD) and ulcerative colitis (UC) (both are types of inflammatory bowel disease [IBD]), common in industrialized nations, were found to share diminished rates with increased LNP status.12,13 In this same time period, diseases in industrialized regions have been observed to follow distinct geographic patterns, with a north-to-south gradient. These include cancers,14,15 hematological malignancies,16,17 multiple sclerosis,18 CD, UC,19,20–22 and other diseases. The putative rate modifier in the north-to-south gradient paradigm may be sunshine, ultraviolet B exposure, and subsequently vitamin D synthesis.14,15
Since the rate-reducing effects of lower latitude, higher ultraviolet B exposure, and higher frequency of LNP populations with some diseases are similar, we hypothesized that the combined effects of ultraviolet B exposure and LP/LNP distribution may have determined recent geographic disease patterns.
In this paradigm, not only is there a north-to-south direction but also some lateral changes in regions. Recent availability of additional data on both IBD rates23 and LNP frequencies24 allow a re-evaluation of these diseases with respect to latitude, ultraviolet B exposure, and distribution of LP/LNP. In this context, IBD offers a model for evaluation of the hypothesis that LP/LNP serves an evolutionary background for a number of modern day diseases.5,25
Materials and methods
Literature search
There are limited data available for matching regional IBD rates with regional lactase phenotypes. Therefore, national data were sought or estimated from the literature. Most data on disease rates for IBD were based on one recent publication.23 Some rates were obtained from other reviews.26–29 PubMed and Google Scholar were also consulted to determine further incidence rates. For IBD, the MeSH words used were: “incidence, prevalence of Crohn’s disease and/or ulcerative colitis around the world” or “international”, “national rates of Crohn’s disease”, ulcerative colitis”, “change in epidemiology or epidemiology” or “inflammatory bowel disease or Crohn’s disease or ulcerative colitis”.
As a control against using average values for latitude and national IBD rates, CD rates for individual European cities as published by Molodecky et al were also correlated with their latitudes.23 Latitudes for individual cities were obtained from the Internet and are presumed to be more focused.
Similarly, a recent review was used for lactase distribution,24 but older reviews were also searched.11,30,31 For further lactase distribution, the terms “lactase persistence or non-persistence”, “lactose maldigestion or intolerance”, or “genetics of lactase” were used. In each topic, individual references listed in relevant papers were also evaluated. Several authors were contacted directly for national estimates either on IBD rates (based on published reports32–34) or LP proportions.35 Finally, for lactase distributions, two online distribution maps were used as a rough estimate of LP/LNP proportions24 (http://www.britannica.com/EBchecked/media/157598/Global-distribution-of-lactose-intolerance-in-humans).
Selection criteria
As stated above, national rates were preferred, failing which regional rates that could be used to estimate national rates were sought. Several publications were also used in which rates could be deduced by the methods described above. These include intestinal biopsies36 and genetic studies for lactase.37,38 These were used to estimate LP/LNP rates.
Handling of data
Nationwide rates were included for the time period described in the Results section. Because some of these were unavailable as single values, available regional data for IBD or ethnic/racial distributions within countries for lactase proportions were used to calculate national rates, using equations 1 and 2. Regional data were matched with populations around the time the data were published. Actual numbers of patients were calculated and added for each available region. Summed populations were calculated as representative of a nation matched for the population during that period. The total numbers were then proportioned to incidence rates in 105.
Estimation of national disease rates (D) based on regional data where Xi is the number of patients with new disease in region or city “i”, Ai is population of region/city, and P is the total population
| [1] |
where and n is the number of cities and/or regions.
Estimation of national LP or LNP rates (L) based on ethnic population percentage, Ei, and fractional ethnic population, fi.
| [2] |
where m is the total number of ethnic groups.
National yearly ultraviolet B (280–315 nm) exposures were deduced from the data of Lee-Taylor and Madronich39 and have been described previously.13 Briefly, monthly surface level radiation based on a radiative transfer model driven by satellite-measured variables was used. Annual averages from the sum of monthly averages for the period 1990–2000 were computed. To obtain a single representative value for each of the countries, population-weighted averages for ultraviolet B surface radiation were calculated for the locations of the largest population centers in each country. A single population-weighted latitude was calculated for each country using the same population weighting as used for calculation of the population-weighted ultraviolet B exposure.
Calculation of national annual average ultraviolet B exposure
| [3] |
where Pi are the populations of the N population centers considered and here .
UVBi is the annual ultraviolet B exposure at population center i. The number of population centers (N) included in the calculation of a national average varied from one for small countries to typically ten or more for the larger countries with many large population centers.
For the results that are presented in terms of a single latitude for each country, a population-weighted latitude was calculated for the country using the same population weighting as applied for calculation of the population-weighted ultraviolet B exposure. So,
| [4] |
where LATi is the latitude of population center i. Local and national populations were obtained from census populations and corresponded to the median of the time range for the published observation periods.
Statistical analysis
Relationships between latitude, ultraviolet B exposure, LP/LNP distribution, and rates of CD and UC were assessed using Spearman correlation coefficients because of the non-normal distributions for most of the variables (especially IBD rates). The relationship between the three global variables and the IBD rates were first assessed using Spearman correlation coefficients, and then by univariate and multivariable analyses. Since correlations between some explanatory variables are very strong (eg, >0.9 between ultraviolet B exposure and latitude), when conducting the multivariable analysis, highly correlated variables (correlations >0.5) were not entered into the model at the same time.
Both the dependent variables, ie, CD rate and UC rate, were positive integers showing an overdispersed Poisson distribution (variance greater than the mean). Hence, linear models were not appropriate, and negative binomial regressions were used in both univariate and multivariable analyses. In a few cases was a negative binomial model did not converge at a specified limit (ie, the relative Hessian convergence criterion was greater than 0.0001), a Poisson model that converged normally and provided similar estimates was used instead. The exponentiation of the parameter estimate (EPE) of the negative binomial regression model can be interpreted in a multiplicative manner, or as a disease rate ratio. An EPE >1 indicates that a higher value for a continuous independent variable is associated with a greater disease rate, and an EPE of <1 indicates the opposite. For example, an EPE of 0.7 for ultraviolet B exposure indicates that the disease rate will decrease by a factor of 0.7 when there is a 10-unit increase in ultraviolet B exposure, if everything else is the same with regard to all other covariates.
Analyses were carried out for three geographic areas, ie, global, Europe, and non-Europe. Sensitivity analyses were carried out for three scenarios. In two cases, the proportions of LNP for three countries (Argentina, Panama, and Malta) were based on line map data only, and as such were deemed less reliable. In the case of Oman, data were also obtained from a more accurate projected map referenced in Itan et al.24 Thus, these data were handled in two ways. In scenario 1, the proportion of LNP was reduced by 50% for three countries (Argentina, Panama, Malta), while in scenario 2, the four countries were removed whenever LNP/LP was involved in the analysis. The third case involves the rate of CD in Hungary. The original analysis used a rate of 2.2 per 105 population based on Bernstein and Shanahan,26 while calculations from Molodecky et al23 based on two regions gave an estimate of 4.6 per 105 population. Thus, in scenario 3, the rate of CD in Hungary was taken as 4.6 instead of 2.2 per 105 population from the original analysis.
As a control for the calculated data, we also analyzed CD rates in specific cities in Europe (from Molodecky et al23) against city latitudes using Pearson’s correlations. All statistical analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA).
Results
Seventeen papers were used for IBD rates and 26 for lactase rates. These included three authors who responded to contact, ie, two for IBD incidence rates (Dr CC Figueroa from Chile, Dr I Hilmi from Malaysia) and one for lactase distribution (Dr J Rocha from Portugal). LP/LNP rates for Argentina, Panama, and Malta were based on an online distribution map as described earlier, and Oman was also derived from a map reference.24
Incidence rates were restricted to study periods between 1980 and 2008. Whenever possible, the more recent data were used. Values for CD (38/52 countries, 73%) and UC (38/48 countries, 88%) were based on data published by Molodecky et al.23 For CD and UC, respectively, 15.4% and 23% of national rates were calculated. The IBD rates based on responses from Chile32 and Malaysia33,34 are listed as estimates (4% of total for each disease).
National lactase phenotype distributions (n=54) were derived from a variety of published reports as outlined, 16/54 (30%) were derived from Itan et al,24 calculated lactase rates were 2%, while national rates for LP/LNP frequencies were estimated in 13%.35 Rates for IBD and lactase distribution are listed in Table 1.
Table 1.
National population-weighted latitudes and average annual calculated ultraviolet B exposures
| Country | Latitude | Longitude | UVB/year (kJ/m2) | LNP% | CD inc/105 | UC inc/105 | Population (in millions) | Median year | Reference for IBD | Reference for lactase |
|---|---|---|---|---|---|---|---|---|---|---|
| Argentina | 34 | 64 | 10,033 | 80λ | 0.06 | 2.17 | 36.95 | 2000 | 23 | Web** |
| China | 32 | 105 | 7,492 | 92 | 0.85 | – | 1,300 | 2003 | 23 | 50 |
| Greece | 39 | 22 | 7,258 | 75 | 2.76* | 3.9* | 10.66 | 1998 | 23 | 51 |
| Iran | 32 | 53 | 9,909 | 86 | 0.29* | 0.42 | 70.58 | 2006 | 42 | 52 |
| Israel | 35 | 34 | 9,951 | 72 | 5 | 5.04 | 5.9 | 2000 | 27 | 24,26 |
| Japan | 36 | 138 | 6,490 | 90 | 0.9 | 0.28 | 125.7 | 1996 | 23 | 53 |
| South Korea | 37 | 137 | 6,562 | 76 | 0.53 | 1.51 | 47.47 | 2000 | 23 | 30 |
| Lebanon | 33 | 35 | 9,213 | 78 | 1.4 | 4.1 | 3.76 | 2007 | 23 | 54 |
| Malaysia | 3 | 102 | 12,420 | 88 | 0.5λ | 0.5λ | 23.95 | 2000 | 33–34 | 55 |
| South Africa | 29 | 24 | 11,074 | 91 | 1.79* | 2.64* | 35.2 | 1990 | 23 | 31 |
| Sri Lanka | 7 | 81 | 13,417 | 72.5 | 0.09 | 0.69 | 19.37 | 2003 | 23 | 56 |
| Taiwan | 25 | 121 | 9,847 | 92 | 2 | – | 23.1 | 2011 | 23 | 50 |
| Tunisia | 37 | 9 | 8,342 | 79.7 | 1.24 | – | 8.79 | 1994 | 43 | 57 |
| Turkey | 40 | 35 | 6,798 | 71.3 | 2.2 | 4.4 | 63.63 | 2000 | 23 | 58 |
| Australia | 34 | 133 | 8,921 | 6 | 6.96 | 17.4* | 19.15 | 2000 | 23 | 59 |
| Austria | 48 | 13 | 4,560 | 19.8 | 6.7 | 4.8 | 8.1 | 2003 | 44 | 24 |
| Barbados | 13 | 59 | 15,300 | – | 0.7 | 1.85 | 0.27 | 2003 | 23 | – |
| Belgium | 51 | 4 | 3,951 | 13 | 4 | 3.22 | 10.13 | 1995 | 23 | 36 |
| Canada | 46 | 95 | 4,809 | 6.6 | 13.4 | 11.8 | 30.69 | 2000 | 23 | 24 |
| Czech Republic | 50 | 15 | 4,138 | 18 | 1.5 | 1.3 | 10.193 | 2000 | 23 | 24 |
| Denmark | 56 | 10 | 3,513 | 4 | 4.6 | 13.2 | 5.33 | 2000 | 23 | 24 |
| Estonia | 59 | 26 | 3,189 | 25 | 1.4 | 1.7 | 1.43 | 2000 | 23 | 60 |
| Finland | 60 | 26 | 2,876 | 17 | 9.2 | 24.8 | 5.3 | 2007 | 45 | 24 |
| Germany | 51 | 9 | 4,054 | 14.6 | 4 | 3.23* | 81.64 | 1995 | 23 | 24 |
| Iceland | 64 | 18 | 1,745 | 4 | 5.5 | 16.5 | 0.26 | 1992 | 23 | 38 |
| Ireland | 53 | 8 | 3,349 | 4 | 5.9 | 14.8 | 3.6 | 1996 | 23 | 24 |
| The Netherlands | 52 | 5 | 3,775 | 4 | 6.9 | 10 | 16.15 | 2003 | 23 | 38 |
| New Zealand | 39 | 174 | 7,004 | 9 | 13.75* | 6.07* | 3.86 | 2000 | 23 | 61 |
| Norway | 60 | 10 | 2,820 | 4 | 5.8* | 12.8* | 4.55 | 2002 | 23 | 62 |
| Slovakia | 48 | 19 | 4,611 | 18 | 6.75 | – | 5.35 | 1994 | 23 | 24 |
| Sweden | 58 | 15 | 3,272 | 8 | 8.9 | – | 8.9 | 2000 | 27 | 63 |
| Switzerland | 47 | 8 | 4,826 | 10 | 1.6 | – | 5.9 | 1965 | 23 | Web** |
| UK | 53 | 2 | 3,657 | 5 | 8 | 2 | 58.17 | 1996 | 23 | 24 |
| USA | 37 | 97 | 7,315 | 28.5* | 6.79* | 11.23* | 287.8 | 2002 | 23 | 24 |
| Malta | 36 | 14 | 8,375 | 34 | 1.29 | 7.88 | 0.39 | 2000 | 23 | Web** |
| Oman | 21 | 57 | 8,375 | 53λ | – | 1.35 | 1.6 | 1991 | 23 | 24 |
| Panama | 9 | 80 | 13,604 | 70λ | 0 | 1.2 | 3.07 | 2002 | 23 | Web** |
| Bosnia | 44 | 18 | 5,556 | 35 | 2.3* | 3.43* | 3.9 | 2011 | 23 | 11 |
| Brazil | 19 | 55 | 11,334 | 57 | 1.48 | 3.96 | 176 | 2002 | 23 | 64 |
| Chile | 32 | 71 | 10,336 | 66 | 1λ | 1λ | 15.15 | 2000 | 32 | 24 |
| Croatia | 45 | 15 | 5,452 | 35 | 1.89 | 1.78* | 4.28 | 2000 | 23 | 11 |
| France | 48 | 2 | 4,920 | 37 | 4.6 | 3.8 | 58.59 | 1998 | 23 | 11 |
| Hungary | 47 | 20 | 4,970 | 37 | 2.2*** | 5.89* | 10.4 | 2000 | 26 | 65 |
| India | 21 | 77 | 12,298 | 67.5 | – | 6.02 | 1,020 | 2001 | 23 | 66 |
| Italy | 42 | 12 | 6,470 | 51 | 2.28 | 5.17 | 56.11 | 1996 | 23 | 24,30 |
| Kuwait | 29 | 47 | 11,270 | 47 | 2.8 | 2.27 | 2.7 | 2010 | 23 | 67 |
| Lithuania | 55 | 24 | 3,640 | 35.6 | 2.01 | 11.9 | 3.4 | 2007 | 29,46 | 68 |
| Mexico | 21 | 102 | 13,692 | 70 | – | 2.13* | 102.6 | 2002 | 47 | 11 |
| Poland | 51 | 20 | 4,011 | 37.5 | 0.1 | 1.8 | 38.2 | 2003 | 29 | 69 |
| Portugal | 40 | 8 | 7,123 | 40λ | 2.99 | 3.6 | 9.9 | 1990 | 23 | 37 |
| Romania | 46 | 25 | 5,367 | 55λ | 0.5 | 0.97 | 21.8 | 2002 | 23 | 29 |
| Saudi Arabia | 25 | 45 | 13,042 | 53 | 1.66 | – | 22.3 | 2003 | 48 | 24 |
| Serbia | 44 | 21 | 5,426 | 35 | 1.84* | 1.31* | 7.7 | 1996 | 23 | 11 |
| Spain | 40 | 4 | 7,156 | 34 | 5.5 | 8 | 40.28 | 2000 | 23 | 24 |
| Uruguay | 33 | 56 | 8,810 | 65 | 0.74 | 4.26 | 3.3 | 2003 | 49 | 70 |
Notes: Lactase nonpersistence frequencies are derived from the indexed references. Incidence/105 of Crohn’s disease and ulcerative colitis are derived from the indexed references. The population data were obtained from on-line information and are matched as closely as possible to median year of disease incidence acquisition.
Countries denoted where national rates are calculated; λ, countries denoted where national rates are estimated
calculation from Molodecky et al23 for Hungary was 4.6.
Abbreviations: CD, Crohn’s disease; IBD, inflammatory bowel disease; inc, incidence; LNP, lactase nonpersistence; UC, ulcerative colitis; UVB, ultraviolet B exposure.
In general, indirect tests described for lactase distributions included intestinal biopsy and predominantly lactose tolerance and lactose breath hydrogen tests. All three have been validated against lactase genotype.40,41 It is also assumed that national lactase rates have changed more slowly over time, especially in the Old World.
Data from 55 countries were obtained, with a few missing data values. Countries with IBD rates, latitude, annual ultraviolet B exposure (kJ/m2), and percent LNP are listed in Table 1. In subsequent statistical analysis, the measure of ultraviolet B exposure was further divided by 100 (ie, ultraviolet B exposure of 7,000 is referred to as 70) in order to avoid small estimates and to facilitate comparisons. The year 2000 was the median for populations (based on median years of observation) regarding national disease rates. As such, the data cover a period of about three decades.
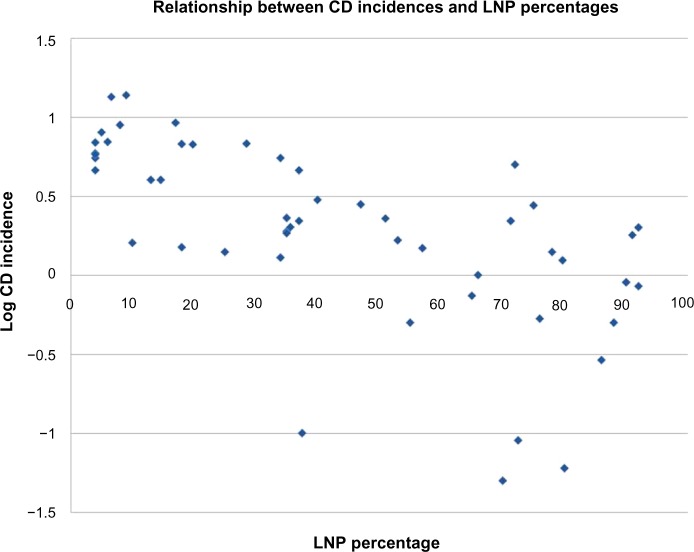
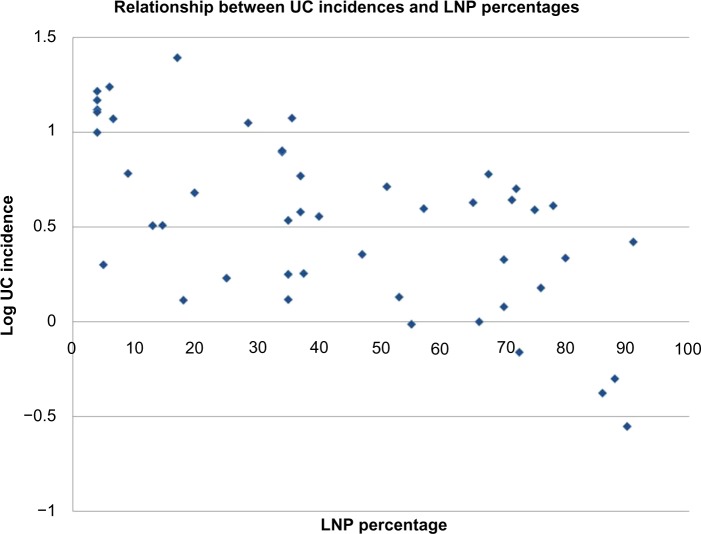
Table 2 shows Spearman correlation coefficients for all variables in the three groups, ie, global, Europe, and non-Europe. Examples of global correlations for LNP and CD and for LNP and UC are shown in Figures 1 and 2. Summary results for r values are shown graphically in Figure 3A–C. In Figure 3C, it can be seen that the influence of latitude and ultraviolet B exposure on LNP in non-Europe decreases dramatically compared with Figure 3A and B. Modest latitudinal effects in non-Europe are evident and less affected by ultraviolet B exposure. In effect, Figure 3A and B show inverse mirror images. In Figure 3C, the modest to moderate influence of LNP on CD and UC is retained in all three geographic groups.
Table 2.
Spearman correlation coefficients of Crohn’s disease (CD), ulcerative colitis (UC), ultraviolet B exposure (UVB), latitude and lactase non persistence (LNP)
| Ulcerative colitis | LNP | UVB | Latitude | |
|---|---|---|---|---|
| Global | ||||
| CD | 0.75 | −0.73 | −0.53 | 0.56 |
| UC | 1 | −0.59 | −0.38 | 0.44 |
| LNP | 1 | 0.74 | −0.76 | |
| UVB | 1 | −0.98 | ||
| Europe | ||||
| CD | 0.68 | −0.59 | −0.38 | 0.39 |
| UC | 1 | −0.42 | −0,41 | 0.41 |
| LNP | 1 | 0.74 | −0.71 | |
| UVB | 1 | −0.99 | ||
| Non Europe | ||||
| CD | 0.79 | −0.54 | −0.40 | 0.49 |
| UC | 1 | −0.68 | −0.35* | 0.55 |
| LNP | 1 | 0.09* | −0.22* | |
| UVB | 1 | −0.86 | ||
Note:
All values were statistically significant except those that have an * mark (ie, P-value >0.05).
Figure 1.
Graphic presentation of the global relationship between CD incidence rates expressed as log CD rate and distributions of LNP as national percentages.
Abbreviations: CD, Crohn’s disease; LNP, lactase nonpersistence.
Figure 2.
Graphic presentation of the global relationship between UC incidence rates expressed as log UC rate and distributions of LNP as national percentages.
Abbreviations: LNP, lactase nonpersistence; UC, ulcerative colitis.
Figure 3.
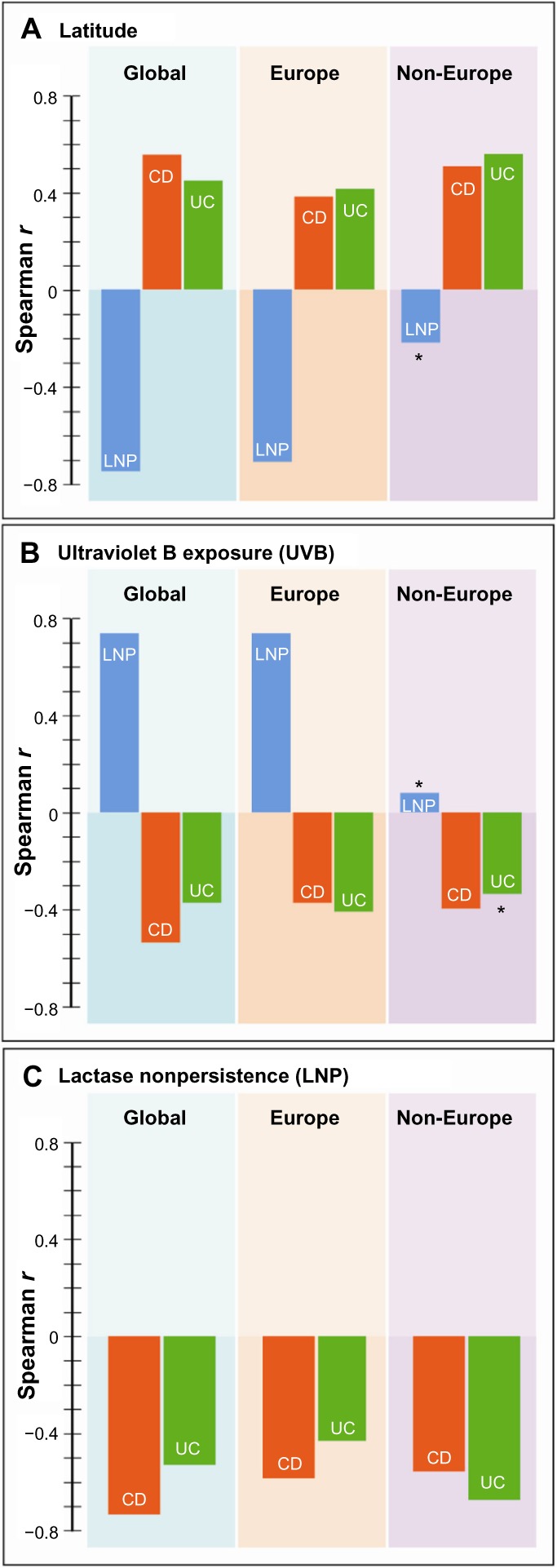
Bar graph distributions of correlations of LNP, CD, or UC with latitude (A), ultraviolet B exposure (B), and relationship of LNP with CD or UC (C) is shown in three geographic domains of global, Europe, or non-Europe.
Notes: (A) and (B) are “mirror images” of the effects of latitude and ultraviolet B exposure on inflammatory bowel disease and lactase distributions. In non-Europe, correlations of LNP with either latitude or ultraviolet B exposure drop to weak correlations which are not statistically significant and the correlation of UC with ultraviolet B exposure becomes nonsignificant. (C) demonstrates that lactase distribution still correlates well with both forms of inflammatory bowel disease. This figure demonstrates an independent mechanistic effect of LP/LNP on inflammatory bowel disease which is different from the effects of latitude/ultraviolet B exposure. *Nonsignificant correlations (P>0.05).
Abbreviations: CD, Crohn’s disease; LNP, lactase nonpersistence; UC, ulcerative colitis; G, global; E, Europe; N, non-Europe; LP, lactase persistence.
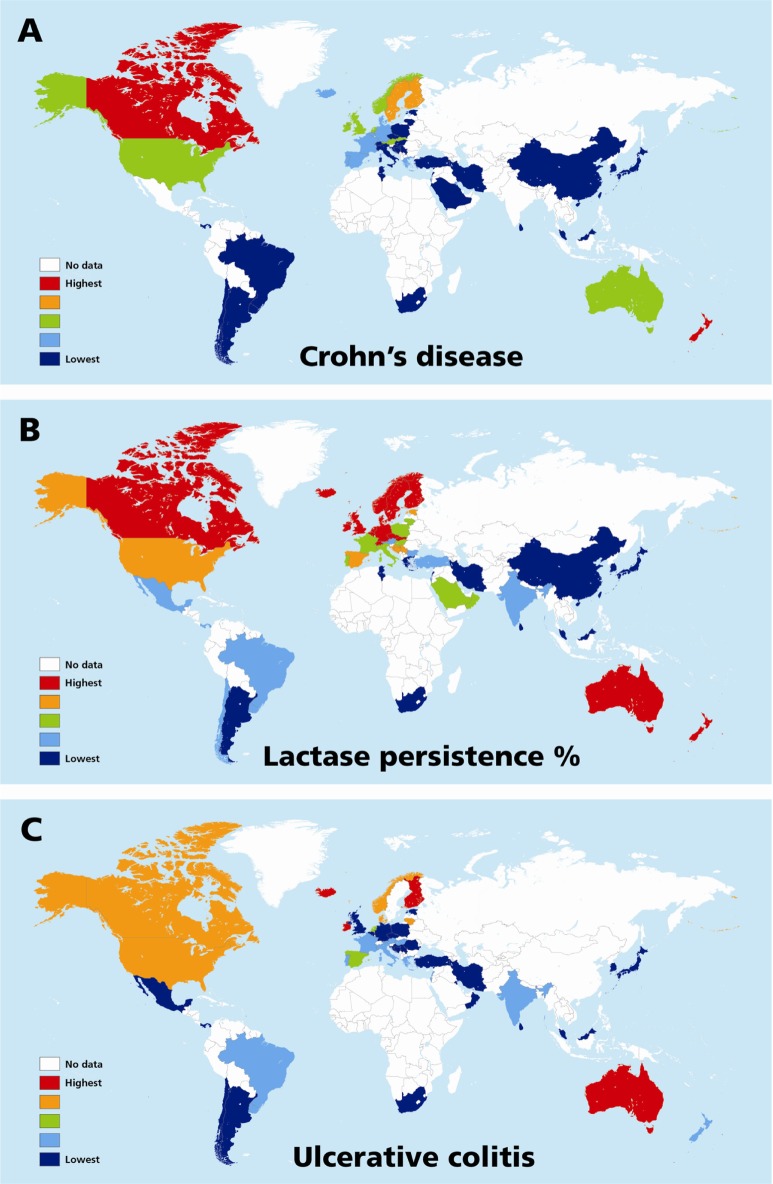
The results of univariate analyses for rates of CD and UC against the three independent variables (latitude, ultraviolet B exposure, and LNP) within the three geographical groupings are shown in Table 3. For both rates, the three variables showed a significant relationship in all three groupings. Table 4 shows the models that provided the best fits from the multivariable analyses. The best-fit model was selected using the Akaike information criterion (a measure of the relative goodness of fit of statistical models). For CD, LNP dominated statistically, both globally and in Europe, while latitude shared dominance with LNP in non-Europe. For UC, LNP dominated statistically, both globally and in non-Europe, while latitude fit the best model in Europe. The values in Tables 3 and 4 are the EPEs from the negative binomial analyses, which can be interpreted in a multiplicative manner, or as a disease rate ratio. For example, the CD rate ratio is 0.78 for LNP in the global data; this means that for every 10-unit increase in the value of LNP (ie, a 10% increase in LNP proportion), the CD rate is decreasing by 0.78 times, or is 22% less. Therefore, populations with 50% LNP are estimated to have 22% lower CD incidence rates than populations with 40% LNP. The opposite effect is true for latitude. The UC rate ratio is 2.01 in Europe for latitude, which means that for every 10-unit increase in latitude (eg, latitude 50 versus 40), the incidence of UC increases 2.01 times, or by 101%. Figure 4A–C shows the recorded global distributions of CD, LP, and LNP, and the incidence of UC. Demarcations are color-divided by quintiles.
Table 3.
Disease rate ratios (95% confidence intervals) from Univariate Negative Binomial Analyses (for every 10-unit change in each variable)
| Disease | Territory | Latitude | UVB | LNP |
|---|---|---|---|---|
| Crohn’s | Global (n 51) | 1.51 (1.24–1.84) | 0.85 (0.79–0.92) | 0.78 (0.72–0.83) |
| Europe (n 27) | 1.49 (1.07–2.07) | 0.83 (0.70–0.98) | 0.79 (0.70–0.89) | |
| Non Europe (n 24) | 2.48 (1.50–4.10) | 0.75 (0.63–0.89) | 0.73 (0.67–0.79) | |
| Ulcerative colitis | Global (n 47) | 1.45 (1.23–1.72) | 0.87 (0.81–0.94) | 0.80 (0.74–0.86) |
| Europe (n 24) | 2.01 (1.36–2.97) | 0.75 (0.62–0.91) | 0.80 (0.67–0.94) | |
| Non Europe (n 23) | 1.71 (1.23–2.38) | 0.84 (0.74–0.96) | 0.78 (0.72–0.84) |
Abbreviations: UVB, ultraviolet B exposure; LNP, lactase nonpersistence.
Table 4.
Disease rate ratios (95% confidence intervals) from Multivariable Negative Binomial Analyses (for every 10-unit change in each variable)
| Disease | Global | Europe | Non-Europe |
|---|---|---|---|
| Crohn’s | n=51 LNP 0.78 (0.72–0.83) |
n=27 LNP 0.79 (0.70–0.89) |
n=24 LNP 0.78 (0.71–0.85) |
| Latitude 1.55 (1.10–2.17) | |||
| Ulcerative colitis | n=47 LNP 0.80 (0.74–0.86) |
n=24 Latitude 2.01 (1.36–2.97) |
n=23 LNP 0.78 (0.72–0.84) |
Abbreviation: LNP, lactase nonpersistence.
Figure 4.
Maps of the world showing distributions of incidence of quintiles of inflammatory bowel disease, ie, Crohn’s disease, (A) ulcerative colitis (C) and percentage lactase persistence (B).
Notes: The map of inflammatory bowel disease covers a time span of about three decades. Color patterns are from highest to lowest frequencies. Quintiles for Crohn’s disease each represent countries with calculated estimate rates of incidence spanning multiples of 2.75/105 cases (overall range 0–13.75), ulcerative colitis 3.48/105 cases (overall range 1–24.8) and lactase persistence 18% of the population (overall range 4–92). Epidemiologically, ulcerative colitis precedes the emergence of Crohn’s disease by about two decades, and in general ulcerative colitis rates are stabilizing in older western societies, but may be rising in regions which are adopting western/industrialized lifestyles. Relatively, LP/LNP distributions may change more slowly. This time difference in disease progression may account for some of the variability in the relationship of ulcerative colitis with LP/LNP. Latitudinal changes in inflammatory bowel disease have also been described in individual countries. Furthermore, it is noted that lactase distributions in (B) show in large scale both latitudinal reduction in LP toward the equator (LNP) as well as lateral changes from politically western toward eastern nations.
Abbreviations: LP, lactase persistence; LNP, lactase nonpersistence.
Sensitivity analysis
In scenarios 1 and 2 (concerning LNP distribution), only non-European countries were affected, so analysis within Europe did not change under these two scenarios. Globally, correlation of LNP with other variables under both scenarios barely changed (all changes are less than 0.03). However, in non- European countries, all changes were small except for the correlation between LNP and CD, which changed from −0.54 to −0.41 when changing the LNP distributions of the three countries. Univariate analyses showed almost no change in the disease rate ratio (changes were no more than 0.01) for either scenario when compared with the original analyses. Multiple variable analyses, which concerned only CD in the non-European theater, showed small changes whereby the disease rate for LNP changed from 0.78 to 0.80 and for latitude from 1.58 to 1.74.
Under scenario 3, whereby rate of CD in Hungary changed from 2.2 to 4.6 per 105 population, only small changes were observed. The correlations between CD with all other variables showed a maximum change of 0.03, while the disease rate ratios showed a maximum change of 0.04. It is expected that changing these results affected the global theater much less than the European or non-European theaters because there were fewer countries for subterritorial analyses.
Finally, to control for outcome in more focused areas, we also did a correlation between CD rates and latitudes in 41 European cities (r=0.55, P=0.002, Table 5). These rate/latitude relationships mimic those found based on extrapolated national correlations in Europe. Lactase distributions were not available for individual cities. The relationship with ultraviolet B exposure would be expected to be similar given that correlations between ultraviolet B exposure and latitude are very high in Europe.
Table 5.
Comparison of latitudes of European cities with incidence
| City | Latitude | CD inc/105 | Population per 105 |
|---|---|---|---|
| Almada | 38.6 | 2.3 | 1.6 |
| Amiens | 49.9 | 8.1 | 1.34 |
| Belgrade | 44.8 | 1.84 | 12 |
| Bologna | 44.5 | 2.7 | 3.8 |
| Braga | 41.5 | 2.5 | 1.09 |
| Brittany | 48 | 2.8 | 447.5 |
| Bucharest | 44.4 | 0.42 | 20 |
| Calais | 50.95 | 4.23 | 0.753 |
| Cardiff | 51.48 | 8.3 | 2.9 |
| Cologne | 50.95 | 5.1 | 10.17 |
| Copenhagen | 55 | 6.6 | 12.3 |
| Crema | 45.36 | 2.7 | 0.58 |
| Dublin | 53.34 | 5.9 | 18.04 |
| Essen | 51.45 | 3.5 | 7.3 |
| Florence | 43.78 | 2.7 | 15 |
| Helsinki | 60.17 | 2.3 | 13.6 |
| Heraklion | 35.3 | 3.9 | 1.73 |
| lonnina | 39.67 | 1 | 1.12 |
| Leicester | 52.6 | 3.7 | 3.3 |
| Liege | 50 | 4.5 | 5.85 |
| Limburg | 50.62 | 6.2 | 11.3 |
| Maastricht | 50.8 | 7.7 | 1.22 |
| Madrid | 40.4 | 7.3 | 40.7 |
| Malta | 35.89 | 1.29 | 4.04 |
| Martinique/Guadalupe | 14.66 | 1.85 | 3.8 |
| Merida | 20.97 | 2.15 | 0.58 |
| Messina | 38.18 | 1.21 | 6.5 |
| Milan | 45.46 | 3.2 | 42.5 |
| Oberpfalze | 49.3 | 6.6 | 10.7 |
| Oslo | 61 | 6.9 | 5.07 |
| Palermo | 38.1 | 5.8 | 7.3 |
| Reggio-E | 44.7 | 4 | 44 |
| Reykjavik | 64 | 8.2 | 1.09 |
| Sabadell | 41.54 | 4.9 | 2.06 |
| Stockholm | 59.3 | 8.3 | 8.71 |
| Tampere | 61.5 | 7.2 | 2.19 |
| Tuzla | 44.5 | 2.3 | 0.607 |
| Upsala | 59.85 | 6.1 | 1.8 |
| Veszprem | 47.09 | 2.23 | 0.643 |
| Vigo | 42.2 | 4.8 | 0.296 |
| Zagreb | 45.8 | 0.7 | 7.9 |
Notes: Populations are based on median from the year 2000. Adapted from Gastroenterology, 142, Molodecky NA, Soon IS, Rabi D, et al, Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on a systematic review, 46–54, copyright (2012), with permission from Elsevier.23
Abbreviations: CD, Crohn’s disease; inc, incidence.
Discussion
While the etiology of IBD remains largely unknown, multiple environmental factors have been implicated.26,71 However, none have been confirmed as causative. Observations of the progression of IBD indicate that UC generally precedes a rise in CD by about two decades. In western countries in general, UC incidence rates are stable or declining, while those of CD are increasing or starting to level off.71 In developing countries and countries adopting the western lifestyle, rates of UC may be rising. Among the variables deemed to be important and relevant, has been the generally consistent finding that incidence and prevalence rates for IBD decrease in a north-to-south direction. The possible role of LP/LNP distribution among variables having an impact has received only occasional mention.12
In the current ecological evaluation, we note modest to moderate correlations between both forms of IBD and latitude and ultraviolet B exposure. The subanalysis of data from European cities with more focused geography and individually more homogeneous populations also supports the north-to-south gradient in Europe. CD and UC are moderately correlated, but there are some differences observed between CD and UC relations. There is a slightly better correlation between CD and either latitude or ultraviolet B exposure in the global domain, but a slightly better correlation between UC with either latitude or ultraviolet B exposure in Europe. There are somewhat divergent correlations with latitude or ultraviolet B exposure in both forms of IBD in non-Europe, such that the correlation with UC is now not statistically significant.
We also note that global correlations between latitude or ultraviolet B exposure and LNP (LP/LNP distribution) drops to very weak levels in non-Europe, suggesting that the global pattern is largely due to that found in Europe. The course of UC likely changed in three decades so that rates may have leveled off, while that of CD is increasing. Further, they reflect the roughly two-decade difference in behavior pattern between UC and CD. While migration and intermarriage change the phenotypes of lactose digestion (LP dominant), these are slower than changes in IBD rates.23 As such, LP/LNP distribution is likely to be more stable than disease distribution.
The impact of LP/LNP on IBD remains robust in all domains including non-Europe and the multivariable analysis supports the primacy of LP/LNP. We interpret this as evidence that the effect of LNP (LP/LNP proportions) is independent from that of latitude and ultraviolet B exposure on IBD.
Studies showing the north-to-south gradient effect on IBD rates
Studies from the latter half of the last century generally observed that IBD rates diminish toward the equator, with rising rates in Australia and New Zealand.71 This pattern of latitudinal change has also been reported from Europe as a whole21,27 and within individual countries. The same gradient has been observed in northern France,72 Scotland,73 and the USA.74,75 In a study of the Nurses Health cohorts I and II, Khalili et al arbitrarily divided the USA into three latitudinal regions, and over the period of the study found that the highest latitudinal region was associated with the highest CD rates.75
No north-to-south gradient is found in Canada, largely because there are few inhabitants in the north. However, the west coast of the country has the lowest rates, while the east coast has the highest.76 The north-to-south gradient for CD appears to be more robust than that for UC. For example, in a study by Nerich et al, no north-to-south gradient for UC was noted, but was noted for CD.72 However, in Finland, no gradient for CD was reported, but a north-to-south gradient was reported for UC. Finland has the highest rates of UC reported to date.77 To our knowledge, there is no detailed analysis from Australia and/or New Zealand, but higher rates would be expected in New Zealand, with a south-to-north gradient in Australia. Part of the observed gradient discrepancies between CD and UC may relate to the fact that UC precedes CD by about 20 years.78
The current report incorporating three decades of disease trends supports the published findings. In addition, a very strong correlation between latitude and ultraviolet B exposure was observed, which in turn was correlated with IBD. This finding provides evidence for this relationship between ultraviolet B exposure and IBD rates.19–21,79,80
IBD, latitude, and the ultraviolet B/vitamin D hypothesis
The main effect of latitude on IBD and other such geographically patterned diseases is thought to be related to sunshine. The effect of ultraviolet B exposure is to increase production of vitamin D in the skin.81,82 Evidence has emerged in the last 15 years that vitamin D modifies the outcome in many diseases, including IBD, through modulation of immunological mechanisms with anticancer and autoimmunity effects.19,81,82 Yet the lack of vitamin D is not the putative cause of IBD and other such diseases. While other variables associated with latitude, such as climate, have also been identified as potentially relevant,83 elements of the western lifestyle are thought to be causative.26 Indeed, current publications on IBD rates show a rise in countries with traditionally high ultraviolet B exposure. Further, differences in IBD rates within regional populations have been found in high latitude northern84 and equatorial or southern hemispheres85,86 with more or less similar ultraviolet B exposure.
Relationship between latitude/ultraviolet B exposure and lactase distribution
In Europe, lack of sunshine and the consequent reduction of vitamin D synthesis in the skin has been postulated to lead to strong selection for LP (the calcium assimilation hypothesis).87 As outlined in the introduction, a counter hypothesis supported by several groups is that LP status evolved in conjunction with pastoralism and herding (the gene-culture coevolution hypothesis).1,8,10 Distribution of lactase phenotypes in the Old World may then have depended both on selection and migrations from central Europe to northern Europe, Russia, and India to the east.8,88,89 Migrations to the New World and South Pacific regions generally were made up of people with LNP, and occurred prior to the emergence of LP genetic dominance. These hypotheses help to explain the more frequent LNP status of indigenous populations in North and South America as well as in New Zealand. The southern continent of Australia and New Zealand was populated much later by LP people from the British Isles.
As seen in Figure 3A–C, the apparent impact of latitude/ultraviolet B exposure is markedly diminished in non-Europe. This effect could be due to the method of assigning single values for each variable to large countries like the USA, People’s Republic of China, and Australia. However, this unusual pattern was also reported previously based on both indirect tests90 and genetic frequencies of LP.9
Relationship between disease and lactase phenotype
If we accept that the current study confirms previous reports of a relationship of latitude and ultraviolet B exposure with IBD and confirms previously reported models of the partial relationship of these variables with LP/LNP proportions, then we should consider the relationship between LP/LNP and IBD. The apparently similar relationship between all three test variables globally and in Europe is challenged by the minimal effect of latitude and ultraviolet B exposure on LNP and weaker correlations between ultraviolet B exposure and IBD. Yet LNP retains its impact on IBD and is in fact more robust. This dichotomy together with the hypothesized different global dispersions of lactase phenotypes suggests an independent LP/LNP mechanism in addition to that of latitude/ultraviolet B exposure.
It is doubtful that LP distributions exert direct pathogenic effects. Rates of IBD have also risen in high LNP populations (eg, Japan, People’s Republic of China, and Korea). It is postulated that any modifier effect of an LP/LNP disease interaction would be more complex than that currently attributed to latitude/ultraviolet B exposure. Several possible modifiers of IBD can be hypothesized and are related to digestion of lactose and its evolution.
Possible modifiers of IBD by lactase distribution
Dairy food consumption
In general, possible modifier mechanisms for LP/LNP status could relate to dairy food consumption. In this instance, a harmful effect of dairy foods for some diseases, including IBD, may occur and LNP status (by virtue of symptomatic lactose intolerance and culture) would reduce the amount of dairy foods consumed. In the case of IBD, a modest increased odds ratio was found on epidemiological grounds and was significant only for UC.13 In another situation, LNP may be protective despite regular dairy food consumption via the effects of undigested lactose on microbial flora. In such cases, lactose could act as a prebiotic, promoting protective bacteria like bifidobacteria.91 This scenario may represent an ecological fallacy on comparison of outcomes in epidemiological and patient-based studies, as has been shown in the case of colorectal cancer.13,92 While mechanisms pertaining to this issue are controversial, dairy food consumption in high LNP countries is much less common than in high LP countries.13 Nevertheless, consumption of dairy foods has been implicated in promoting development of IBD,93 but further studies are required because outcomes are inconsistent. For example, a British study suggested a possible protective effect of unpasteurized milk consumption in CD.94
Coevolutionary genetics
Coevolution of other genes with LP status could predispose to IBD in modern environments. Emergence of LP status is hypothesized to have incurred an increased risk of intestinal infections and death as a result of drinking unpasteurized milk. A number of genes may have coevolved with LP. For example, HLA types, leading to immune signaling,95 and the NOD2/CARD15 system are hypothesized to have evolved in response to drinking milk and the threat of infection.96 Interestingly, genetic mutations in the NOD2 system, which are associated mainly with terminal ileal CD, are largely confined to Caucasians. In vitro at least, normal function of non-mutated NOD2 requires adequate 1,25(OH)2D3 to be present in cell culture medium. However, mutations in NOD2 cannot be overcome with increasing vitamin D.97 This in vitro study by Wang et al potentially links vitamin D with IBD, and may provide a plausible explanation as to how low ultraviolet B exposure may promote IBD at high latitude.97
Cystic fibrosis, which in the homozygous state is a lethal disease in Europeans, is hypothesized to have incurred a selective advantage in limiting diarrhea in the heterozygous form.98 However, reports of possible protective effects of heterozygous mutations against IBD are conflicting.99,100
Overall, there are only a few contradictory studies examining the impact of lactase phenotype on IBD.101–104 However, almost two dozen other immune-related diseases share genotypes with IBD,105 possibly linking a common epidemiology.
Possible modification of IBD by influence of geography on socioeconomic factors
Countries with higher LNP frequency populations tend to be economically disadvantaged. This is true largely in South America, Africa, and south Asia. Countries like the People’s Republic of China, South Korea, and Japan have already or are adopting more western lifestyles. Most of these countries had lower rates of IBD until towards the end of the last century. It is therefore possible that reporting of IBD rates is influenced by lack of experience with IBD or in economically less favored countries by lack of doctors and inexperience with the disease.
In the middle of the last century IBDs had higher mortality rates. UC initially had a six-fold mortality rate compared with CD, but in the two decades following, the mortality of CD increased.78 If such mortality rates were encountered in countries with previously low IBD rates, their reporting would likely have been more thorough. However, no increased mortality has been reported in low IBD-incidence countries. This could suggest that mortality of IBD has spontaneously receded or that with reduced mortality these diseases are less recognized by local medical communities.
Initial observations suggested that IBD was more common in higher socioeconomic groups. Indeed, the study by Nerich et al from northern France did show a negative association between CD and farming and households below the poverty level.72 However, other reports have refuted this claim.106 Along similar lines, deprivation scores were not related to juvenile onset of CD in Scotland.73 Therefore, the relationship has not been settled. It is possible that failure to diagnose or failure to get medical assistance in low socioeconomic countries nevertheless contribute to low reported incidence rates. The appropriate reporting of outcomes of diseases more common in higher LNP populated countries (eg, stomach cancer, nasopharyngeal cancer, and hepatocellular cancer) suggests that unfamiliarity with IBD might contribute somewhat to under-reporting. More research on the impact of socioeconomic parameters on IBD rates would need to be carried out.
Other possible effects on IBD
Independent of dairy foods and genetic predisposition, observations that infectious diseases are still rampant in areas inhabited by large LNP populations107 raise the question of their role in infections modifying host immunity. Immune reactions to such agents have been postulated to protect against modern day allergic and autoimmune diseases, including IBD.108
Limitations
A limitation of this analysis is the lack of ability to match data for the five variables (ie, latitude, ultraviolet B exposure, LP/LNP distribution, CD, and UC) in specific regions. As a consequence, it was felt that the best match would be to extrapolate data to a national level. Another limitation of this method is that large countries were identified by unique values for each of the five variables. This technique could hide existing relationships, such as the reports of north-to-south gradients in countries like the USA74,75 and France.72 For example, the nonsignificant latitude/ultraviolet B exposure relationship with LP/LNP and UC in non-Europe may reflect this potential problem. A third limitation is the wide time period chosen to evaluate IBD rates. The primary reason for this is the variable time periods for IBD rates reported from different locales.
However, on examining the five evaluated variables, it should be noted that latitudes and ultraviolet B exposure are stable over time. Single values used for national descriptions take into account measurements from different parts of countries and are population-weighted. These unique descriptors do have a relationship to “national average” yearly ultraviolet B exposure and latitude. In addition, ultraviolet B exposure is more independent of the direction of latitude north or south of the equator.
Although national lactase distributions are even less well defined, they are potential estimates of percentages of populations in local regions and may represent the majority of the country. However, in general, lactase distributions are more stable in Asia, Africa, and Australia, and to some extent in Europe. The most frequent disparity within a region would occur in Africa where tribal differences exist, but for our purposes, no matching IBD data are reported in such countries (eg, South Africa). It is true that migrations in the last decade and a half might have changed the landscape somewhat, but more North Americans than Asians are able to digest lactose. This large population split results in lateral polarity outside Europe. The rates of change in IBD are likely greater than changes in national lactase proportions.23 As such, disease rates are the predominant altering variables. The effect of these changes in disease rate is to reduce correlative findings because of stabilizing UC rates and expanding CD rates in different countries. The different times of onset and change in incidence rates between the two forms of IBD could be separated by as much as 20 years.78 This time differential could account for the somewhat different relationships of CD or UC with LP/LNP distributions.
Strengths
Our original hypothesis was that the LP/LNP evolutionary divide millennia ago has continued to have an impact on the geographic distribution of modern diseases in the last six decades. As a result, a general pattern correlation may be sufficient without precise regional effects. Within-country relationships will require far more appropriate data to be available in multiple regions of the world. Our findings are based on data that were independently published from different sources, free of bias.
We also emphasize in the current paradigm that neither the concept of the disease rate-reducing effects of latitude/ultraviolet B exposure or LNP population frequency nor the relationship between LNP and latitude/ultraviolet B exposure are new. The most important finding here is that for the first time we relate these three independent variables simultaneously to rates of IBD.
The precise effect of lactase status on IBD and other diseases is not obvious (as was perceived for ultraviolet B exposure and vitamin D), but it is difficult to ignore when evaluating different disease rates among different racial groups (eg, first nations in North America, African Americans in North America, and Maoris in New Zealand, which are all predominantly comprised of LNP people).
Conclusion
This analysis relates modern lactase phenotype distributions to patterns of geographic distributions for IBD in the last 60 years. In the process, the previously observed relationships of latitude and IBD are supported. The close correlation between ultraviolet B exposure and latitude is confirmed, and lends support to the ultraviolet B exposure/vitamin D hypothesis and its impact on IBD. Similarly, the previously hypothesized and observed relationships between geographic distribution of LP/LNP and latitude/ultraviolet B exposure are supported. The findings of this study support the conclusion that the evolution of LP together with pre and post divide migrations contributed to the observed geographic spread of modern diseases currently common in western/industrialized societies.
We propose that the relationship between latitudinal polarity (north-to-south, south-to-north) and LP/LNP distributions mimic each other’s disease-modifying effects through global LP–LNP polarity on a large scale as well. This is most clear in Europe, but there is polarity in non-Europe as well, where LP–LNP polarity generally exists from north-to-south or south-to-north, but seemingly less robustly so than in Europe. Further, polarity exists more evidently in lateral directions, and the current analysis shows that LP/LNP distributions can have independent effects from ultraviolet B exposure. We do not use the term “longitudinal” because this requires reference points; however, in geographic terms, there is a west-to-east and east-to-west polarity implied. In Europe, the polarity appears to be restricted to north-to-south and west-to-east.
IBD represents a model for other diseases associated with polar distributions. The implications of the effects of LP/LNP are likely complex and would require investigation, perhaps for each related disease and on multiple levels. However, coevolution of genes predisposing to modern disease with evolution of lactase is at least one such likely contributing variable.
Acknowledgments
We would like to thank Dr Ian Shrier, Department of Epidemiology, Jewish General Hospital, McGill University, for his insightful advice and help in setting up data. We would also like to thank Professor Brian Smith, Desautel Department of Management, McGill University, for reviewing and commenting on the manuscript. Finally, we thank Ms Melissa Szilagyi for assistance with calculations of national disease and lactase distribution rates.
Footnotes
Disclosure
This work was supported by a two-year stipend awarded to AS by the Department of Medicine, Jewish General Hospital. AS receives nonspecific general research funds from the Department of Medicine, Jewish General Hospital. In the past 2 years, AS has participated in an advisory board meeting for the Dairy Bureau of Canada. The other authors have no conflicts of interest to declare.
References
- 1.Swallow DM. Genetics of lactase persistence and lactose intolerance. Ann Hum Genet. 2003;37(4):197–219. doi: 10.1146/annurev.genet.37.110801.143820. [DOI] [PubMed] [Google Scholar]
- 2.Kruse TA, Bolund L, Grzeschik KH, et al. The human lactase-phlorizin gene is located on chromosome 2. FEBS Lett. 1988;240(1–2):123–126. doi: 10.1016/0014-5793(88)80352-1. [DOI] [PubMed] [Google Scholar]
- 3.Enattah NS, Sahi T, Savilahti E, et al. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30(2):233–237. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- 4.Tishkoff SA, Reed FA, Ranciaro A, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007;39(1):31–40. doi: 10.1038/ng1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones BL, Swallow DM. The impact of cis-acting polymorphisms on the human phenotype. Hugo J. 2011;5(1–4):13–23. doi: 10.1007/s11568-011-9155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forster P, Matsumura S. Did early humans go north or south? Science. 2005;308(5724):965–966. doi: 10.1126/science.1113261. [DOI] [PubMed] [Google Scholar]
- 7.Burger J, Kirchner M, Bramanti B, Haak W, Thomas MG. Absence of the lactase-persistence-associated allele in early Neolithic Europeans. Proc Natl Acad Sci U S A. 2007;104(10):3736–3741. doi: 10.1073/pnas.0607187104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itan Y, Powell A, Beaumont MA, Burger J, Thomas MG. The origins of lactase persistence in Europe. PLoS One Comput Biol. 2009;5(8):e1000491. doi: 10.1371/journal.pcbi.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerbault P, Moret C, Currat M, Sanchez-Mazas A. Impact of selection and demography on the diffusion of lactase persistence. PLoS One. 2009;4(7):e6369. doi: 10.1371/journal.pone.0006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoons FJ. Primary adult lactose intolerance and the milking habit: a problem in biologic and cultural interrelations. II. A culture historical hypothesis. Am J Dig Dis. 1970;15(8):695–710. doi: 10.1007/BF02235991. [DOI] [PubMed] [Google Scholar]
- 11.Cramer DW, Xu H. Lactase persistence, galactose metabolism and milk consumption as risk factors for ovarian cancer. In: Aurichio S, Semenza G, editors. Common Food Intolerances 2: Milk in Human Nutrition and Adult-Type Hypolactasia. Basel, Switzerland: Karger; 1993. [Google Scholar]
- 12.Nanji AA, Denardi FG. Primary adult lactose intolerance protects against development of inflammatory bowel disease. Med Hypotheses. 1986;19(1):1–6. doi: 10.1016/0306-9877(86)90131-3. [DOI] [PubMed] [Google Scholar]
- 13.Shrier I, Szilagyi A, Correa JA. Impact of lactose containing foods and the genetics of lactase on diseases: an analytical review of population data. Nutr Cancer. 2008;60(3):292–300. doi: 10.1080/01635580701745301. [DOI] [PubMed] [Google Scholar]
- 14.Grant WB. A meta-analysis of second cancers after a diagnosis of non-melanoma skin cancer: additional evidence that solar ultraviolet -B irradiance reduces the risk of internal cancers. J Steroid Biochem Mol Biol. 2007;103(3–5):668–674. doi: 10.1016/j.jsbmb.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Grant WB. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? Dermatoendocrinol. 2009;1(1):17–24. doi: 10.4161/derm.1.1.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Leeuwen MT, Turner JJ, Falster MO, et al. Latitude gradients for lymphoid neoplasm subtypes in Australia support an association with ultraviolet radiation exposure. Int J Cancer. 2013;133(4):944–951. doi: 10.1002/ijc.28081. [DOI] [PubMed] [Google Scholar]
- 17.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Ultraviolet light B and incidence rates of leukemia worldwide. Am J Prev Med. 2011;41(1):68–74. doi: 10.1016/j.amepre.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Simpson S, Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(10):1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 19.Ananathakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142(3):482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyrin-Biroulet L, Oussalah A, Bigard M-A. Crohn’s disease: the hot hypothesis. Med Hypotheses. 2009;73(1):94–96. doi: 10.1016/j.mehy.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Shivinanda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south ? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39(5):690–697. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juste RA. Crohn’s disease and ruminant farming. Got lactase? Med Hypotheses. 2010;75(1):7–13. doi: 10.1016/j.mehy.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 23.Molodecky NA, Soon IS, Rabi D, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on a systematic review. Gastroenterology. 2012;142(1):46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Itan Y, Jones BL, Ingram CJE, Swallow DM, Thomas MG. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol Biol. 2010;10:36. doi: 10.1186/1471-2148-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szilagyi A. Determinants of geographic patterns of diseases: interaction of lactose/lactase status and sunshine exposure. Med Hypotheses. 2010;75(5):466–470. doi: 10.1016/j.mehy.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57(9):1185–1191. doi: 10.1136/gut.2007.122143. [DOI] [PubMed] [Google Scholar]
- 27.Economou M, Pappas G. New global map of Crohn’s disease: genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis. 2008;14(5):709–720. doi: 10.1002/ibd.20352. [DOI] [PubMed] [Google Scholar]
- 28.Thia KT, Loftus EV, Sandborn WJ, Yang S-K. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103(12):3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 29.Lakatos L, Lakatos PL. Is the incidence and prevalence of inflammatory bowel diseases increasing in Eastern Europe? Postgrad Med J. 2006;82(967):332–337. doi: 10.1136/pgmj.2005.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahi T. Genetics and epidemiology of adult-type hypolactasia. Scand J Gastroenterol. 1994;29( Suppl 202):S7–S20. doi: 10.3109/00365529409091740. [DOI] [PubMed] [Google Scholar]
- 31.Scrimshaw N, Murray EB. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am J Clin Nutr. 1988;48( Suppl 4):S1083–S1159. doi: 10.1093/ajcn/48.4.1142. [DOI] [PubMed] [Google Scholar]
- 32.Figueroa CC, Quera PR, Valenzuela EJ, Jensen BC. Enfermedades inflamatorias intestinales: Experiencia de dos centros chilenos. [Inflammatory bowel disease: experience of two Chilean centers] Rev Med Chil. 2005;133(11):1295–1304. Spanish. [PubMed] [Google Scholar]
- 33.Hilmi I, Singh R, Ganesananthan S, et al. Demography and clinical course of ulcerative colitis in a multiracial Asian population: a nationwide study from Malaysia. J Dig Dis. 2009;10(1):15–20. doi: 10.1111/j.1751-2980.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- 34.Chua KH, Hilmi I, Ng CC, et al. Identification of NOD2/CARD15 mutations in Malaysian patients with Crohn’s disease. J Dig Dis. 2009;10(2):124–130. doi: 10.1111/j.1751-2980.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- 35.Coelho M, Luiselli Bertorelle G, Lopes AI, Seixas S, Destro-Bisol G, Rocha J. Microsatellite variation and evolution of human lactase persistence. Hum Genet. 2005;117(4):329–339. doi: 10.1007/s00439-005-1322-z. [DOI] [PubMed] [Google Scholar]
- 36.Blomme B, Gerlo E, Hauser B, Vandenplas Y. Disaccharidase activities in Belgian children: reference intervals and comparison with non-Belgian Caucasian children. Acta Pediatr. 2003;92(7):806–810. doi: 10.1080/08035250310002803. [DOI] [PubMed] [Google Scholar]
- 37.Timpson NJ, Brennan P, Gaborieau V, et al. Can lactase persistence genotype be used to reassess the relationship between renal cell carcinoma and milk drinking? Potentials and problems in the application of Mendelian randomization. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1341–1348. doi: 10.1158/1055-9965.EPI-09-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg RB. Apolipoprotein A-IV-2 allele: association of its worldwide distribution with adult persistence of lactase and speculation on its function and origin. Genet Epidemiol. 1999;17(4):285–297. doi: 10.1002/(SICI)1098-2272(199911)17:4<285::AID-GEPI4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Lee-Taylor J, Madronich S. Climatology of UV-A, UV-B, and Erythemal Radiation at the Earth’s Surface, 1979–2000. Technical Note TN-474+STR. Boulder, CO, USA: National Center for Atmospheric Research; 2007. [Google Scholar]
- 40.Ennattah NS, Kuokkanen M, Forsblom C, et al. Correlation of intestinal disaccharidase activities with the C/T-13910 variant and age. World J Gastroenterol. 2007;13(25):3508–3512. doi: 10.3748/wjg.v13.i25.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marton A, Xue X, Szilagyi A. Meta-analysis: the diagnostic accuracy of lactose breath hydrogen or lactose tolerance tests for predicting the North European lactase polymorphism C/T-13910. Aliment Pharmacol Ther. 2012;35(4):429–440. doi: 10.1111/j.1365-2036.2011.04962.x. [DOI] [PubMed] [Google Scholar]
- 42.Vahedi H, Merat S, Momtahen S, et al. Epidemiologic characteristics of 500 patients with inflammatory bowel disease in Iran studied from 2004 through 2007. Arch Iran Med. 2009;12(5):454–460. [PubMed] [Google Scholar]
- 43.Zouiten-Mekki L, Zaouali H, Boubaker J, et al. Card15/Nod2 in a Tunisian population with Crohn’s disease. Dig Dis Sci. 2005;50(1):130–135. doi: 10.1007/s10620-005-1290-0. [DOI] [PubMed] [Google Scholar]
- 44.Petritsch W, Fuchs S, Berghold A, et al. Incidence of inflammatory bowel disease in the province of Styria, Austria, from 1997 to 2007: a population-based study. J Crohns Colitis. 2013;7(1):58–69. doi: 10.1016/j.crohns.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Jussila A, Virta LJ, Kautiainen H, et al. Increasing incidence of inflammatory bowel diseases between 2000 and 2007: a nationwide register study in Finland. Inflamm Bowel Dis. 2012;18(3):555–561. doi: 10.1002/ibd.21695. [DOI] [PubMed] [Google Scholar]
- 46.Kiudelis G, Zvirbytiene A, Sventoraitye J, Kupecnikas L. Referral epidemiology of ulcerative colitis and Crohn’s disease in Kaunas region Lithuania: one year prospective study. J Crohns Colitis. 2008;2( Suppl 1):S70. [Google Scholar]
- 47.Yammamoto-Furusho JK. Clinical epidemiology of ulcerative colitis in Mexico: a single hospital-based study in a 20-year period (1987–2006) J Clin Gastroenterol. 2009;43(3):221–224. doi: 10.1097/MCG.0b013e31817a76b4. [DOI] [PubMed] [Google Scholar]
- 48.Fadda MA, Peedikayil MC, Kagevi I, Kahtani KA, Ben AA, Al HI. Inflammatory bowel disease in Saudi Arabia: a hospital-based clinical study of 312 patients. Ann Saudi Med. 2012;32(3):276–282. doi: 10.5144/0256-4947.2012.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buenavida G, Casañias A, Vásquez C, et al. National Inflammatory Bowel Disease Registry. Acta Gastroenterol Latinoam. 2011;41(4):281–287. [PubMed] [Google Scholar]
- 50.Wang YG, Yan YS, Xu JJ, Du RF, Kuhnau W, Flatz G. Prevalence of primary adult lactose malabsorption in three populations of northern China. Hum Genet. 1984;67(1):103–106. doi: 10.1007/BF00270566. [DOI] [PubMed] [Google Scholar]
- 51.Ladas S, Papanikos J, Arapakis G. Lactose malabsorption in Greek adults: correlation of small bowel transit time with the severity of lactose intolerance. Gut. 1982;23(11):968–973. doi: 10.1136/gut.23.11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadre M, Karbasi K. Lactose intolerance in Iran. Am J Clin Nutr. 1979;32(9):1948–1954. doi: 10.1093/ajcn/32.9.1948. [DOI] [PubMed] [Google Scholar]
- 53.Nose O, Iida Y, Kai H, Harada T, Ogawa M, Yabuuchi H. Breath hydrogen test for detecting lactose malabsorption in infants and children. Prevalence of lactose malabsorption in Japanese children and adults. Arch Dis Child. 1979;54(6):436–440. doi: 10.1136/adc.54.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasrallah SM. Lactose intolerance in the Lebanese population and in Mediterranean lymphoma. Am J Clin Nutr. 1979;32(10):1994–1996. doi: 10.1093/ajcn/32.10.1994. [DOI] [PubMed] [Google Scholar]
- 55.Aswami MZ, Seppo L, Vapaatalo H, Korpela R. Hypolactasia and lactose intolerance among three ethnic groups of Malaysia. Indian J Med Res. 2006;124(6):697–704. [PubMed] [Google Scholar]
- 56.Senewiratne B, Thambipillai S, Pereira H. Intestinal lactase deficiency in Ceylon (Sri Lanka) Gastroenterology. 1977;72(6):1257–1259. [PubMed] [Google Scholar]
- 57.Filali A, Hassine B, Dhouib H, Matri S, Ben Ammar A, Garoui H. Study of malabsorption of lactose by the hydrogen breath test in a population of 70 Tunisian adults. Gastroenterol Clin Biol. 1987;11(8–9):554–557. [PubMed] [Google Scholar]
- 58.Flatz G, Henze HJ, Palabiyikoglu E, Dagalp K, Turkkan T. Distribution of the adult lactase phenotypes in Turkey. Trop Geogr Med. 1986;38(3):255–258. [PubMed] [Google Scholar]
- 59.Bolin TD, Morrison RM, Steel JE, Davis AE. Lactose intolerance in Australia. Med J Aust. 1970;1(26):1289–1292. doi: 10.5694/j.1326-5377.1970.tb84589.x. [DOI] [PubMed] [Google Scholar]
- 60.Lember M, Torniainen S, Kull M, et al. Lactase non-persistence and milk consumption in Estonia. World J Gastroenterol. 2006;12(45):7329–7331. doi: 10.3748/wjg.v12.i45.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbott WG, Tasman-Jones C. Incidence of acquired primary hypolactasia in three New Zealand racial groups. N Z Med J. 1985;98(776):228–229. [PubMed] [Google Scholar]
- 62.Farup PG, Monsbakken KW, Vandvik PO. Lactose malabsorption in a population with irritable bowel syndrome: prevalence and symptoms. A case-control study. Scand J Gastroenterol. 2004;39(7):645–649. doi: 10.1080/00365520410005405. [DOI] [PubMed] [Google Scholar]
- 63.Kuokkanen M, Butzow R, Rasinpera H, et al. Lactase and ovarian carcinoma risk in Finland, Poland and Sweden. Int J Cancer. 2005;117(1):90–94. doi: 10.1002/ijc.21130. [DOI] [PubMed] [Google Scholar]
- 64.Mattar R, Monteiro MS, Villares CA, Santos AF, Silva JM, Carrilho FJ. Frequency of LCT-13910C.T single nucleotide polymorphism associated with adult-type hypolactasia/lactase persistence among Brazilians of different ethnic groups. Nutr J. 2009;8:46. doi: 10.1186/1475-2891-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Czeizel A, Flatz G, Flatz SD. Prevalence of primary adult lactose malabsorption in Hungary. Hum Genet. 1983;64(4):398–401. doi: 10.1007/BF00292375. [DOI] [PubMed] [Google Scholar]
- 66.Babu J, Kumar S, Babu P, Prasad JH, Ghoshal UC. Frequency of lactose malabsorption among healthy southern and northern Indian populations by genetic analysis and lactose hydrogen breath and tolerance tests. Am J Clin Nutr. 2010;91(1):140–146. doi: 10.3945/ajcn.2009.27946. [DOI] [PubMed] [Google Scholar]
- 67.Al-Sanae H, Saldanha W, Sugathan TN, Majid Molla A. Comparison of lactose intolerance in healthy Kuwaiti and Asian volunteers. Med Princ Pract. 2003;12(3):160–163. doi: 10.1159/000070752. [DOI] [PubMed] [Google Scholar]
- 68.Khabarova YA, Torniainen ST, Nurmi HA, Jarvela IE, Isokoski MK, Mattila KJ. Prevalence of lactase persistent/non-persistent genotypes and milk consumption in young population in north-west Russia. World J Gastroenterol. 2009;15(15):1849–1853. doi: 10.3748/wjg.15.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Socha J, Ksiazyk J, Flatz G, Flatz SD. Prevalence of primary adult lactose malabsorption in Poland. Ann Hum Biol. 1984;11(4):311–316. doi: 10.1080/03014468400007211. [DOI] [PubMed] [Google Scholar]
- 70.Maggi R, Sayagues B, Fernandez A, et al. Lactose malabsorption and intolerance in Uruguayan population by breath hydrogen test (H2) J Pediatr Gastroenterol Nutr. 1987;6(3):373–376. doi: 10.1097/00005176-198705000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62(4):630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 72.Nerich V, Monnet E, Weill A, et al. Fine scale geographic variations of inflammatory bowel disease in France: correlation with socioeconomic and house equipment variables. Inflamm Bowel Dis. 2010;16(5):813–821. doi: 10.1002/ibd.21122. [DOI] [PubMed] [Google Scholar]
- 73.Armitage EL, Aldhous MC, Anderson N, et al. Incidence of juvenile-onset Crohn’s disease in Scotland: association with northern latitude and affluence. Gastroenterology. 2004;127(4):1051–1057. doi: 10.1053/j.gastro.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 74.Sonnenberg A, McCarty DJ, Jacobsen SJ. Geographic variation of inflammatory bowel disease within the United States. Gastroenterology. 1991;100(1):143–149. doi: 10.1016/0016-5085(91)90594-b. [DOI] [PubMed] [Google Scholar]
- 75.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61(12):1686–1692. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population based study. Am J Gastroenterol. 2006;101(7):1559–1568. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 77.Jussila A, Virta LJ, Salomaa V, Maki J, Jula A, Farkkila MA. High and increasing prevalence of inflammatory bowel disease in Finland with a clear north-south difference. J Crohns Colitis. 2013;7(7):e256–e262. doi: 10.1016/j.crohns.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 78.Sonnenberg A. Time trends of mortality from Crohn’s disease and ulcerative colitis. Int J Epidemiol. 2007;36(4):890–899. doi: 10.1093/ije/dym034. [DOI] [PubMed] [Google Scholar]
- 79.Garg M, Lubel JS, Sparrow MP, Holt SG, Gibson PR. Review article: vitamin D and inflammatory bowel disease – established concepts and future directions. Aliment Pharmacol Ther. 2012;36(4):324–344. doi: 10.1111/j.1365-2036.2012.05181.x. [DOI] [PubMed] [Google Scholar]
- 80.Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn’s disease – a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32(3):377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 81.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229(11):1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 82.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22( Suppl 2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 83.Aamodt G, Bengtson MB, Vatn MH. Can temperature explain the latitudinal gradient of ulcerative colitis? Cohort of Norway. BMC Public Health. 2013;13:530. doi: 10.1186/1471-2458-13-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murdoch TB, Bernstein CN, El-Gabalawy H, et al. Prevalence of genetic variants associated with inflammatory bowel disease in a healthy First Nations cohort. CMAJ. 2012;184(8):E435–E441. doi: 10.1503/cmaj.110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shapira M, Tamir A. Crohn’s disease in the Kinneret sub-district, Israel, 1960–1990. Incidence and prevalence in different ethnic subgroups. Eur J Epidemiol. 1994;10(2):231–233. doi: 10.1007/BF01730377. [DOI] [PubMed] [Google Scholar]
- 86.Gearry RB, Lea RA, Roberts RL, Chambers GK, Barclay ML, Kennedy MA. CARD15 allele frequency differences in New Zealand Maori: ancestry specific susceptibility to Crohn’s disease in New Zealand? Gut. 2006;55(4):580. doi: 10.1136/gut.2005.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flatz G, Rotthauwe HW. Lactose nutrition and natural selection. Lancet. 1973;2(7820):76–77. doi: 10.1016/s0140-6736(73)93267-4. [DOI] [PubMed] [Google Scholar]
- 88.Heyer E, Brazier L, Segurel L, et al. Lactase persistence in central Asia: phenotype, genotype, and evolution. Hum Biol. 2011;83(3):379–392. doi: 10.3378/027.083.0304. [DOI] [PubMed] [Google Scholar]
- 89.Gallego Romero I, Basu Mallick C, Liebert A, et al. Herders of Indian and European cattle share their predominant allele for lactase persistence. Mol Biol Evol. 2012;29(1):249–260. doi: 10.1093/molbev/msr190. [DOI] [PubMed] [Google Scholar]
- 90.Szilagyi A, Leighton H, Burstein B, Shrier I. Significant positive correlation between sunshine and lactase nonpersistence in Europe may implicate both in similarly altering risks for some diseases. Nutr Cancer. 2011;63(7):991–999. doi: 10.1080/01635581.2011.596641. [DOI] [PubMed] [Google Scholar]
- 91.Szilagyi A. Lactose: a potential prebiotic. Aliment Pharmacol Ther. 2002;16(9):1591–1602. doi: 10.1046/j.1365-2036.2002.01321.x. [DOI] [PubMed] [Google Scholar]
- 92.Szilagyi A, Nathwani U, Vinokuroff C, Correa JA, Shrier I. The effect of lactose maldigestion on the relationship between dairy food intake and colorectal cancer: a systematic review. Nutr Cancer. 2006;55(2):141–150. doi: 10.1207/s15327914nc5502_4. [DOI] [PubMed] [Google Scholar]
- 93.Spooren CE, Pierik MJ, Zeegers MP, Feskens EJ, Masclee AA, Jonkers DM. Review article: the association of diet with onset and relapse in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38(10):1172–1187. doi: 10.1111/apt.12501. [DOI] [PubMed] [Google Scholar]
- 94.Abubakar I, Myhill DJ, Hart AR, et al. A case control study of drinking water and dairy products in Crohn’s disease: further investigation of the possible role of Mycobacterium avium paratuberculosis. Am J Epidemiol. 2007;165(7):776–783. doi: 10.1093/aje/kwk067. [DOI] [PubMed] [Google Scholar]
- 95.Alfonso-Sanchez MA, Perez-Miranda AM, Garcia-Obregon S, Pena JA. An evolutionary approach to the high frequency of the delta F508 CFTR mutation in European populations. Med Hypotheses. 2010;74(6):989–992. doi: 10.1016/j.mehy.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 96.Gasche C, Nemeth M, Grundtner P, Willheim-Polli C, Ferenci P, Schwarzenbacher R. Evolution of Crohn’s disease-associated Nod2 mutations. Immunogenetics. 2008;60(2):115–120. doi: 10.1007/s00251-008-0274-6. [DOI] [PubMed] [Google Scholar]
- 97.Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn’s disease. J Biol Chem. 2010;285(4):2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Modiano G, Ciminellui BM, Pignatti PF. Cystic fibrosis and lactase persistence: a possible correlation. Eur J Hum Genet. 2007;15(3):255–259. doi: 10.1038/sj.ejhg.5201749. [DOI] [PubMed] [Google Scholar]
- 99.Bresso F, Askling J, Astegiano M, et al. Potential role for the common cystic fibrosis ΔF508 mutation in Crohn’s disease. Inflamm Bowel Dis. 2007;13(5):531–536. doi: 10.1002/ibd.20067. [DOI] [PubMed] [Google Scholar]
- 100.Bahmanyar S, Ekbom A, Askling J, Johanneson M, Montgomery SM. Cystic fibrosis gene mutations and gastrointestinal disease. J Cyst Fibros. 2010;9(4):288–291. doi: 10.1016/j.jcf.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 101.Buning C, Ockenga JK, Jurga J, et al. The C/C(-13910) and G/G9-22018) genotypes for adult-type hypolactasia are not associated with inflammatory bowel disease. Scand J Gastroenterol. 2003;38(5):538–542. [PubMed] [Google Scholar]
- 102.Elguezabal N, Chamorro S, Molina E, et al. Lactase persistence, NOD2 status and Mycobacterium avium subsp. Paratuberculosis infection associations to inflammatory bowel disease. Gut Pathog. 2012;4(1):6. doi: 10.1186/1757-4749-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nolan DJ, Han DY, Lam WJ, et al. Genetic adult lactase persistence is associated with risk of Crohn’s disease in a New Zealand population. BMC Res Notes. 2010;3:339. doi: 10.1186/1756-0500-3-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Juste RA, Elguezabal N, Chamorro S, et al. Associations between CARD15 polymorphisms, MAPDNA in blood and lactase persistence in a Crohn’s disease case-control study in north Spain. Proceedings of the Tenth International Colloquium on Paratuberculosis. 2009;269(2):9–14. [Google Scholar]
- 105.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 106.Bernstein CN, Kraut A, Blanchard JF, Rawsthorne P, Yu N, Walld R. The relationship between inflammatory bowel disease and socioeconomic variables. Am J Gastroenterol. 2001;96(7):2117–2125. doi: 10.1111/j.1572-0241.2001.03946.x. [DOI] [PubMed] [Google Scholar]
- 107.Ross AG, Olds GR, Cripps AW, Farrar JJ, McManus DP. Enteropathogens and chronic illness in returning travelers. N Engl J Med. 2013;368(19):1817–1825. doi: 10.1056/NEJMra1207777. [DOI] [PubMed] [Google Scholar]
- 108.Okada H, Kuhn C, Feillet H, Bach J-F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]