Abstract
The notion that adolescence is characterized by dramatic changes in behavior, and often by emotional upheaval, is widespread and longstanding in popular western culture. In recent decades, this notion has gained increasing support from empirical research showing that the peri- and post-pubertal developmental stages are associated with a significant rise in the rate of psychiatric symptoms and syndromes. As a result, interest in adolescent development has burgeoned among researchers focused on the origins of schizophrenia and other psychotic disorders. Two factors have fueled this trend: 1) increasing evidence from longitudinal research that adolescence is the modal period for the emergence of “prodromal” manifestations, or precursors of psychotic symptoms, and 2) the rapidly accumulating scientific findings on brain structural and functional changes occurring during adolescence and young adulthood. Further, gonadal and adrenal hormones are beginning to play a more prominent role in conceptualizations of adolescent brain development, as well as in the origins of psychiatric symptoms during this period (Walker and Bollini, 2002; Walker et al., 2008). In this paper, we begin by providing an overview of the nature and course of psychotic disorders during adolescence/young adulthood. We then turn to the role of hormones in modulating normal brain development, and the potential role they might play in the abnormal brain changes that characterize youth at clinical high-risk (CHR) for psychosis. The activational and organizational effects of hormones are explored, with a focus on how hormone-induced changes might be linked with neuropathological processes in the emergence of psychosis.
Keywords: psychosis, prodrome, puberty, adolescence, hypothalamic-pituitary-adrenal [HPA] axis, hypothalamic-pituitary-gonadal [HPG] axis, brain development
Introduction
Schizophrenia and other psychotic disorders (e.g., schizoaffective disorders, and mood disorders with psychotic features) continue to be among the most serious and debilitating of all mental illnesses (Geller et al., 2000; Murray and Lopez, 1996; Wu et al., 2005). With the advent of antipsychotic medications, especially the atypical antipsychotic medications, the prognosis for patients has improved, and more are able to live in the general community. Nonetheless, most individuals diagnosed with a psychotic disorder are not completely self-sufficient as adults (Murray and Lopez, 1996), and the course of psychotic illness is often characterized by periodic relapses that persist throughout the life span (Yung and McGorry, 1996). The social and medical costs of psychotic disorders are, therefore, extremely high (Greenblatt et al., 2011; Khaykin et al., 2010; Wu et al., 2005). Schizophrenia continuously ranks among the top 10 causes of disability in developed countries worldwide (Geller et al., 2000; Murray and Lopez, 1996; Wu et al., 2005). On a personal level, the burdens are equally disturbing: patients are typically diagnosed in adolescence or young adulthood and their occupational and social aspirations are derailed (Walker et al., 2008).
In this paper, we provide an overview of the course of psychotic disorders, beginning in the prodromal phase—when subclinical signs first emerge—through the first onset of the clinical syndrome. Because these events almost always unfold between the onset of puberty and the early 20s, this developmental period has come under intense scrutiny by clinical investigators and it is now assumed that a confluence of factors give rise to the onset of psychosis during this period (Walker et al., 2008). We then turn our attention to recent scientific advances in adolescent neuromaturation, as these findings provide a framework for interpreting the rapidly accumulating evidence on neuromaturational abnormalities that precede and accompany the transition to psychosis. Finally, we examine the hormonal and brain changes observed in both psychotic patients and individuals at clinical high risk (CHR) for psychosis. Taken together, the findings suggest that hormonal factors may be involved in neuropathological processes that give rise to the onset of psychosis. This assumption has significant implications for future approaches to preventive intervention.
Background: Current Understanding of the Nature and Course of Psychosis
By contemporary definition, psychotic disorders entail the presence of symptoms which suggest impairment in the individual’s understanding of, or contact with, reality (DSM-IV-TR, 2000). The key symptoms of psychosis are referred to as positive symptoms, and include delusions, hallucinations, and thought disorder. One or more positive symptoms must be present for a psychotic diagnosis. In addition, psychotic syndromes often include negative symptoms which entail deficits in functioning. Among the most common negative symptoms are social withdrawal, anhedonia (reduced emotional experience), and blunted affect (reduced emotional expression). The current psychiatric taxonomy, the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR, 2000) has multiple categories of psychotic diagnoses: schizophrenia, schizoaffective disorder, schizophreniform disorder and mood disorder with psychotic features. It is anticipated that these categories will undergo revision in the next edition of the DSM (DSM V), now in preparation, largely due to research advances on the origins of psychosis.
Indeed etiologic and diagnostic conceptualizations of psychoses have undergone significant changes over the past two decades. It is now generally accepted that the contemporary DSM diagnostic boundaries distinguishing among psychotic disorders, especially between schizophrenia and other psychotic disorders, do not converge with recent advances in our understanding of genetic and environmental factors that contribute to the diathesis. For example, rapidly accumulating research findings in the field of genetics indicate that many genes contribute incrementally to vulnerability, and these genes are not specific to a single DSM psychotic disorder, but rather confer vulnerability for a range of psychotic illnesses, including schizoaffective disorder and mood disorders with psychotic features (Craddock et al., 2009). Similarly, bio-environmental risk factors, such as prenatal complications (Buka and Fan, 1999) and cannabis use (Moore et al., 2007), appear to be associated with heightened vulnerability for both schizophrenia and the affective psychoses. Given these empirical trends, the term psychosis is used in this paper to refer to both schizophrenia and affective psychoses.
The Developmental Course of Psychosis
The ‘diathesis-stress’ model has dominated conceptualizations of psychoses etiology for over four decades (Walker and Diforio, 1997). The basic underlying assumption is that psychotic disorders arise from interaction of pre-existing vulnerability (i.e., “diathesis”) with exposure to various psychosocial and biological stressors that have the potential to trigger its expression.
Vulnerability to psychosis is assumed to originate from genetic factors and fetal brain development abnormalities (Keshavan et al., 2005). Subsequently, adolescent neuromaturational processes are posited to play a role in the clinical expression of psychotic illness, as its clinical onset is typically in late adolescence/early adulthood (Adams et al., 2000; Feinberg, 1982; Keshavan et al., 1994; Keshavan et al., 2005). It has been suggested, for example, that aberrant neural connectivity, acting in concert with dopamine (DA) circuitry abnormalities, arise during adolescence and set the stage for the first psychotic episode (Maccabe, 2008; Walker, 1994; Walker and Bollini, 2002). Psychotic illnesses are, therefore, viewed as neurodevelopmental disorders (Brennan and Walker, 2001).
The Prodromal Stage
The period of functional decline and gradual emergence of attenuated positive symptoms preceding the first psychotic episode can last from a few months to several years, and is referred to as the psychosis prodrome. The prodromal syndrome usually becomes apparent in adolescence, and is characterized by subclinical manifestations of positive and negative symptoms, as well as nonspecific indicators such as impaired attention, dysphoric mood and declines in role functioning (Ang and Tan, 2004; Fuller et al., 2002; Lencz et al., 2004; Walker et al., 1993; Yung et al., 2004).
Many view the prodromal phase as affording the greatest opportunity for preventive intervention (Addington, 2003; Compton et al., 2007; McGlashan, 2005; Yung and McGorry, 2007), and it has therefore become the focus of more intense research. As a result, several structured approaches to prospectively assess prodromal features have been developed (Correll et al., 2010). These structured interviews yield symptom ratings and utilize standard criteria for designating prodromal status. The term clinical high-risk (CHR) is generally used to refer to individuals manifesting clinical signs that conform to the prodromal criteria used in these measures.
In the US, the Scale of Prodromal Symptoms (SOPS) is the most widely used, and, like other prodromal interview schedules, it enhances positive predictive power for designating risk (Miller et al., 2002; Miller et al., 1999). Of those who meet psychosis prodrome criteria, based on the SOPS and similar measures, approximately 20% to 40% develop a psychotic illness within 2 to 4 years (Cannon et al., 2008; McFarlane, 2011; Walker et al., 2010a; Yung et al., 2006). While this level of predictive power is superior to that obtained solely on the basis of having a biological relative with psychosis (Gottesman, 1991; Hans et al., 2004; Kendler and Gardner, 1997), the level of false positives is nonetheless substantial. Thus, by intensifying the focus on CHR individuals who do and do not develop a psychotic disorder, researchers hope to develop measures and algorithms to enhance predictive power. The North American Prodrome Longitudinal Study (NAPLS), for example, is a multi-site project examining a range of biological, behavioral, and clinical factors to enhance prediction, elucidate underlying mechanisms, and identify malleable treatment targets (Addington et al., 2007; Cannon et al., 2008). Hormones are among the biological measures currently under investigation in CHR youth, primarily because of rapidly accumulating evidence of hormonal effects on neuromaturational processes, particularly in adolescence and young adulthood.
Background: Normal Developmental Processes in Hormones and Brain Structure and Function
In recent years, the pervasive role of hormones in modulating neuronal activity and structure has come into clearer focus. Although a comprehensive review is beyond the scope of this article, it is relevant to highlight the emerging trends. Numerous lines of research have elucidated the effects of various hormones on neural activity via binding and modulation of both membrane and intracellular receptors (Guerriero, 2009; Melcangi et al., 2011). Nongenomic mechanisms in hormone activity entail rapid changes via membrane-associated receptors and signaling cascades, whereas slower genomic mechanisms are mediated by nuclear receptors that can regulate gene expression (Falkenstein et al., 2000; McEwen, 1991). Another distinction that has relevance for neuromaturation is that between the activational and organizational effects of hormones (Brown and Spencer, 2012; Sisk and Zehr, 2005). With respect to the brain, organizational effects are conceptualized as those that result in enduring structural changes, while activational effects of hormones entail temporary changes that affect neuronal function. For example, temporary activational effects of adrenal and gonadal hormones on neuronal spine density (Dumitriu et al., 2010; Komatsuzaki et al., 2012; Mendez et al., 2011) and excitability (Groeneweg et al., 2012) have been demonstrated. Although distinguishing between activational and organizational effects of hormones is complex, especially in research on human brain development, research with animals indicates that both effects play a role in adolescent brain function and development in nonhuman species (Brown and Spencer, 2012).
Peri- and post-pubertal development is associated with dramatic increases in hormone levels and activity, thus advances in our understanding of neurohormonal signaling has implications for our understanding of adolescent brain development. As in the prenatal period, it is now assumed that hormonal changes in adolescence have both activational and organizational effects on the brain (Arnold and Breedlove, 1985; Brown and Spencer, 2012; Charmandari et al., 2003; Peper et al., 2009; Peper et al., 2011a). These effects not only provide a framework for exploring normative adolescent neuromaturation, but also hold promise for shedding light on the mechanisms that contribute to the increased risk for serious mental disorders in the adolescent and young adult stages.
Adolescent Hormonal and Brain Development
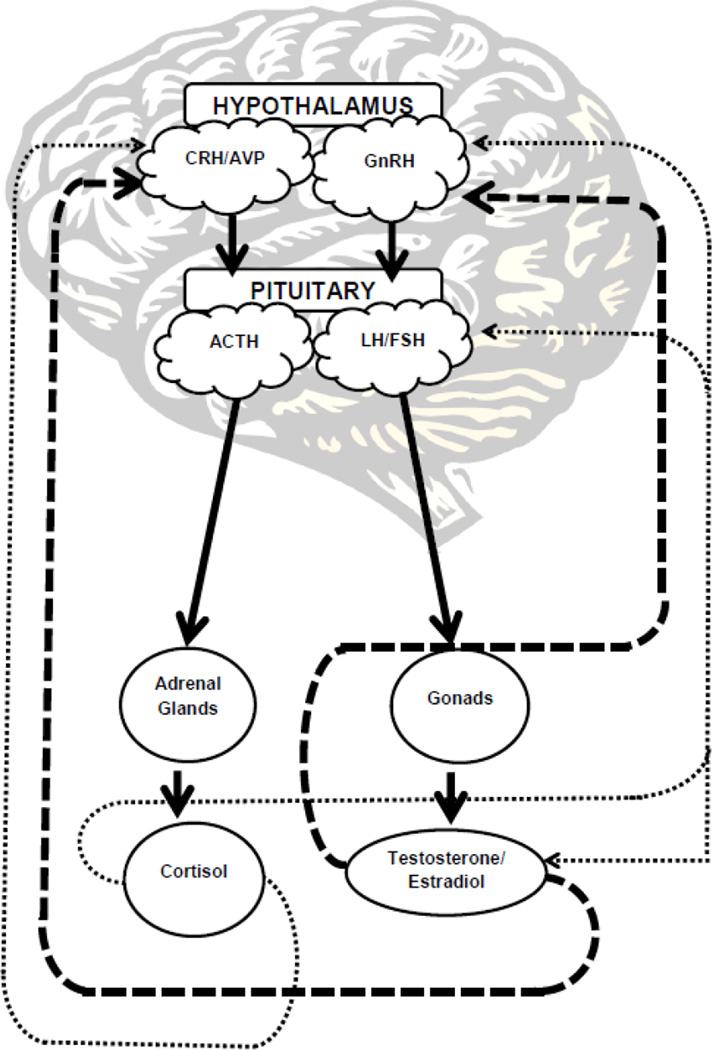
Reproductive hormone changes accompanying puberty are well documented. Pubertal onset, marked by reactivation of the hypothalamic-pituitary-gonadal (HPG) axis (Brook, 1999; Dorn et al., 2006), sets the stage for a cascade of hormonal changes that lead to sharp increases in estradiol, progesterone, and testosterone. As illustrated in Figure 1, the hypothalamus releases gonadotropin releasing hormone (GnRH), which triggers an increase in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland (Brook, 1999). LH and FSH then stimulate the release of gonadal hormones. Estradiol and testosterone subsequently modulate the release of GnRH and, thereby, the activity of the HPG axis (Dorn and Biro, 2011; Susman and Dorn, 2009).
Figure 1. The Brain Regions and Chemical Messengers involved in the HPA and HPG axes.
The dashed lines indicate inhibitory pathways and the solid lines represent activational pathways. In the psychosis prodrome, the increase in HPA activity may be suppressing the HPG axis, and contributing to abnormalities in the balance and progression of hormonal changes that influence normal adolescent brain changes.
More recently, research has revealed adolescent developmental changes in the hypothalamic-pituitary-adrenal (HPA) axis. These include a post-pubertal increase in basal and stress-induced cortisol secretion that occurs in conjunction with both age and advancing stages of puberty and appears to extend into the 3rd decade of life in humans (Adam, 2006; Gunnar et al., 2009; Kenny et al., 1966; Lupien et al., 2002; Shirtcliff et al., 2011; Walker et al., 2008; Walker et al., 2001; Walker et al., 2010b). While both males and females manifest an increase in cortisol secretion with age through adolescence, there is some evidence that females show a more pronounced increase (Shirtcliff et al., 2011). A recent review of the literature on pubertal maturation and stress reactivity across species concludes that the pubertal period is associated with an increased biobehavioral reactivity to stress exposure (Foilb et al., 2011; Romeo, 2010). For example, experimental studies of performance and social stress induction indicate that, when compared to children, healthy adolescents manifest a higher baseline and greater cortisol response to both types of stress (Stroud et al., 2009). Thus the HPA axis appears to undergo a maturational increase in baseline activity as well as sensitivity.
Bidirectional influences in HPA and HPG activity during adolescence are apparent, though the specific nature of this relationship is not well understood. As illustrated in Figure 1, it appears that cortisol elevations can inhibit the HPG axis at multiple levels by suppressing GnRH neurons in the hypothalamus, pituitary release of LH and FSH, and gonadal hormone production (Hiller-Sturmhofel and Bartke, 1998; Kerdelhue et al., 2002; Shi et al., 2011). Increased HPA activation may also play a role in modulating the hormonal cascade that precipitates the onset of puberty. In human females, higher prepubertal cortisol levels are associated with later pubertal growth spurt and menarche onset (Shi et al., 2011). Animal studies suggest that stress exposure can lead to changes in testosterone levels, but only after puberty (Foilb et al., 2011). This suggests that both environmental factors and pubertal stage may influence the relation of HPA and HPG activity. Conversely, the HPG axis is shown to modulate HPA activity. For example, animal studies demonstrate regulatory effects of estrogen on the glucocorticoid and mineralocorticoid receptors, and these effects are moderated by menstrual phase (Carey et al., 1995; Redei et al., 1994). Testosterone also appears to modulate HPA activity, although the mechanisms are not yet clear (Viau, 2002).
Across species, the adolescent period is characterized by changes in brain structure and function, and neuroimaging advances have greatly contributed to our understanding of human brain development (Adams et al., 2000; Blakemore, 2012; De Bellis et al., 2001; Giedd et al., 1999; Giedd et al., 1996; Sowell et al., 2001; Spear, 2000). Adolescent maturational changes are widespread, especially in the limbic structures and the cortex (Giedd et al., 1996; Rapoport et al., 1999; Suzuki et al., 2005), and they entail both regressive and progressive processes (Sowell et al., 2001). Normative adolescent neuromaturation includes reductions in cortical gray matter volume (Giedd et al., 1999), increases in white matter and the volume of some limbic regions (e.g., amygdala, hippocampus) (Peper et al., 2011a), synaptic pruning, and increased myelination and neurogenesis (Giedd, 2004). Further, prefrontal and parietal gray matter volume is inversely correlated with pubertal stage, indicating that pubertal development is associated with processes that entail some regression (Bramen et al., 2011; Peper et al., 2009).
At the functional level, when compared to adult patterns of brain activity, adolescence is characterized by immature executive processing circuits and elevated subcortical activity (e.g., in the striatum) (Luna et al., 2010; Van Leijenhorst et al., 2010). This activational pattern is assumed to shift as development proceeds and neural connectivity changes (i.e., changes in the nature and extent of interconnectivity in neural circuits). Studies using Diffusion Tensor Imaging (DTI) have revealed adolescent increases in white matter integrity and connectivity, as indexed by fractional anisotropy (FA) (Asato et al., 2010). More advanced pubertal development in humans is associated with increased white matter fibers connecting the frontal and temporal regions, as well as between frontal and subcortical regions (Asato et al., 2010). Thus, adolescent white matter maturation is likely associated with enhanced corticalsubcortical connectivity (Asato et al., 2010). Females show earlier and more protracted white matter maturation compared to males, which may be a consequence of the earlier pubertal onset in girls (Asato et al., 2010; Gatzke-Kopp, 2011; Ladouceur et al., 2012). Recent findings from animal studies have also highlighted changes in DA systems during adolescence, with increased connections from DA neurons to the frontal lobes (Spear, 2000). Interestingly, stressful experiences during adolescence have been shown to contribute to sensitivity of the mesolimbic DA system (Gatzke-Kopp, 2011). Further, mesolimbic DA activity is related to psychotic symptoms (Epstein et al., 1999).
Extensive experimental animal research suggests that adrenal and gonadal hormonal changes affect brain organization during adolescence (Cashion et al., 2003; Schulz et al., 2009; Sisk and Zehr, 2005; Vigil et al., 2011; Yates and Juraska, 2008). More recently, studies of human subjects have yielded findings suggesting similar effects. A comprehensive review on the relation of gonadal hormones with brain structure in human adolescents concluded that decreases in prefrontal, parietal, and temporal gray matter were associated with increased gonadal hormones (estradiol in girls and testosterone in boys) (Peper et al., 2011a). Thus, rising gonadal hormones may be contributing to some normative ‘regressive’ processes in the cortex. In contrast, increasing gonadal hormones were associated with greater amygdala, thalamus, hippocampal, and parahippocampal volume. In this case, there are ‘progressive’ effects of gonadal hormone increases on subcortical volume. Further, estradiol was positively correlated with gray matter volume in some cortical regions, including the middle frontal gyrus, the inferior temporal gyrus and the middle occipital gyrus (Peper et al., 2011a).
Although there have been relatively few studies of white matter volume and gonadal hormones, a review of the results suggests that testosterone in males and luteinizing hormone in both males and females are linked with puberty-related increases in global white matter and regional white matter growth in frontal and temporal connections (Peper et al., 2011a). A study published subsequent to this review used DTI to investigate white matter microstructure in children and young-to-mid adolescents and showed that FA, a measure of white matter integrity, was positively associated with testosterone in boys and estradiol in girls, indicating that rising gonadal hormone levels are facilitating white matter development (Herting et al., 2011).
There is currently only limited research on the relation of cortisol with regional brain volumes in humans, and most of the research has been conducted with clinical populations. Nonetheless, the results are consistent in showing an inverse relation of baseline cortisol with cortical gray matter and hippocampal volume in adults and adolescents (Castro-Fornieles et al., 2009; Mondelli et al., 2010; Tessner et al., 2007). Thus, the increase in cortisol secretion during adolescence may also play a role in brain maturational processes that involve volumetric reductions. The inhibition of neurogenesis may be one mechanism through which glucocorticoids are associated with volumetric reductions. A recent review concluded that elevations in glucocorticoids decrease the rate of neurogenesis in the dentate gyrus of adult rodents (Schoenfeld and Gould, 2012). Relatedly, strategies for reducing or minimizing stressors in adult squirrel monkeys have been shown to facilitate neurogenesis in the hippocampus, presumably through decreasing the levels of circulating cortisol (Lyons et al., 2010).
Research has not yet elucidated the mechanisms mediating the relation between hormones and brain structure in humans, but, as described above, it has been proposed that genomic processes are at least partially involved (Peper et al., 2011b). Thus, hormones may be triggering the expression of genes that influence normal neuromaturational processes, such as gray matter reduction and increased connectivity. Pubertal hormonal changes might also impact both timing and organization of white matter changes, and genetic factors may mediate this process (Ladouceur et al., 2012). It is important to note, however, that the research on hormone-brain relations in humans presents interpretational challenges, as the processes subserving the relation could reflect either activational or organizational effects of hormones. Further, it is possible that relations between hormone levels and brain characteristics in adolescents are not directly causally linked, but instead are a consequence of other agerelated factors that are associated with adolescent development. Nonetheless, the findings highlight the importance of further research aimed at elucidating the mechanisms involved in hormone-brain relations in adolescence. Clearly, elucidating the cascade of hormonal and genetic processes will require longitudinal studies that allow us to characterize individual differences in brain developmental trajectories (Asato et al., 2010).
Hormones and Psychosis
Gonadal Hormones
As noted above, it is assumed that brain maturational processes are playing a role in the expression of the neuropathophysiology subserving psychosis; risk for onset is very low in childhood, then increases with age following puberty and through the third decade of life (Walker et al., 2008). The fact that adolescence/young adulthood is the modal period for the onset of the psychosis prodrome, coupled with the well-established later age at onset of psychotic disorders among females, has generated interest in the relation of gonadal hormone levels with psychosis. It is well established that the timing and pace of pubertal maturation is associated with psychological adjustment (Marceau et al., 2011), and this relation may be mediated by increased exposure or reactivity to stressful events (Ellis et al., 2011; Shi et al., 2011). The research findings to date indicate no association between timing of puberty and risk for psychosis (Golub et al., 2008), however there is evidence that gonadal hormones modulate the expression of psychosis.
Recent comprehensive reviews of the research findings (Hayes et al., 2012; Markham, 2011) concluded the following about estrogen: 1) women with schizophrenia manifest reduced estrogen levels compared to healthy same-sex controls, 2) peak bone mass, an indicator of cumulative estrogen exposure, is significantly lower in women with first episode schizophrenia than in matched controls, 3) in adulthood, risk for psychosis is higher during periods of low estrogen (e g., during the low estrogen follicular phase of the menstrual cycle and postmenopausal), and 4) adjunctive estradiol may reduce symptom severity in psychotic women. Although the mechanisms involved in the relation of estrogen with psychotic disorders are not understood, it has been suggested that they may reflect anti-dopaminergic and/or neuroprotective effects (Arad and Weiner, 2009). For example, one study showed that estrogen depletion via ovariectomy in rats increased DA D2 receptor densities in the nucleus accumbens and caudate nucleus, and estrogen treatment reversed the increase and further reduced DA D2 receptor levels (Chavez et al., 2010). This is consistent with evidence that the behavioral response to antipsychotic drugs, which are DA D2 antagonists, are decreased following ovariectomy in female rats (Arad and Weiner, 2012) and that estrogen treatment ameliorates psychotic symptoms in humans (Hayes et al., 2012).
Similarly, the review by Markham (2011) concludes that the preponderance of studies find lower testosterone levels in men with schizophrenia, especially those with negative symptoms. Reductions in the free androgen index, a measure of biologically active testosterone, were recently found in a study of first episode antipsychotic-naïve men with psychosis (Fernandez-Egea et al., 2011). Similarly, lower testosterone levels were reported in a recent study of CHR male adolescents, suggesting that lower levels may precede illness onset (van Rijn et al., 2011).
The origins of the association between gonadal hormone levels and psychosis are not known, and reduced hormone levels could be a consequence of behavioral factors or symptoms. While we are aware of no controlled research on testosterone administration in psychotic patients, as mentioned above, there is accumulating evidence that estrogen administration reduces symptom severity in female patients, and one recent report indicates it may also be beneficial for male patients (Kulkarni et al., 2011).
Adrenal Hormones
Previous reviews have documented heightened cortisol levels in patients with schizophrenia and other psychotic disorders, especially those who are unmedicated (Walker and Diforio, 1997; Walker et al., 2008). These reviews support five general conclusions: 1) indices of HPA activity (cortisol and adrenocorticotropic hormone [ACTH]) are elevated in some patients with schizophrenia and other psychoses, especially in unmedicated and firstepisode patients; 2) antipsychotic medications typically reduce cortisol, with more pronounced reductions in drug responders; 3) both prescription and recreational drugs that exacerbate or induce psychotic symptoms also increase HPA activity; 4) glucocorticoid receptors appear to be down-regulated in psychotic patients, suggesting reduced negative feedback on the HPA axis, and 5) reduced hippocampal volume, a correlate of hypercortisolemia, is among the most consistently reported brain abnormality in psychotic patients.
Subsequent to these reviews, several other studies have reported heightened cortisol levels in psychotic patients (Guest et al., 2011; Steen et al., 2011; Yildirim et al., 2011). For example, Steen and colleagues (2011) found increased activity of cortisol metabolism in diagnosed schizophrenia patients. Similarly, Guest and colleagues (2011) found elevated cortisol in a large sample of over 200 first- and recent-onset patients. Among patients with mood disorders, those with psychotic symptoms manifest the greatest elevation in cortisol (Stetler and Miller, 2011). Recently, a study of first-episode psychotic patients revealed no differences from control participants in cortisol levels, but the magnitude of the decrease in cortisol over the course of 12 weeks was associated with the decline in severity of psychotic, negative, and mood symptoms (Garner et al., 2011). Further, abnormalities in the HPA axis may be associated with genetic risk for psychosis. Heightened cortisol levels (Yildirim et al., 2011) and more pronounced cortisol reactivity to daily stress (Collip et al., 2011) have been observed in first degree relatives of psychotic patients.
Indices of the biological stress response are of particular interest during the prodromal phase, given that stress is assumed to play a role in triggering symptom expression. To date, only a few published reports have addressed the relation of the HPA system, or its activity, with conversion to psychosis in a CHR or “prodromal” sample. In one study, pituitary volume was measured via magnetic resonance imaging in a CHR group (Garner et al., 2005). The subjects who later developed psychosis (n = 31) had a significantly larger baseline pituitary volume compared with those who did not develop psychosis (n = 63). The authors suggested that the larger pituitary volume may be indicative of heightened HPA activation. However, in another report on 23 CHR participants, this same research group found that plasma cortisol showed no significant relationship with global psychopathology, psychotic symptoms, or pituitary and hippocampal volumes (Thompson et al., 2007b). It should be noted that this study had limited statistical power; cortisol was only available for 18 participants in the study, and of these only 5 developed a psychotic disorder. These investigators conducted another study in which they administered the dexamethasone corticotrophin releasing hormone (DEX/CRH) test to twelve CHR individuals (M age = 19.4 years, SD = 3.6; range = 15–25) at baseline; three of the twelve developed psychosis within 2 years (Thompson et al., 2007a). Again, the sample size was small, and given this statistical analyses were not conducted, but the authors reported that, contrary to findings on studies of psychotic patients (Walker et al., 2008), participants who did not develop psychosis nevertheless showed a trend toward higher plasma cortisol levels at the latter stages of the test, when compared to the three participants who did develop psychosis.
In contrast, studies of larger samples demonstrate that CHR participants who develop psychosis do manifest higher cortisol levels compared to those who do not (Walker et al., 2001; Weinstein et al., 1999). For example, a recent study of 56 CHR youth (age: 12 to 18) found that those who go on to develop a psychotic disorder showed significantly higher cortisol levels (Walker et al., 2010a). Thus, when compared to the CHR subjects who did not develop a psychotic disorder within the 4-year follow-up period, the 14 later diagnosed with psychosis manifested higher cortisol levels over the first year following baseline. This study had the advantage of multiple saliva samples over a period of one year to enhance cortisol estimation reliability. As in previous studies of adolescent HPA activity, an age-related increase in cortisol secretion was observed in both the healthy and CHR groups, consistent with the evidence reviewed above that the developmental period of heightened risk for prodromal symptom onset may also be characterized by greater stress sensitivity (Stroud et al., 2009). Using similar methods, a recent investigation of baseline cortisol levels from the ongoing NAPLS project reported significantly higher salivary cortisol levels in CHR subjects (n = 260) than healthy controls matched on age and sex (Walker et al., submitted). Cortisol levels were also positively—though modestly—correlated with ratings of positive, negative, disorganized, and general prodromal symptoms.
The above findings raise important questions about the significance of elevated cortisol in individuals at risk for psychosis. A recent review of the extensive literature on stress and psychosis concludes that, when compared to controls, psychotic patients and pre-psychotic CHR individuals do not report significantly increased numbers of stressful life events, but they do report more subjective distress in response to stressors (Holtzman et al., 2013). Thus elevated cortisol levels in psychotic and CHR groups may reflect a greater sensitivity to stress, rather than greater stress exposure. Further, there is evidence of a causal relation between cortisol and psychosis: both administration of steroids and Cushing’s disease, which involves heightened cortisol, are associated with increased risk of psychotic disorders (Ross and Cetas, 2012). Also noteworthy, case studies of Cushing’s patients indicate that psychotic symptoms remit after treatment is successful in reducing cortisol levels (Chana et al., 2011).
In summary, there is evidence of reduced HPG activity, but increased HPA activity in psychosis (Davidson and Heinrichs, 2003; Hill et al., 2004). The origins of these abnormalities and the interactions between these two axes remain unclear. Nevertheless, the importance of this area of investigation has been highlighted by advances in our understanding of neurohormonal signaling, epigenetic processes, and brain abnormalities in the etiology of mental disorders. Rapidly increasing evidence suggests that abnormalities in brain maturation characterize CHR individuals, and elucidating the pathways that affect these neuromaturational abnormalities is a high priority.
Brain Development and Psychosis
A large body of research has documented a range of abnormalities in brain structure in psychoses. These include decreased volumes in the hippocampus (Adriano et al., 2012) and superior temporal gyrus (Siever and Davis, 2004), decreased frontal functioning (Davidson and Heinrichs, 2003; Hill et al., 2004), and increased pituitary volume (Garner et al., 2005; Mondelli et al., 2008; Pariante et al., 2005; Pariante et al., 2004; Takahashi et al., 2009). Recent systematic reviews on volumetric differences between psychotic patients and healthy controls conclude that there is a generalized reduction in cortical gray matter volume in most regions, and this characterizes both first episode and chronic patients (Arnone et al., 2009; Levitt et al., 2010). These abnormalities appear to increase over the course of illness, in at least some patients (Hulshoff Pol and Kahn, 2008). Further, studies of patients with psychosis onset during adolescence indicate that the gray matter volumetric reductions, relative to sameage healthy controls, are more pronounced the earlier the onset of the illness (Douaud et al., 2009). Finally, comparisons of monozygotic twins discordant for schizophrenia indicate that the volumetric reductions are more pronounced in the affected co-twin, especially for the perihippocampal region, indicating that the brain abnormalities in psychotic patients are partially independent of genetic factors (Borgwardt et al., 2010; van Haren et al., 2004).
More recently, volumetric abnormalities have been documented in CHR individuals, with more significant volumetric reductions in those who develop psychosis (Fusar-Poli et al., 2011; Puri, 2010; Smieskova et al., 2010). Consistent with evidence of heightened HPA activity preceding and following the onset of psychoses, the hippocampus appears to be a brain region with marked volumetric reduction in patients with psychotic disorders, as well as in those at elevated risk for psychosis (Witthaus et al., 2009). For example, a meta-analysis of data from over 700 healthy control and 800 CHR subjects revealed reduced gray matter volume in the parahippocampal/hippocampal regions, anterior cingulate, right superior temporal gyrus, left precuneus, left medial frontal gyrus, and right middle frontal gyrus for the CHR group (Fusar-Poli et al., 2011). Among the CHR participants, those who later developed psychosis showed lower baseline gray matter volume in the right inferior frontal gyrus and in the right superior temporal gyrus (Fusar-Poli et al., 2011).
In light of the normative decrease in gray matter volume associated with adolescent and young adult development, the more pronounced gray matter declines in CHR individuals have led some to propose that risk for psychosis involves an abnormal acceleration in the brain developmental processes associated with adolescence (Fusar-Poli et al., 2011; Walker et al., 2008). Thus, brain organizational processes may go awry and disrupt neural circuitry (Whitford et al., 2011). Further, it has been suggested that this aberrant process, as well as the brain abnormalities associated with psychosis, may partially reflect the adverse effects of persistent glucocorticoid elevations on brain structure (Walker et al., 2008).
Neurodevelopmental Models of Schizophrenia and Other Psychoses
Given evidence that the prodrome to psychosis is associated with increased activity of the HPA axis and, potentially, decreased HPG activity, the role of hormones in the expression of psychosis is becoming more salient. The scientific community has long recognized the relation between the onset of puberty and risk rates for many forms of psychopathology (Adams et al., 2000; Graber et al., 1997), and recent developmental models of psychosis posit that hormones modulate the expression of vulnerability to psychiatric disorders (van Wingen et al., 2011; Walker et al., 2008). Further, because DA, more than any other neurotransmitter, has played a prominent role in the neurobiological theories of psychosis (Carlsson, 1988; Meltzer and Stahl, 1976; Winterer, 2006), it has been featured heavily in neurodevelopmental models (Murray et al., 2002; Tan, 2009; Walker and Diforio, 1997). The preponderance of evidence suggests that heightened DA activity is associated with both psychosis and psychosis risk. Maturational increases in DA activity during adolescence have thus been cited to account for the modal age at onset of schizophrenia and other psychoses (Benes, 2003; Walker, 1994; Walker and Bollini, 2002).
Aberrant functional connectivity, acting in concert with increased activity in DA circuitry during adolescence, may be the final common pathway setting the stage for the onset of psychosis (Maccabe, 2008; Walker, 1994; Walker and Bollini, 2002). Of course, neurotransmitter systems interact, and glutamate, GABA, and serotonin have also been implicated in etiology, in part due to their effects on the DA system (Howes and Kapur, 2009).
Stress has been a central feature in many theories of the neurodevelopment of schizophrenia, and research evidence supporting a role for HPA hyperactivity in psychosis is substantial (Holtzman et al., 2013). The adverse effects of persistent glucocorticoid elevations on brain structure/function are also well documented (Arnsten, 2009; Frodl and O'Keane, 2012), thus recent models of psychosis etiology have hypothesized that cortisol elevations in CHR individuals may be contributing to brain structural abnormalities that precede psychosis. Such effects may be organizational, in that they permanently compromise brain structure. Of course, the results of research on Cushing’s patients, as described above, suggests that there are also adverse activational effects of elevated cortisol that can be reversed.
As noted above, with respect to the HPG axis, theorists have speculated on how estrogen might influence the expression of psychosis by suppressing DA activity, thereby dampening its adverse effects, delaying psychosis onset, and improving prognosis in women (Riecher-Rossler and Kulkarni, 2011). Coupled with the evidence that symptom severity changes in conjunction with estrogen levels during the menstrual cycle, and that estrogen administration reduces symptom severity in both sexes, these findings suggest activational effects of estrogen that modulate the neuropathophysiology underlying psychosis.
The complex interactions between the HPG and HPA axes should also be considered with regard to the emergence of psychosis. There is extensive evidence that stress exposure and glucocorticoids affect HPG activity (Hiller-Sturmhofel and Bartke, 1998; Kerdelhue et al., 2002; Shi et al., 2011; Viau, 2002). In fact, it is well established that glucocorticoid secretion exerts inhibitory effects on reproduction and acts at multiple levels of the HPG axis. Specifically, as illustrated in Figure 1, glucocorticoids can suppress GnRH neurons, the pituitary gonadotropin secretion, and production of gonadal hormones. It is possible that reduced gonadal hormone levels observed in psychotic patients and CHR individuals reflect the suppression of HPG function by elevated glucocorticoid secretion.
Research findings on the effects of HPG activity on HPA function in humans are more limited, although some findings suggest that estrogens may suppress glucocorticoid secretion (Patacchioli et al., 2006). If this is the case, then glucocorticoid-induced reductions in HPG activity may, in turn, further contribute to glucocorticoid elevations. The cumulative effects could be a disruption in adolescent neuromaturational processes that appear to be exaggerated in psychosis; namely, the more pronounced developmental increase in cortisol secretion (Walker et al., 2010a), decrease in gray matter volume (Sowell et al., 1999), and pruning of synapses (McGlashan and Hoffman, 2000).
Summary and Conclusions
As our scientific understanding of adolescent brain development and neurohormonal mechanisms has grown, the range of processes that have the potential to derail neuromaturation and give rise to psychosis have become more apparent. As described above, steroid hormones may contribute to the pathogenesis of psychosis at the genomic level, disrupting brain development by triggering the expression of latent vulnerability genes or by altering the expression of genes that govern normal brain development (Walker and Bollini, 2002). At this point, our understanding of hormonal and brain changes in the prodromal phase of psychosis is too limited to posit specific genes or neural mechanisms. Future examinations of the interaction of adrenal and gonadal hormones and their relationship with aberrant brain development in the psychosis prodrome are the next steps for delineating these mechanisms.
Along with advances in our understanding of the etiology of psychosis, the possibilities for preventive intervention are coming into clearer view. If it is the case that the adolescent hormonal milieu is playing a role in the pathogenesis of psychosis, then prevention may involve modulations aimed at normalizing or constraining aberrant hormonal processes. Furthermore, there is reason to believe that identification of specific neurohormonal triggers during adolescence could possibly hasten treatment onset, thereby increasing positive outcomes in individuals at greatest risk for psychosis (Drake et al., 2000; Johannessen et al., 2001; McGlashan et al., 2001).
Highlights.
An overview of the nature and course of psychosis during adolescence is provided.
We review the potential role of hormones in abnormal brain changes in adolescence.
We discuss hormones in relation to neuropathological processes in psychosis onset.
Acknowledgments
This research was supported in part by Grant U01MHMH081988 from the National Institute of Mental Health awarded to Dr. Walker.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adams J, Barone S, Jr, LaMantia A, Philen R, Rice DC, Spear L, Susser E. Workshop to identify critical windows of exposure for children's health: neurobehavioral work group summary. Environ Health Perspect. 2000;108(Suppl 3):535–544. doi: 10.1289/ehp.00108s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J. The prodromal stage of psychotic illness: observation, detection or intervention? J Psychiatry Neurosci. 2003;28:93–97. [PMC free article] [PubMed] [Google Scholar]
- Addington J, Cadenhead K, Cannon T, Cornblatt B, McGlashan T, Perkins D, Seidman L, Tsuang M, Walker E, Woods S, Heinssen R, Study NAPL. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophrenia bulletin. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Ang Y, Tan H. Academic deterioration prior to first episode schizophrenia in young Singaporean males. Psychiatry research. 2004;121:303–307. doi: 10.1016/s0165-1781(03)00257-9. [DOI] [PubMed] [Google Scholar]
- Arad M, Weiner I. Disruption of latent inhibition induced by ovariectomy can be reversed by estradiol and clozapine as well as by co-administration of haloperidol with estradiol but not by haloperidol alone. Psychopharmacology (Berl) 2009;206:731–740. doi: 10.1007/s00213-009-1464-0. [DOI] [PubMed] [Google Scholar]
- Arad M, Weiner I. Abnormally rapid reversal learning and reduced response to antipsychotic drugs following ovariectomy in female rats. Psychoneuroendocrinology. 2012;37:200–212. doi: 10.1016/j.psyneuen.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cerebral cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Why does psychosis develop during adolescence and early adulthood? Current Opinion in Psychiatry. 2003;16:317–319. [Google Scholar]
- Blakemore SJ. Imaging brain development: the adolescent brain. NeuroImage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T, Murray R, McGuire PK. Regional gray matter volume in monozygotic twins concordant and discordant for schizophrenia. Biological psychiatry. 2010;67:956–964. doi: 10.1016/j.biopsych.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Walker EF. Vulnerability to Schizophrenia Risk Factors in Childhood and Adolescence. In: Ingram RE, Price JM, editors. Vulnerability to Psychopathology: Risk Across the Lifespan. New York: The Guilford Press; 2001. p. 476. [Google Scholar]
- Brook CG. Mechanism of puberty. Horm Res. 1999;51(Suppl 3):52–54. doi: 10.1159/000053162. [DOI] [PubMed] [Google Scholar]
- Brown GR, Spencer KA. Steroid hormones, stress and the adolescent brain: A comparative perspective. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Buka SL, Fan AP. Association of prenatal and perinatal complications with subsequent bipolar disorder and schizophrenia. Schizophr Res. 1999;39:113–119. doi: 10.1016/s0920-9964(99)00109-7. discussion 160-111. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM. The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology. 2003;144:274–280. doi: 10.1210/en.2002-220711. [DOI] [PubMed] [Google Scholar]
- Castro-Fornieles J, Bargallo N, Lazaro L, Andres S, Falcon C, Plana MT, Junque C. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. Journal of psychiatric research. 2009;43:331–340. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Chana LF, Vaidyab M, Westphalc B, Allgrovea J, Martina L, Afshard F, Hindmarshf PC, Savagea MO, Grossmane AB, Storra HL. Use of Intravenous Etomidate to Control Acute Psychosis Induced by the Hypercortisolaemia in Severe Paediatric Cushing’s Disease. Hormone Research in Paediatrics. 2011;75:441–446. doi: 10.1159/000324419. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos G. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–59. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychological medicine. 2011;41:2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- Compton MT, McGlashan TH, McGorry PD. Toward Prevention Approaches for Schizophrenia: An Overview of Prodromal States, the Duration of Untreated Psychosis, and Early Intervention Paradigms. Psychiatric Annals. 2007;37:340–348. [Google Scholar]
- Correll CU, Hauser M, Auther AM, Cornblatt BA. Research in people with psychosis risk syndrome: a review of the current evidence and future directions. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2010;51:390–431. doi: 10.1111/j.1469-7610.2010.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, O'Donovan MC, Owen MJ. Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or "schizoaffective") psychoses. Schizophrenia bulletin. 2009;35:482–490. doi: 10.1093/schbul/sbp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry research. 2003;122:69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. 4th ed. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- Dorn LD, Biro FM. Puberty and Its Measurement: A Decade in Review. Journal of Research on Adolescence. 2011;21:180–195. [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the Boundaries of Early Adolescence: A User's Guide to Assessing Pubertal Status and Pubertal Timing in Research With Adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, Connell J, Roberts N, Crow TJ, Matthews PM, Smith S, James A. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132:2437–2448. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- Drake R, Haley C, Akhtar S, Lewis S. Causes and consequences of duration of untreated psychosis in schizophrenia. Br J Psychiatry. 2000;177:511–515. doi: 10.1192/bjp.177.6.511. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: effects depend on biological sensitivity to context. Dev Psychopathol. 2011;23:85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Stern E, Silbersweig D. Mesolimbic activity associated with psychosis in schizophrenia. Symptom-specific PET studies. Ann N Y Acad Sci. 1999;877:562–574. doi: 10.1111/j.1749-6632.1999.tb09289.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of psychiatric research. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Garcia-Rizo C, Miller B, Parellada E, Justicia A, Bernardo M, Kirkpatrick B. Testosterone in newly diagnosed, antipsychotic-naive men with nonaffective psychosis: a test of the accelerated aging hypothesis. Psychosom Med. 2011;73:643–647. doi: 10.1097/PSY.0b013e318230343f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Lui P, Romeo RD. The transformation of hormonal stress responses throughout puberty and adolescence. J Endocrinol. 2011;210:391–398. doi: 10.1530/JOE-11-0206. [DOI] [PubMed] [Google Scholar]
- Frodl T, O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho B, Andreasen N. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, Mc Guire P, Sacchetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biological psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Garner B, Phassouliotis C, Phillips LJ, Markulev C, Butselaar F, Bendall S, Yun Y, McGorry PD. Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. Journal of psychiatric research. 2011;45:249–255. doi: 10.1016/j.jpsychires.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM. The canary in the coalmine: the sensitivity of mesolimbic dopamine to environmental adversity during development. Neurosci Biobehav Rev. 2011;35:794–803. doi: 10.1016/j.neubiorev.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, Delbello MP, Soutullo CA. Diagnostic characteristics of 93 cases of a prepubertal and early adolescent bipolar disorder phenotype by gender, puberty and comorbid attention deficit hyperactivity disorder. Journal of child and adolescent psychopharmacology. 2000;10:157–164. doi: 10.1089/10445460050167269. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, Sharpe RM, Skakkebaek NE, Toppari J. Public health implications of altered puberty timing. Pediatrics. 2008;121(Suppl 3):S218–S230. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- Gottesman I. Schizophrenia genesis: The origins of madness. 1991 [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Harmatz JS, Shader RI. Psychotropic drug prescribing in the United States: extent, costs, and expenditures. Journal of clinical psychopharmacology. 2011;31:1–3. doi: 10.1097/JCP.0b013e318209cf05. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol Cell Endocrinol. 2012;350:299–309. doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Guerriero G. Vertebrate sex steroid receptors: evolution, ligands, and neurodistribution. Annals of the New York Academy of Sciences. 2009;1163:154–168. doi: 10.1111/j.1749-6632.2009.04460.x. [DOI] [PubMed] [Google Scholar]
- Guest PC, Martins-de-Souza D, Vanattou-Saifoudine N, Harris LW, Bahn S. Abnormalities in metabolism and hypothalamic-pituitary-adrenal axis function in schizophrenia. Int Rev Neurobiol. 2011;101:145–168. doi: 10.1016/B978-0-12-387718-5.00006-7. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans SL, Auerbach JG, Styr B, Marcus J. Offspring of parents with schizophrenia: mental disorders during childhood and adolescence. Schizophrenia bulletin. 2004;30:303–315. doi: 10.1093/oxfordjournals.schbul.a007080. [DOI] [PubMed] [Google Scholar]
- Hayes E, Gavrilidis E, Kulkarni J. The role of oestrogen and other hormones in the pathophysiology and treatment of schizophrenia. Schizophr Res Treatment. 2012;2012:540273. doi: 10.1155/2012/540273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The Impact of Sex, Puberty, and Hormones on White Matter Microstructure in Adolescents. Cerebral cortex. 2011 doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophrenia research. 2004;68:49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- Hiller-Sturmhofel S, Bartke A. The endocrine system: an overview. Alcohol Health Res World. 1998;22:153–164. [PMC free article] [PubMed] [Google Scholar]
- Holtzman CW, Trotman HD, Goulding SM, Ryan AT, McDonald AN, Shapiro DI, Brasfield JL, Walker EF. Stress and Neurodevelopmental Processes in the Emergence of Psychosis. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia bulletin. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophrenia bulletin. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen JO, McGlashan TH, Larsen TK, Horneland M, Joa I, Mardal S, Kvebaek R, Friis S, Melle I, Opjordsmoen S, Simonsen E, Ulrik H, Vaglum P. Early detection strategies for untreated first-episode psychosis. Schizophrenia research. 2001;51:39–46. doi: 10.1016/s0920-9964(01)00237-7. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychological medicine. 1997;27:411–419. doi: 10.1017/s003329179600445x. [DOI] [PubMed] [Google Scholar]
- Kenny FM, Gancayco GP, Heald FP, Hung W. Cortisol production rate in adolescent males in different stages of sexual maturation. J Clin Endocrinol Metab. 1966;26:1232–1236. doi: 10.1210/jcem-26-11-1232. [DOI] [PubMed] [Google Scholar]
- Kerdelhue B, Brown S, Lenoir V, Queenan JT, Jr, Jones GS, Scholler R, Jones HW., Jr Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology. 2002;75:158–163. doi: 10.1159/000048233. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Anderson S, Pettegrew J. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of psychiatric research. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Diwadkar V, Rosenberg D. Developmental biomarkers in schizophrenia and other psychiatric disorders: common origins, different trajectories? Epidemiologia e psichiatria sociale. 2005;14:188–193. doi: 10.1017/s1121189x00007934. [DOI] [PubMed] [Google Scholar]
- Khaykin E, Eaton WW, Ford DE, Anthony CB, Daumit GL. Health insurance coverage among persons with schizophrenia in the United States. Psychiatric services. 2010;61:830–834. doi: 10.1176/ps.2010.61.8.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki Y, Hatanaka Y, Murakami G, Mukai H, Hojo Y, Saito M, Kimoto T, Kawato S. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS One. 2012;7:e34124. doi: 10.1371/journal.pone.0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Headey B, Marston N, Sinclair K, Lee S, Gurvich C, Fitzgerald PB, Burger H. Estrogens and men with schizophrenia: is there a case for adjunctive therapy? Schizophr Res. 2011;125:278–283. doi: 10.1016/j.schres.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Peper JS, Crone EA, Dahl RE. White Matter Development in Adolescence: The Influence of Puberty and Implications for Affective Disorders. Dev Cogn Neurosci. 2012;2:36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith C, Auther A, Correll C, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophrenia research. 2004;68:37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Bobrow L, Lucia D, Srinivasan P. A selective review of volumetric and morphometric imaging in schizophrenia. Current Topics in Behavioral Neurosciences. 2010;4:243–281. doi: 10.1007/7854_2010_53. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Menard C, Ng Ying Kin NM, Nair NP. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Buckmaster PS, Lee AG, Wu C, Mitra R, Duffey LM, Buckmaster CL, Her S, Patel PD, Schatzberg AF. Stress coping stimulates hippocampal neurogenesis in adult monkeys. Proc Natl Acad Sci U S A. 2010;107:14823–14827. doi: 10.1073/pnas.0914568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccabe JH. Population-based cohort studies on premorbid cognitive function in schizophrenia. Epidemiol Rev. 2008;30:77–83. doi: 10.1093/epirev/mxn007. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys' and girls' timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol. 2011;47:1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA. Sex steroids and schizophrenia. Rev Endocr Metab Disord. 2011 doi: 10.1007/s11154-011-9184-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci. 1991;12:141–147. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- McFarlane WR. Prevention of the first episode of psychosis. Psychiatr Clin North Am. 2011;34:95–107. doi: 10.1016/j.psc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH. Early detection and intervention in psychosis: an ethical paradigm shift. Br J Psychiatry Suppl. 2005;48:s113–s115. doi: 10.1192/bjp.187.48.s113. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophrenia bulletin. 2001;27:563–570. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Panzica G, Garcia-Segura LM. Neuroactive steroids: focus on human brain. Neuroscience. 2011;191:1–5. doi: 10.1016/j.neuroscience.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Meltzer H, Stahl S. The dopamine hypothesis of schizophrenia: a review. Schizophrenia bulletin. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM, Muller D. Estradiol promotes spine growth and synapse formation without affecting pre-established networks. Hippocampus. 2011;21:1263–1267. doi: 10.1002/hipo.20875. [DOI] [PubMed] [Google Scholar]
- Miller T, McGlashan T, Rosen J, Somjee L, Markovich P, Stein K, Woods S. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miller T, McGlashan T, Woods S, Stein K, Driesen N, Corcoran C, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Gabilondo A, Tournikioti K, Walshe M, Marshall N, Schulze KK, Murray RM, McDonald C, Pariante CM. Pituitary volume in unaffected relatives of patients with schizophrenia and bipolar disorder. Psychoneuroendocrinology. 2008;33:1004–1012. doi: 10.1016/j.psyneuen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Pariante CM, Navari S, Aas M, D'Albenzio A, Di Forti M, Handley R, Hepgul N, Marques TR, Taylor H, Papadopoulos AS, Aitchison KJ, Murray RM, Dazzan P. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophrenia research. 2010;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Murray RM, McDonald C, Bramon E. Neurodevelopmental impairment, dopamine sensitisation, and social adversity in schizophrenia. World Psychiatry. 2002;1:137–145. [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, Fearon P, Orr K, Hutchinson G, Pantelis C, Velakoulis D, Jones PB, Leff J, Murray RM. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. 2005;30:1923–1931. doi: 10.1038/sj.npp.1300766. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, Brewer W, Smith DJ, Dazzan P, Yung AR, Zervas IM, Christodoulou GN, Murray R, McGorry PD, Pantelis C. Pituitary volume in psychosis. British Journal of Psychiatry. 2004;185:5–10. doi: 10.1192/bjp.185.1.5. [DOI] [PubMed] [Google Scholar]
- Patacchioli FR, Simeoni S, Monnazzi P, Pace M, Capri O, Perrone G. Menopause, mild psychological stress and salivary cortisol: influence of long-term hormone replacement therapy (HRT) Maturitas. 2006;55:150–155. doi: 10.1016/j.maturitas.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Peper J, Brouwer R, Schnack H, van Baal G, van Leeuwen M, van den Berg S, Delemarre-Van de Waal H, Boomsma D, Kahn R, Hulshoff Pol H. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011a;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011b;36:1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Puri BK. Progressive structural brain changes in schizophrenia. Expert review of neurotherapeutics. 2010;10:33–42. doi: 10.1586/ern.09.142. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, Nicolson R, Bedwell J, Lenane M, Zijdenbos A, Paus T, Evans A. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Redei E, Li L, Halasz I, McGivern RF, Aird F. Fast glucocorticoid feedback inhibition of ACTH secretion in the ovariectomized rat: effect of chronic estrogen and progesterone. Neuroendocrinology. 1994;60:113–123. doi: 10.1159/000126741. [DOI] [PubMed] [Google Scholar]
- Riecher-Rossler A, Kulkarni J. Estrogens and gonadal function in schizophrenia and related psychoses. Curr Top Behav Neurosci. 2011;8:155–171. doi: 10.1007/7854_2010_100. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Ross DA, Cetas JS. Steroid psychosis: a review for neurosurgeons. J Neurooncol. 2012;109:439–447. doi: 10.1007/s11060-012-0919-z. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Molenda-Figueira H, Sisk C. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wudy SA, Buyken AE, Maser-Gluth C, Hartmann MF, Remer T. Prepubertal glucocorticoid status and pubertal timing. J Clin Endocrinol Metab. 2011;96:E891–E898. doi: 10.1210/jc.2010-2935. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental psychobiology. 2011 doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever L, Davis K. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. American Journal of Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Sisk C, Zehr J. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rossler A, Borgwardt SJ. Neuroimaging predictors of transition to psychosis--a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing agerelated changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steen NE, Methlie P, Lorentzen S, Hope S, Barrett EA, Larsson S, Mork E, Almas B, Lovas K, Agartz I, Melle I, Berg JP, Andreassen OA. Increased systemic cortisol metabolism in patients with schizophrenia and bipolar disorder: a mechanism for increased stress vulnerability? J Clin Psychiatry. 2011;72:1515–1521. doi: 10.4088/JCP.10m06068yel. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosomatic medicine. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD. Puberty: Its role in development, Handbook of Adolescent Psychology. New York, NY: John Wiley & Sons, Inc.; 2009. pp. 116–151. [Google Scholar]
- Suzuki M, Zhou SY, Hagino H, Niu L, Takahashi T, Kawasaki Y, Matsui M, Seto H, Ono T, Kurachi M. Morphological brain changes associated with Schneider's first-rank symptoms in schizophrenia: a MRI study. Psychological medicine. 2005;35:549–560. doi: 10.1017/s0033291704003885. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- Tan CO. Anticipatory changes in regional cerebral hemodynamics: a new role for dopamine? J Neurophysiol. 2009;101:2738–2740. doi: 10.1152/jn.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Dhruv SH, Hochman K, Hamann S. The relation of cortisol levels with hippocampus volumes under baseline and challenge conditions. Brain research. 2007;1179:70–78. doi: 10.1016/j.brainres.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Thompson KN, Berger G, Phillips LJ, Komesaroff P, Purcell R, McGorry PD. HPA axis functioning associated with transition to psychosis: combined DEX/CRH test. Journal of psychiatric research. 2007a;41:446–450. doi: 10.1016/j.jpsychires.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Thompson KN, Phillips LJ, Komesaroff P, Yuen HP, Wood SJ, Pantelis C, Velakoulis D, Yung AR, McGorry PD. Stress and HPA-axis functioning in young people at ultra high risk for psychosis. Journal of psychiatric research. 2007b;41:561–569. doi: 10.1016/j.jpsychires.2006.05.010. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Picchioni MM, McDonald C, Marshall N, Davis N, Ribchester T, Hulshoff Pol HE, Sharma T, Sham P, Kahn RS, Murray R. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biological psychiatry. 2004;56:454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, de Sonneville L, Sprong M, Ziermans T, Schothorst P, van Engeland H, Swaab H. Neuroendocrine markers of high risk for psychosis: salivary testosterone in adolescent boys with prodromal symptoms. Psychological medicine. 2011;41:1815–1822. doi: 10.1017/S0033291710002576. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Ossewaarde L, Backstrom T, Hermans EJ, Fernandez G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Vigil P, Orellana RF, Cortes ME, Molina CT, Switzer BE, Klaus H. Endocrine modulation of the adolescent brain: a review. J Pediatr Adolesc Gynecol. 2011;24:330–337. doi: 10.1016/j.jpag.2011.01.061. [DOI] [PubMed] [Google Scholar]
- Walker E. Developmentally moderated expressions of the neuropathology underlying schizophrenia. Schizophrenia bulletin. 1994;20:453–480. doi: 10.1093/schbul/20.3.453. [DOI] [PubMed] [Google Scholar]
- Walker E, Bollini AM. Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophrenia research. 2002;54:17–23. doi: 10.1016/s0920-9964(01)00347-4. [DOI] [PubMed] [Google Scholar]
- Walker E, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychological Review. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker E, Walder D, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and Psychopathology. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. Journal of Abnormal Psychology. 2010a;119:401–408. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol. 2010b;119:401–408. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Grimes KE, Davis DM, Smith AJ. Childhood precursors of schizophrenia: facial expressions of emotion. Am J Psychiatry. 1993;150:1654–1660. doi: 10.1176/ajp.150.11.1654. [DOI] [PubMed] [Google Scholar]
- Weinstein DD, Diforio D, Schiffman J, Walker E, Bonsall R. Minor physical anomalies, dermatoglyphic asymmetries, and cortisol levels in adolescents with schizotypal personality disorder. American Journal of Psychiatry. 1999;156:617–623. doi: 10.1176/ajp.156.4.617. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Shenton ME. Diffusion tensor imaging, structural connectivity, and schizophrenia. Schizophr Res Treatment. 2011;2011:709523. doi: 10.1155/2011/709523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G. Cortical microcircuits in schizophrenia--the dopamine hypothesis revisited. Pharmacopsychiatry. 2006;39(Suppl 1):S68–S71. doi: 10.1055/s-2006-931498. [DOI] [PubMed] [Google Scholar]
- Witthaus H, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, Gallinat J, Ruhrmann S, Brune M, Heinz A, Klingebiel R, Juckel G. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry research. 2009;173:163–169. doi: 10.1016/j.pscychresns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Wu EQ, Birnbaum HG, Shi L, Ball DE, Kessler RC, Moulis M, Aggarwal J. The economic burden of schizophrenia in the United States in 2002. Journal of Clinical Psychiatry. 2005;66:1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- Yates MA, Juraska JM. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol. 2008;209:284–287. doi: 10.1016/j.expneurol.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim O, Dogan O, Semiz M, Kilicli F. Serum cortisol and dehydroepiandrosterone-sulfate levels in schizophrenic patients and their first-degree relatives. Psychiatry Clin Neurosci. 2011;65:584–591. doi: 10.1111/j.1440-1819.2011.02252.x. [DOI] [PubMed] [Google Scholar]
- Yung A, Phillips L, Yuen H, McGorry P. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophrenia research. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust N Z J Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. Prediction of psychosis: setting the stage. Br J Psychiatry Suppl. 2007;51:s1–s8. doi: 10.1192/bjp.191.51.s1. [DOI] [PubMed] [Google Scholar]
- Yung AR, Stanford C, Cosgrave E, Killackey E, Phillips L, Nelson B, McGorry PD. Testing the Ultra High Risk (prodromal) criteria for the prediction of psychosis in a clinical sample of young people. Schizophrenia research. 2006;84:57–66. doi: 10.1016/j.schres.2006.03.014. [DOI] [PubMed] [Google Scholar]