Abstract
Background
Biodegradable polymers have been applied as bulk or coating materials for coronary artery stents. The degradation of polymers, however, could induce endothelial dysfunction and aggravate neointimal formation. Here we use polymeric microparticles to simulate and demonstrate the effects of degraded stent materials on phagocytic activity, cell death and dysfunction of macrophages and endothelial cells.
Methods
Microparticles made of low molecular weight polyesters were incubated with human macrophages and coronary artery endothelial cells (ECs). Microparticle-induced phagocytosis, cytotoxicity, apoptosis, cytokine release and surface marker expression were determined by immunostaining or ELISA. Elastase expression was analyzed by ELISA and the elastase-mediated polymer degradation was assessed by mass spectrometry.
Results
We demonstrated poly(D,L-lactic acid) (PLLA) and polycaprolactone (PCL) microparticles induced cytotoxicity in macrophages and ECs, partially through cell apoptosis. The particle treatment alleviated EC phagocytosis, as opposed to macrophages, but enhanced the expression of vascular cell adhesion molecule-1 (VCAM) along with decreased nitric oxide production, indicating ECs were activated and lost their capacity to maintain homeostasis. The activation of both cell types induced release of elastase or elastase-like protease, which further accelerated polymer degradation.
Conclusions
This study revealed that low molecule weight PLLA and PCL microparticles increased cytotoxicity and dysregulated endothelial cell function, which in turn enhanced elastase release and polymer degradation. These indicate polymer or polymer-coated stents impose a risk of endothelial dysfunction after deployment which can potentially lead to delayed endothelialization, neointimal hyperplasia and late thrombosis.
Keywords: poly(L-lactic acid), PLLA, polycaprolactone, PCL, stent, coronary, polymer degradation, macrophage, endothelial cell, dysfunction, elastase
1. Introduction
Drug-eluting stents (DES) have remarkably reduced acute in-stent thrombosis and the occurrence of revascularization. However, late stent thrombosis associated with DES still remains unexpectedly high, which can be partially attributed to endothelial dysfunction and vasomotor disorders caused by stent, drug or polymer coatings.[1] Besides the stent geometry, the influence of incorporated drugs on inducing endothelial dysfunction has been studied in both animals and humans over the past decade.[2-4] In contrast, polymer coating-induced endothelial dysfunction has yet been reported despite of their proven hypersensitive effect. Biodegradable and resorbable polymers provide appealing substitutes to durable polymer coatings, since they are less likely inducing severe chronic inflammatory response and their degradation products can be cleared out of the human body. Coronary artery stents coated with or completely made of biodegradable polyesters, i.e. poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) and to a lesser degree, poly(ε -caprolactone) (PCL), have been developed.[5-9] These polymers undergo hydrolysis and enzyme-mediated degradation (“biodegradation”),[10, 11] resulting in a release of low molecular weight degradation products. The released polymer debris is then removed by leukocytes and foreign body giant cells (“bioresoprtion”), as indicated by enhanced phagocytosis and cell activation. Activated leukocytes subsequently alter the phenotype of surrounding cells (i.e. endothelial cells) and make them actively involved in clearing and remodeling processes. Endothelial cells (ECs) are normally quiescent and maintain vascular homeostasis. The activation of endothelium promotes the adhesion of platelets and leukocytes while losing its cell-cell integrity, which gradually leads to vascular malfunctions such as atherosclerosis and stenosis.[12] For example, neointimal formation has been found with the degradation of polymer stents, [13, 14] as revealed by locally elevated inflammation and cell growth to intima. Although it can be alleviated by timed-release anti-proliferative drugs, locally delivered therapeutics often induce EC apoptosis/necrosis, impair re-endothelialization and lead to late thrombosis.[15, 16] It is unclear, however, whether there is a casual relationship between polymer debris and endothelial activation/dysfunction. Therefore, the objective of this study is to understand the interaction between polymer debris and ECs, which is particularly meaningful for polymer coronary stents. Here we use low molecular weight poly(D,L-lactic acid) (PLLA) and PCL microparticles as model molecules of degradation products to facilitate cellular uptake and to elucidate how macrophage and arterial endothelial cell functions can be regulated by degraded polymers. Result of this study will help interpret the occurring restenosis and late-thrombosis in polymer-coated stents and warrant more careful evaluation of endovascular polymer devices in general. It will enlighten both clinicians and biomedical engineers to improve the design of polymer-involved coronary stents from a perspective of minimizing the deteriorating effect of polymers on endothelium.
2. Methods
2.1 Preparation of stainless steel, PLLA and PCL microparticles
PLLA (RESOMER R202S, 10-18k Da, Sigma, St, Louis, MO) or PCL (1.25k Da, Sigma) was dissolved in dichloromethane (10% w/w) and the polymer solution was added to 1% poly(vinyl acid) (PVA) water solution drop-wise under continuous stirring at 3000 rpm (Silverson L4RT homogenizer, East Longmeadow, MA). The polymer solution was then slowly stirred at room temperature overnight to evaporate the organic solvent. The PVA was removed by repeated washing with DI water and the particles were collected by centrifugation and lyophilizing overnight. Particles less than 38 μm were obtained by sieving (H&C Sieving Systems, Columbia, MD). Stainless steel (SS) particles with around 10 μm in diameter were prepared by grinding and served as controls throughout this study. Particle size and morphology were visualized using a Hitachi S4200 high resolution scanning electron microscope (SEM, Schaumburg, IL) (Fig. 1).
Figure 1.

SEM images of microparticles: (a) stainless steel, (b) PLLA and (c) PCL. Scale bar: 150 (a and b) or 37.5 (c) μm.
2.2 Cell culture
Human blood-derived monocytes were obtained from Advanced Biotechnologies (Columbia, MD) and differentiated into human blood monocyte-derived macrophages.[17] Monocytes at a density of 2x106 cells/ mL were incubated with 10 mL DMEM (Invitrogen, Carlsbad, CA) with 20% fetal calf serum (Invitrogen), 10% human serum (Sigma), and 5 ng/ mL macrophage colony-stimulating factor (Sigma) for 9 days to differentiate into macrophages.[18] Human coronary artery ECs (HCAECs, Cell Applications, San Diego, CA) were cultured in endothelial cell growth medium (Cell Applications) until 80% confluent before seeding. Polymer or SS particles were UV sterilized for 1 hour prior to cell seeding. Macrophages (3 × 105 cells/ mL) were cultured with polymer particles (400μg/ mL of PLLA or PCL) or SS particles (8000μg/ mL) for 3 days before endpoint assays.
2.3 Phagocytosis and superoxide assays
After 3 days culture, macrophages or HCAECs were treated with green-fluorescent Escherichia coli (E. coli) particles for 2 hours according to the manufacturer's protocol (Vybrant® Phagocytosis assay kit, Life Technologies, Carlsbad, CA). Cells were stained with dihydroethidium (1μg / mL DHE, Life Technologies) for 15 minutes. Cell nuclei were counterstained with Hoechst and imaged with an inverted Nikon Ti fluorescent microscope (Nikon Instruments, Melville, NY). Fluorescence intensities of E. coli particles phagocytized by activated cells and superoxide as indicated by DHE were measured using a plate reader (Tecan Group, Ltd Männendorf, Switzerland). Phagocytic activity and superoxide production were normalized to cell number quantified by Hoechst nuclear staining (n = 4 per polymer condition).
2.4 Cytotoxicity
Macrophage and endothelial cell cytotoxicity was measured using Cytotox One Homogeneous Membrane Assay (Promega, Madison, WI) by quantifying lactate dehydrogenase released from damaged cells according to supplier's protocol. Fluorescence values were normalized to cell samples without material treatment (n=4 per polymer condition).
2.5 TNF-α and nitric oxide expression
After 3 day culture, media samples were collected and analyzed for secretion of tumor necrosis factor (TNF)-α, elastase, and nitric oxide (NO). TNF-α secretion was measured using Human TNF-α ELISA Max Standard Kit (Biolegend, San Deigo, CA) according to supplier's protocol. NO secretion was measured by incubating 100 μL 1X modified Greiss reagent (Sigma) with 100 μL media or nitrate standards for 15 minutes before reading the absorbance at 540 nm using a plate reader (Tecan). Nitrate standards (0-65 μmol) were made using sodium nitrate (Sigma) (n=4 per polymer condition).
2.6 Immunostaining of VCAM-1 and Annexin V
Cells were fixed with 4% paraformaldehyde (PFA) for 15 minutes, blocked with 10% goat serum in Dulbecco's phosphate-buffered saline (DPBS) for 1 hour, and incubated with allophycocyanin-conjugated VCAM-1 antibodies (1:100 dilution, Abcam, Cambridge, MA) for 2 hours in DPBS. For Annexin-V staining, HCAECs were fixed with 4% PFA for 15 minutes, permeabilized with 0.25% TritonX-100 for 10 minutes, blocked with 10% goat serum for 1 hour, incubated with rabbit anti-human Annexin V antibodies (1:200 dilution, Abcam) overnight at 4°C, and then incubated with Alexa 488 goat anti-rabbit antibodies (1:00 dilution, Abcam) for 1 hour in DPBS. After washing twice with DPBS, cells were imaged on a LSM 710 META inverted confocal microscope (Zeiss, Thornwood, NY). The number of cells that were positive to each staining was counted and divided by the total number of cells per image field (n=6 images from three replicate experiments).
2.7 Elastase activity
In order to measure elastase activity, elastase standards (0-10 nmol) were made using human neutrophil elastase (Sigma). 200 μL of media samples or standards were incubated with 200 μL of 1mM granulocyte elastase substrate (Sigma) in DPBS for 1 hour at 37°C and glacial acetic acid was added to stop the reaction. Absorbance was measured at 415 nm by a plate reader (Tecan) (n=4 per polymer condition).[19]
2.8 Polymer degradation by elastase
PDLLA particles were suspended in DPBS with or without elastase (0.08U/mL, Sigma), and incubated at 37°C for 7 days. Insoluble polymer particles were filtered off using centrifugation units (Amicon-0.5ml 3K MWCO, Millipore, Billerica, MA) at 12,000g for 20 min. Degradation products in the filtered solution were confirmed by liquid chromatography coupled with mass spectrometry (LC-MS) (Variant 1000, Agilent, Santa Clara, CA) using a C18 column (Kinetex, Phenomenex, Torrance, CA) with an acetonitrile-H2O mobile phase system. (n=3 per polymer condition).
2.9 Statistical analysis
In all experiments, analytical results are expressed as means ± standard error of the mean (SEM). One-way ANOVA was used to determine if statistical differences exist between groups. Comparisons of individual sample groups were performed using unpaired Student's t-test. For all experiments, p < 0.05 was considered statistically significant.
3. Results
3.1 Cell number and cytotoxicity of macrophages and ECs
The numbers of macrophages and ECs were determined by measuring the fluorescence intensity of Hoechst staining cell nuclei. The cell number significantly decreased in both cell types after being cultured with PLLA, PCL or SS microparticles for 3 days (p<0.05, Fig. 2a) compared to no polymer control of the corresponding cell type. However, no significant difference in cell number was observed among the three test material types. The cytotoxic effect of these microparticles, presented by lactase dehydrogenase release from cells resulting from a damaged membrane, was shown in Fig. 2b. Apparently, the decrease of cell number correlates to the increase of cytotoxicity of materials. Compared to no polymer control, PLLA, PCL and SS microparticles showed a remarkable increase of cytotoxicity to macrophages (p<0.05, Fig. 2b). Interestingly, there was no significant difference in cytotoxicity between PLLA and PCL microparticles, although PCL but not PLLA microparticles considerably increased cytotoxicity to ECs. As a negative control, SS microparticles has higher cytotoxicity to both cell types than PLLA and PCL microparticles (p<0.05, Fig. 2b). These results demonstrate that the macrophages are more susceptible to cytotoxic effects of these microparticles than ECs as more severe cytotoxicity of the materials was seen in macrophages (Fig. 2b).
Figure 2.
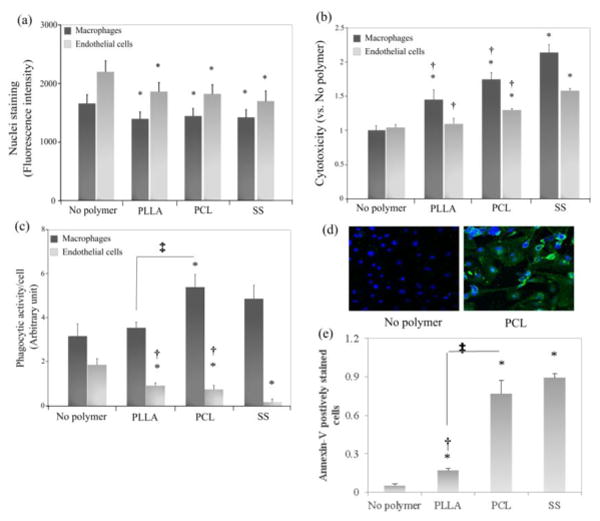
Cytotoxicity, phagocytosis and apoptosis by interaction with microparticles: (a) Cell number of each group indicated by Hoechst florescence intensity; (b) cytotoxicity measured by lactate dehydrogenase released from damaged cells and normalized to no polymer control; (c) phagocytosis of fluorescent-tagged E. coli particles by activated macrophages and ECs. The fluorescence intensity emitted from E. coli particles was normalized to the corresponding cell number measured by Hoechst staining; (d) apoptosis in untreated (left) and PCL microparticle-treated ECs (right), blue: Hoechst, green: Annexin V; (e) quantification of apoptosis presented as the ratio of Annexin V-positive cells relative to the total cell number. *p<0.05 versus no polymer control; †p<0.05 versus SS control; ‡p<0.05 between PLLA and PCL groups connected by line.
3.2 Phagocytic activity of macrophages and ECs
Phagocytic activity of cells was measured by quantitating the intensity of intracellular fluorescence emitted from green fluorescent-tagged E. coli particles with or without the treatment of microparticles. The addition of PCL and SS microparticles enhanced the phagocytic activity of macrophages compared to no polymer control (p<0.05, Fig. 2c), whereas PLLA microparticles had no further effect on macrophage phagocytosis (p<0.05). In contrast, the phagocytosis of E. coli particles by ECs was significantly decreased after adding PLLA, PCL and SS microparticles (p<0.05, Fig. 2c), indicating the phagocytosis stimulated by these microparticles is cell type-dependent.
3.3 Apoptosis of ECs
Apoptosis of ECs induced my microparticles was measured by Annexin-V staining. Treatment of microparticles significantly promoted EC apoptosis compared to no particle control (p<0.05, Fig. 2d-e). Apoptosis was even more dramatically increased with addition of PCL or SS microparticles, indicating a detrimental effect of these microparticles on EC survival.
3.4 VCAM-1 expression of ECs
The expression of VCAM-1 on ECs induced by microparticles was shown in Fig. 3a. Compared to ECs cultured with PLLA microparticles or no polymer control, ECs treated with PCL microparticles increasingly expressed VCAM-1 in both mono- and co-culture with macrophages. The quantitation of VCAM-1 positive cells showed that treatment of PCL and SS microparticles resulted in a significantly higher number of VCAM-1 positive ECs compared to no polymer controls in both mono- and co-culture with macrophages (p<0.05, Fig. 3b). In contrast, the addition of PLLA microparticles only slightly increased VCAM expression in both culture conditions compared to no polymer control.
Figure 3.
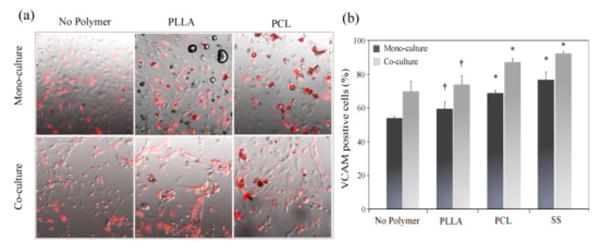
Microparticle-induced EC activation : (a) Merged images of APC-VCAM staining (red) and phase contrast; (b) VCAM-1 positive cells (%) relative to the total cell number in each sample. *p<0.05 versus no polymer control; †p<0.05 versus SS control.
3.5 Expression of TNF-α, superoxide, and NO in macrophages and/or ECs
The TNF-α concentration in cell culture supernatants was determined by ELISA. Generally, the TNF-α expression increased in both macrophages and ECs by adding PLLA and PCL microparticles, although there was no significant difference compared to no polymer controls or between PLLA and PCL groups (Fig. 4a). The production of ROS was determined by staining cells with dihydroethidium that relocates from cytosol to nuclei after being oxidized to oxyethidium and emits red fluorescence. Results showed no significant differences among the test groups in each cell type (Fig. 4b).
Figure 4.
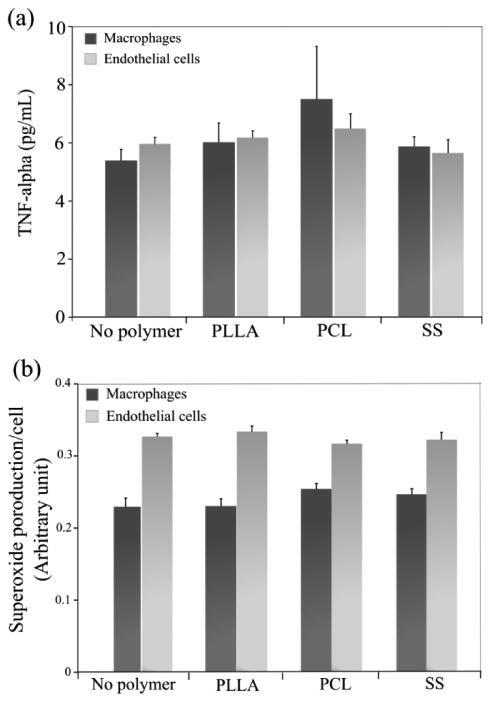
Microparticle-mediated macrophage activation: (a) TNF-α release into cell culture media which was determined by ELISA; (b) An intracellular superoxide level which was stained by DHE and normalized to the corresponding cell number measured by Hoechst staining.
NO production in EC mono-culture was quantified. Results showed the addition of PLLA, PCL and SS microparticles significantly reduced NO production in ECs, with the least NO production in ECs treated with SS microparticles (Fig. 5). PCL induced lower NO production than PLLA microparticles. However, no statistically difference was observed among these test materials.
Figure 5.
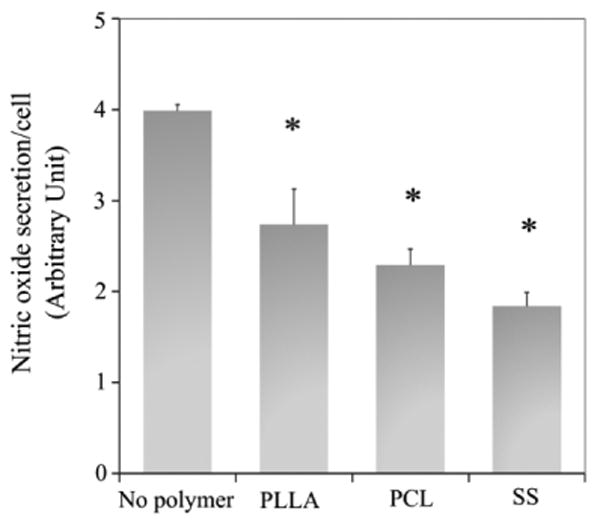
EC dysfunction induced by microparticles was determined by quantitating the amount of nitric oxide in supernatant (Greiss reagent). The absorbance was measured at 540nm and normalized to the cell number measured by Hoechst staining. *p<0.05 versus no polymer control.
3.6 Elastase activity and polymer degradation
The activity of elastase in cell culture supernatant was determined. The three types of microparticles promoted elastase secretion from both macrophages and ECs (p<0.05, Fig. 6), compared to no polymer control. However, there was no significant difference between PLLA and PCL groups, although SS and PCL microparticles induced the highest elastase expression in ECs and macrophages, respectively. ECs also generally had a higher elastase activity than macrophages (Fig. 6).
Figure 6.
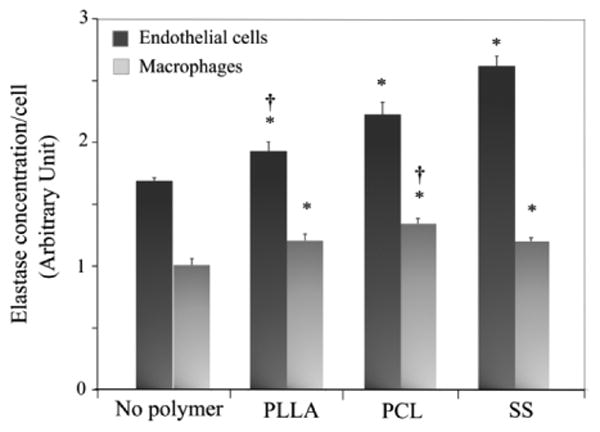
Elastase production from cells after incubation with microparticles. Elastase production in cell culture media was determined by reacting with elastase substrate and comparing with the elastase standard at 415 nm. The absorbance value was normalized to the corresponding cell number measured by Hoechst staining. *p<0.05 versus no polymer control; †p<0.05 versus SS control.
The elastase-mediated degradation of PLLA microparticles was demonstrated by LC-MS (Fig. 7). Slow degradation of PLLA microparticles was observed in DPBS without elastase as identified by various lengths of PLLA polymer chain (repeating unit m/z =72) at m/z 185, 257, 329, 401, 473, 545 and 617[20, 21] (Fig. 7a). When elastases were added (Fig. 7b), the degradation of PLLA microparticles was greatly enhanced with an increase in the relative abundance of molecules at the various m/z values mentioned above.
Figure 7.
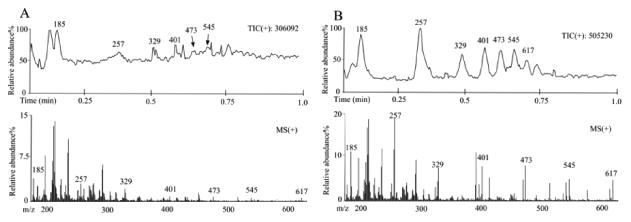
Elastase-mediated polyester degradation. Mass spectrum of PLLA microparticle and degradation products. PLLA particles were suspended in DPBS without (a) or with (b) elastase and incubated at 37°C for 7 days. Degradation products in filtered solutions were confirmed by LC-MS with an acetonitrile-H2O mobile phase system (positive ion mode).
4. Discussion
As an important group of implantable biomaterials, polyesters have been widely used for biomedical and clinical applications. Here we report the treatment of representative polyesters (PLLA and PCL) can lead to dysfunctional activation of ECs and macrophages, which in turn promotes further elastase-mediated polymer degradation and contribute to this degradation-phagocytosis-dysfunction loop. Low molecular weight microparticles were used in this study as model molecules of degradation products that could be quickly accessed by cells.
PLLA is generally considered non-toxic and non-inflammatory. For example, no cytotoxicity to macrophages and Schwann cells was observed during the interaction with PLLA films and micro-/nanofibers.[22, 23] In other studies, microparticles with a diameter of ∼1.6 μm were made from PLLA (100kDa) and PLGA (100kDa) blends which did not decrease cell viability of human lung cancer cells, showing PLLA/PLGA microparticles at these specific molecular weights and particle sizes are not cytotoxic to cancer cells.[24] However, PLLA microparticles of small molecular weight (10kDa) with ∼5 μm in diameter decreased cell viability of both human primary and murine RAW macrophages in a dose-dependent manner.[25] Moreover, pre-degraded PLLA microparticles (<40 μm) with a range of molecular weight from 99 to 53kDa induced phagocytosis and cell death of macrophages in mice.[26] These results indicate that the cytotoxic effect of PLLA on macrophages is closely related to phagocytosis, geometry, molecular weight and dose. Similarly, apoptosis or necrosis of macrophages mediated by the uptake of PCL nanoparticles (42kDa) was dose- and size-dependent, evidenced by the fact that a higher dose and a smaller particle size resulted in lower cell viability.[27] When high molecular weight PCL underwent hydrolytic degradation, macrophages phagocytized smaller-molecular-weight degradation products of PCL.[28] Especially, the fragments less than 3kDa could be completely degraded in phagosomes of macrophages.[29] In this study, we showed the low molecular weight PLLA and PCL microparticles (10k and 1.25kDa) increased phagocytic activity of macrophages and exhibited higher cytotoxicity than no treatment groups (Fig. 2b-c). As PCL degrades slowly, lower levels of macrophage activation and accumulation were expected compared to fast degrading PLLA and PLGA, thus causing less phagocytosis. However, both phagocytic activity and cytotoxicity of macrophages were enhanced in PCL groups (Fig. 2b-c), indicating the hydrolytic degradation of PCL in this study is possibly less important than the elevated particle uptake. The PCL microparticles being used in this study had a molecular weight of 1.25kDa which could be readily phagocytized by macrophages.[29] Moreover, the surface properties of PCL microparticles might contribute to the higher intracellular uptake. It was reported the cellular uptake of particles could be enhanced by surface-coated bovine serum albumin.[30] The adsorption of serum proteins on PCL is theoretically higher than on PLLA due to the higher hydrophobicity of PCL, which may enhance the uptake of PCL particles compared to PLLA particles. In summary, the increased phagocytic activities associated with low molecular weight polyester microparticles, PCL in particular, played a key role in the loss of macrophage viability and cell number in this study (Fig. 2a-c).
Although PLLA is considered non-toxic to ECs, its unfavorable surface properties can decrease EC adhesion and dysregulate cell functions. For example, PLLA (800kDa) membrane reduced the viability of human umbilical vein ECs (HUVECs)[31] and PLLA (126kDa) nanofibers slightly reduced the number of adherent bovine aortic ECs after 3 days of culture[32]. In this study, the addition of PLLA and PCL microparticles decreased both cell number and viability of human coronary artery ECs (Fig. 2a-b). Polyesters have been reported to induce EC and macrophage apoptosis.[33, 34] Here the loss of cell number and viability seems partially due to cell apoptosis induced by polymer particles, as evidenced by Annexin V positive ECs after treatment with PCL particles (Fig. 2d,e). Again, polymer molecular weight, particle size, and surface modification may be responsible for the increased apoptosis observed with PLLA and PCL particles.
ECs phagocytize dead cells and cell debris, e.g. apoptosis bodies[35], senescent neutrophils[36] and red blood cells[37]. The phagocytosis of necrotic cells induces activation of ECs by releasing TGF-β1 and IL-6 [38, 39] and increases ICAM-1 expression to facilitate macrophage adhesion.[40] In this scenario, phagocytosis dysreguates EC function and eventually leads to cell death.[37] ECs have also been shown to phagocytize polymer particles. [41-43] In contrast to enhanced macrophage phagocytosis, the phagocytic activity of ECs decreased upon microparticle treatment (Fig. 2c). However, as a marker of EC activation[44, 45], VCAM-1 expression increased significantly in ECs upon particle treatment (Fig. 3). Thus the phagocytic activity of ECs inversely correlates to their activation (Fig. 2c and 3). In order to understand the regulatory mechanism, we further quantified the production of TNF-α and superoxide since they are critical to endothelial activation. For example, autocrine TNF-α induces the expression of VCAM-1 in ECs and activates ECs to release other cytokines such as IL-6, IL-8, GM-CSF.[46, 47] TNF-α also contributes to EC activation via ROS production.[48, 49] In this study, we demonstrate the addition of PLLA and PCL microparticles did not significantly alter the production of TNF-α and ROS in either macrophages or ECs (Fig. 4), indicating a non-TNF-α nor ROS-dependent mechanism to regulate VCAM-1 expression. NO keeps the endothelium quiescent, inhibits the adhesion and activation of platelets and leukocyte and maintain the normal endothelium function. Therefore, we further revealed that the production of NO from ECs was significantly reduced by adding microparticles (p<0.05, Fig. 5). Along with the increase of VCAM-1 expression, the loss of ability to produce NO leads to a pro-inflammatory vascular phenotype and indicates EC dysfunction after the microparticle treatment.[50] Therefore the reduced phagocytosis of microparticles in ECs (Fig. 2c) can be further considered as a consequence of NO-dependent EC activation (Fig. 2d and 3).
In addition to cytokines, extracellularly released proteases could also induce EC dysfunction. Elastase is one of such proteases that actively participate in vascular wall remodeling after injury by degrading extracellular matrices such as elastin.[51] Furthermore, it was shown that the enzymatic activity of leucocyte elastase led to endothelial cell damage. [52-54] Similar to leucocytes such as neutrophils and macrophages, ECs secret an elastase-like enzyme which breaks down the same substrate that leucocyte elastase can degrade.[55] In this study, ECs exhibited a higher elastase activity than macrophages, and the treatment of cells with PLLA or PCL microparticles significantly enhanced elastase expression in both cell types (Fig. 6), which potentially contributed to EC dysfunction as discussed above. On the other hand, elastase expressed by macrophages and ECs could degrade polyesters and further enhance the cellular uptake of degradation products and activate cells. The degradation of PDLLA by neutrophil elastase as a model was demonstrated by mass spectrometry (Fig. 7), showing the active involvement of elastase in cell-material interactions and degradation product-induced EC activation and dysfunction.
This study enriches our knowledge on polymer-mediated endothelial dysfunction, which is particular important for polymer-coated drug-eluting coronary stents since late-thrombosis has been persistently observed after the drug is exhausted, indicating a possible role of polymer in preventing endothelial healing. Characterized by elevated inflammation and cell ingrowth from tunica media and adventitia, neointimal formation has been found with the degradation of polymer stents [13, 14]. Although the acute thrombosis and neointimal formation can be alleviated by applying an anti-proliferative drug, as a consequence, locally delivered therapeutics often induce EC apoptosis/necrosis, impair re-endothelialization and lead to late thrombosis.[15, 16] Endothelial dysfunction causes plaque formation and plays a key role in atherosclerosis.[56] In parallel, neoatherosclerosis has been found in drug-eluting stents which occurred earlier with higher frequency than in bare metal stents, again indicating a drug and/or polymer effect on incomplete endothelialization.[57, 58] Here the impairment of coronary artery endothelial function by biocompatible polymers such as PLLA and PCL was demonstrated.
4.1 Limitations
The major limitation of this study is lack of demonstration of endothelial dysfunction in an animal model using, for example, an acetylcholine assay. We realized this and chose low molecular weight polymer microparticles to mimic the effect of degraded polymer debris in vitro. The size of polymer microparticles ranged approximately from 1 to 38μm in this study, which might limit the accurate interpretation of size effect of particles on cellular response, although it was obvious in this study that microparticles within this range elicited cell dysfunction, making fine-tuning particle size unnecessary. The third limitation is we selected PLLA and PCL and drew the conclusions based on these polymers due to their clinical potential as a degradable stent material. However, possibilities cannot be excluded that other degradable biopolymers may be less cytotoxic with reduced dysregulating effects on macrophages and ECs. More careful examinations need to be performed to evaluate degradable polymers for coronary stent applications.
5. Conclusions
A delayed re-endothelialization has been observed with polymer-coated DES, for which the local delivery of anti-proliferative drugs is considered to be responsible. However in this study, we report for the first time that endothelial cell dysfunction can be also induced by biodegradable polymers such as PLLA and PCL, which are widely used as biocompatible materials. Moreover, microparticles made of these materials could lead to apoptosis and death of macrophages and endothelial cells. Upon exposure to polymer microparticles, cells increased the release of proteases such as elastase for polymer degradation, which in turn activated both cell types and resulted in cell dysfunction. The results of this study add important knowledge to cellular/tissue response to polymer-coated DES and warrant more careful evaluation of biodegradable polymers for coronary stent application.
Acknowledgments
This study was supported by NIH HL091465, NIH 1UH2 TR000491, NSF DMR 1006558, and NSF CAREER CBET 1056046. Confocal imaging was performed in part through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource (supported by NIH grants CA 68485, DK 20593, DK58404, HD15052, DK 59637, EY 08126). SEM imaging was performed through the use of the Vanderbilt Institute for Nanoscale Science and Engineering (VINSE) which is housed through in facilities renovated under NSF ARI-R2 DMR-0963361. There are no conflicts of interest associated with this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minami Y, Kaneda H, Inoue M, Ikutomi M, Morita T, Nakajima T. Endothelial dysfunction following drug-eluting stent implantation: a systematic review of the literature. Int J Cardiol. 2013;165:222–8. doi: 10.1016/j.ijcard.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 2.Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:166–70. doi: 10.1093/eurheartj/ehi571. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez-Valero S, Moreno R, Sanchez-Recalde A. Very Late Drug-Eluting Stent Thrombosis Related to Incomplete Stent Endothelialization: In-Vivo Demonstration by Optical Coherence Tomography. Journal of Invasive Cardiology. 2009;21:488–90. [PubMed] [Google Scholar]
- 4.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Acharya G, Lee CH, Lee Y. Optimization of cardiovascular stent against restenosis: factorial design-based statistical analysis of polymer coating conditions. PLoS One. 2012;7:e43100. doi: 10.1371/journal.pone.0043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Jing Q, Xu B, Yang L, Liu H, Shang X, et al. Safety and efficacy of biodegradable polymer-coated sirolimus-eluting stents in “real-world” practice: 18-month clinical and 9-month angiographic outcomes. JACC Cardiovasc Interv. 2009;2:303–9. doi: 10.1016/j.jcin.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 8.Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 9.Xi T, Gao R, Xu B, Chen L, Luo T, Liu J, et al. In vitro and in vivo changes to PLGA/sirolimus coating on drug eluting stents. Biomaterials. 2010;31:5151–8. doi: 10.1016/j.biomaterials.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Mueller RJ. Biological degradation of synthetic polyesters - Enzymes as potential catalysts for polyester recycling. Process Biochemistry. 2006;41:2124–8. [Google Scholar]
- 11.Yuan XY, Mak AFT, Yao KD. In vitro degradation of poly(L-lactic acid) fibers in phosphate buffered saline. Journal of Applied Polymer Science. 2002;85:936–43. [Google Scholar]
- 12.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 13.van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DR, Jr, et al. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94:1690–7. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 14.Lincoff AM, Furst JG, Ellis SG, Tuch RJ, Topol EJ. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol. 1997;29:808–16. doi: 10.1016/s0735-1097(96)00584-0. [DOI] [PubMed] [Google Scholar]
- 15.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans - Delayed healing and late thrombotic risk. Journal of the American College of Cardiology. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Sheehy A, Hsu S, Bouchard A, Lema P, Savard C, Guy LG, et al. Comparative vascular responses three months after paclitaxel and everolimus-eluting stent implantation in streptozotocin-induced diabetic porcine coronary arteries. Cardiovascular Diabetology. 2012;11:75. doi: 10.1186/1475-2840-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachman AL, Crowder SW, Ortiz O, Zienkiewicz KJ, Bronikowski CM, Yu SS, et al. Pro-angiogenic and anti-inflammatory regulation by functional peptides loaded in polymeric implants for soft tissue regeneration. Tissue Eng Part A. 2013;19:437–47. doi: 10.1089/ten.tea.2012.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1503–8. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dent G, Rabe KF, Magnussen H. Relationship between bronchoalveolar lavage neutrophil numbers and lavage fluid elastase and antielastase activities. Lung. 1995;173:165–75. doi: 10.1007/BF00175657. [DOI] [PubMed] [Google Scholar]
- 20.Andersson SR, Hakkarainen M, Albertsson AC. Long-term properties and migration of low molecular mass compounds from modified PLLA materials during accelerated ageing. Polymer Degradation and Stability. 2012;97:914–20. [Google Scholar]
- 21.Rydz J, Adamus G, Wolna-Stypka K, Marcinkowski A, Misiurska-Marczak M, Kowalczuk MM. Degradation of polylactide in paraffin and selected protic media. Polymer Degradation and Stability. 2013;98:316–24. [Google Scholar]
- 22.Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, et al. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12:1900–11. doi: 10.1021/bm200248h. [DOI] [PubMed] [Google Scholar]
- 23.Sangsanoh P, Waleetorncheepsawat S, Suwantong O, Wutticharoenmongkol P, Weeranantanapan O, Chuenjitbuntaworn B, et al. In vitro biocompatibility of schwann cells on surfaces of biocompatible polymeric electrospun fibrous and solution-cast film scaffolds. Biomacromolecules. 2007;8:1587–94. doi: 10.1021/bm061152a. [DOI] [PubMed] [Google Scholar]
- 24.Kang Y, Wu J, Yin G, Huang Z, Yao Y, Liao X, et al. Preparation, characterization and in vitro cytotoxicity of indomethacin-loaded PLLA/PLGA microparticles using supercritical CO2 technique. Eur J Pharm Biopharm. 2008;70:85–97. doi: 10.1016/j.ejpb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Walter E, Merkle HP. Microparticle-mediated transfection of non-phagocytic cells in vitro. J Drug Target. 2002;10:11–21. doi: 10.1080/10611860290007478. [DOI] [PubMed] [Google Scholar]
- 26.Lam KH, Schakenraad JM, Esselbrugge H, Feijen J, Nieuwenhuis P. The effect of phagocytosis of poly(L-lactic acid) fragments on cellular morphology and viability. J Biomed Mater Res. 1993;27:1569–77. doi: 10.1002/jbm.820271214. [DOI] [PubMed] [Google Scholar]
- 27.Eidi H, Joubert O, Attik G, Duval RE, Bottin MC, Hamouia A, et al. Cytotoxicity assessment of heparin nanoparticles in NR8383 macrophages. Int J Pharm. 2010;396:156–65. doi: 10.1016/j.ijpharm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735–40. doi: 10.1016/j.biomaterials.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Woodward SC, Brewer PS, Moatamed F, Schindler A, Pitt CG. The Intracellular Degradation of Poly(Epsilon-Caprolactone) Journal of Biomedical Materials Research. 1985;19:437–44. doi: 10.1002/jbm.820190408. [DOI] [PubMed] [Google Scholar]
- 30.Cu Y, LeMoellic C, Caplan MJ, Saltzman WM. Ligand-modified gene carriers increased uptake in target cells but reduced DNA release and transfection efficiency. Nanomedicine. 2010;6:334–43. doi: 10.1016/j.nano.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Gao C, Liu Y, Shen J. Endothelial cell functions in vitro cultured on poly(L-lactic acid) membranes modified with different methods. J Biomed Mater Res A. 2004;69:436–43. doi: 10.1002/jbm.a.30007. [DOI] [PubMed] [Google Scholar]
- 32.Francois S, Chakfe N, Durand B, Laroche G. A poly(L-lactic acid) nanofibre mesh scaffold for endothelial cells on vascular prostheses. Acta Biomater. 2009;5:2418–28. doi: 10.1016/j.actbio.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Can E, Udenir G, Kanneci AI, Kose G, Bucak S. Investigation of PLLA/PCL Blends and Paclitaxel Release Profiles. Aaps Pharmscitech. 2011;12:1442–53. doi: 10.1208/s12249-011-9714-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potnis PA, Tesfamariam B, Wood SC. Induction of Nicotinamide-Adenine Dinucleotide Phosphate Oxidase and Apoptosis by Biodegradable Polymers in Macrophages: Implications for Stents. Journal of Cardiovascular Pharmacology. 2011;57:712–20. doi: 10.1097/FJC.0b013e31821a4f1e. [DOI] [PubMed] [Google Scholar]
- 35.Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, et al. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108(Pt 3):967–73. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 36.Gao C, Xie R, Li W, Zhou J, Liu S, Cao F, et al. Endothelial cell phagocytosis of senescent neutrophils decreases procoagulant activity. Thromb Haemost. 2013;109 doi: 10.1160/TH12-12-0894. [DOI] [PubMed] [Google Scholar]
- 37.Fens MH, van Wijk R, Andringa G, van Rooijen KL, Dijstelbloem HM, Rasmussen JT, et al. A role for activated endothelial cells in red blood cell clearance: implications for vasopathology. Haematologica. 2012;97:500–8. doi: 10.3324/haematol.2011.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Q, Chen L, Liu B, Vialli C, Stone P, Ching LM, et al. The role of autocrine TGFbeta1 in endothelial cell activation induced by phagocytosis of necrotic trophoblasts: a possible role in the pathogenesis of pre-eclampsia. J Pathol. 2010;221:87–95. doi: 10.1002/path.2690. [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Stone P, Ching LM, Chamley L. A role for interleukin-6 in spreading endothelial cell activation after phagocytosis of necrotic trophoblastic material: implications for the pathogenesis of pre-eclampsia. J Pathol. 2009;217:122–30. doi: 10.1002/path.2425. [DOI] [PubMed] [Google Scholar]
- 40.Chen Q, Stone PR, McCowan LM, Chamley LW. Phagocytosis of necrotic but not apoptotic trophoblasts induces endothelial cell activation. Hypertension. 2006;47:116–21. doi: 10.1161/01.HYP.0000196731.56062.7c. [DOI] [PubMed] [Google Scholar]
- 41.Hara S, Ishiguro S, Mizuno K. Phagocytosis of polystyrene spheres in the rabbit corneal endothelium: contribution of lysosomal enzymes to the endothelial degeneration. Invest Ophthalmol Vis Sci. 1985;26:1631–4. [PubMed] [Google Scholar]
- 42.Serda RE, Gu J, Burks JK, Ferrari K, Ferrari C, Ferrari M. Quantitative mechanics of endothelial phagocytosis of silicon microparticles. Cytometry A. 2009;75:752–60. doi: 10.1002/cyto.a.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo JW, Doshi N, Mitragotri S. Endocytosis and Intracellular Distribution of PLGA Particles in Endothelial Cells: Effect of Particle Geometry. Macromol Rapid Commun. 2010;31:142–8. doi: 10.1002/marc.200900592. [DOI] [PubMed] [Google Scholar]
- 44.Andresen TK, Svennevig JL, Videm V. Soluble VCAM-1 is a very early marker of endothelial cell activation in cardiopulmonary bypass. Perfusion. 2002;17:15–21. doi: 10.1191/0267659102pf531oa. [DOI] [PubMed] [Google Scholar]
- 45.Videm V, Albrigtsen M. Soluble ICAM-1 and VCAM-1 as markers of endothelial activation. Scand J Immunol. 2008;67:523–31. doi: 10.1111/j.1365-3083.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 46.Iademarco MF, Barks JL, Dean DC. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest. 1995;95:264–71. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Q, Wang J, Wang XL, VandeBerg JL. Comparative analysis of vascular endothelial cell activation by TNF-alpha and LPS in humans and baboons. Cell Biochem Biophys. 2004;40:289–303. doi: 10.1385/CBB:40:3:289. [DOI] [PubMed] [Google Scholar]
- 48.Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, et al. TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2007;27:1269–75. doi: 10.1161/ATVBAHA.107.142521. [DOI] [PubMed] [Google Scholar]
- 49.Gao X, Zhang H, Belmadani S, Wu J, Xu X, Elford H, et al. Role of TNF-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H2242–9. doi: 10.1152/ajpheart.00587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudic RD, Sessa WC. Nitric oxide in endothelial dysfunction and vascular remodeling: clinical correlates and experimental links. Am J Hum Genet. 1999;64:673–7. doi: 10.1086/302304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 52.Chignard M, Balloy V, Renesto P. Leucocyte elastase-mediated release of von Willebrand factor from cultured endothelial cells. Eur Respir J. 1993;6:791–6. [PubMed] [Google Scholar]
- 53.Tull SP, Bevins A, Kuravi SJ, Satchell SC, Al-Ani B, Young SP, et al. PR3 and elastase alter PAR1 signaling and trigger vWF release via a calcium-independent mechanism from glomerular endothelial cells. PLoS One. 2012;7:e43916. doi: 10.1371/journal.pone.0043916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blann AD, Seigneur M, Adams RA, McCollum CN. Neutrophil elastase, von Willebrand factor, soluble thrombomodulin and percutaneous oxygen in peripheral atherosclerosis. Eur J Vasc Endovasc Surg. 1996;12:218–22. doi: 10.1016/s1078-5884(96)80110-9. [DOI] [PubMed] [Google Scholar]
- 55.Menashi S, Hornebeck W, Robert L, Legrand Y. Elastase-like activity in cultured aortic endothelial cells. Thromb Res. 1989;53:11–8. doi: 10.1016/0049-3848(89)90111-4. [DOI] [PubMed] [Google Scholar]
- 56.Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314–22. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J Am Coll Cardiol. 2012;59:2051–7. doi: 10.1016/j.jacc.2011.10.909. [DOI] [PubMed] [Google Scholar]


