Abstract
The BH3-only BID (BH3-interacting domain death agonist) protein has a critical function in the death-receptor pathway in the liver by triggering mitochondrial outer membrane permeabilization (MOMP). Here we show that MTCH2/MIMP (mitochondrial carrier homologue 2/Met-induced mitochondrial protein), a novel truncated BID (tBID)-interacting protein, is a surface-exposed outer mitochondrial membrane protein that facilitates the recruitment of tBID to mitochondria. Knockout of MTCH2/MIMP in embryonic stem cells and in mouse embryonic fibroblasts hinders the recruitment of tBID to mitochondria, the activation of Bax/Bak, MOMP, and apoptosis. Moreover, conditional knockout of MTCH2/MIMP in the liver decreases the sensitivity of mice to Fas-induced hepatocellular apoptosis and prevents the recruitment of tBID to liver mitochondria both in vivo and in vitro. In contrast, MTCH2/MIMP deletion had no effect on apoptosis induced by other pro-apoptotic Bcl-2 family members and no detectable effect on the outer membrane lipid composition. These loss-of-function models indicate that MTCH2/MIMP has a critical function in liver apoptosis by regulating the recruitment of tBID to mitochondria.
Programmed cell death or apoptosis is critical for both the development and maintenance of tissues. Caspases, a family of cysteine proteases, are the major executioners of the apoptotic process, whereas the Bcl-2 family members are the major regulators of this process1,2. The Bcl-2 family is composed of both pro-apoptotic and anti-apoptotic proteins. A subset of the pro-apoptotic factors are the BH3-only proteins, which act as sentinels of internal damage.
The BH3-only BID protein is a critical activator of the mitochondrial apoptotic program. Activation of the tumour necrosis factor (TNF)/Fas death receptors leads to caspase-8-mediated cleavage of BID to the truncated form, tBID, that translocates to the mitochondria to activate Bax and Bak, resulting in MOMP3–5. The requirement for BID in the Fas death pathway was demonstrated in BID-deficient mice, which are resistant to Fas-induced hepatocellular apoptosis6; the requirement for Bax and Bak was demonstrated in Bax/Bak double-knockout cells, which are resistant to multiple apoptotic stimuli as well as to tBID and several other BH3-only molecules7,8.
The molecular mechanism by which the outer mitochondrial membrane (OMM) is permeabilized by tBID-induced activation of Bax and Bak is poorly understood. This process has been proposed to be regulated by several resident mitochondrial proteins and by the OMM lipid composition9–14. We previously demonstrated that in TNF-α-activated haematopoietic FL5.12 cells, tBID becomes part of a roughly 45 kDa crosslinkable mitochondrial complex that comprises mitochondrial carrier homologue 2 (MTCH2 (refs 15, 16); also named Met-induced mitochondrial protein (MIMP)17). MTCH2/MIMP is a novel and previously uncharacterized 33 kDa protein related to the mitochondrial carrier (MC) protein family18. Here we show that MTCH2/MIMP is an OMM protein with a critical function in the MOMP process by facilitating the recruitment of tBID to mitochondria.
RESULTS
MTCH2/MIMP is an OMM protein
MTCH2/MIMP is related to members of the MC protein family and was therefore likely to be localized to the inner mitochondrial membrane (IMM). To determine the mitochondrial location of MTCH2/MIMP, we exposed purified mouse liver mitochondria to proteinase K and found that MTCH2/MIMP was sensitive to cleavage15 (Fig. 1a, left panel). As controls, apoptosis-inducing factor (AIF, localized to the intermembrane space) and adenine nucleotide translocator (ANT, localized to the IMM) remained largely resistant to cleavage (Fig. 1a, middle and right panels). Thus, these results suggested that MTCH2/MIMP is exposed on the surface of mitochondria and is therefore most probably localized to the OMM. To assess its location further, we performed submitochondrial membrane fractionation studies as described previously19. We found that MTCH2/MIMP is most prominent in the low-density fractions (enriched in OMM vesicles; Fig. 1b), indicating that it is enriched at the outer membrane.
Figure 1.
MTCH2/MIMP is an outer mitochondrial membrane protein. (a) MTCH2/MIMP is exposed on the surface of mitochondria. Mouse liver mitochondria were either left untreated (−) or treated with a low (0.1 μg ml−1; +) or a high (1 μg ml−1; ++) concentration of proteinase K (Prot K), lysed, size-fractionated by SDS–PAGE and analysed by western blotting with anti-MTCH2/MIMP antibodies (left panel), anti-AIF antibodies (middle panel) or anti-ANT antibodies (right panel). IMS, intermembrane space. (b) MTCH2/MIMP is enriched in the OMM. Submitochondrial membrane vesicles were prepared from rat liver mitochondria, lysed, size-fractionated by SDS–PAGE and analysed by western blotting with anti-cytochrome c oxidase subunit IV (Cyt Oxi) antibodies, anti-ANT antibodies, anti-TOM20 antibodies and anti-MTCH2/MIMP antibodies. OMM, low-density fractions enriched in outer-membrane vesicles; IMM, high-density fractions enriched in inner-membrane vesicles. (c) MTCH2/MIMP is degraded by trypsin after import into yeast mitochondria. Radiolabelled MTCH2/MIMP was imported into purified wild-type yeast mitochondria for 30 min at 30 °C in the presence or absence of a membrane potential (ΔΨm). Non-imported precursor was removed by treatment with protease, and the imported proteins were resolved by 15% SDS–PAGE and detected by autoradiography. Also included were 5% input standards (STD) of MTCH2/MIMP and Su9-DHFR. For Su9-DHFR, the locations of precursor (p) and imported mature (m) protein are indicated. (d) MTCH2/MIMP is a membrane-associated protein. Radiolabelled MTCH2/MIMP and AAC were imported into purified wild-type yeast mitochondria for 30 min at 30 °C in the presence of membrane potential (ΔΨm). After import, the samples were extracted with carbonate at the indicated pH values. The supernatants were precipitated with trichloroacetic acid. Total (T), pellets (P) and supernatants (S) were resolved by 13% SDS–PAGE and detected by autoradiography. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
As a third approach to confirm the OMM localization of MTCH2/MIMP we used in vitro import into yeast mitochondria. Radiolabelled MTCH2/MIMP was imported into wild-type yeast mitochondria for 30 min. Trypsin was then added to degrade non-imported protein. As expected, MTCH2/MIMP, and not Su9-DHFR (matrix protein) control, was degraded (Fig. 1c). Assembly in the OMM was confirmed by repeating the import assay followed by carbonate extraction over a pH gradient. In agreement with the prediction that MTCH2/MIMP is imported and is an OMM protein, the imported MTCH2/MIMP protein remained associated with the membrane fraction up to the basic pH of 12.5, similarly to the ADP/ATP carrier (AAC) of the inner membrane (Fig. 1d). In addition, mitochondrial import was time-dependent (Supplementary Information, Fig. S1a). Furthermore, loss of the membrane potential impaired AAC import (Supplementary Information, Fig. S1b), but import of MTCH2/MIMP was not affected because its import does not require the inner-membrane translocons. Consistent with these data, we have also performed in vitro imports into yeast mitochondria with temperature-sensitive (ts) mutations in the mitochondrial carrier-dependent pathway, specifically Tim10p. Import of AAC has been decreased in this mutant (as demonstrated by the increased fraction in the supernatant20), but import of MTCH2/MIMP was not significantly decreased, suggesting that Tim10p is not required for import of MTCH2/MIMP (Supplementary Information, Fig. S1b). Taken together, all three approaches support the localization of MTCH2/MIMP to the mitochondrial outer membrane.
Loss of MTCH2/MIMP in embryonic stem cells decreases the sensitivity to tBID-induced MOMP
To determine the role and importance of MTCH2/MIMP in vivo, we disrupted the MTCH2/MIMP gene in mice. In the targeted allele, the first three exons were replaced by a neomycin-resistant cassette, thereby creating a functional null allele (Fig. 2a). Embryonic stem (ES) cell clones containing the targeted MTCH2/MIMP allele were isolated, and independent lines of genetically modified mice were generated. Analysing the offspring of heterozygote intercrosses revealed that heterozygotes for the targeted allele (MTCH2/MIMP+/−) are viable and fertile and show no obvious phenotypic abnormalities. However, no homozygote null (MTCH2/MIMP−/−) animals were observed, and timed pregnancies revealed that loss of MTCH2/MIMP results in embryonic lethality at embryonic day (E)7.5 (Supplementary Information, Fig. S2a, b). Histological analysis and RNA in situ hybridization of the E7.5 wild-type and MTCH2/MIMP−/− embryos identified multiple defects in the knockout embryos that are likely to account for the embryonic lethality (Supplementary Information, Fig. S2c–e).
Figure 2.
Loss of MTCH2/MIMP in ES cells decreases the sensitivity to tBID-induced MOMP. (a) The MTCH2/MIMP gene spans about 23 kilobases (kb) on chromosome 2 and consists of 13 exons. In the targeted allele, the first three exons were replaced by a neomycin (Neo)-resistant cassette, thereby creating a functional null allele. Also indicated are the short homology (SH) and long homology (LH) regions, the thymidine kinase (TK) negative selection cassette, restriction enzyme sites (NcoI and SpeI) and the positions of external probes. (b) Generation of MTCH2/MIMP−/− ES stable lines. Left: wild-type and MTCH2/MIMP−/− E3.5 blastocytes were lysed, subjected to SDS–PAGE and western blot analysed with anti-MTCH2/MIMP antibodies. Right: the MTCH2/MIMP knockout ES cells were transfected with either an empty pcDNA3.1 vector (KO) or a pcDNA3.1 vector carrying MTCH2/MIMP-MH (R). Cells from four single stable clones (KO1, KO2, R1 and R2 clones) were lysed and analysed as above. Porin was used as an internal standard. (c) R cells are more sensitive than KO cells to tBID-induced mitochondrial depolarization. Presented are tetramethylrhodamine ethyl ester fluorescence (excitation wavelength 545 nm, emission wavelength 580 nm) recordings of KO1 (black) and R1 (red) clones treated with 0, 1 and 40 nM recombinant tBID. The actual raw data from a representative experiment appears in the top panel, and the data in the bottom panel represent the means and s.e.m. for three independent recordings. (d) R cells are more sensitive than KO cells to tBID-induced cytochrome c release. At the end of the recordings in c, the suspensions of the KO1 and R1 clones were centrifuged and the supernatants were subjected to SDS–PAGE, followed by western blot analysis with anti-cytochrome c antibodies. Actin was used as an internal standard. rectBID, recombinant tBID. (e) Bak is homodimerized in the R cells at the low concentration of tBID. KO1 and R1 clones were treated as in c, and after centrifugation the pellet fractions were treated with the crosslinker 1,6-bismaleimidohexane (BMH), lysed and western blot analysed with anti-Bak antibodies. Porin was used as an internal standard. Similar results were obtained with the two additional pairs of KO and R clones. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Next we prepared wild-type and MTCH2/MIMP−/− ES cells from E3.5 blastocytes. Western blot analysis with anti-MTCH2/MIMP antibodies confirmed the absence of MTCH2/MIMP from the knockout cells (Fig. 2b, left panel). The MTCH2/MIMP−/− ES cells were then transfected with either an empty vector or a vector carrying MTCH2/MIMP fused to a Myc–His (MH) tag, and several stable lines carrying either the empty vector (KO) or MTCH2/MIMP-MH (R for rescue) were generated (Fig. 2b, right panel; two of the KO and two of the R clones are shown). Next we examined the sensitivity of the KO and R cells to tBID-induced mitochondria depolarization, cytochrome c release and Bak/Bax dimerization. Both KO and R cells responded to 40 nM recombinant tBID by mitochondria depolarization (Fig. 2c), cytochrome c release (Fig. 2d) and Bak dimerization (Fig. 2e; similar results were obtained for Bax (data not shown)). However, the R cells were more sensitive than the KO cells to lower levels of tBID (1 nM; Fig. 2c–e). Similar results were obtained from two more sets of KO and R clones (Supplementary Information, Fig. S3). Thus, the presence of MTCH2/MIMP sensitizes mitochondria to tBID-induced MOMP.
Conditional knockout of MTCH2/MIMP in mouse embryonic fibroblasts (MeFs) reduces the sensitivity to tBID-induced apoptosis
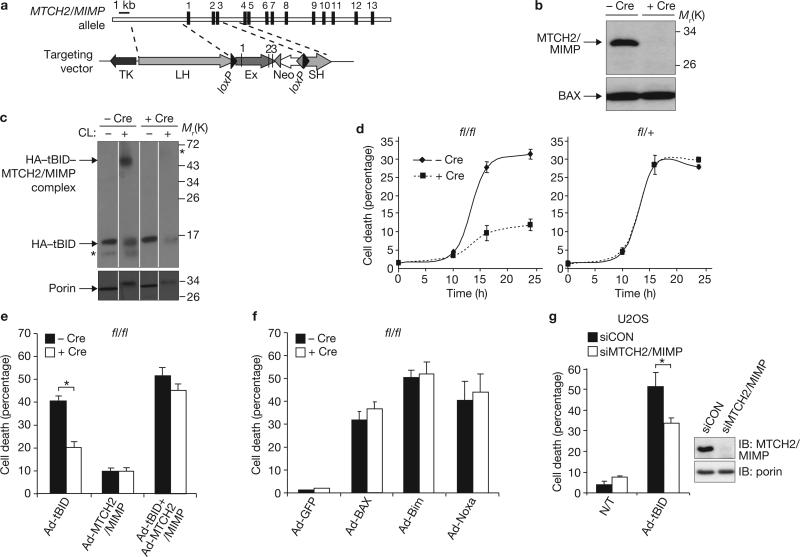
To dissect the role of MTCH2/MIMP in viable mice and cells, we used the Cre/loxP system to generate a conditional gene knockout mouse (Fig. 3a). ES cell clones containing the targeted MTCH2/MIMP allele were isolated, and independent lines of genetically modified mice were generated (Methods and Supplementary Information, Fig. S4). To define the functional consequences of a deletion of MTCH2/MIMP, MEFs were isolated from homozygous MTCH2/MIMPfl/fl embryos and transduced with purified Cre recombinase, leading to efficient deletion of MTCH2/MIMP in vitro (Fig. 3b). Crosslinking experiments confirmed that tBID forms the roughly 45 kDa tBID–MTCH2/MIMP crosslinkable complex in MTCH2/MIMPfl/fl cells but not in the same cells transduced with purified Cre recombinase (Fig. 3c).
Figure 3.
Conditional knockout of MTCH2/MIMP in MEFs decreases the sensitivity to tBID-induced apoptosis. (a) Generation of the MTCH2/MIMP conditional targeting vector. Indicated are loxP sites (black triangles), Frt sites (grey triangles), the neomycin (Neo) positive selection cassette, and the thymidine kinase (TK) negative selection cassette. (b) Conditional deletion of MTCH2/MIMP in MEFs. MTCH2/MIMPfl/fl MEFs in the absence or presence of Cre recombinase were lysed, and the mitochondria-enriched fractions were western blot analysed for MTCH2/MIMP. Bax was used as an internal standard. (c) The tBID crosslinked complex is not generated in MTCH2/MIMP-deficient MEFs. MTCH2/MIMPfl/fl MEFs were treated as in b, infected with Ad-tBID, and the mitochondria-enriched fractions were treated in the absence or presence of the crosslinker bis(2-(sulfosuccinimidooxy-carbonyloxy)ethyl)sulphone (BSOCOES) followed by western blotting analysis. CL, crosslinker. Asterisks mark crossreactive bands. Porin was used as an internal standard (bottom panel) and all samples were run on the same gel. (d) MTCH2/MIMP-deficient MEFs are less sensitive to Ad-tBID-induced apoptosis. MTCH2/MIMPfl/fl (fl/fl; left panel) and MTCH2/MIMPfl/+ (fl/+; right panel) MEFs in the absence or presence of Cre recombinase were infected with Ad-tBID and cell death was monitored by propidium iodide dye exclusion. Data are means and s.d. for three independent experiments. (e) The reduced susceptibility of MTCH2/MIMP-deficient MEFs to Ad-tBID is due to the absence of MTCH2/MIMP. fl/fl MEFs in the absence or presence of Cre recombinase were infected with the indicated adenoviruses, and cell death was monitored as above. Data are the means and s.d. for three independent experiments; asterisk, P < 0.00005. (f) MTCH2/MIMP deletion has no effect on apoptosis induced by other pro-apoptotic Bcl-2 family members. fl/fl MEFs in the absence or presence of Cre recombinase were infected with the indicated adenoviruses, and cell death was monitored as above. Data are means and s.d. for three independent experiments. GFP, green fluorescent protein. (g) Knocking down MTCH2/MIMP in U2OS cells decreases the sensitivity to Ad-tBID. MTCH2/MIMP was knocked down in U2OS cells as described in Methods, and cells were either left untreated (N/T) or infected with Ad-tBID and cell death was monitored as above. Data are the means and s.d. for three independent experiments; asterisk, P < 0.005. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
To assess the sensitivity to tBID-induced cell death, homozygous MTCH2/MIMPfl/fl and heterozygous MTCH2/MIMPfl/+ MEFs were transduced with Cre recombinase and infected with haemagglutinin (HA)-tagged tBID (Ad-tBID21), and cell death was monitored. Whereas Cre transduction did not affect sensitivity to apoptosis in heterozygous MTCH2/MIMPfl/+ (fl/+) cells, MTCH2/MIMPfl/fl (fl/fl) cells transduced with Cre recombinase were significantly less sensitive to Ad-tBID (Fig. 3d). Reintroduction of MTCH2/MIMP into fl/fl MEFs transduced with Cre recombinase fully restored susceptibility to tBID-induced cell death (Fig. 3e). Thus, MTCH2/MIMP is important in tBID-induced cell death.
To assess whether the effects described above were specific to tBID, we infected cells with recombinant adenoviruses carrying Bax, Bim or Noxa vectors. Infection with all three viruses induced high levels of cell death in fl/fl MEFs (in comparison with control Ad-GFP); however, Cre recombinase transduction had no effect on their ability to induce cell death (Fig. 3f). Thus, MTCH2/MIMP does not have a function in the pro-apoptotic action of other Bcl-2 family members. Finally, to assess whether the effect of MTCH2/MIMP is conserved in different mammalian species, MTCH2/MIMP was knocked down in human U2OS cells, and this knockdown resulted in a roughly 40% decrease in Ad-tBID-induced cell death (Fig. 3g). Thus, MTCH2/MIMP has a function in the pro-apoptotic action of tBID in human cells also.
Conditional knockout of MTCH2/MIMP in MeFs hinders the recruitment of tBID to mitochondria
To assess their sensitivity to apoptotic signals, fl/fl MEFs were treated as above and exposed to Fas, TNF-α, staurosporine, etoposide, cisplatin and ultraviolet. This analysis demonstrated that fl/fl cells after Cre transduction were less sensitive to cell death induced by etoposide and cisplatin but equally sensitive to the other death reagents (Fig. 4a, left panel; Supplementary Information, Fig. S5a). The decreased sensitivity to etoposide and cisplatin was due to deletion of MTCH2/MIMP, because fl/+ MEFs transduced with Cre recombinase did not show decreased sensitivity to these stimuli (Fig. 4a, right panel). Caspase-3 cleavage analysis confirmed that deletion of MTCH2/MIMP decreases apoptosis induced by Ad-tBID or etoposide (Supplementary Information, Fig. S5b).
Figure 4.
Conditional knockout of MTCH2/MIMP in MEFs hinders the recruitment of tBID to mitochondria. (a) MTCH2/MIMP-deficient MEFs are less sensitive to apoptosis induced by DNA-damaging reagents. fl/fl and fl/+ MEFs in the absence or presence of Cre recombinase were treated with each of the indicated apoptotic stimuli for 14 h: Fas (1 ng ml−1) and cycloheximide (CHX; 1 μg ml−1), etoposide (Etop; 100 μM), and cisplatin (Cis; 33 μM). Cell death was monitored as above. Data are means and s.d. for three independent experiments; asterisk, P < 0.0005. (b) Deletion of MTCH2/MIMP hinders the recruitment of tBID to mitochondria. fl/fl MEFs in the absence or presence of Cre recombinase were either infected with Ad-tBID, treated with etoposide (100 μM; 8 h) or treated with Fas (5 ng ml−1) and cycloheximide (CHX; 1 μg ml−1; 6 h). Cells were then lysed, and the mitochondria-enriched fractions, the cytosolic fractions and total cell lysates were western blot analysed with anti-HA (top left panel) or anti-BID (all other panels) antibodies. Asterisks mark crossreactive bands. Porin and actin were used as internal standards. (c) Deletion of MTCH2/MIMP decreases the levels of N-terminal exposed/activated Bax. fl/fl MEFs were treated as in b and lysed, and the mitochondria-enriched fractions were treated with trypsin as described in Methods, followed by western blot analysis. The middle panel shows a short exposure of the blot shown in the top panel. Porin was used as an internal standard. (d) Deletion of MTCH2/MIMP reduces cytochrome c release. fl/ fl MEFs in the absence or presence of Cre recombinase were treated with the indicated death stimuli. Cells were then fixed and immunostained for cytochrome c, and the percentage of cells with cytochrome c released was quantified. Data are means and s.d. for three independent experiments. About 300 cells of each treatment were analysed. Asterisk, P < 0.05; two asterisks, P < 0.01. (e) fl/fl MEFs are type I cells. fl/fl MEFs were either infected or not with Ad-Bcl-2 and then treated with the indicated death stimuli for 14 h: Fas and cycloheximide (as in a), and etoposide (10 μM). Cell death was monitored as in a. Data are means and s.d. for three independent experiments; asterisk, P < 0.005. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Next, we examined the status of tBID and found that deletion of MTCH2/MIMP significantly hindered the recruitment of tBID to mitochondria after treatment with Ad-tBID, etoposide and Fas (Fig. 4b, top panels). Western blot analysis of the cytosolic fractions and of total cell lysates indicated that deletion of MTCH2/MIMP had no effect on the levels of either full-length BID or tBID (Fig. 4b, middle and bottom panels, respectively). Thus, the presence of MTCH2/MIMP facilitates the recruitment of tBID to mitochondria.
Next, we assessed the effect of MTCH2/MIMP deletion on Bax activation and cytochrome c release. Bax undergoes an activating conformational change before membrane integration and oligomerization that includes exposure of its amino terminus, which becomes accessible to protease cleavage22. We found that fl/fl MEFs transduced with Cre recombinase showed significantly less cleavage of Bax by trypsin after treatment with all three stimuli (Fig. 4c). We also found that deletion of MTCH2/MIMP significantly decreased the formation of Bax homodimers in cells treated with all three stimuli (Supplementary Information, Fig. S5c). On the basis of these results we predicted that MTCH2/MIMP deletion would also decrease MOMP, and indeed we found that fl/fl MEFs transduced with Cre recombinase showed significantly less cytochrome c release after treatment with all three stimuli (Fig. 4d; Supplementary Information, Fig. S5d). Thus, deletion of MTCH2/MIMP hinders the recruitment of tBID to mitochondria, resulting in less Bax activation and MOMP.
Finally, the fact that Cre transduction had little effect on apoptosis induced by Fas but hindered Fas-induced tBID recruitment, Bax activation and cytochrome c release suggested that fl/fl MEFs are type I cells (that is, cells in which the mitochondrial pathway does not determine the time course of death-receptor-induced apoptosis). Indeed, we found that fl/fl MEFs infected with recombinant adenoviruses carrying the Bcl-2 vector were protected from etoposide but not from Fas-induced cell death (Fig. 4e).
MTCH2/MIMP deletion in the liver decreases the sensitivity of mice to Fas-induced hepatocellular apoptosis and hinders the recruitment of tBID to mitochondria
It was previously shown that BID is a critical substrate in vivo for signalling by death-receptor agonists, which mediates a mitochondrial amplification loop essential for the apoptosis of hepatocytes6. To determine whether MTCH2/MIMP is a critical component of the tBID-death pathway in vivo, we generated MTCH2/MIMP liver-specific knockout mice by using the Alb-Cre transgene (Methods and Supplementary Information, Fig. S6). MTCH2/ MIMPfl/Δ;Alb-Cre (liver-specific knockout) and MTCH2/MIMPfl/+;Alb-Cre (liver-specific heterozygote) were used for these studies, and western blotting confirmed efficient and specific deletion of the MTCH2/MIMP allele in the liver of the knockout animals but not in that of the heterozygous animals (Fig. 5a). In addition, analysis of serum liver enzymes indicated that MTCH2/MIMP deletion in the liver does not significantly alter the function of hepatocytes (Supplementary Information, Fig. S7).
Figure 5.
MTCH2/MIMP deletion in the liver decreases the sensitivity of mice to Fas-induced hepatocellular apoptosis and hinders the recruitment of tBID to mitochondria. (a) Western blot analysis of MTCH2/MIMP in liver lysates demonstrates its absence in livers prepared from MTCH2/MIMPfl/Δ;Alb-Cre (fl/Δ) mice. Bcl-XL was used as an internal standard (bottom panel). The asterisk marks a crossreactive band. (b) Conditional knockout of MTCH2/MIMP in the liver significantly decreases the sensitivity of mice to Fas-induced hepatocellular apoptosis. Left: Kaplan–Meier survival curves of MTCH2/MIMPfl/+;Alb-Cre mice (fl/+; n = 15) and MTCH2/MIMPfl/Δ;Alb-Cre mice (fl/Δ; n = 14) in response to a single intraperitoneal injection of 0.55 μg g−1 anti-Fas antibody (Jo2; Pharmingen). The Kaplan–Meier survival curves were compared by using the long-rank test and were found statistically different from each other (P < 0.05). Right: haematoxylin/eosin staining of paraffin-embedded liver sections from a fl/+ mouse (top) and a fl/Δ mouse (bottom) 4 h after injection of anti-Fas antibody. Note the condensed and fragmented nuclei and the haemorrhage in the fl/+ liver. Scale bars, 50 μM. (c) A significant decrease in caspase-3 activation in fl/Δ liver cytosolic fractions in response to anti-Fas antibody. fl/+ and fl/Δ mice (four of each) were either left untreated (−) or injected with anti-Fas antibodies for the indicated durations. After treatment, S100 fractions (the supernatant resulting from 100,000g centrifugation of cytosolic fractions) were prepared from all eight livers and analysed by western blotting for caspase-8 cleavage/activation (top panel; p18 represents the activated protease), BID cleavage/activation (middle panel), and caspase-3 (Casp-3) activity with the fluorogenic peptide substrate DEVD-AMC (bottom panel; the results are presented in arbitrary units (AU) as means and s.d. for three independent experiments; asterisk, P < 0.02; two asterisks, P < 0.01). Actin was used in both blots as an internal standard. IB, immunoblot. (d) A significant decrease in tBID recruitment to mitochondria and Bax activation in fl/Δ liver mitochondrial fractions in response to anti-Fas antibody. Top: liver mitochondrial fractions prepared from the mice described in c were lysed, and western blot analysed with anti-BID antibodies. Bottom: liver mitochondrial fractions treated with trypsin, lysed, and western blot analysed with anti-Bax antibodies. Porin was used in both blots as an internal standard. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
To determine the sensitivity of these mice to Fas, MTCH2/MIMPfl/+;Alb-Cre (fl/+) and MTCH2/MIMPfl/Δ;Alb-Cre (fl/Δ) mice were injected intraperitoneally with anti-Fas antibody (Jo2; 0.55 μg g−1). Most fl/+ mice (12/15; 80%) died within about 5 h from acute liver failure associated with massive hepatic apoptosis and haemorrhagic necrosis (Fig. 5b). In contrast, only 21% (3/14) of the fl/Δ mice died within 5 h of injection with anti-Fas antibody, and the rest either died with delayed kinetics (5/14; 35%) or survived (6/14; 43%) with no apparent liver injury (Fig. 5b).
To determine the molecular basis for the differences in sensitivity, three fl/+ and three fl/Δ mice were injected with anti-Fas antibodies, and the mice were killed at three time points after injection (the fl/Δ mice were killed at a 2-h delay owing to their delayed death). After removal, the eight livers (including livers from two non-injected mice) were lysed, and the cytosolic and mitochondrial fractions were taken for analysis. Analysis of the cytosolic fractions of the fl/+ livers indicated that caspase-8, BID and caspase-3 had been activated (Fig. 5c, top, middle and bottom panels, respectively). Analysis of the cytosolic fractions of the fl/Δ livers indicated that caspase-8 and BID had been activated in all three mice to a similar extent to that in the fl/+ mice (Fig. 5c, top and middle panels). However, there was significantly less caspase-3 activation in all three fl/Δ cytosols (Fig. 5c, bottom right panel). The results obtained here with caspase-8 and caspase-3 are similar to those obtained with BID-deficient mice6, indicating that MTCH2/MIMP acts downstream of BID cleavage and upstream of caspase-3 activation.
To determine whether MTCH2/MIMP acts at the level of tBID recruitment to mitochondria, we examined the liver mitochondrial fractions for tBID content. We found that the mitochondrial levels of tBID were significantly lower in all three fl/Δ mitochondrial fractions than in the three fl/+ mitochondrial fractions (Fig. 5d, top panel). We also examined the state of Bax activation and found that Bax cleavage by trypsin was also significantly decreased in all three fl/Δ mitochondrial fractions (Fig. 5d, bottom panel). MTCH2/MIMP deficiency results in increased levels of mitochondrial Bax, which may indicate an attempt to compensate for the lack of MTCH2/MIMP. However, despite this increase, mitochondrial Bax remained largely inactive.
MTCH2/MIMP deletion in the liver prevents the in vitro import of tBID
The results described above suggest that MTCH2/MIMP has a critical function in the recruitment of tBID to liver mitochondria in vivo. To verify these findings we performed in vitro import of tBID into liver mitochondria. Cytosolic fractions of 293T cells expressing HA–tBID were incubated with purified intact mouse liver mitochondria prepared from either fl/+ or fl/Δ mice, followed by centrifugation to separate the mitochondria from the soluble fraction. At 15, 30 and 60 min after the addition of HA–tBID to mitochondria, a substantial amount of HA–tBID was incorporated into fl/+ mitochondria, whereas significantly less was incorporated into the fl/Δ mitochondria (Fig. 6a). As expected, the decreased recruitment of HA–tBID to fl/Δ mitochondria resulted in less or delayed cytochrome c release (Fig. 6b). Thus, conditional knockout of MTCH2/MIMP in the liver prevents the recruitment of tBID to liver mitochondria both in vivo and in vitro.
Figure 6.
MTCH2/MIMP deletion in the liver prevents the in vitro import of tBID. Kinetics of HA–tBID import into mitochondria (a) and cytochrome c release (b). Cytosolic fractions of 293T cells expressing HA–tBID and depleted of cytochrome c (using anti-cytochrome c antibodies) were incubated with purified, intact mitochondria isolated from mouse liver prepared from either fl/+ mice (top panels) or fl/Δ mice (bottom panels). At the indicated time points, mitochondria were separated from the soluble fraction by centrifugation, and both fractions were lysed and analysed by western blotting with anti-HA (a) or anti-cytochrome c (b) antibodies. Actin and porin were used as internal standards for the soluble and cytosolic fractions and the mitochondrial fraction, respectively. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
MTCH2/MIMP deletion in the liver has no detectable effect on the levels of cardiolipin or other lipid species in the outer mitochondrial membrane
It has previously been proposed that cardiolipin (CL) acts as a major facilitator of the incorporation of tBID into the OMM13. It was therefore important to determine whether the absence of MTCH2/MIMP has an effect on the content and distribution of CL in liver mitochondria. To address this issue, we initially prepared liver mitochondria from fl/+ and fl/Δ mice and analysed their acyl composition and amount of CL by using high-performance liquid chromatography (HPLC) (tandem) mass spectrometry. This analysis revealed that there were no significant differences between the fl/+ and fl/Δ samples (Fig. 7a). Next, we purified OMM from liver mitochondria prepared from fl/+ and fl/Δ mice. Using TOM20 and MTCH2/MIMP (both localized to the outer membrane) and cytochrome c oxidase (localized to the inner membrane) we could show that the resulting mitochondrial fractions were largely enriched for outer membranes but still contaminated with inner membranes (Fig. 7b). The OMM fractions were then analysed for their acyl composition and amount of CL, and this analysis revealed that there were no significant differences between the fl/+ and fl/Δ samples (Fig. 7c, left and right panels); the levels of CL in the OMM fractions are significantly lower than in whole mitochondria although they seem similar in the figure (see Methods for details). Moreover, OMM fractions prepared from fl/+ and fl/Δ mice injected with anti-Fas antibodies showed no significant differences (Fig. 7c, right panel +Fas). We also analysed the amount of other lipid species including phosphatidylglycerol, phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositol in the OMM fractions and in whole mitochondria samples and found no significant differences between the fl/+ and fl/Δ samples (data not shown). Thus, MTCH2/MIMP deletion in the liver has no detectable effect on the levels of CL and other lipid species in whole mitochondria or in the OMM.
Figure 7.
MTCH2/MIMP deletion in the liver has no effect on the levels of CL in whole mitochondria and the OMM. (a) CL spectra of liver mitochondria. CL spectra were acquired by HPLC mass spectrometry in whole mitochondria prepared from livers of fl/+ and fl/Δ mice. Three major clusters of CLs (C70, C72 and C74) are shown, where the cluster designated C72 contains the major CL tetralinoleoylcardiolipin (C7x corresponds to the number of carbon atoms in the four acyl side chains of the molecule). Tetramyristoylcardiolipin (C56 = IS) was used as internal standard. The almost identical abundances of the different clusters and the distribution of the peaks within the clusters implies that the acyl composition is not different in fl/+ and fl/Δ mice. m/z, mass to charge ratio. (b) Purification of OMM from liver mitochondria. Samples from the purified mitochondria (Mito), and outer-membrane fraction (OMM) were lysed, size-fractionated by SDS–PAGE and analysed by western blotting with anti-TOM20 antibodies, anti-MTCH2/MIMP antibodies and anti-cytochrome c oxidase subunit IV (Cyt Oxi) antibodies. (c) CL levels and spectra of OMM fractions from liver. CL levels and spectra were determined by HPLC mass spectrometry in enriched OMM fractions prepared from livers of fl/+ and fl/Δ mice either left untreated or injected with 0.55 μg g−1 anti-Fas antibody (+Fas; 2 h). Left panel: as for whole mitochondria in a, the abundances of the different CL clusters and the distributions of the peaks within the clusters show no differences when comparing fl/+ and fl/Δ mice. Right panel: data are means and s.d. for three independent experiments.
DISCUSSION
It remains poorly understood how Bcl-2 family members regulate MOMP. Several of the Bcl-2 family members such as tBID and Bax need to be recruited to the mitochondria and to go through several conformational changes to reach full activation. Recruitment and transition between the different states are expected to be regulated by factors that would either enhance or retard the process. Here we show that MTCH2/MIMP, a resident OMM protein, has a critical function in the MOMP process by facilitating the recruitment of tBID to mitochondria.
To determine the functional importance of MTCH2/MIMP we used several experimental systems. In the first system, permeabilized ES cells, we found that MTCH2/MIMP positively regulates tBID-induced MOMP (Fig. 2). In the second system, conditional knockout MEFs, we established a physiological role for MTCH2/MIMP that is specific for the pro-apoptotic activity of tBID (Fig. 3). We also showed that MTCH2/MIMP-deficient MEFs are less sensitive to apoptosis induced by etopo-side or cisplatin, as previously demonstrated for BID-deficient cells23,24. Most revealing was the finding that MTCH2/MIMP-deficient MEFs showed a significant decrease in tBID recruitment to mitochondria after treatment with Ad-tBID, etoposide or Fas (Fig. 4). MTCH2/MIMP-deficient MEFs also hindered Bax activation and cytochrome c release after all three stimuli. Thus, MTCH2/MIMP acts as a tBID receptor-like protein in the OMM to facilitate tBID recruitment, resulting in accelerated Bax activation and MOMP.
Using MTCH2/MIMP liver-specific knockout mice we showed that the absence of MTCH2/MIMP decreases the sensitivity of mice to anti-Fas antibody-induced hepatocellular apoptosis (Fig. 5). We also performed a biochemical analysis of livers after treatment with Fas and found that MTCH2/MIMP deficiency significantly decreased the mitochondrial recruitment of tBID and the activation of Bax. Moreover, using purified liver mitochondria, we demonstrated that MTCH2/MIMP deletion in the liver prevents the in vitro import of tBID (Fig. 6). These results establish a physiological role for MTCH2/MIMP in the Fas-death pathway in vivo.
It was previously reported that about 80% of the BID-deficient mice injected with anti-Fas antibody were resistant to the injection6, whereas we found that only about 45% of the MTCH2/MIMP liver-deficient mice were resistant and most of the rest died with delayed kinetics. These results suggest that either the absence of MTCH2/MIMP is less cytoprotective than the absence of BID or that Fas might kill mice not only by effects on hepatocytes but also by effects on non-hepatocytes. In favour of the first hypothesis, it was previously demonstrated that caspase-8 liver-conditional knockout mice (also generated with the Alb-Cre transgene) are resistant to the injection of anti-Fas antibody25. Thus, the absence of MTCH2/MIMP is less cytoprotective than the absence of either BID or caspase-8.
The fact that MTCH2/MIMP deficiency decreases but does not eliminate tBID recruitment suggested that MTCH2/MIMP might affect the interaction of tBID with mitochondria by indirect effects, such as regulating the levels of CL. However, we found that MTCH2/MIMP deficiency had no significant effect on the levels of CL and other lipid species in the OMM (Fig. 7). Thus, MTCH2/MIMP does not seem to facilitate tBID recruitment by regulating the OMM lipid composition. However, the possibility cannot be excluded that MTCH2/MIMP regulates the transport of CL from the inner membrane to microdomains of the OMM.
In summary, we have shown that MTCH2/MIMP acts at the very early stages of MOMP by facilitating the recruitment of tBID, the initiator of this process. The studies in mice show that MTCH2/MIMP is an indispensable participant in the tBID death pathway required for effective hepatocellular apoptosis.
METHODS
Generation of MTCH2/MIMP knockout mice
The targeting vector was designed to delete 2.4 kb, encompassing exons 1–3 of MTCH2/MIMP. The targeting vector was constructed by PCR on ES genomic DNA26. First the short homology (SH), which contains 2.1 kb upstream to exon-1 was ligated into the XhoI site in the pRapidflirt vector. Next, a polylinker made of a double-stranded oligonucleotide containing an NheI and an AsisI site was inserted into the NotI site of this vector. Finally, the long homology (LH), which contained 5.1 kb downstream of exon-3, was ligated into the NheI and AsisI sites of this vector by PCR. The linearized targeting vector was introduced into R1 ES cells (derived from 129/ola mice) by electroporation, and neomycin-resistant clones were picked. The individual clones described above were screened for homologous recombination by Southern blot analysis. Two homologous recombinant R1 clones were identified, aggregated with tetraploid embryos and implanted into separate white-coated ICR foster-mother mice. The first generation of black-coated mice were born, and bred again to white ICR mice to obtain the second generation of MTCH2/MIMP+/− animals. Intercross of MTCH2/MIMP+/− animals resulted in offspring homozygous for the MTCH2/MIMP knockout (MTCH2/MIMP−/−).
Timed pregnancies, isolation of embryos, PCR analysis, and histological analysis
Timed pregnancies were conducted with MTCH2/MIMP+/− mice. Pregnant females were killed at different time points of gestation, and embryos were dissected from maternal tissue. Decidua were separated, embryos were dissected out under a binocular microscope, and pictures were taken. For genotyping of the E6.5 and E7.5 embryos, DNA was prepared with the Epicentre MasterPure purification kit and then analysed by PCR. For the E8.5, E9.5 and E10.5 embryos, the yolk sac was separated and lysed with the REDExtract-N-Amp Tissue PCR Kit (Sigma), and the sets of primers that were used for genotyping of pups were employed. For histological preparation, the whole uterus was fixed in 4% paraformaldehyde for 48 h at room temperature (22–24°C). Sections were cut from paraffin blocks and stained with haematoxylin/eosin.
Generation and studies with ES cells
Pregnant females from MTCH2/MIMP+/− intercrosses were killed at E3.5, and blastocysts were collected and cultured as described previously27. For the generation of ES stable clones, MTCH2/MIMP KO ES cells were transfected with either an empty pcDNA3.1 vector or a pcDNA3.1 vector carrying MTCH2/MIMP by using Lipofectamine 2000 (Invitrogen). The cells were then cultured under selective conditions, and surviving clones were expanded and used as stable clones for the experiments described. Studies with the permeabilized ES cells were performed as described previously28.
Generation of MTCH2/MIMP conditional knockout mice
To generate the MTCH2/MIMP floxed targeting vector, a construct designed to excise the first three exons of MTCH2/MIMP was assembled in the pRapidflirt vector29. The long homology (LH) arm, which consists of 7 kb upstream of exon-1, was ligated into XhoI and FseI sites in the pRapidflirt vector downstream of the TK cassette. A 2.9 kb DNA fragment consisting of the 5′ untranslated region (UTR), the first three exons of MTCH2/MIMP and a small portion of the third intron (named Ex; Fig. 3a; Supplementary Information, Fig. S4) was ligated into SalI and SbfI sites of the pRapidflirt vector between the two loxP sites (the first loxP site is located downstream of the 5′ UTR of MTCH2/MIMP and the other one is located upstream to the Frt site of the PGKneo cassette). The short homology (SH) arm, containing 2 kb of the third intron, was ligated into the NotI and ClaI restriction sites of the pRapidflirt vector. Subsequently, the complete targeting vector was subjected to sequence analysis, the roughly 18.8 kb linearized vector was introduced into R1 ES cells by electroporation, and homologous recombinant candidates were screened by Southern blot analysis. Two homologous recombinant R1 clones were identified, aggregated with tetraploid embryos and implanted into separate white-coated ICR foster-mother mice. Confirmed chimaeras with germline transmission were mated to wild-type mice from the 129/SVJ line.
Generation of MTCH2/MIMP liver-specific knockout mice. The existing Cre systems provide variable efficiencies, which are rather weak in most cases. To ensure a high efficiency in this system we generated mice in which one of the MTCH2/ MIMP alleles was fully deleted and the other was knocked out only in the organ of target. These mice were generated by first mating the MTCH2/MIMPfl/+ mice with mice bearing Pgk-Cre, a general deleter transgene30 to create MTCH2/MIMP+/Δ mice. These mice were then mated with mice bearing Alb-Cre, a transgene for Cre recombinase under control of the liver albumin promoter31. Offspring that expressed both the MTCH2/MIMP deleted allele and the Alb-Cre transgene (MTCH2/MIMP+/Δ;Alb-Cre) were mated with MTCH2/MIMPfl/fl homozygous mice, to generate the MTCH2/MIMPfl/Δ;Alb-Cre and MTCH2/MIMPfl/+;Alb-Cre mice (liver-specific knockout and its control littermate, respectively).
Preparation and transduction of MEFs with Cre recombinase
MTCH2/MIMPfl/fl and MTCH2/MIMPfl/+ primary MEFs were prepared from 11–13-day-old embryos and transformed with the SV40 whole genome as described previously21. All the studies with MEFs described in the paper were performed with SV40-immortalized MEFs. Recombinant His-TAT-NLS-Cre (Cre recombinase) fusion protein was expressed and purified as described previously32. Cre recombinase was diluted in DMEM/PBS to a final concentration of 3 μM, sterile-filtered, and incubated with the cells for 16 h. The cells were then washed with PBS, supplemented with growth medium, and grown for a further 5 days before use in experiments.
Preparation of recombinant adenoviruses and infection of MEFs
tBID and GFP recombinant adenoviruses were prepared as described previously21. MTCH2/MIMP, Bax, Noxa and Bcl-2 recombinant adenoviruses were prepared as described previously for preparing tBID recombinant adenoviruses21. Bim recombinant adenoviruses were prepared as described previously for Nbk recombinant adenoviruses33. Infection of MEFs was performed as described previously21.
Recombinant tBID and crosslinking
Purified recombinant histidine-tagged murine tBID was prepared as described previously34. Crosslinking with BSOCOES (bis(2-(sulphosuccinimido-oxycarbonyloxy)ethyl)sulphone), and BMH (1,6-bismaleimidohexane) (both from Pierce) was performed as described previously15.
Isolation of mitochondria, treatment with proteases, and in vitro import assays
Mitochondria were prepared from mouse liver or cultured cells and treated with proteinase K as described previously15. Mitochondria were isolated from rat liver and subfractionated as described previously19. Treatment of mitochondria from mouse liver or cultured cells with trypsin was performed as described previously35. Purification of yeast mitochondria and in vitro import assays were performed as described previously20,36.
Isolation and purification of the OMM from mouse liver mitochondria
Livers from four female mice were used to prepare each OMM sample. Livers were excised and mitochondria were prepared as described previously15, with several modifications. The final mitochondria-enriched pellet was purified on a discontinuous Nycodenz (Sigma) gradient as described in ref. 37 and the OMM was isolated by the swell–shrink–sonication procedure as described in ref. 19.
Cell viability and caspase-3 activity assays
Cell viability was determined by propidium iodide (PI) dye exclusion, and caspase-3 activity assays were performed as described previously21.
Immunofluorescence and imaging
For imaging, cells on coverslips were fixed with 3% paraformaldehyde in PBS and permeabilized with 0.2% Triton X-100 in PBS. Cells were immunostained with anti-cytochrome c 6H2.B4 monoclonal antibodies (Pharmingen) followed by Cy3-conjugated goat anti-IgG (Jackson ImmunoResearch). Nuclei were stained with 4′,6-diamidino-2-phenylin-dole (DAPI; 10 μg ml−1). Images were collected on an Olympus IX70 microscope, equipped with a Deltavision imaging system, using a 40× PLAN-APO 1.42 numerical aperture objective. Images were processed by constrained iterative deconvolution on softWoRxTM software (Applied Precision).
Phospholipid analysis of liver mitochondria samples
CLs and other phospholipids were analysed by HPLC (tandem) mass spectrometry (MS). Phospholipids were extracted from the equivalent of 0.5 mg of protein with the use of a one-phase extraction as described in ref. 38. The HPLC–MS system was operated as described previously39 and phospholipids were measured essentially as described in ref. 40. For quantification of CL levels, the area under the curve of the HPLC profile corresponding to the mass spectra of CL species was integrated, as well as that of the internal standard tetramyristoylcardiolipin. CL concentration was calculated on the basis of the internal standard, assuming an identical response, and expressed as nmol per mg protein. It appears in Fig. 7 that there are equal amounts of CL in the OMM and in whole mitochondria (or in the IMM for that matter) but it should be taken into consideration that the amount of protein in the IMM is much higher than in the OMM and therefore the absolute amount of CL in the IMM and/or whole mitochondria is much higher than in the OMM.
Knockdown of MTCH2/MIMP in U2OS cells
Human MTCH2/MIMP was knocked down by using short interfering RNA (siRNA) On-TargetPlus smart pools (Dharmacon). Cells were transfected with siRNA (44 nM) with the Dharmafect 1 reagent in accordance with the manufacturer's instructions.
Western blot analysis and antibodies
Western blot analysis was performed as described previously15. Antibodies used for western blotting included anti-MTCH2/MIMP antibody15, anti-BID antibody15, anti-mBax antibody (651), anti-Bak antibody (Upstate), anti-HA monoclonal antibody (3F10; Roche), anti-cytochrome c monoclonal antibody (7H8.2C12, Pharmingen), anti-TOM20 antibody (a present from Gordon Shore), anti-ANT antibody (Santa Cruz), anti-AIF antibody (Santa Cruz), anti-cytochrome c oxidase subunit IV (Cyt Oxi) antibody (a present from Jim Hare), anti-cleaved caspase-3 antibody (Cell Signaling), anti-Bcl-XL antibody (Santa Cruz), anti-caspase-8 antibody (Alexis), anti-porin antibody (Calbiochem) and anti-actin antibody (Santa Cruz).
Statistical analysis
Data are presented as means and s.d. Student's unpaired two-tailed t-test was performed with statistical analysis functions in Microsoft Excel. Differences were considered statistically significant at P < 0.05. The Kaplan–Meier survival curves were compared by using the long-rank test (PASW Statistics 17.0 software).
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to S. Jung for advice regarding the animal studies; A. Harmelin, R. Haffner, A. Maizenberg, G. Damari and T. Berkutzki for help with the animal and ES cell studies; H. van Lenthe and F. Stet for technical assistance with the phospholipid analysis; T. Langer (University of Cologne) for the TAT-Cre plasmid; T. S. Tanaka (University of Illinois at Urbana-Champaign) for DNA probes for Brachyury; D. Wallach (Weizmann Institute) for anti-Fas antibodies and Richard Marcellus (McGill University) for recombinant adenoviruses. This study was supported in part by the USA–Israel Binational Science Foundation, the Ministero dell’Università e della Ricerca, the Apulia Region, and the University of Bari. It was also supported by a grant of the Prinses Beatrix Fonds to F.M.V. and grants of the Barth Syndrome Foundation to W.K. and F.M.V. A.G. is the incumbent of the Armour Family Career Development Chair of cancer research. E.A.J. is funded by a National Institutes of Health (NIH) Minority Access to Research Careers U*STAR Scholarship, and S.W. is funded by a NIH National Research Service Award.
Footnotes
Supplementary Information is available on the Nature Cell Biology website.
AUTHOR CONTRIBUTIONS
Y.Z., L.S, N.Y.-O., M.S. and M.M. performed most of the experiments that characterized the knockout mice and cells. R.H.H and F.M.V. performed the phospholipid analysis. F.D.L, G.F. and F.P. assessed the carrier activity of MTCH2/MIMP. B.G. and P.T.D. prepared the Bim recombinant adenoviruses. E.J., S.W. and C.M.K. performed the studies of import into yeast mitochondria. Y.Z., L.S. and A.G. wrote the paper. All authors discussed the results and commented on the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 3.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 4.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 5.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin XM, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 7.Wei MC, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roucou X, Montessuit S, Antonsson B, Martinou JC. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem. J. 2002;368:915–921. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovell JF, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Schafer B, et al. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol. Biol. Cell. 2009;20:2276–2285. doi: 10.1091/mbc.E08-10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutter M, et al. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 14.Lucken-Ardjomande S, Montessuit S, Martinou JC. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ. 2008;15:929–937. doi: 10.1038/cdd.2008.9. [DOI] [PubMed] [Google Scholar]
- 15.Grinberg M, et al. Mitochondrial carrier homolog 2 is a target of tBID in cells signaled to die by tumor necrosis factor α. Mol. Cell. Biol. 2005;25:4579–4590. doi: 10.1128/MCB.25.11.4579-4590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross A. Mitochondrial carrier homolog 2: a clue to cracking the BCL-2 family riddle? J. Bioenerg. Biomembr. 2005;37:113–119. doi: 10.1007/s10863-005-6222-3. [DOI] [PubMed] [Google Scholar]
- 17.Leibowitz-Amit R, et al. Mimp, a mitochondrial carrier homologue, inhibits Met-HGF/SF-induced scattering and tumorigenicity by altering Met-HGF/SF signaling pathways. Cancer Res. 2006;66:8687–8697. doi: 10.1158/0008-5472.CAN-05-2294. [DOI] [PubMed] [Google Scholar]
- 18.Palmieri F. Diseases caused by defects of mitochondrial carriers: a review. Biochim. Biophys. Acta. 2008;1777:564–578. doi: 10.1016/j.bbabio.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J. Biol. Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 20.Koehler CM, et al. Import of mitochondrial carriers mediated by essential proteins of the intermembrane space. Science. 1998;279:369–373. doi: 10.1126/science.279.5349.369. [DOI] [PubMed] [Google Scholar]
- 21.Sarig R, et al. BID-D59A is a potent inducer of apoptosis in primary embryonic fibroblasts. J. Biol. Chem. 2003;278:10707–10715. doi: 10.1074/jbc.M210296200. [DOI] [PubMed] [Google Scholar]
- 22.Goping IS, et al. Regulated targeting of BAX to mitochondria. J. Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamer I, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Shelton SN, Shawgo ME, Robertson JD. Cleavage of Bid by executioner caspases mediates feed forward amplification of mitochondrial outer membrane permeabilization during genotoxic stress-induced apoptosis in Jurkat cells. J. Biol. Chem. 2009;284:11247–11255. doi: 10.1074/jbc.M809392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan KO, et al. MAP-1 is a mitochondrial effector of Bax. Proc. Natl Acad. Sci. USA. 2005;102:14623–14628. doi: 10.1073/pnas.0503524102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli-Taliadoros LA, Sedgwick JD, Wood SA, Korner H. Gene knock-out technology: a methodological overview for the interested novice. J. Immunol. Methods. 1995;181:1–15. doi: 10.1016/0022-1759(95)00017-5. [DOI] [PubMed] [Google Scholar]
- 27.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo. Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 28.Madesh M, Antonsson B, Srinivasula SM, Alnemri ES, Hajnoczky G. Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J. Biol. Chem. 2002;277:5651–5659. doi: 10.1074/jbc.M108171200. [DOI] [PubMed] [Google Scholar]
- 29.Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 30.Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- 31.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β-cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 32.Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc. Natl Acad. Sci. USA. 2002;99:4489–4494. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillissen B, et al. Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway. EMBO J. 2003;22:3580–3590. doi: 10.1093/emboj/cdg343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grinberg M, et al. tBID homooligomerizes in the mitochondrial membrane to induce apoptosis. J. Biol. Chem. 2002;277:12237–12245. doi: 10.1074/jbc.M104893200. [DOI] [PubMed] [Google Scholar]
- 35.Ruffolo SC, Shore GC. BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J. Biol. Chem. 2003;278:25039–25045. doi: 10.1074/jbc.M302930200. [DOI] [PubMed] [Google Scholar]
- 36.Claypool SM, McCaffery JM, Koehler CM. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J. Cell Biol. 2006;174:379–390. doi: 10.1083/jcb.200605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Cruz S, et al. Proteomic analysis of the mouse liver mitochondrial inner membrane. J. Biol. Chem. 2003;278:41566–41571. doi: 10.1074/jbc.M304940200. [DOI] [PubMed] [Google Scholar]
- 38.Vaz FM, Houtkooper RH, Valianpour F, Barth PG, Wanders RJ. Only one splice variant of the human TAZ gene encodes a functional protein with a role in cardiolipin metabolism. J. Biol. Chem. 2003;278:43089–43094. doi: 10.1074/jbc.M305956200. [DOI] [PubMed] [Google Scholar]
- 39.Houtkooper RH, et al. Identification and characterization of human cardiolipin synthase. FEBS Lett. 2006;580:3059–3064. doi: 10.1016/j.febslet.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 40.Valianpour F, et al. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J. Lipid Res. 2005;46:1182–1195. doi: 10.1194/jlr.M500056-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.