Abstract
To examine whether microbial community structure differs across rice genotypes, automated ribosomal intergenic spacer analysis (ARISA) was conducted. Nine cultivars of Oryza sativa ssp. indica or japonica and seven lines of other Oryza species were grown in paddy fields with low, standard, and high levels of N fertilization. Multidimensional scaling plots of bacterial ARISA for aerial parts of rice (shoots) revealed that the structure of shoot bacterial communities was significantly affected by plant genotype (indica or japonica) based on similarity tests, whereas root microbiomes were largely affected by the N fertilization level.
Keywords: automated ribosomal intergenic spacer analysis, nitrogen fertilization, microbial community structure, plant genotype, rice
A wide range of microorganisms, including bacteria and fungi, have been found in the phyllosphere and rhizosphere (21, 25, 26), and reside in and on plants as endophytes and epiphytes (22, 25, 30). These symbiotic microbes could be important components of the proximate mechanisms underlying plant functional traits such as nutrient acquisition, plant defense, plant morphology and abiotic stress tolerance (7); however, many questions remain about the driving forces for shaping a community structure of plant-associated microbes (9).
In studies of how plant genetic factors control microbial community structure, artificially generated genetic variations are an important basic resource (11). In rice, Ikeda et al. (14) reported significant impacts of the OsCCaMK gene on the diversity of root-associated bacteria under both paddy and upland field conditions using OsCCaMK mutants screened from a Tos17 mutant panel (2). CCaMK plays an important role in a common symbiosis pathway that leads to successful rhizobial and arbuscular mycorrhizal symbioses in plants (18). In addition, naturally occurring genetic variation in plants is another important resource (17). Genotypes derived from natural plant variations have been shown to affect root-associated bacterial communities in rice (10). Hardoim et al. (10) demonstrated that plant genotypes helped to shape bacterial community structures in rice roots; however, the effects of plant genotype on aerial parts of rice (shoot)-associated bacterial communities have not been analyzed by culture-independent methods.
Nitrogen is the most important mineral nutrient for crop production, and an adequate supply of nitrogen fertilizer is essential for sustaining high yields. Previous studies have shown that nitrogen application influences rice-associated microbial communities using polymorphisms of 16S rRNA and nifH genes (5, 15, 29).
In general, genetic fingerprinting techniques allow high throughput and comparative profiling of numerous samples for microbial community analyses (3, 26). Automated ribosomal intergenic spacer analysis (ARISA) is a highly sensitive method due to the laser detection of fluorescently labeled DNA, and it is a rapid and effective technique that can be used in conjunction with more accurate but labor-intensive methods (26). Using this method, Ikeda et al. (13) showed that the bacterial community structure in soybean roots could be classified into three groups according to the plant genotype.
In the present study, ARISA was used to reveal the effects of inter- and intraspecific genetic variations in the genus Oryza on bacterial communities. Nine cultivars of O. sativa and seven lines of other Oryza species were used for shoot-associated bacterial community analysis, and five cultivars of O. sativa were used for root-associated bacterial community analysis (Table 1).
Table 1.
Rice plant materials used in this study
| Cultivar/line | Species | Genomea |
|---|---|---|
| Nipponbare | O. sativa L. ssp. japonica | AA |
| Sasanishiki | O. sativa L. ssp. japonica | AA |
| Taichung 65 | O. sativa L. ssp. japonica | AA |
| Gemdjah Beton | O. sativa L. ssp. japonica | AA |
| Koshihikari | O. sativa L. ssp. japonica | AA |
| Habataki | O. sativa L. ssp. indica | AA |
| Kasalath | O. sativa L. ssp. indica | AA |
| IR24 | O. sativa L. ssp. indica | AA |
| IR36 | O. sativa L. ssp. indica | AA |
| WK21 | O. glaberrima Steud. | AA |
| W106 | O. rufipogon sensu lato | AA |
| W1965 | O. rufipogon sensu lato | AA |
| W1967 | O. rufipogon sensu lato | AA |
| W630 | O. rufipogon sensu lato | AA |
| W1515 | O. punctata Kotschy ex Steud. | BB |
| W1527 | O. eichingeri Peter | CC |
Cultivars and lines were classified based on the genome composition according to the degree of meiotic chromosome pairing in hybrid plants (23).
A total of 48 seedlings of each cultivar and line were planted and grown in paddy fields at Kashimadai, Miyagi, Japan, as described by Obara et al. (24). Low nitrogen (LN) and standard nitrogen (SN) fields were managed with 0 and 30 kg N ha−1 of nitrogen fertilization, respectively. In the high nitrogen (HN) field, 270 kg N ha−1 of ammonium sulfate (Ube Agri-Materials, Tokyo, Japan) was applied in addition to basal fertilizer. To reach 270 kg N ha−1, 30 or 60 kg N ha−1 of ammonium sulfate was applied as additional fertilization every 2 weeks. Plants were sampled 90 days after transplanting. Each rice plant was dug up in a square (30 cm × 30 cm) to a depth of about 30 cm from the soil surface. The roots were washed with running tap water in the laboratory until the soil was removed from roots, and around 20 g shoots and a whole root in each plant were stored separately at −80°C until molecular analysis. A total of 20 g shoots and whole root tissue from one plant were separately ground to a powder in liquid nitrogen with a mortar and pestle. DNA was extracted from 200 to 300 mg powdered tissue by the DNA extraction method developed by Ikeda et al. (12). The remaining shoot samples were dried and then digested with sulfuric acid to quantify total nitrogen by the Kjeldahl method (Table S1).
The multidimensional scaling (MDS) method was used in conjunction with ARISA to evaluate similarities of bacterial communities in shoot and roots. The bacterial primer set ITSF/ITSReub (3) was used for ARISA. PCR amplification was carried out as described by Ikeda et al. (13). PCR products (1 μL) were mixed with 1 μL LIZ1200 internal size standard (Applied Biosystems, Foster City, CA, USA), and 20 μL deionized formamide was added. The mixture was denatured at 95°C for 5 min and cooled on ice. Next, the PCR product was placed in an ABI 3730xl DNA Analyzer (Applied Biosystems). The profile data (up to 1,200 bases) obtained by ARISA were initially analyzed with ABI GeneMapper software (Applied Biosystems) and were processed further with the RIBOSORT program (27) to assign fragment size and calculate the relative abundance of each ribotype. Fragments with fewer than 100 fluorescence units were eliminated from the analyses. Using the R program (27), an MDS plot was generated from the ribotypes using a similarity matrix produced by the RIBOSORT program with default parameters and the vegan package with the Bray-Curtis index of dissimilarity. Analyses of similarities (ANOSIM) were performed to test for significant differences using 1000 permutation tests. The resulting test statistic R indicates the degree of separation, ranging from 0 (no separation) to 1 (complete separation). Numbers of operational taxonomic units in each sample are shown in Table S2.
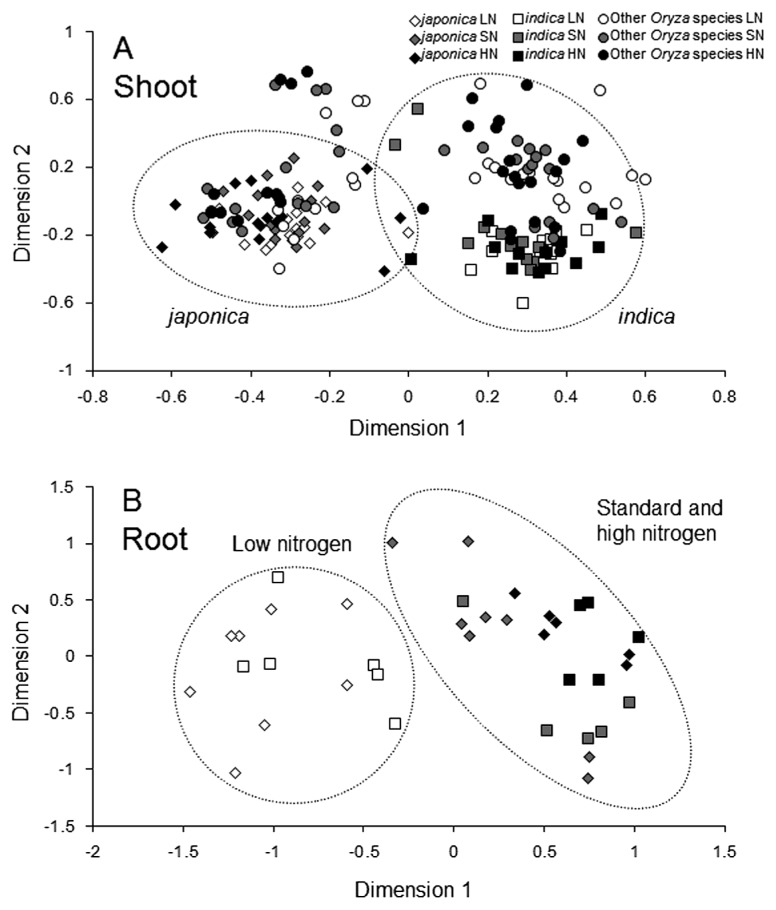
MDS was performed to compare the complex ARISA profiles of the bacterial community structures among the plant genotypes within the genus Oryza (Table 1). In O. sativa, MDS plots of the bacterial ARISA of shoot samples indicate that the bacterial communities were apparently separated between japonica and indica groups (Fig. 1A), which were statistically significant by similarity tests (ANO-SIM) (Table S3). MDS plots of the bacterial ARISA of shoot samples from other Oryza species (O. glaberrima, O. rufipogon, O. punctata, and O. eichingeri) (Table 1) did not form a distinct cluster, but were widely dispersed (Fig. 1A), suggesting that the diversity of bacterial community in shoots of O. sativa was lower than those of other Oryza species (Fig. 1A). This may reflect the presence of more genetic variations found in these Oryza species than in those of O. sativa (16).
Fig. 1.
Multidimensional scaling plots generated from ARISA profiles with primers ITSF and ITSReub for rice (A) shoot- and (B) root-associated bacterial communities under low (LN), standard (SN), and high (HN) nitrogen conditions. Nine cultivars of O. sativa and seven lines of other Oryza species were used for shoot-associated bacterial community analysis with three replications, and five cultivars of O. sativa were used for root-associated bacterial community analysis with at least two replications (Table 1).
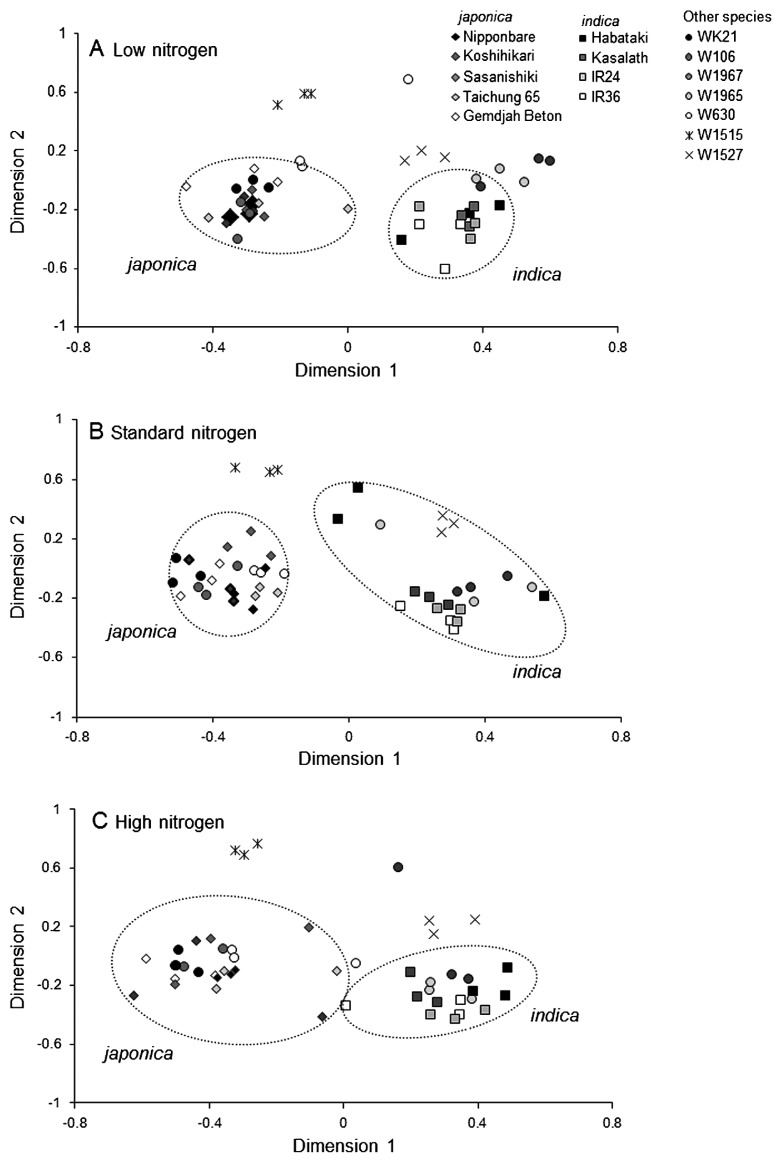
MDS plots of the bacterial ARISA of shoot samples indicated that the community structures for japonica and indica genotypes formed clear clusters along dimension 1 under all nitrogen conditions (Fig. 2), suggesting that the effect of a plant genotype in O. sativa species on the shoot-associated bacterial community is greater than that of the nitrogen fertilization level. Although the nitrogen concentrations of rice shoots under HN conditions were apparently higher than those under other conditions (SN and LN), no correlation was observed between the N concentration and microbial community (Table S1).
Fig. 2.
Multidimensional scaling plots generated from ARISA profiles with primers ITSF and ITSReub for rice shoot-associated bacterial communities under (A) low, (B) standard, and (C) high nitrogen conditions. japonica and indica indicate O. sativa spp. japonica and indica, respectively (Table 1). W106, W1967, W1965 and W630 belongs to O. rufipogon (Table 1) (n=3).
Diverse environmental factors control the establishment of microbial communities in the phyllosphere, but recently it has been recognized that a plant genotype plays an important role in selecting phyllosphere communities (30). There are considerable variations between indica and japonica cultivars in the concentrations of chemical components, such as nitrate and heavy metals (1, 6). In addition, De Costa et al. (4) found that leaves from different rice genotypes have different culturable bacterial communities and concluded that the difference was significantly correlated with anatomical and physiological differences, such as leaf hair length and density, leaf temperature, stomatal density, and transpiration rate. Among these traits, the variation between indica and japonica cultivars in stomatal density and size and canopy temperature is well recognized, and the genetic factors underlying these traits have been identified (19, 28). Thus, these variations in the chemical characteristics and microstructures between indica and japonica might affect shoot-associated bacterial communities.
For root-associated bacterial community analysis, Sasanishiki, Taichung 65, Gemdjah Beton, IR24, and IR36 were examined (Table S2). MDS plots for bacterial ARISA of root samples indicated that root bacterial communities under SN and HN conditions formed a tight cluster (Fig. 1B), suggesting that the effect of nitrogen fertilization on the bacterial community associated with roots was greater than that of the plant genotypes examined. Demba Diallo et al. (5) showed that nitrogen treatment had a strong effect on the composition and diversity of expressed nifH pools that shifted towards methylotroph-related nitrogenases. In another study, colonization of sugarcane by Acetobacter diazotrophicus has been inhibited by high nitrogen fertilization (8). The bacterial community of bulk soil would help to understand the effect of nitrogen fertilization on the paddy field ecosystem (31).
Different genotypes of Arabidopsis thaliana have been used for structural comparisons of root microbial communities to improve our understanding of plant-microbe interactions (20). To the best of our knowledge, the present study is the first report that shoot-associated microbial communities are largely dependent on rice genotypes (O. sativa ssp. japonica and indica).
Supplementary Material
Acknowledgements
This work was supported by the Ministry of Agriculture, Forestry and Fisheries of Japan (PMI-0002); BRAIN; two Grants-in-Aid for Scientific Research, (A) 23248052 and (C) 22580074, from the Ministry of Education, Science, Sports and Culture of Japan; and the Japanese Government Scheme for the Development of Mitigation and Adaptation Techniques to Global Warming in the Sectors of Agriculture, Forestry and Fisheries. Grateful acknowledgement is extended to H.S. Lee, E. Hanzawa Y. Kazama, and S. Inaba (Graduate School of Life Sciences, Tohoku University) for their kind cooperation in conducting this study.
References
- 1.Abe T, Taguchi-Shiobara F, Kojima Y, Ebitani T, Kuramata M, Yamamoto T, Yano M, Ishikawa S. Detection of a QTL for accumulating Cd in rice that enables efficient Cd phytoextraction from soil. Breed Sci. 2011;61:43–51. [Google Scholar]
- 2.Banba M, Gutjahr C, Miyao A, Hirochika H, Paszkowski U, Kouchi H, Imaizumi-Anraku H. Divergence of evolutionary ways among common sym genes: CASTOR and CCaMK show functional conservation between two symbiosis systems and constitute the root of a common signaling pathway. Plant Cell Physiol. 2008;49:1659–1671. doi: 10.1093/pcp/pcn153. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale M, Brusetti L, Quatrini P, Borin S, Puglia AM, Rizzi A, Zanardini E, Sorlini C, Corselli C, Daffonchio D. Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl Environ Microbiol. 2004;70:6147–6156. doi: 10.1128/AEM.70.10.6147-6156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Costa DM, Rathnayake RMPS, De Costa WAJM, Kumari WMD, Dissanayake DMN. Variation of phyllosphere microflora of different rice varieties in Sri Lanka and its relationship to leaf anatomical and physiological characters. J Agron Crop Sci. 2006;192:209–220. [Google Scholar]
- 5.Demba Diallo M, Reinhold-Hurek B, Hurek T. Evaluation of PCR primers for universal nifH gene targeting and for assessment of transcribed nifH pools in roots of Oryza longistaminata with and without low nitrogen input. FEMS Microbiol Ecol. 2008;65:220–228. doi: 10.1111/j.1574-6941.2008.00545.x. [DOI] [PubMed] [Google Scholar]
- 6.Fan X, Jia L, Li Y, Smith SJ, Miller AJ, Shen Q. Comparing nitrate storage and remobilization in two rice cultivars that differ in their nitrogen use efficiency. J Exp Bot. 2007;58:1729–1740. doi: 10.1093/jxb/erm033. [DOI] [PubMed] [Google Scholar]
- 7.Friesen ML, Porter SS, Stark SC, von Wettberg EJ, Sachs JL, Martinez-Romero E. Microbially mediated plant functional traits. Annu Rev Ecol Evol Syst. 2011;42:23–46. [Google Scholar]
- 8.Fuentes-Ramírez LE, Caballero-Mellado J, Sepúlveda J, Martínez-Romero E. Colonization of sugarcane by Acetobacter diazotrophicus is inhibited by high N-fertilization. FEMS Microbiol Ecol. 1999;29:117–128. [Google Scholar]
- 9.Hardoim PR, van Overbeek LS, van Elsas JD. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008;16:463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Hardoim PR, Andreote FD, Reinhold-Hurek B, Sessitsch A, van Overbeek LS, van Elsas JD. Rice root-associated bacteria: insights into community structures across 10 cultivars. FEMS Microbiol Ecol. 2011;77:154–164. doi: 10.1111/j.1574-6941.2011.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirochika H. Contribution of the Tos17 retrotransposon to rice functional genomics. Curr Opin Plant Biol. 2001;4:118–122. doi: 10.1016/s1369-5266(00)00146-1. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda S, Watanabe KN, Minamisawa K, Ytow N. Evaluation of soil DNA from arable land in Japan using a modified direct-extraction method. Microbes Environ. 2004;19:301–309. [Google Scholar]
- 13.Ikeda S, Rallos LEE, Okubo T, Eda S, Inaba S, Mitsui H, Minamisawa K. Microbial community analysis of field-grown soybeans with different nodulation phenotypes. Appl Environ Microbiol. 2008;74:5704–5709. doi: 10.1128/AEM.00833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda S, Okubo T, Takeda N, et al. The genotype of the calcium/calmodulin-dependent protein kinase gene (CCaMK) determines bacterial community diversity in rice roots under paddy and upland field conditions. Appl Environ Microbiol. 2011;77:4399–4405. doi: 10.1128/AEM.00315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knauth S, Hurek T, Brar D, Reinhold-Hurek B. Influence of different Oryza cultivars on expression of nifH gene pools in roots of rice. Environ Microbiol. 2005;7:1725–1733. doi: 10.1111/j.1462-2920.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Hurwitz B, Yu Y, et al. Construction, alignment and analysis of twelve framework physical maps that represent the ten genome types of the genus Oryza. Genome Biol. 2008;9:R45. doi: 10.1186/gb-2008-9-2-r45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koornneef M, Alonso-Blanco C, Vreugdenhil D. Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol. 2004;55:141–172. doi: 10.1146/annurev.arplant.55.031903.141605. [DOI] [PubMed] [Google Scholar]
- 18.Kouchi H, Imaizumi-Anraku H, Hayashi M, Hakoyama T, Nakagawa T, Umehara Y, Suganuma N, Kawaguchi M. How many peas in a pod? Legume genes responsible for mutualistic symbioses underground. Plant Cell Physiol. 2010;51:1381–1397. doi: 10.1093/pcp/pcq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laza MRC, Kondo M, Ideta O, Barlaan E, Imbe T. Quantitative trait loci for stomatal density and size in lowland rice. Euphytica. 2011;172:149–158. [Google Scholar]
- 20.Lundberg DS, Lebeis SL, Paredes SH, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mano H, Morisaki H. Endophytic bacteria in the rice plant. Microbes Environ. 2008;23:109–117. doi: 10.1264/jsme2.23.109. [DOI] [PubMed] [Google Scholar]
- 22.Mano H, Tanaka F, Nakamura C, Kaga H, Morisaki H. Culturable endophytic bacterial flora of the maturing leaves and roots of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ. 2007;22:175–185. [Google Scholar]
- 23.Nonomura KI, Morishima H, Miyabayashi T, Yamaki S, Eiguchi M, Kubo T, Kurata N. The wild Oryza collection in National BioResource Project (NBRP) of Japan: history, biodiversity and utility. Breed Sci. 2010;60:502–508. [Google Scholar]
- 24.Obara M, Sato T, Sasaki S, Kashiba K, Nagano A, Nakamura I, Ebitani T, Yano M, Yamaya T. Identification and characterization of a QTL on chromosome 2 for cytosolic glutamine synthetase content and panicle number in rice. Theor Appl Genet. 2004;110:1–11. doi: 10.1007/s00122-004-1828-0. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblueth M, Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact. 2006;19:827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- 26.Saito A, Ikeda S, Ezura H, Minamisawa K. Microbial community analysis of the phytosphere using culture-independent methodologies. Microbes Environ. 2007;22:93–105. [Google Scholar]
- 27.Scallan U, Liliensiek A, Clipson N, Connolly J. RIBOSORT: a program for automated data preparation and exploratory analysis of microbial community fingerprints. Mol Ecol Res. 2008;8:95–98. doi: 10.1111/j.1471-8286.2007.01901.x. [DOI] [PubMed] [Google Scholar]
- 28.Takai T, Yano M, Yamamoto T. Canopy temperature on clear and cloudy days can be used to estimate varietal differences in stomatal conductance in rice. Field Crops Res. 2010;115:165–170. [Google Scholar]
- 29.Tan Z, Hurek T, Reinhold-Hurek B. Effect of N-fertilization, plant genotype and environmental conditions on nifH gene pools in roots of rice. Environ Microbiol. 2003;5:1009–1015. doi: 10.1046/j.1462-2920.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 30.Whipps JM, Hand P, Pink D, Bending GD. Phyllosphere microbiology with special reference to diversity and plant genotype. J Appl Microbiol. 2008;105:1744–1755. doi: 10.1111/j.1365-2672.2008.03906.x. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Ma K, Li Q, Ke X, Lu Y. Composition of archaeal community in a paddy field as affected by rice cultivar and N fertilizer. Microb Ecol. 2009;58:819–826. doi: 10.1007/s00248-009-9554-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.